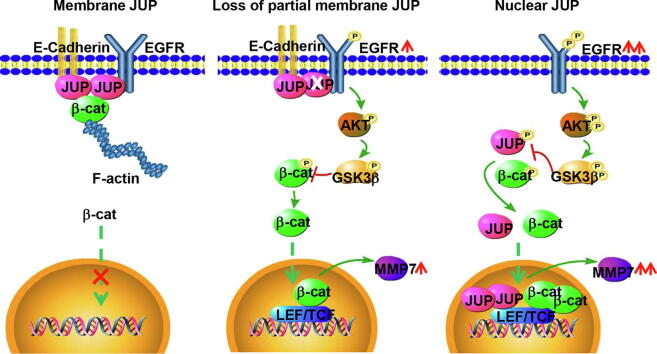
Graphical abstract
Keywords: JUP, EGFR-AKT-GSK3β signaling, β-catenin stability, Gastric cancer
Abstract
JUP, a homologue of β-catenin, is a cell-cell junction protein involved in adhesion junction and desmosome composition. JUP may have a controversial role in different malignancies dependence of its competence with or collaboration with β-catenin as a transcription factor. In this study, we reveal that the function of JUP is related to its cellular location in GC development process from epithelium-like, low malignant GC to advanced EMT-phenotypic GC. Gradual loss of membrane and/or cytoplasm JUP is closely correlated with GC malignancy and poor prognostics. Knockdown of JUP in epithelium-like GC cells causes EMT and promotes GC cell migration and invasion. Ectopic expression of wild JUP in malignant GC cells leads to an attenuated malignant phenotype such as reduced cell invasive potential. In mechanism, loss of membrane and/or cytoplasm JUP abolishes the restrain of JUP to EGFR at cell membrane and results in increased p-AKT levels and AKT/GSK3β/β-catenin signaling activity. In addition, nuclear JUP interacts with nuclear β-catenin and TCF4 and plays a synergistic role with β-catenin in promoting TCF4 transcription and its downstream target MMP7 expression to fuel GC cell invasion.
Introduction
Gastric cancer (GC) is one of the most common malignant tumor and has a high carcinogenic mortality rate with a 5-year overall survival of less than 25%, suffering an approximately 70% of disease recurrence or metastasis [1], [2]. The occurrence of gastric cancer is a continuous process of progress from superficial gastritis to intestinal metaplasia, carcinoma in situ, eventually to invasive adenocarcinoma [3]. There are many signal pathways, including Wnt/β-catenin signaling, involved in the progression and development of gastric cancer. Invasion and metastasis are the cancer cells escaped from the basement membrane to distant tissues and continue to grow metastases [4]. However, the mechanisms underlying metastasis of GC are not well defined.
Escaping the cell adhesion system is an important mechanism for tumor cells to obtain the abilities of migration and invasion [5]. JUP, a member of catenin families, contains the conserved armadillo repeat protein. It acts as a desmosome connection and an adhesive component whose classical function is to attach cadherin to the cytoskeleton in maintenance of cell structure stability [6]. JUP can also act as a cross-talk between cell-cell junction and signal transduction as same as its homologue β-catenin. They can share the same complexes with APC and AXIN, which can be both degraded by the proteasome-mediated degradation [7]. Besides, JUP also has a TCF/LEF-dependent transcriptional activity, same as β -catenin in β-catenin-deficient cells [8]. Conversely, several lines of evidence suggest that JUP and β-catenin have different activities in regulating tumorigenesis. JUP was shown to be a novel regulator of HAI-1 that regulates HGF/c-MET-signal in a P53-dependent manner which induces anti-migratory effects [9]. Furthermore, JUP has a tumor suppressive activity through inhibition of β -catenin/TCF-dependent transcription in non-small-cell lung cancer whereas β-catenin has an oncogenic signaling activity [10]. Moreover, previous studies revealed a controversial role of JUP in tumors. Ectopic expression of JUP can sustain the expression of markers of naïve pluripotency in embryonic stem cell [11]. An aberrant JUP expression and higher level of nuclear JUP are uncovered to be associated with poor clinical outcome in acute myeloid leukemia [12]. However, other study displays that overexpression of JUP can decrease the expression of oncogenic STAB1 which inhibits the migration and invasion of the human tongue squamous cell carcinoma [13]. Here, we focused on the role of JUP in tumor progress and metastasis of GC.
Materials and methods
Cell culture, RNA interference, plasmids and engineered cells
MGC-803, NUGC-3 and NCL-87 cell lines were kindly donated by Prof. Yang Ke of the Beijing Institute of Cancer Research (Beijing, China). NCI-87, NUGC-3 cells were cultured in RPMI-1640 medium with 10% FBS (Gibco, Australia); MGC-803 cells were cultured in DMEM with 10% FBS. The shRNA against JUP and its control shRNA were obtained from GenePharma (Shanghai, China). The sequences of these shRNA are listed in Supplementary Table 1. The construct encoding JUP were purchased from Addgene (Plasmid #32228), and the corresponding 11-13 armadillo repeats mutant JUP (Mut JUP ARM11-13) and pcDNA-β-catenin constructs were generated by GenePharma (Shanghai, China). The TOP-Flash reporter and control reporter plasmids were the gifts from Dr. Richard Pestell Lab. The engineered NCI-87, NUGC-3, and MGC-803 with a stable expression of shJUP or control shRNA; the engineered MGC-803, NUGC-3 with ectopic JUP or control construct were established using the lentivirus infection as described elsewhere. The transduced cells were selected with puromysin.
RNA extraction and quantitative real time PCR (qRT-PCR)
RNA extraction and qRT-PCR were done as previously described [14]. Briefly, Trizol (Cat# 15596018; Invirogen) was used to isolate total RNA according to the manufacture’s instruction. qRT-PCR was performed using the SYBR Premix Ex Taq II (Cat# RR820A; TaKaRa, Dalian, China) after the purified RNAs being reverse transcription (Cat# RR037B; TaKaRa, Dalian, China) according to the manufacture’s protocol. The β-actin was used as a normalized standard. The used primers were listed in Supplementary Table 2. All experiments were done at least three times. Relative gene expression was measured as 2Ct (internal control) – Ct (gene).
Wound-healing and cell invasion assay
Wound-healing was done following the previously described method [15]. The mobility of the cells was determined by measuring the wounded area using Image J Software as previously described at the designed time point [16].
Tumor cell invasion assay was performed using the transwell assay as described previously [15]. Cells (5 × 104) in 200 μL of FBS-free media were seeded on the upper Boyden chambers (8 μm, Millipor, MA, USA) coated with ECM (1:7.5) (Sigma, St. Louis, MO). The invaded cells were captured and counted under the microscopy (Nikon TE-2000U, Tokyo, Japan).
Cell proliferation
The proliferation rate of tumor cells was determined by CCK8 (Cell counting kit-8) experiment. Cells were seeded in 96-well-plates at a density of 2 × 103 cells per well. Next day, 10 μL of CCK8 reagents (Cat# C0038; Beyotime, China) were added into each well in dark and incubated for 2 h at 37 °C. Then the absorbance was read by the ultraviolet spectrophotometric reader at the wavelength of 450 nm. The experiments were repeated three times.
Tissue specimens
Tissue microarrays (TMAs) derived from progressive gastric carcinoma tissue were purchased from Shanghai Outdo Biotech Co. Ltd., which were approved by the Medical Ethics Committee for the Use of Human or Animal Subjects of Taizhou Hospital, Zhejiang Province. And the investigation was also approved by the ethics committee of Chongqing Medical University. These TMAs include 34 normal adjacent tissues (NATs), and 182 gastric adenocarcinomas. Of these cases, 164 patients with complete clinical information and 70 cases with survival data were used to statistically analysis. Gastric tumor tissues with different degrees of differentiation used in this study were obtained from patients with gastric cancer without previous radiotherapy or chemotherapy at the First Affiliated Hospital of Chongqing Medical University. The investigation was approved by the ethics committee of Chongqing Medical University.
Immunofluorescence (IF) and immunohistochemistry (IHC) analysis
Immunofluorescence was performed as described previously [16]. Cells were grown on sterilized glass coverslips for 24 h. After fixed in 4% paraformaldehyde, penetrated using the 0.1% Triton and treated with 10% goat serum, cells were incubated with primary antibodies at 37 °C overnight. The used antibodies are as follow: anti-JUP antibody (Cat# ab15153, Abcam, 1:150), anti-β-catenin antibody (Cat# ab16051, Abcam, 1:200); normal rabbit IgG was used as a negative control. The coverslips were then incubated with a FITC-labeled secondary antibody (Cat# ZF-0311, ZSBiO, 1:1000) and seated with 70% glycerin containing DAPI. Immunofluorescent images were captured using a Nikon Eclipse 80i microscope (Tokyo, Japan).
