Abstract
Aim
The present study aimed both to gain knowledge on the distinctive clinical characteristics of older adults with coronavirus disease 2019 (COVID‐19), in comparison with those of younger patients, and to identify risk factors for mortality.
Methods
A retrospective observational study was carried out of patients consecutively admitted to Doctor Peset University Hospital, Valencia (Spain) for COVID‐19 from 11 March to 28 April 2020. Every case was diagnosed by reverse transcription polymerase chain reaction or by serology test to detect antibodies. Demographic details, clinical characteristics, laboratory findings on admission and complications of each case were collected from electronic medical records.
Results
The dataset comprised 340 patients. Of them, 152 (44.6%) were aged >70 years. Comorbidities were more common in the older groups. Confusion was more common in older adults, whereas typical symptoms of COVID‐19, such as fever, cough and myalgia, were less common. Oxygen saturation ≤93% on room air, neutrophilia, D‐dimer >0.5 μg/mL, creatinine >1.5 mg/dL, lactate dehydrogenase ≥250 U/L and elevation of creatine kinase were higher in the older adult groups.
Complications during hospitalization, such as acute respiratory distress syndrome (53.3% vs 33.2%, P < 0.001), acute kidney injury (11.8% vs 5.3%; P = 0.030) and mortality (28.9% vs 6.5%; P < 0.001) were more common in patients aged >70 years. Oxygen saturation ≤93% on room air on admission was a predictor of mortality (odds ratio 11.65, 95% confidence interval 3.26–41.66, P < 0.001) in patients aged >70 years.
Conclusions
Older adults with COVID‐19 have more atypical presentation, more complications and higher mortality. Oxygen saturation ≤93% on room air on admission is a predictive factor of death. Geriatr Gerontol Int 2021; 21: 60–65.
Keywords: clinical characteristics, coronavirus, COVID‐19, mortality, older adults
Introduction
Older adults are particularly affected by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), responsible for coronavirus disease 2019 (COVID‐19), causing a more severe spectrum of clinical manifestations of this disease and higher mortality. 1 , 2 The case fatality rate of COVID‐19 rises rapidly with age. Thus, the case fatality rate is <1% for people aged <50 years, and rises to 1.3% for 50‐year‐olds, 3.6% for 60‐year‐olds, 8% for septuagenarians and 14.8% for octogenarians. 1 In the first case series of COVID‐19 published from China, the proportion of older adults was 15.1%. 3 However, in Spain, one of the longest‐living countries in the world with a life expectancy of 83 years, the percentage of patients aged >70 years with COVID‐19 has been reported as 36.9%. 4 Making an accurate clinical diagnosis of COVID‐19 might be difficult in older adults because of certain clinical specificities: (i) cognitive and behavioral disorders that might be more difficult to compile in medical records; (ii) the decompensation of underlying chronic diseases that can mimic clinical manifestations of COVID‐19; and (iii) clinical presentation with non‐specific or atypical symptoms. 5 Furthermore, the data on clinical manifestations in older adults with COVID‐19 and the differences between older adults and younger adults are limited. 6 , 7 , 8 , 9 , 10 , 11
In addition, the knowledge of the factors related with poor prognosis in older people is still scarce. We do not know yet if this poor prognosis in older people is due to the higher prevalence of comorbidity 1 or if it is associated with alterations of the biological background of that group. Indeed, these aspects could complicate the course of disease, even much more than age. 12 The present study aimed both to gain knowledge of the clinical characteristics of older adults with COVID‐19 in comparison with those in younger adults and to identify the risk factors for mortality present on admission to hospital.
Methods
Study design and study population
A retrospective study was carried out of patients with SARS‐CoV2 infection included in the SEMI‐COVID19 network, thematic network of the Spanish Society of Internal Medicine, admitted to the Doctor Peset University Hospital, a teaching tertiary hospital in Valencia, Spain. All confirmed cases of COVID‐19 admitted to hospital from 11 March to 28 April were included. Consecutive patients with COVID‐19 were recruited; the final sample comprised 340 patients. To compare outcomes, patients were divided into two groups: the older group (aged ≥70 years) and non‐older group (aged <70 years).
Research methods
For diagnosis of COVID‐19, patients need to have both clinical manifestations (fever and/or respiratory symptoms; together with the radiological imaging characteristics of pneumonia) and confirmed SARS‐CoV‐2 infection by testing positive on either polymerase chain reaction of a nasopharyngeal sample or serology test for antibodies.