For IHC, briefly, tumor tissues (4 μm) were gradually dewaxed in xylene and rehydrated in graded alcohols and distilled water. After cooking for 5 min in a specific cooker with 0.1 mol/L sodium citrate (pH 6.0) at 70 kPa pressure for antigen retrieval, and blocked with 3% hydrogen peroxide to neutralize the endogenous peroxidase activity, sections were then incubated with primary antibody JUP (Cat# ab15153; Abcam, 1:200) or antibody β-catenin (Cat# ab16051; Abcam, 1:200) at 4 °C overnight. Then tissues were sequentially incubated with HRP-conjugated goat anti-rabbit IgG (Cat# ZDR-5306; ZSBiO, 1:1000), Diaminobenzidine (DAB) (Cat# ZLI-9017; ZSBiO) and hematoxylin (Baso, China). After mounted with neutral resins, pictures were taken with Nikon Eclipse 80i microscope (Tokyo, Japan).
Co-Immunoprecipitation (Co-IP)
Co-IP was performed according to the manufacturer protocol using recombinant Protein A/G immune precipitation magnetic beads (Cat# B23202; Biotool, China). Cell lysates were incubated with 30 μL of recombinant Protein A/G beads and 4 μL anti-EGFR antibody (Cat# ab52894; Abcam, 1:50) overnight at 4 °C. The complexity of beads-antibody-protein was collected and washed with PBST, and western blotting was used to detect the co-immunoprecipitated proteins with anti-JUP antibody (Cat# ab15153; Abcam, 1:1000).
Luciferase reporter assay
For promoter reporter assay, cells were seeded at a density of 1x105 cells in 24-well plates. TOP-Flash reporter or control reporter plasmid, β-catenin, JUP and mutant JUP were cotransfected alone or combined into 293 T cells; or TOP-Flash reporter plasmid, were cotransfected with Renilla luciferase pRL-TK (internal control for transfection efficiency) into JUP-knocked down NUGC-3, MGC-803 cells and their control cells. Luciferase activities were performed by using a Dual-Luciferase Reporter System (Promega, USA) after 36–48 h.
Western blot analysis
Western blot was performed as described previously [17]. Briefly, total cell proteins were obtained using RIPA lysis buffer (P0013B, Beyotime, China), quantified with the BCA protein assay kit (Cat# P0012; Beyotime). 50 μg of cell lysates in RIPA buffer were separated by electrophoresis with 8% to 10% SDS-PAGE gel and subjected to Western blotting. The specific primary antibodies used in this study included rabbit anti-JUP (Cat# ab15153; Abcam, 1:1000), rabbit anti-AKT (Cat# ab8805; Abcam, 1:1000), rabbit anti-p-AKT (Cat# ab38449; Abcam, 1:1000), rabbit anti–GAPDH (Cat# 5174; Cell Signaling Technology, 1:1000); mouse anti-E-cadherin (Cat# ab1416; Abcam, 1:1000); rabbit anti-Fibronectin (Cat# ab2413; Abcam, 1:1000), rabbit anti-β-catenin (Cat# ab16051; Abcam, 1:1000), rabbit anti-p-β-catenin (Cat# ab27798; Abcam, 1:1000), rabbit anti-EGFR (Cat# ab52894; Abcam, 1:1000), rabbit anti-p-EGFR (Cat# ab40815; Abcam, 1:1000), rabbit anti-p-GSK3β (Cat# ab75745; Abcam, 1:1000); rabbit anti-GSK3β (Cat# ab32391; Abcam, 1:1000); rabbit anti-MMP7 (Cat# ab5706; Abcam, 1:1000); rabbit anti-Vimentin (Cat# ab92547; Abcam, 1:1000), rabbit anti-β-tublin (Cat# ab179513; Abcam, 1:1000), rabbit anti-Histone H3 (Cat# ab1791; Abcam, 1:1000); rabbit anti-TCF4 (Cat# ab217668; Abcam, 1:1000); rabbit anti-PCNA (Cat# BS1289; Bioworld, 1:1000). The appropriate horseradish peroxidase (HRP)-conjugated anti-mouse or rabbit IgG (ZSGBBIO, China) was used as secondary antibodies. The protein bands were visualized using the enhanced chemiluminescence system (Amersham Pharmacia Biotech, Tokyo, Japan).
Statistical analysis
Statistical analysis was performed using the SPSS 17.0. The independent Student’s t-test was used to compare the continuous variables between two groups. The association analysis of JUP with clinical features was investigated using chi-square test. A p-value less than 0.05 was considered to be statistically significant.
Results
Distribution of JUP in gastric tumor tissues is closely related to malignant pathological features and worse prognostics of gastric cancer
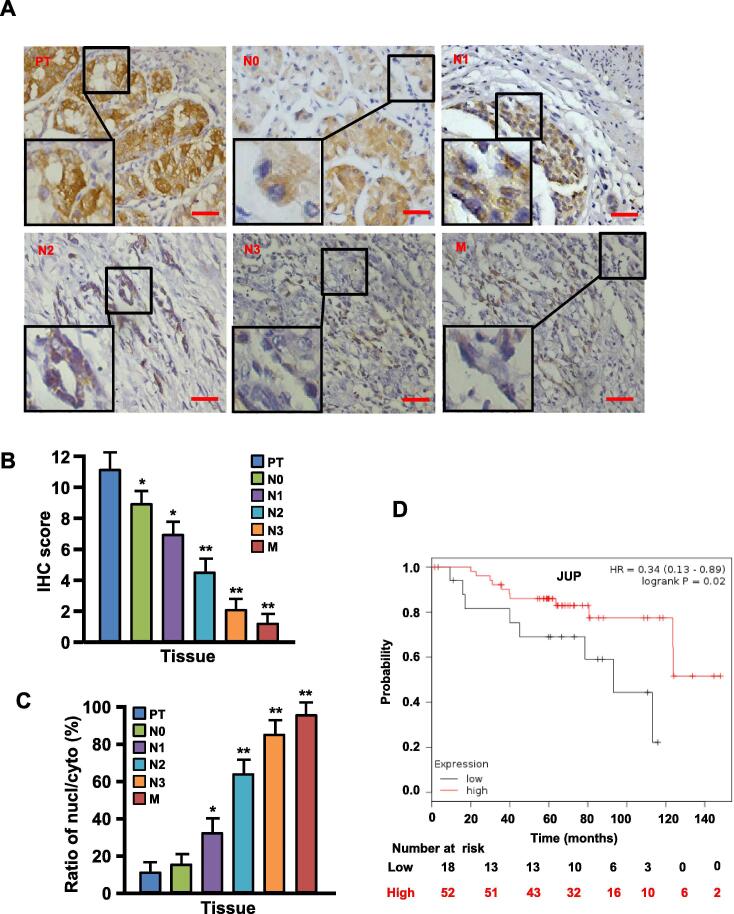
To understand the relationship among JUP expression, distribution and malignancy of gastric tumors, we assessed JUP protein levels in gastric tumor tissues by IHC staining. We found that JUP expression was gradually decreased accompanied with the increased malignancy of GC (Fig. 1 and Table 1). Compared with carcinoma in situ (PTs), the gradually decreased membrane/cytoplasmic JUP protein and enhanced nuclear JUP protein were detected from lymph node metastasis tissues (N0–N3) to distant metastasis tissues (M) (Fig. 1A–C). Furthermore, it was found that GC patients with lower level of JUP had worse 10-year survival rate than those with higher level of JUP evaluated by Kaplan-Meier Survival curves (Fig. 1D).
Fig. 1.
JUP expression and its location in gastric cancer tissues. (A) IHC staining to detect expression and location of JUP in different stages of gastric cancer tissues, such as carcinoma in situ (PT), lymph node metastasis tissues (N0-N3), distant metastasis tissues (M) (Scale bars, 100 µm). (B) Quantification of JUP expression was determined by IHC scores for carcinoma in situ (PT), lymph node metastasis tissues (N0-N3), distant metastasis tissues (M). (C) The ratio of nuclear JUP/cytoplasmic JUP in PT, N0-N3 and M. (D) The Kaplan-Meier survival analysis reveals lower expression of JUP to be related with poor prognosis.
Table 1.
Correlation between clinicopathological features and cytomembrane/cytoplasmic/nuclear JUP expressions.
| Characteristic | No. of patients | Cytomembrane JUP |
Cytoplasmic JUP |
Nuclear JUP |
|||
|---|---|---|---|---|---|---|---|
| Total cases (N=164) | X2 Negative | P value Positive | X2 Negative | P value Positive | X2 Negative | P value Positive | |
| Gender | 0.93 | 0.336 | 0.003 | 0.867 | 0.13 | 0.719 | |
| Male | 93 | 45 | 48 | 42 | 51 | 55 | 38 |
| Female | 71 | 29 | 42 | 33 | 38 | 40 | 31 |
| Age (mean ± S.D.) | 52.0 ± 10.3 | 56.1 ± 11.2 | 51.5 ± 10.7 | 49.5 ± 11.5 | 50.3 ± 9.7 | 53.3 ± 10.9 | 52.2 ± 10.7 |
| Tumor size(cm) | 47.79 | <0.001 | 6.03 | 0.149 | 9.45 | 0.009 | |
| T1 | 45 | 5 | 40 | 12 | 33 | 23 | 22 |
| T2 | 87 | 41 | 46 | 38 | 49 | 35 | 52 |
| T3 | 32 | 29 | 3 | 17 | 15 | 23 | 9 |
| Node status | 1.64 | 0.650 | 4.33 | 0.228 | 6.02 | 0.111 | |
| N0 | 81 | 40 | 41 | 37 | 44 | 52 | 29 |
| N1 | 27 | 15 | 12 | 10 | 17 | 15 | 12 |
| N2 | 32 | 20 | 12 | 18 | 14 | 16 | 16 |
| N3 | 24 | 13 | 11 | 15 | 9 | 9 | 15 |
| Distant metastasis | 10.59 | 0.001 | 0.52 | 0.471 | 40.24 | <0.001 | |
| M0 | 123 | 48 | 75 | 61 | 62 | 97 | 26 |
| M1 | 41 | 28 | 13 | 23 | 18 | 10 | 31 |
| Histologic grade | 19.95 | <0.001 | 3.37 | 0.338 | 11.38 | 0.010 | |
| 1 | 35 | 11 | 24 | 18 | 17 | 27 | 28 |
| 2 | 58 | 23 | 35 | 21 | 37 | 34 | 24 |
| 3 | 52 | 33 | 19 | 26 | 26 | 18 | 34 |
| 4 | 19 | 16 | 3 | 7 | 12 | 4 | 15 |
| Clinical stage | 34.06 | <0.001 | 8.90 | 0.031 | 48.50 | <0.001 | |
| I | 57 | 15 | 42 | 19 | 38 | 48 | 9 |
| II | 36 | 22 | 14 | 11 | 25 | 13 | 23 |
| III | 44 | 34 | 10 | 23 | 21 | 14 | 30 |
| IV | 27 | 21 | 6 | 16 | 11 | 4 | 23 |
P values <0.05 in bold were considered statistically significant.
After analyzing JUP levels and pathological indicators of GC, we also found that membrane JUP levels were inversely associated with tumor size (χ2 = 47.79, P < 0.001), distant metastasis (χ2 = 10.59, P < 0.001), histologic grade (χ2 = 19.95, P < 0.01) and clinical stage (χ2 = 34.06, P < 0.01) (Table 1). Cytoplasmic JUP was negative correlation with tumor size (χ2 = 6.03, P = 0.049) and clinical stage (χ2 = 8.90, P = 0.031). Loss of membrane JUP or cytoplasmic JUP, which accompanied with increased nuclear JUP, was significantly related with tumor size (χ2 = 9.45, P = 0.009), distant metastasis (χ2 = 40.24, P < 0.001), histologic grade (χ2 = 11.38, P = 0.010) and clinical stage (χ2 = 48.50, P < 0.001). These data suggest that lower level of membrane/cytoplasmic JUP or higher level of nuclear JUP is closely related to clinical malignant pathological features of GC.
The differentially distributed JUP has a heterogeneous function in different differentiated-GC cells and tumor tissues
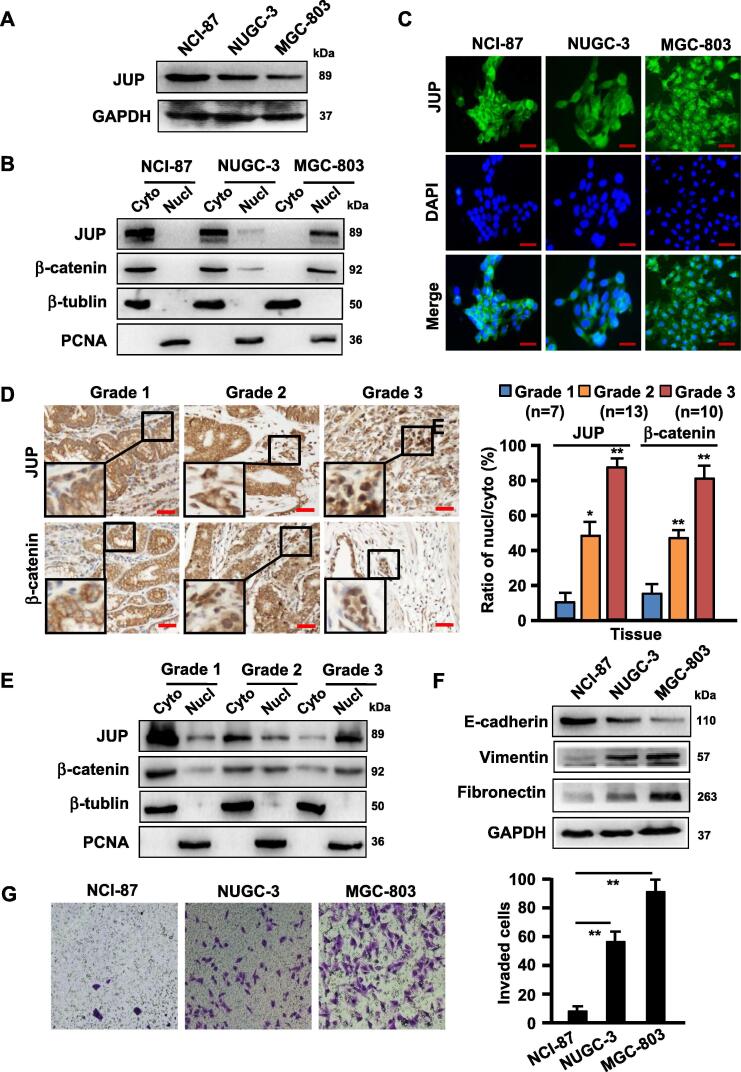
Based on the aforementioned pathological findings in GC tissues, we detected JUP expression and distribution in three representative gastric cancer cells in different differentiated stage, NCI-87 (well differentiated GC cell), NUGC-3 (moderately differentiated GC cell) and MGC-803 (poorly differentiated cell). We found that higher level of JUP protein in NCI-87, but lower in NUGC-3 and MGC-803 (Fig. 2A). Interestingly, a gradually reduced cytoplasmic JUP, while increased nuclear JUP, was detected from NCI-87 to NUGC-3 and MGC-803 dependent on poorly differentiated GC status (Fig. 2B). Similar results were proved by immunofluorescence staining in GC cells, in which a strong membrane JUP was in NCI-87, cytoplasmic JUP in NUGC-3, weakly expressed cytoplasmic JUP with strong nuclear JUP in MGC-803, which is the most malignant cell among these GCs (Fig. 2C). To confirm these findings acquired from in vitro cells, the JUP expression and distribution in cohort of clinical GC tissues were assessed using IHC (Fig. 2D) and western blotting (Fig. 2E). As expected, JUP, like its homologue of β-catenin, showed a gradually decreased membrane/cytoplasmic distribution and increased nuclear distribution from G1 (well differentiated), to G2 (moderately differentiated) and G3 (poorly differentiated), confirming that the decreased membrane/cytoplasmic JUP and enhanced nuclear JUP are closely related with GC development and tumor differentiation status. JUP is a component of cell-cell adhesion and is related to EMT (epithelial-mesenchymal transition) [18], thus EMT bio-markers were analyzed in GC cells. Indeed, the epithelial marker E-cadherin was gradually reduced, however, the mesenchymal marker Vimentin and Fibronectin were gradually acquired in these cells (Fig. 2F). Accordingly, cell invasive potentials of these GC cells were increased in company with loss of membrane and/or cytoplasm JUP and acquisition of nuclear JUP from NCI-87, NUGC-3 to MGC-803 (Fig. 2G). These data indicate that JUP distribution affects EMT status and invasive potentials of GC cells.
Fig. 2.
Expression and localization of JUP in three differently differentiated gastric cancer cells and tissues. (A) Western blot was done to show protein levels of JUP in NCI-87 (well differentiated GC cells), NUGC-3 (moderately differentiated GC cells), MGC-803 (poorly differentiated GC cells) cells. (B) Subcellular expression levels of JUP and β -catenin in different differentiated gastric cancer cells were analyzed by western blotting. PCNA is the loading control for nuclear proteins. β-tubulin, a loading control of the cytoplasmic proteins. (C) Immunofluorescence staining was performed to detect the expression and localization of JUP in GC cells (Scale bars, 50 µm). (D) IHC staining to detect expression and location of JUP and β -catenin in gastric cancer tissues with different degrees of differentiation (G1, well differentiated, n = 7; G2, moderately differentiated, n = 13; G3, poorly differentiated, n = 10). Representative images of IHC staining are presented in left panel (Scale bars, 100 µm). The ratio of nuclear/cytoplasmic JUP and β -catenin in right panel (*P < 0.05, **P < 0.01). (E) Western blot to show subcellular levels of JUP and β -catenin in gastric cancer tissues with different degrees of differentiation (G1, G2, and G3). PCNA is the loading control for nuclear proteins. β-tubulin, a loading control of the cytoplasmic proteins. (F) The protein levels of E-cadherin, Vimentin and Fibronectin were detected by Western blotting. GAPDH is the loading control. (G) Cell migration was determined by Transwell assay for NCI-87, NUGC-3 and MGC-803 cells. The experiment was repeated three times (**P < 0.01, vs NCI-87 cells).