The clinical symptoms or signs, and laboratory findings on admission were extracted from electronic medical records. All laboratory testing was carried out according to the clinical care needs of each patient. Laboratory assessments consisted of a complete blood count, blood chemical analysis, coagulation testing, assessment of liver and renal function, and measures of electrolytes, C‐reactive protein, lactate dehydrogenase (LDH) and creatine kinase.
Acute respiratory distress syndrome (ARDS) was classified as mild, moderate or severe by using arterial partial pressure of oxygen to fraction of inspired oxygen thresholds of 300, 200 and 100 mm Hg, respectively. 13
The Charlson Comorbidity Index (CCI) adjusted for age predicts 10‐year survival in patients with multiple comorbidities, and is used as a measure of total comorbidity burden. It includes a correction factor for age by decade: from 50 years onwards, 1 point is added for every decade. 14 Hence, an absolute number can be obtained and used as a comparison. The obtained values for adjusted CCI were classified into four categories scores of 0, 1–2, 3–4 and >4, respectively.
Statistical analysis
Continuous variables were represented by the mean and standard deviation, and categorical variables are represented by numbers and percentages. Student's t‐tests or anova and χ2‐test were used to analyze continuous and categorical variables, respectively. Finally, in patients aged ≥70 years, a logistic regression model was used to identify the risk factors for mortality. Mortality was the dependent variable in the model, and the independent variables were those that showed statistically significant differences in the univariate analyses (P < 0.20), when comparing patients aged ≥70 years and <70 years. Age was modeled as a categorical variable, and the baseline category in the model was patients <70 years. The empirical analysis was carried out using the Statistical Software Package of Social Sciences (spss 22.0; IBM Corporation, Armonk, NY, USA), and we used p value <0.05 for statistical significance.
Ethical aspects
Scientific and ethical permission to carry out the present study was obtained from the Medicinal Research Ethics Committee at the Regional University Hospital in Málaga, and this was approved by the Doctor Peset University Hospital in Valencia, Spain (registration number 61/20). Informed consent was obtained from each patient for the purpose of publication.
Results
The dataset comprised 340 consecutively recruited patients with COVID‐19, with a mean age of 63 ± 15 years. Demographic details, clinical characteristics and laboratory findings according to patients’ age are shown in Tables 1 and 2.
Table 1.
Clinical characteristics and outcomes for patients hospitalized with COVID‐19 by age
| Total 340 (100) | 18–49 years 52 (15.3) | 50–59 years 65 (19.2) | 60–69 years 71 (20.9) | 70–79 years 93 (27.1) | >80 years 59 (17.4) | P‐value † | |
|---|---|---|---|---|---|---|---|
| Mean age (±SD) | 65.5 (15.0) | 40.1 (7.0) | 55.1 (3.0) | 65.4 (3.0) | 74.7 (2.9) | 85.1 (4.2) | |
| Male, n (%) | 193 (56.6) | 27 (51.9) | 34 (52.3) | 37 (52.1) | 59 (63.4) | 36 (61.0) | 0.438 |
| Comorbidities, n (%) | |||||||
| Hypertension | 162 (47.8) | 3 (5.8) | 20 (31.3) | 31 (46.5) | 62 (66.7) | 44 (74.6) | <0.001 |