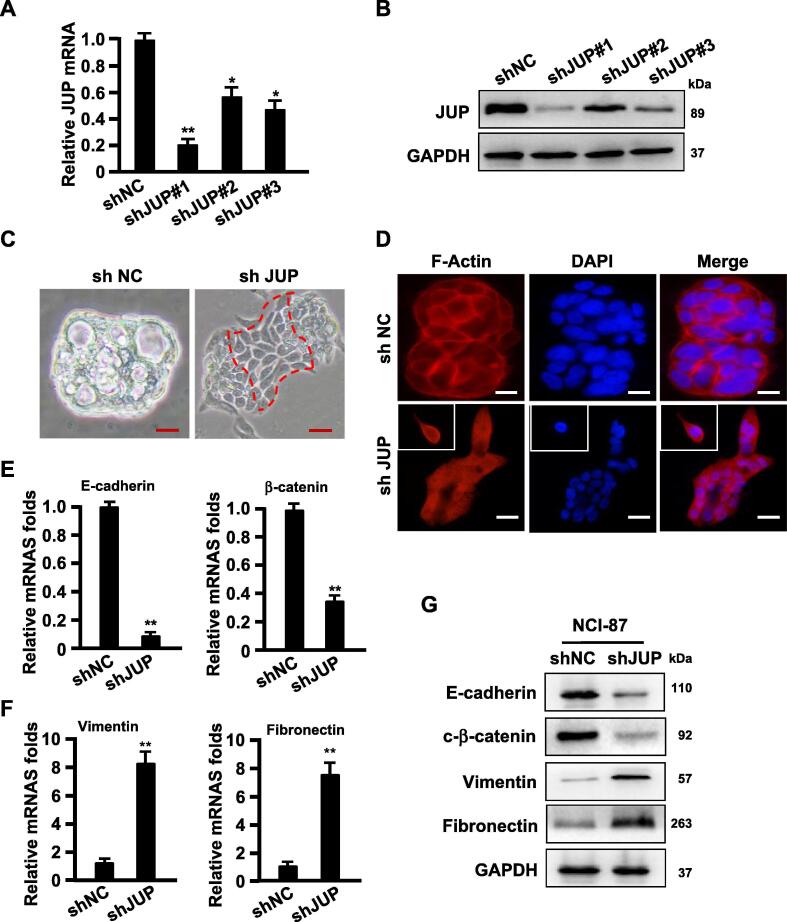
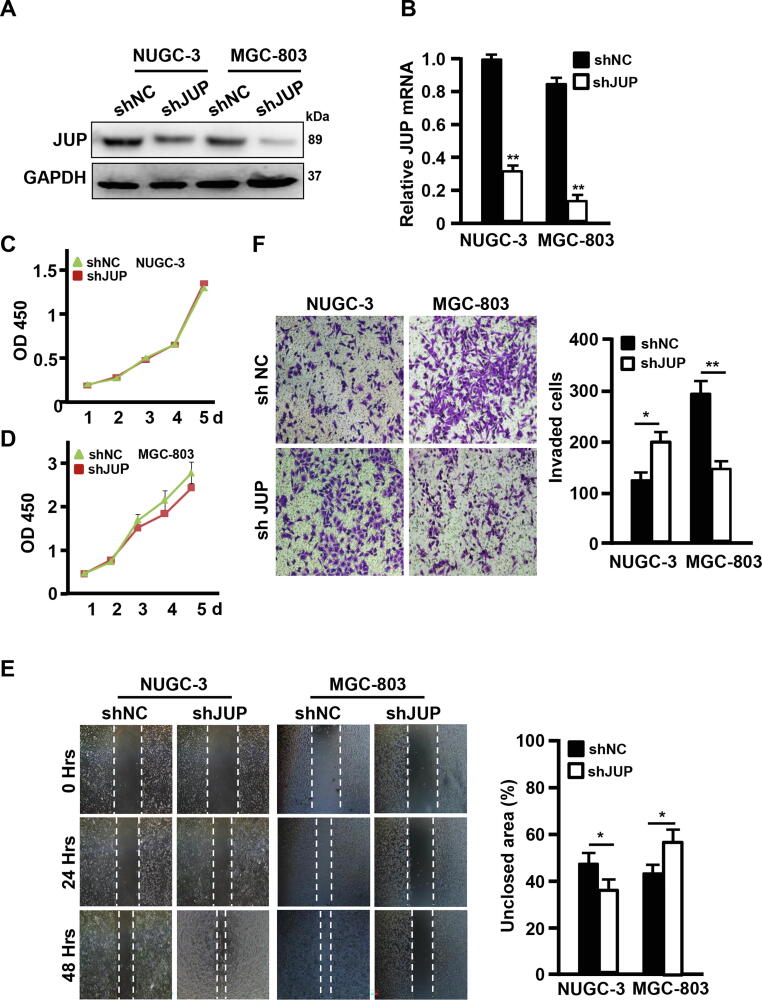
Efficient knockdown of JUP by lentivirus-mediated shRNA (Fig. 3A–B) led to a spindle-like morphologic change in comparison with control NCI-87 with cobblestone-like (epithelium-like) morphology (Fig. 3C). F-actin, the major component of the cytoskeleton microfilament [19], was displayed to be tentacle-like and more polarity detected by phalloidin staining in JUP-knocked down NCI-87 cells (Fig. 3D), which accompanied with notably decreased epithelial marker E-cadherin and β-catenin and enhanced mesenchymal marker Vimentin and Fibronectin in the JUP-knocked down NCI-87 cells (Fig. 3E–G). Although knockdown of JUP by shRNA in NUGC-3 and MGC-803 (Fig. 4A–B) almost had no effects on cell proliferation (Fig. 4C–D), but caused a heterogeneous effects on cell mobility and invasion. Silence of JUP increased cell mobility and invasion potentials in NUGC-3, however, obviously decreased cell mobility and invasion abilities in MGC-803 (Fig. 4E–F). Together, these data indicate a different JUP function in GC, in which membrane JUP endows NCI-87 cells an epithelial-like phenotype, cytoplasmic JUP in NUGC-3 blunts malignancy in some degree, and nuclear JUP in advanced MGC-803 cells acts as a promoter to tumor malignancy.
Fig. 3.
Knockdown of JUP in NCI-87 induces EMT phenotypes. (A, B) Interference efficiencies of JUP gene by shRNA in NCI-87 was measured by qRT-PCR (A) and Western blot (B) (*P < 0.05, **P < 0.01 vs control shRNA). GAPDH acts as a loading control. (C) A picture to show the morphological changes of NCI-87 treated with shJUP in comparison with cells with control shRNA (Scale bars, 100 µm). (D) The expression and localization of F-actin was stained with phalloidine (Scale bars, 50 µm). (E–G) The indicated mRNA and protein levels of EMT-related markers were detected by qRT-PCR (E, F) and Western blot (G). GAPDH worked as loading control (**P < 0.01, vs control).
Fig. 4.
Knockdown of JUP promotes Gastric cancer cell migration and invasion. (A, B) Knockdown efficiencies of JUP by shJUP in NUGC-3 and MGC-803 were detected by qRT-PCR (A) and Western blot (B) (**P < 0.01 vs control shNC). GAPDH is a loading control. (C, D) Growth curves of NUGC-3 (C) and MGC-803 (D) with shJUP or shNC were performed with CCK-8 assay for 5 days. (E, F) The cell mobility and invasion potentials were tested by Wound healing assay (E) and Transwell assay (F) (*P < 0.05, **P < 0.01 vs control shRNA).