| Diabetes | 59 (17.4) | 4 (7.7) | 9 (13.8) | 12 (16.9) | 21 (22.8) | 13 (22.0) | 0.149 |
| Obesity | 84 (27.5) | 17 (35.4) | 9 (15.5) | 19 (29.7) | 24 (28.6) | 15 (29.4) | 0.201 |
| Smoking history | 122 (36.4) | 11(21.2) | 22 (33.9) | 22(30.9) | 42 (46.2) | 25 (43.9) | 0.023 |
| Chronic kidney disease | 13 (3.8) | 0(0) | 1 (1.5) | 1 (1.4) | 2 (2.2) | 9 (15.5) | <0.001 |
| Heart failure | 13 (3.8) | 0 (0) | 0 (0) | 2 (2.8) | 3 (3.3) | 8 (13.6) | <0.001 |
| COPD | 14 (4.1) | 0 (0) | 0 (0) | 0 (0) | 11 (12.0) | 2 (5.1) | <0.001 |
| Dementia | 15 (4.4) | 0 (0) | 1 (1.3) | 0 (0) | 4 (4.3) | 11 (16.9) | <0.001 |
| Age‐adjusted Charlson Comorbidity Index >4, n (%) | 66 (19.8) | 2 (3.9) | 0 (0) | 6 (8.6) | 19 (21.1) | 39 (68.4) | <0.001 |
| Moderate or severe dependency, n (%) | 25 (39.1) | 1(1.9) | 2 (3.1) | 1 (1.4) | 4 (4.3) | 17 (28.8) | <0.001 |
| Symptoms, n (%) | |||||||
| Mean symptoms onset to admission, days (SD) | 7.81 (5.8) | 7.1 (5.3) | 8.5 (3.8) | 8.1 (5.0) | 7.4 (5.7) | 8.0 (7.3) | 0.681 |
| Temperature >38°C | 205 (60.3) | 42 (80.8) | 417 (63.1) | 44 (62.0) | 55 (54.1) | 23 (39.0) | <0.001 |
| Dry cough | 215 (63.2) | 39 (7.5) | 48 (73.8) | 42 (59.2) | 54 (58.1) | 32 (54.2) | 0.087 |
| Myalgia | 113 (33.2) | 26 (50.0) | 29 (44.6) | 22 (31.0) | 25 (26.9) | 11 (18.6) | 0.001 |
| Fatigue | 146 (42.9) | 19 (36.5) | 31 (47.7) | 28 (39.4) | 42 (45.2) | 26 (44.1) | 0.728 |
| Anorexia | 52 (15.3) | 4 (7.8) | 13 (20.0) | 7 (9.9) | 16 (17.2) | 12 (20.3) | 0.176 |
| Shortness of breath | 195 (57.4) | 31 (54.6) | 38 (58.5) | 39 (54.9) | 50 (53.8) | 37 (62.7) | 0.828 |
| Hyposmia | 31 (9.1) | 8 (15.4) | 11 (16.9) | 8 (11.3) | 4 (4.3) | 0 (0) | 0.003 |
| Diarrhea | 78 (23) | 13 (25.0) | 20 (30.8) | 22 (31.0) | 18 (19.6) | 5 (8.5) | 0.014 |
| Nausea | 39 (11.5) | 4 (7.8) | 5 (7.7) | 10 (14.1) | 16 (17.4) | 4 (6.8) | 0.167 |
| Confusion | 59 (17.4) | 1 (1.9) | 0 (0) | 1 (1.4) | 11 (12.0) | 19 (32.2) | <0.001 |
| Vital signs, n (%) | |||||||
| Respiratory rate >24 breaths/min | 118 (36.1) | 14 (28.0) | 10 (16.1) | 17 (24.39) | 38 (42.7) | 39 (69.6) | <0.001 |
| Heart rate ≥100 b.p.m. | 73 (24.2) | 20 (38.5) | 14 (21.5) | 12 (17.1) | 14 (15.1) | 13 (22.0) | 0.018 |
| SBP <90 mmHg | 0(0) | 1 (1.7) | 0 (0) | 2 (2.3) | 1 (1.7) | 4 (1.2) | 0.652 |
| SpO2 ≤93% on room air | 133 (39.1) | 10 (19.2) | 17 (26.2) | 30 (42.3) | 44 (47.3) | 32 (54.2) | <0.001 |
| Clinical outcomes, n (%) | |||||||
| ARDS | 142 (42.1) | 17 (32.7) | 17(26.2) | 28 (40.0) | 44 (47.8) | 36 (62.1) | 0.001 |
| ICU | 50 (14.7) | 6 (11.5) | 8 (12.3) | 17 (23.9) | 19 (20.4) | 0 (0) | 0.001 |
| IMV | 39 (11.2) | 3 (5.9) | 4 (6.2) | 13 (18.6) | 18 (19.4) | 0 (0) | 0.001 |
| Death | 53 (16.2) | 2 (3.8) | 2 (3.1) | 8 (11.6) | 15 (17.6) | 26 (45.6) | <0.001 |
Data are presented as mean ± SD or n (%).
Differences between older adults and younger adults groups examined using the Fisher's exact test for categorical data, and an unpaired t‐test used for the comparison of means.
ARDS, acute respiratory distress syndrome; COPD, chronic obstructive pulmonary disease; ICU, intensive care unit; IMV, invasive mechanical ventilation; SBP, systolic blood pressure; SD, standard deviation; SpO2, oxygen saturation.
Table 2.