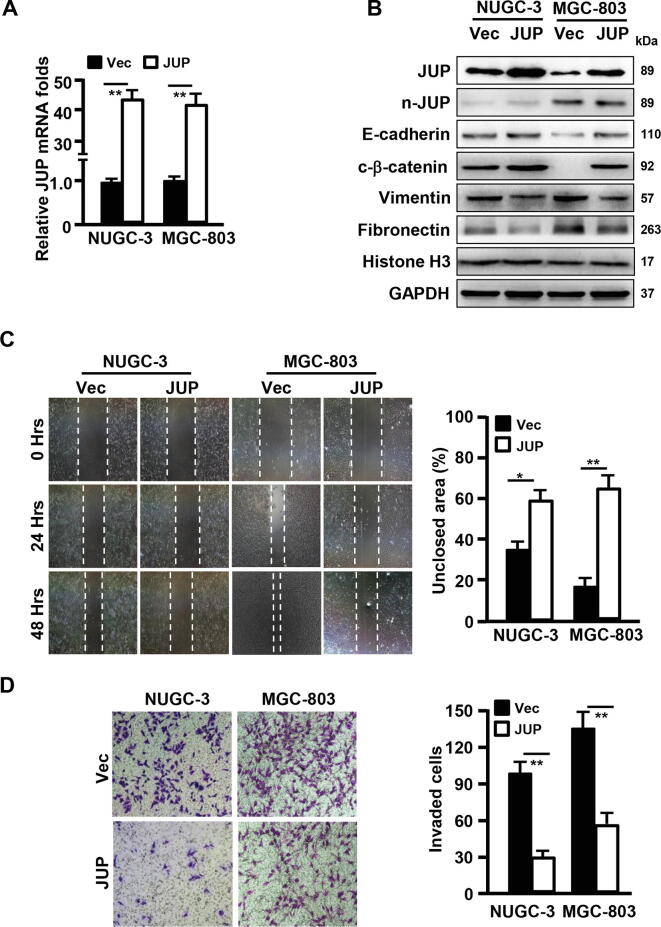
Next, ectopic JUP was stably transfected into NUGC-3 and MGC-803 using lentivirus-mediated encoding constructer (Fig. 5A–B). We found that ectopic JUP had no significant influence on nuclear JUP accumulation in comparison with the vector (Fig. 5B), which mainly located at cell membrane or in cytoplasm of NUGC-3 and MGC-803 cells checked by IF staining (data not shown). Correspondingly, the cytoplasmic β-catenin and epithelial marker E-cadherin were increased, while the mesenchymal marker Vimentin and Fibronectin were down-regulated in ectopic JUP-expressing NUGC-3 and MGC-803 cells (Fig. 5B). Furthermore, ectopic JUP in NUGC-3 and MGC-803 led to a compromised cell mobility and invasion compared with their control cells (Fig. 5C–D). These data suggest that JUP location at membrane and/or in cytoplasm halts malignancy of gastric carcinoma.
Fig. 5.
Overexpression of JUP in NUGC-3 and MGC-803 inhibits gastric cancer cell migration and invasion during the process of MET. (A) qRT-PCR (A) was done to show the overexpression efficiency of JUP in NUGC-3 and MGC-803 (**P < 0.01 vs vector). (B) Western blotting was used to detect the indicated protein levels of EMT-related markers. GAPDH acts as a loading control. (C, D) Wound healing assay (C) and Transwell assay (D) were performed to analyze the cell migration and invasion capacities of gastric cancer cells transfected with JUP or Vector (*P < 0.05, **P < 0.01 vs vector).
Differentially distributed JUP affects β-catenin localization and activity to regulate MMP7 expression
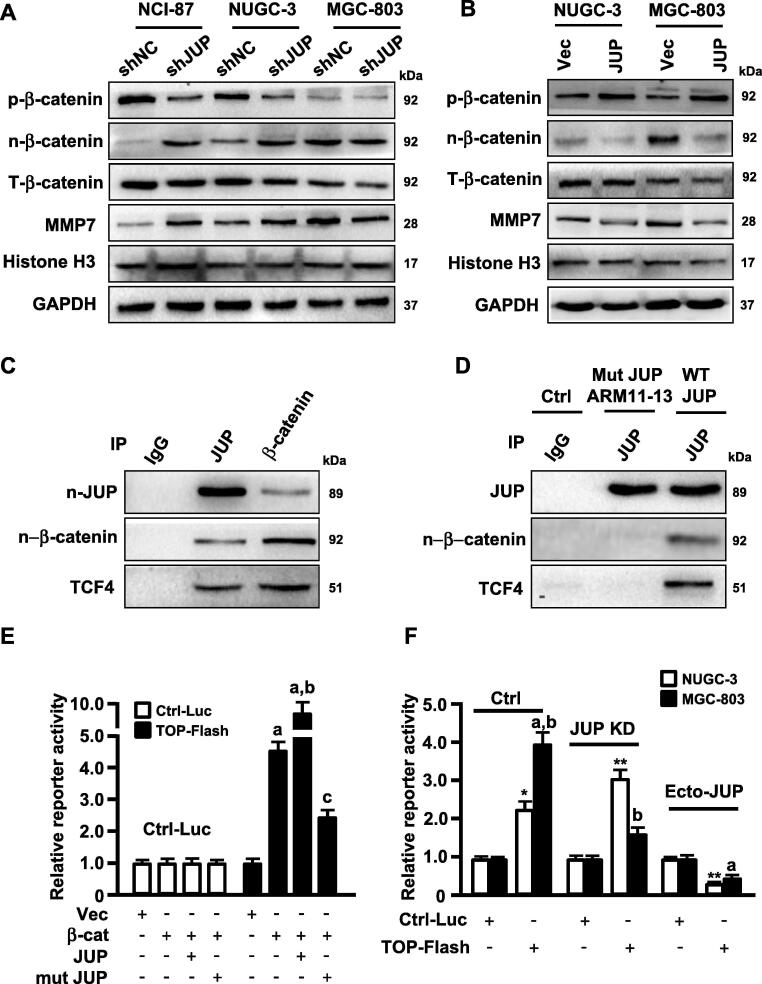
JUP is known as a homolog of β-catenin and collaborates with β -catenin to maintain cell-cell tight-junction [20]. Given previous observations, changed JUP may affect β-catenin stability and its nuclear translocation in AML cells [12]. We asked whether JUP directly affects the activity and function of β-catenin in GC cells, thus plays a role on GC malignancy. Actually, interfering JUP expression by shRNA in NCI-87 and NUGC-3 cells obviously reduced phosphorylated β-catenin (p-β-catenin) and promoted nuclear translocation of β-catenin (n-β-catenin) to enhance MMP7 levels (Fig. 6A, left and middle panels), a known downstream target of β-catenin signaling in charge of tumor cell invasion [21]. Interestingly, loss of JUP in MGC-803 resulted in non-significant change of phosphorylated β-catenin in cytoplasm and nuclear β-catenin levels, but decreased MMP-7 proteins (Fig. 6A). Over-expression of ectopic JUP in NUGC-3 and MGC-803 cells increased phosphorylated β-catenin to result in notable reduce of nuclear β -catenin and MMP7 (Fig. 6B), indicating that location of JUP might play a role to β-catenin signaling or its activity in different differentiated GC cells.
Fig. 6.
JUP mediates MMP7 expression via the interaction of JUP/β -catenin/TCF4. (A, B) Levels of phosphorylated β-catenin (p-β-catenin), nuclear β-catenin (n-β-catenin), total β-catenin (T-β-catenin) and MMP7 were determined by Western blot in the JUP knocked-down (A) and overexpressing (B) gastric cancer cells and its control cells. Histone H3 and GAPDH are the loading control. (C, D) Whole-cell lysates from WT MGC-803 (C) and JUP ARM11-13 mutant MGC-803 cell (D) were immunoprecipitated with anti-JUP and anti-β-catenin antibodies. Western blot showed the interaction of JUP, β-catenin and TCF4. IgG was used as a control antibody. (E) 293 T cells were co-transfected with control luciferase reporter or TOP-Flash reporter and indicated constructs, relative reporter activity were measured for TCF4 (a, P < 0.01 vs vector; b, P < 0.01, β -catenin/JUP vs β -catenin alone; c, P < 0.01, β -catenin/mutant JUP vs β -catenin/JUP). (F) NUGC-3 and MGC-803 and their engineered cells (JUP-knocked down cells and ectopic JUP-overexpressed cells) were transfected with control luciferase reporter or TOP-Flash reporter. Endogenous TCF4 transcript activity was detected using luciferase assay (*P < 0.05, **P < 0.01 vs Control reporter; a, P < 0.01, TOP-Flash reporter vs control reporter; b, P < 0.01, MGC-803 vs NUGC-3).
Next, we tried to understand why nuclear JUP, the homolog of β -catenin, can positively activate β-catenin signaling. We found that nuclear JUP could interact with nuclear β-catenin and TCF4, a key transcription factor of Wnt/β-catenin signaling, in MGC-803 cells (Fig. 6C). Both JUP and β-catenin have a conserved 13 amino acid repeats, the armadillo (ARM)-type domain, which is predicted to interact with another protein or nucleic acid, and the armadillo of 11 to 13 is essential for the protein interaction [20]. Thus, we guessed that 11-13 armadillo may mediate the collaboration of nuclear JUP and β-catenin in synergistic regulating TCF4 transcript activities. As expected, mutation of 11-13 armadillo repeats (Mut JUP ARM11-13) dissociated JUP with TCF-4 (Fig. 6D). Furthermore, we proved JUP and β-catenin could synergistically promote TCF4 transcript activity compared with β-catenin or JUP alone, however, mutant JUP (Mut JUP ARM11-13) cancelled the synergistic function of JUP and β-catenin in regulating TCF4 transcription activity (Fig. 6E) checked by luciferase reporter assay. In parent MGC-803 cells with high level of nuclear β-catenin or JUP, robust transcript activity was detected in comparison with NUGC-3 (Fig. 6F). Silence of endogenous JUP in NUGC-3 promoted transcript activity of TCF4, and notable decreased-TCF4 luciferase activity in MGC-803; ectopic JUP could hamper TCF4 luciferase activities in NUGC-3 and MGC-803 (Fig. 6F), suggesting an impedimental role of cytoplasmic JUP to Wnt/β-catenin signaling in NUGC-3 and synergistic function of nuclear JUP to Wnt/β-catenin signaling in MGC-803. Taken together, these data demonstrate that different distribution of JUP in GC cells endows JUP a different role to Wnt/β-catenin signaling activity.
JUP causes β-catenin stability and nuclear translocation via AKT/GSK3β signaling axis
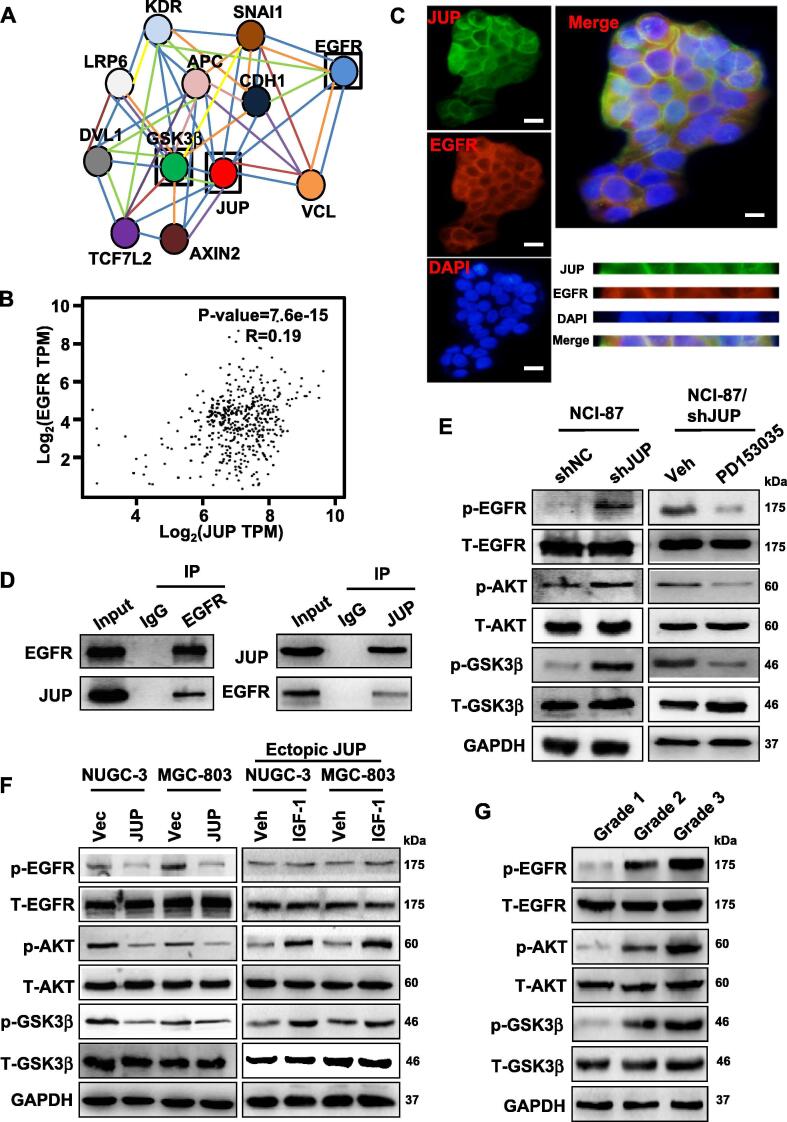
To further gain insights into the mechanism of JUP on β-catenin stability thus fueling GC cell migration and invasion, we investigated the potential interacting proteins of JUP using bioinformatics. Based on the bio-information analysis using the database from protein interaction network (https://string-db.org/) and our previous LC-MS/MS data, we identified 12 proteins including EGFR, GSK3β, AXIN3 and APC which may have a close relationship with JUP (Fig. 7A). EGFR is a known membrane protein and induces tyrosine-phosphorylation of JUP in an EGF dependent manner [22]. Thus, we detected the direct interaction between JUP and EGFR. After analyzing their potential affinity using GEPIA database (http://gepia.cancer-pku.cn/), a close interaction of JUP and EGFR was identified (Fig. 7B). Indeed, JUP and EGFR were found to co-locate at cell membrane of NCI-87 cells by immunofluorescence staining (Fig. 7C). And the direct binding between JUP and EGFR was further confirmed using Co-IP western blotting (Fig. 7D).
Fig. 7.
JUP regulates the activity of EGFR/AKT/GSK3β to stable β-catenin. (A) A network to show the interaction of JUP and other proteins identified by TOF-MS. Red represents JUP, blue represents EGFR, green represents GSK3β. (B) The correlation coefficient of JUP and EGFR was calculated by Pearson’s correlation analysis. (C) JUP co-localizing with EGFR at cellular membrane in NCI-87 was determined by immunofluorescence (Green: JUP; Red: EGFR; Blue: DAPI. Scale bars, 50 µm). (D) IP-Western blot to show the interaction of JUP and EGFR in cell lysates of NCI-87. IgG was used as a control antibody. (E, F) Western blotting was used to determine the protein levels of p-EGFR, T-EGFR, p-AKT, T-AKT, p-GSK3 β and T-GSK3 β in the indicated cells. NCI-87 with shJUP was treated with EGFR inhibitor, PD153035 (10 μmol/L); NUGC-3 and MGC-803 with ectopic JUP were treated with IGF-1 (100 ng/mL). GAPDH was used as a loading control. (G) The total and phosphorylated EGFR, AKT, and GSK3β levels in representative gastric cancer tissues with different degrees of differentiation (G1, G2 and G3) were analyzed by western blotting. GAPDH was used as a loading control. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Next, we wondered whether JUP interacting with EGFR has an effect on EGFR and its downstream signaling activities. Interestingly, we found the enhanced phosphorylated EGFR (p-EGFR) was in JUP-knocked down NCI-87 cells (Fig. 7E, left panel), while decreased in ectopic JUP-overexpressing NUGC-3 and MGC-803 cells (Fig. 7F, left panel). Administration of JUP-knocked down NCI-87 cells with EGFR inhibitor PD153035 (10 μM) decreased p-EGFR levels (Fig. 7F, right panel). It has been known that GSK3β/APC/AXIN/β-catenin complex closely affects Wnt/β-catenin activity via GSK3β-mediated phosphorylation and ubiquitinylation of cytoplasmic β-catenin in tumor cells. Thus, activities GSK3β in JUP-knocked down and JUP-overexpressing GC cells were analyzed. As expected, p-GSK3β was increased in JUP-knocked down NCI-87 cells (Fig. 7E, left panel), and decreased in JUP-overexpressing NUGC-3 and MGC-803 cells (Fig. 7F, left panel). AKT is a downstream target of EGFR, which directly regulates GSK3β activity through phosphorylating serine 9 site of GSK3β, and lead to inactive GSK3β [23]. In line with previous findings, loss of JUP relieved its binding effect on EGFR and led to augmented p-AKT levels in NCI-87; otherwise, overexpression of ectopic JUP decreased p-AKT levels in NUGC-3 and MGC-803 cells (Fig. 7E–F, left panels). Inhibiting EGFR activity using PD153035 (10 μM) in JUP-knocked down NCI-87 partially eliminated the effect of silenced JUP on downstream AKT signaling activity (Fig. 7E, right panel). Inversely, IGF-1 (an agonist of PI3K/AKT, 100 ng/mL) antagonized the inactivation of AKT signaling in ectopic JUP overexpressed-NUGC-3 or MGC-803 cells. To further validate the molecular mechanism in vivo, we measured the total and phosphorylated protein levels of EGFR/AKT/GSK3β signaling molecules in representative tumor tissues with different degrees of differentiation. Consistently, accompanied with progressively reduce of membrane/cytoplasmic JUP, phosphorylated EGFR, AKT and GSK3β were gradually increased in GC tissues from G1, G2 to G3 (Fig. 7G). Collectively, loss of JUP leads to activation of EGFR/AKT/GSK3β signaling and β-catenin stability, nuclear JUP facilitates β-catenin to enhance TCF4 transcript activity in GC to promote tumor invasion and metastasis (see Fig. 8).