Laboratory findings of patients hospitalized with COVID‐19 by age
| Total 340 (100) | 18–49 years 52 (15.3) | 50–59 years 65 (19.2) | 60–69 years 71 (20.9) | 70–79 years 93 (27.1) | >80 years 59 (17.4) | P‐value | |
|---|---|---|---|---|---|---|---|
| Neutrophil count | |||||||
| >8 × 109/L, n (%) | 20 (5.9) | 1 (1.9) | 1 (1.5) | 4 (5.6) | 4 (4.3) | 10 (16.9) | 0.002 |
| <2 × 109/L, n (%) | 116 (34.1) | 22 (42.3) | 27 (41.5) | 23 (32.4) | 29 (31.4) | 15 (25.4) | 0.233 |
| Lymphocyte count <1.5 × 109/L, n (%) | 274 (80.6) | 40 (76.9) | 53 (81.6) | 53 (74.6) | 75 (80.6) | 53 (89.8) | 0.257 |
| Hemoglobin concentration (g/dL), n (%) | 13.88 (1.66) | 13.27 (1.67) | 13.16 (1.50) | 12.81 (1.61) | 12.52 (1.57) | 12.34 (1.63) | 0.006 |
| Platelet count <150 × 109/L, n (%) | 88 (25.9) | 10 (19.2) | 11 (16.9) | 19 (26.8) | 33 (35.5) | 15 (25.4) | 0.077 |
| Prothrombin time ≥16 s, n (%) | 25 (7.4) | 1 (1.9) | 2 (3.1) | 3 (4.2) | 8 (8.8) | 11 (18.6) | 0.003 |
| D‐dimer ≥0.5 μg/mL, n (%) | 97 (29.4) | 5 (10.0) | 14(21.9) | 19 (27.1) | 29 (32.6) | 30 (52.6) | <0.001 |
| Alanine aminotransferase >40 U/L, n (%) | 94 (28.3) | 15 (28.8) | 18 (28.1) | 26 (36.6) | 22 (24.2) | 13 (24.1) | 0.453 |
| Creatinine ≥1.5 mg/dL, n (%) | 32 (9.4) | 1 (1.9) | 1 (1.5) | 2 (2.8) | 14 (15.1) | 14 (23.7) | <0.001 |
| Lactate dehydrogenase ≥250 U/L, n (%) | 218 (72.2) | 29 (59.2) | 33 (60) | 54 (84.4) | 56 (66.7) | 46 (92.0) | <0.001 |
| Creatine kinase ≥200 U/L, n (%) | 62 (20) | 10 (20.8) | 5 (3.5) | 11 (15.9) | 22 (26.5) | 14 (27.5) | 0.048 |
| C‐reactive protein ≥10 mg/L, n (%) | 302 (89.6) | 45 (86.5) | 54 (84.4) | 63 (90) | 85 (91.4) | 55 (94.8) | 0.350 |
Data are presented as n (%).
When comparing older adults (aged ≥70 years) and non‐older adults (aged <70 years) in a univariate analyses, on admission, confusion was more common in the older adult groups than non‐older adults (19.9% vs 1.1%; P < 0.001). By contrast, typical symptoms associated with viral illness, such as fever (67.6% vs 51.3%; P < 0.001), dry cough (68.6% vs 56.6%; P = 0.014), myalgia (41% vs 23.7%; P = 0.001) or highly suspicious symptoms of COVID‐19, such as hyposmia (13.8% vs 2.6%; P < 0.001), were more common in non‐older adults. The percentage of patients with respiratory rate >24 breaths/min and oxygen saturation (SpO2) ≤93% on room air was higher in older adults (P < 0.001).
The most common laboratory alterations found were lymphopenia, high C‐reactive protein and high LDH. There were some differences between older patients and non‐older patients. Older patients had a higher proportion of neutrophilia and thrombocytopenia (9.2% vs 3.2%, P = 0.019 and 31.6% vs 21.2%, P = 0.031 respectively). We could not find any differences in lymphocyte counts. The proportion of patients with D‐dimer >0.5 mg/L was higher among older adults (40.4% vs 20.7%, P < 0.001), as well as with prothrombin time >16 s (19% vs 6%, P = 0.001). There was no difference in the proportion of patients with C‐reactive protein >10 mg/L. The proportion of patients with creatinine >1.5 mg/dL and creatine kinase was higher among older adults (18.4% vs 2.1%, P < 0.001 and 26.9% vs 14.8%, P = 0.008, respectively).
A comparison of complications in patients aged >70 years and ≤70 years is shown in Figure 1. The most common form of organ damage that arose due to SARS‐CoV2 was ARDS (142 patients, 42.1%) and acute kidney injury (AKI) in 28 patients (8.2%). Of the total of 152 older adults, 60 (39.5%) had ARDS on admission, and 20 (13.2%) developed ARDS during their hospitalization. Risk factors on admission for ARDS in older adults were neutrophilia (13.8% vs 4.3%, P = 0.047), LDH ≥250 U/L (84.3% vs 66.7%, P = 0.018) and D‐dimer ≥0.5 μg/mL (49.3% vs 29%, P = 0.013). Mortality among older patients with ARDS was 51.3%.