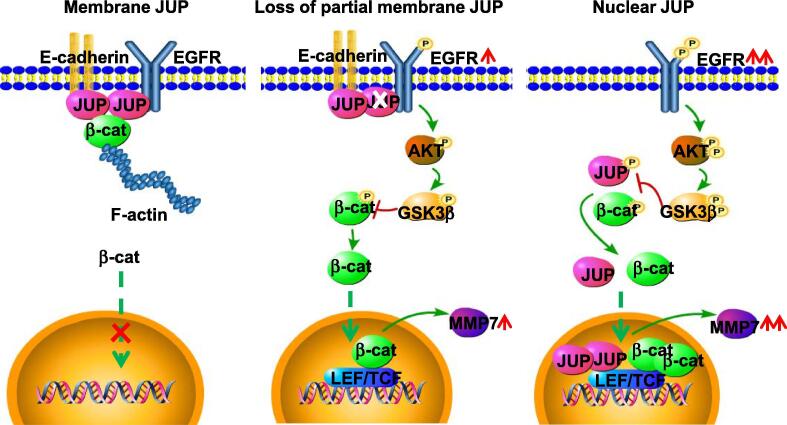
Fig. 8.
A working model of different distributed-JUP in regulating cell invasion via activation of β-catenin in gastric cancer cells. The proposed model shows the role of JUP in differently differentiated GC cells. In the well differentiated GC cells (left panel), JUP locates at cell membrane and links with E-cadherin and -catenin to block activation of EGFR and its downstream signaling. In moderately differentiated GC cells (middle panel), loss of partial membrane JUP leads phosphorylated EGFR and activation of downstream AKT/GSK3β/β-catenin signaling. In the poorly differentiated GC cells (right panel), complete loss of membrane JUP triggers an enhanced EGFR/AKT/GSK3β/β-catenin signaling, and location of JUP in nuclear, which collaborates with nuclear β-catenin, further promotes MMP7 expression and cell invasion potential.
Discussion
GC is a highly fatal disease with a very poor 5-year overall survival of less than 35% due to its high invasion and metastasis [24], [25]. In the current study, we demonstrated that gradual loss of JUP, a major component of cell-cell adhesion, is closely related with differentiation status and malignancy of GC. Our data showed that JUP located at membrane or cytoplasma is positively correlated with lower-grade, well-differentiated and well prognostic GC; however, nuclear JUP is more likely to be expressed in patients with poorly clinical stage of GC. Loss of membrane or cytoplasma JUP partially or completely activated EGFR/AKT/GSK3β signaling which is involved in EMT and cell motility of GC cells. Thus, our data may provide a new insight on the biological function of JUP associated with its distribution in GC.
As a homologue of β-catenin, JUP is a cytoplasmic peripheral membrane protein whose classical function is as a cell junction component both in adhesion junction and desmosome assembly [26]. At the adherence junction, JUP links the transmembrane protein E-cadherin to the actin cytoskeleton, whereas it links desmogleins and desmocollins to intermediate filaments of the actin cytoskeleton at the desmosomes. Overexpression of JUP upregulated E-cadherin, which in turn catches JUP located at cytoplasma. Cell-cell junction is essential for maintaining cellular polarity, creates barriers for gastric tissues and provides proper functionality [27]. To fully understand the impact of JUP on cell-cell adhesion, we knocked down JUP located at membrane of NCI-87, which induced EMT phenotype. And this is coincided with enhanced nuclear β -catenin which is well confirmed involving in EMT [28]. Our data suggest that as a cell adhesive member, loss of membrane JUP affects cell polarity and induces EMT.
Both JUP and β -catenin are armadillo family proteins. It has been reported that β-catenin has an abnormal distribution and appears to have pathologic and prognostic significance in hepatocellular carcinoma [29]. Many studies have concentrated on β-catenin and defined it as an oncogenic; however, the role of JUP is often overlooked. Previous reports have divided JUP into soluble and insoluble pool, which could be differently phosphorylated and endowed various functions [30]. Interestingly, here we found that membrane or cytoplasmic JUP plays a tumor suppressor; however, nuclear JUP synergizing nuclear β-catenin allows GC cells with an advanced migration and invasion ability. These data suggest that JUP might have various functions in different differentiated GC cells associated with its distribution.
In the current study, we demonstrate that JUP/EGFR/AKT/GSK3β is involved in tumor metastasis via inducing nuclear β-catenin translocation to up-regulation of MMP7 expression in gastric cancer. Loss of membrane or cytoplasmic JUP activates EGFR, which induces phosphorylation of AKT and GSK3β to inhibit phosphorylation of β-catenin, thus induces nuclear localization of β-catenin and promotes EMT of GC cells to acquire an advanced invasion potential. Ectopic JUP could interact with EGFR, which decreases phosphorylation of AKT and GSK3β, enhances phosphorylation of β-catenin and reduces its nuclear accumulation. Our data support previous finding that loss of JUP activates AKT/GSK3β/β-catenin signaling to promote the progression of cardiomyopathy [6].
Our data provide evidence that the membrane JUP interacts with EGFR to blunt EGFR activation. Loss of membrane JUP led to high level of phosphorylated EGFR which triggers the downstream EGFR/AKT/GSK3β signaling. The cytoplasmic, non-junctional JUP acts as a tumor suppressor. Loss of cytoplasmic JUP promoted translocation of cytoplasmic β-catenin to nuclear thus induced GC invasion. Ectopic overexpression of JUP mitigated the accumulation of nuclear β-catenin and tumor cell invasion. This is supported by a previous study, in which overexpression of JUP resulted in enhanced phosphorylation of β-catenin by GSK3β/APC/AXIN complex, thus lead to its degradation by the ubiquitin-proteasome system [31]. Furthermore, cytoplasmic JUP was confirmed as a tumor suppressor which interacts with metastasis suppressor Nm23 or transcription factor p53 to repress the expression of oncogenes [13], [32]. In addition, our study reveal that the nuclear JUP acts as an oncogenic function, in which nuclear JUP interacted with nuclear β-catenin through armadillo domain and enhanced nuclear β-catenin function in regulating TCF4 transcriptional activation in GC. Our study supports the finding in AML, in which the aberrant overexpressed nuclear JUP promoted nuclear localization of β-catenin and its oncogenic functions.
In summary, our results provide a new insight into the role of JUP beyond cell-cell adhesion involving in tumor invasion and metastasis. Different distribution of JUP was endowed heterogeneous functions to GC biological properties. In the mechanism, EGFR/AKT/GSK3β signaling pathway is involved in JUP-mediated tumor invasion. Targeting JUP may be a novel approach for clinical treatment. Clearly, more clinical investigations are required to further explore the role of JUP in both junctional and non-junctional functions.
Conclusion
In the current study, we unraveled that JUP expression and distribution are closely related with GC differentiation and malignant stages. JUP is mainly located in cell membrane or cytoplasm of high or medium differentiation GC; while in low differentiation GC, JUP is in nucleus. The membrane/cytoplasmic JUP affects β-catenin stability and nuclear translocation through EGFR/AKT/GSK3β signal axis. Nuclear JUP has a synergic role with β-catenin to promote GC cell invasion and EMT by up-regulating MMP7.
Compliance with ethics requirements
All Institutional and National Guidelines for the care and use of human were followed.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This work was supported in part by National Natural Science Foundation of China (NSFC 81773078) for Yixuan Hou, and National Natural Science Foundation of China (NSFC 31671481, NSFC 81172296, NSFC 81472476) for Manran Liu; And also supported in part by the Chongqing education committee of Chongqing Graduate Research and Innovation Project (2016) (CYS16137) for Yanlin Chen.
Footnotes
Peer review under responsibility of Cairo University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jare.2020.06.026.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Rugge M., Meggio A., Pravadelli C., Barbareschi M., Fassan M., Gentilini M. Gastritis staging in the endoscopic follow-up for the secondary prevention of gastric cancer: a 5-year prospective study of 1755 patients. Gut. 2018 doi: 10.1136/gutjnl-2017-314600. [DOI] [PubMed] [Google Scholar]
- 2.Romano F., Garancini M., Uggeri F., Degrate L., Nespoli L., Gianotti L. Surgical treatment of liver metastases of gastric cancer: state of the art. World J Surg Oncol. 2012;10:157. doi: 10.1186/1477-7819-10-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Correa P., Haenszel W., Cuello C., Tannenbaum S., Archer M. A model for gastric cancer epidemiology. Lancet. 1975;2:58–60. doi: 10.1016/s0140-6736(75)90498-5. [DOI] [PubMed] [Google Scholar]
- 4.Yilmazand M., Christofori G. Mechanisms of motility in metastasizing cells. Mol Cancer Res MCR. 2010;8:629–642. doi: 10.1158/1541-7786.MCR-10-0139. [DOI] [PubMed] [Google Scholar]
- 5.Breault J.E., Shiina H., Igawa M., Ribeiro-Filho L.A., Deguchi M., Enokida H. Methylation of the gamma-catenin gene is associated with poor prognosis of renal cell carcinoma. Clin Cancer Res. 2005;11:557–564. [PubMed] [Google Scholar]
- 6.Li J., Swope D., Raess N., Cheng L., Muller E.J., Radice G.L. Cardiac tissue-restricted deletion of plakoglobin results in progressive cardiomyopathy and activation of {beta}-catenin signaling. Mol Cell Biol. 2011;31:1134–1144. doi: 10.1128/MCB.01025-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kodama S., Ikeda S., Asahara T., Kishida M., Kikuchi A. Axin directly interacts with plakoglobin and regulates its stability. J Biol Chem. 1999;274:27682–27688. doi: 10.1074/jbc.274.39.27682. [DOI] [PubMed] [Google Scholar]
- 8.Maeda O, Usami N, Kondo M, Takahashi M, Goto H, Shimokata K, et al. Plakoglobin (gamma-catenin) has TCF/LEF family-dependent transcriptional activity in beta-catenin-deficient cell line. Oncogene 2004; 23: 964-972. [DOI] [PubMed]
- 9.Sechler M, Borowicz S, Van Scoyk M, Avasarala S, Zerayesus S, Edwards MG, et al. Novel Role for gamma-catenin in the regulation of cancer cell migration via the induction of hepatocyte growth factor activator inhibitor type 1 (HAI-1), J Biol Chem 2015; 290: 15610–15620. [DOI] [PMC free article] [PubMed]
- 10.Winn R.A., Bremnes R.M., Bemis L., Franklin W.A., Miller Y.E., Cool C. gamma-Catenin expression is reduced or absent in a subset of human lung cancers and re-expression inhibits transformed cell growth. Oncogene. 2002;21:7497–7506. doi: 10.1038/sj.onc.1205963. [DOI] [PubMed] [Google Scholar]
- 11.Mahendram S., Kelly K.F., Paez-Parent S., Mahmood S., Polena E., Cooney A.J. Ectopic gamma-catenin expression partially mimics the effects of stabilized beta-catenin on embryonic stem cell differentiation. PLoS ONE. 2013;8 doi: 10.1371/journal.pone.0065320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morgan R.G., Pearn L., Liddiard K., Pumford S.L., Burnett A.K., Tonks A. gamma-Catenin is overexpressed in acute myeloid leukemia and promotes the stabilization and nuclear localization of beta-catenin. Leukemia. 2013;27:336–343. doi: 10.1038/leu.2012.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aktaryand Z., Pasdar M. Plakoglobin represses SATB1 expression and decreases in vitro proliferation, migration and invasion. PLoS ONE. 2013;8 doi: 10.1371/journal.pone.0078388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou M., Hou Y., Yang G., Zhang H., Tu G., Du Y.E. LncRNA-Hh strengthen cancer stem cells generation in twist-positive breast cancer via activation of hedgehog signaling pathway. Stem Cells. 2016;34:55–66. doi: 10.1002/stem.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang X., Hou Y., Yang G., Wang X., Tang S., Du Y.E. Stromal miR-200s contribute to breast cancer cell invasion through CAF activation and ECM remodeling. Cell Death Differ. 2016;23:132–145. doi: 10.1038/cdd.2015.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang L., Hou Y., Sun Y., Zhao L., Tang X., Hu P. c-Ski activates cancer-associated fibroblasts to regulate breast cancer cell invasion. Mol Oncol. 2013;7:1116–1128. doi: 10.1016/j.molonc.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu T., Yang G., Hou Y., Tang X., Wu C., Wu X.A. Cytoplasmic GPER translocation in cancer-associated fibroblasts mediates cAMP/PKA/CREB/glycolytic axis to confer tumor cells with multidrug resistance. Oncogene. 2017;36:2131–2145. doi: 10.1038/onc.2016.370. [DOI] [PubMed] [Google Scholar]
- 18.Goto W., Kashiwagi S., Asano Y., Takada K., Takahashi K., Hatano T. Circulating tumor cell clusters-associated gene plakoglobin is a significant prognostic predictor in patients with breast cancer. Biomark Res. 2017;5:19. doi: 10.1186/s40364-017-0099-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naumann H., Rathjen T., Poy M.N., Spagnoli F.M. The RhoGAP Stard13 controls insulin secretion through F-actin remodeling. Mol Metab. 2018;8:96–105. doi: 10.1016/j.molmet.2017.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhurinsky J., Shtutman M., Ben-Ze'ev A. Plakoglobin and beta-catenin: protein interactions, regulation and biological roles. J Cell Sci. 2000;113(Pt 18):3127–3139. doi: 10.1242/jcs.113.18.3127. [DOI] [PubMed] [Google Scholar]
- 21.Han J., Gao B., Jin X., Xu Z., Li Z., Sun Y. Small interfering RNA-mediated downregulation of beta-catenin inhibits invasion and migration of colon cancer cells in vitro. Med Science Monit. 2012;18:BR273-280. doi: 10.12659/MSM.883205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gaudry C.A., Palka H.L., Dusek R.L., Huen A.C., Khandekar M.J., Hudson L.G. Tyrosine-phosphorylated plakoglobin is associated with desmogleins but not desmoplakin after epidermal growth factor receptor activation. J Biol Chem. 2001;276:24871–24880. doi: 10.1074/jbc.M102731200. [DOI] [PubMed] [Google Scholar]
- 23.Solberg N.T., Waaler J., Lund K., Mygland L., Olsen P.A., Krauss S. TANKYRASE inhibition enhances the antiproliferative effect of PI3K and EGFR inhibition, mutually affecting beta-CATENIN and AKT signaling in colorectal cancer. Mol Cancer Res: MCR. 2018;2018(16):543–553. doi: 10.1158/1541-7786.MCR-17-0362. [DOI] [PubMed] [Google Scholar]
- 24.Virgilio E., Giarnieri E., Giovagnoli M.R., Montagnini M., Proietti A., D'Urso R. Gastric cancer cells in peritoneal lavage fluid: a systematic review comparing cytological with molecular detection for diagnosis of peritoneal metastases and prediction of peritoneal recurrences. Anticancer Res. 2018;38:1255–1262. doi: 10.21873/anticanres.12347. [DOI] [PubMed] [Google Scholar]
- 25.Fujiwara Y., Doki Y., Taniguchi H., Sohma I., Takiguchi S., Miyata H. Genetic detection of free cancer cells in the peritoneal cavity of the patient with gastric cancer: present status and future perspectives. Gastric Cancer. 2007;10:197–204. doi: 10.1007/s10120-007-0436-5. [DOI] [PubMed] [Google Scholar]
- 26.Parker H.R., Li Z., Sheinin H., Lauzon G., Pasdar M. Plakoglobin induces desmosome formation and epidermoid phenotype in N-cadherin-expressing squamous carcinoma cells deficient in plakoglobin and E-cadherin. Cell Motil Cytoskeleton. 1998;40:87–100. doi: 10.1002/(SICI)1097-0169(1998)40:1<87::AID-CM8>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 27.Wickline E.D., Du Y., Stolz D.B., Kahn M., Monga S.P. gamma-Catenin at adherens junctions: mechanism and biologic implications in hepatocellular cancer after beta-catenin knockdown. Neoplasia. 2013;15:421–434. doi: 10.1593/neo.122098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao J.H., Luo Y., Jiang Y.G., He D.L., Wu C.T. Knockdown of beta-Catenin through shRNA cause a reversal of EMT and metastatic phenotypes induced by HIF-1alpha. Cancer Invest. 2011;29:377–382. doi: 10.3109/07357907.2010.512595. [DOI] [PubMed] [Google Scholar]
- 29.Wong C.M., Fan S.T., Ng I.O. beta-Catenin mutation and overexpression in hepatocellular carcinoma: clinicopathologic and prognostic significance. Cancer. 2001;92:136–145. doi: 10.1002/1097-0142(20010701)92:1<136::aid-cncr1301>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 30.Pasdar M., Li Z., Chlumecky V. Plakoglobin: kinetics of synthesis, phosphorylation, stability, and interactions with desmoglein and E-cadherin. Cell Motil Cytoskeleton. 1995;32:258–272. doi: 10.1002/cm.970320403. [DOI] [PubMed] [Google Scholar]
- 31.Aktary Z., Alaee M., Pasdar M. Beyond cell-cell adhesion: Plakoglobin and the regulation of tumorigenesis and metastasis. Oncotarget. 2017;8:32270–32291. doi: 10.18632/oncotarget.15650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aktary Z., Kulak S., Mackey J., Jahroudi N., Pasdar M. Plakoglobin interacts with the transcription factor p53 and regulates the expression of 14-3-3sigma. J Cell Sci. 2013;126:3031–3042. doi: 10.1242/jcs.120642. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.