Figure 1.
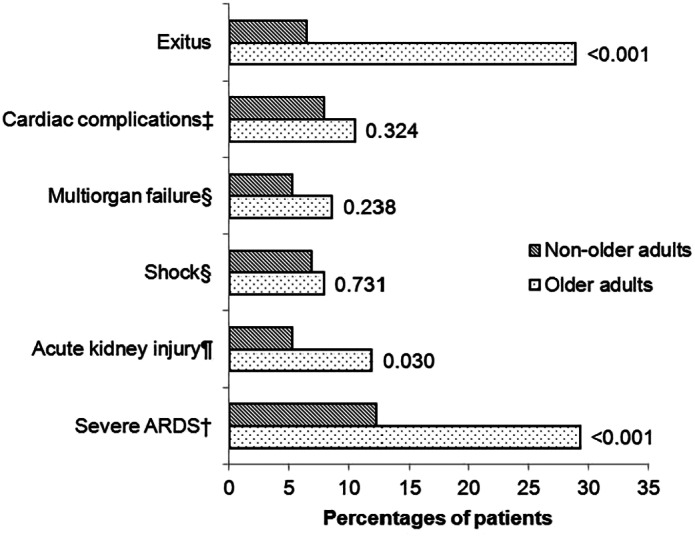
Complications during admission (in percentages) of older patients and non‐older patients and P‐value. The variables were expressed as the percentage and compared with the χ2‐test. ‡Cardiac complications: heart failure, auricular arrhythmia, ventricular arrhythmia, myocardial infarction or myocarditis. §Multiorgan failure and shock are defined as the third international consensus definitions for sepsis and septic shock (Sepsis‐3). 15 ¶Acute kidney injury was identified as an increase in serum creatinine by >0.3 mg/dL within 48 h or an increase in serum creatinine to >1.5‐fold baseline within the prior 7 days compared with the preceding 1 year of data in acute care medical records. †Acute respiratory distress syndrome (ARDS) is classified as severe by using an arterial partial pressure of oxygen to fraction of inspired oxygen threshold of 100 mmHg. 13
AKI occurred more often in older adults (11.8% vs 5.3%; P = 0.030). Among older adults, AKI was related to ARDS (94.4% vs 47.7%, P < 0.001). We could not find any association with hypertension, diabetes, chronic kidney disease or age >80 years.
Other complications, including cardiac complications, multiorgan failure and shock requiring vasoactive drugs, arose in similar proportions in both groups (Fig. 1).
A total of 50 patients (14.7%) were admitted to the intensive care unit (ICU) with no differences between older and non‐older adults (12.6% vs 16.5%, P = 0.302). No patient aged >80 years was admitted to the ICU.
The mean length of hospital stay was 11.4 ± 8.8 days, with no differences between the two groups (12.1 ± 9.6 days for older adults vs 10.8 ± 9.1 days for non‐older adults; P = 0.201). A total of 12 patients remained in hospital at the time of data cut‐off, and these cases were not included in the death analysis.
A total of 53 patients (16.2%) died: 41 (28.9%) in the older adult group, and 12 (6.5%) in the non‐older adult group (P < 0.001). All 41 older adults who died had ARDS either on admission or developed it during hospitalization. Furthermore, those who died had a higher proportion of other complications than survivors: shock requiring vasoactive drugs (12.2% vs 0%; P < 0.001), AKI (24.4% vs 5%, P = 0.001) and multiorgan failure (19.5% vs 0%; P < 0.001).
The mean time in days from initial symptoms to death was 17.67 ± 12.85 days in older adults, and 21.50 ± 9.18 days in non‐older adults (P = 0.263). Risk factors for mortality in older adults are shown in Tables 3 and 4. Age >80 years, dependency, confusion, SpO2 ≤93% on room air, CCI >4 and D‐dimer≥0.5 μg/mL were associated with mortality by univariate analysis. Only SpO2 ≤93% on room air showed a significant relationship with death in the multivariate analysis (see Table 4).
Table 3.
Risk factors for mortality in COVID‐19 patients aged >70 years by logistic regression univariate analysis
| Univariate analysis | ||
|---|---|---|
| Risk factors | OR (95% CI) | P‐value |
| Age >80 years † | 3.91 (1.82–8.40) | <0.001 |
| Male | 1.35 (0.65–2.83) | 0.420 |
| Confusion | 4.06 (1.71–9.65) | 0.001 |
| SpO2 ≤93% on room air | 11.37 (3.75–34.11) | <0.001 |
| Moderate or severe dependency | 3.75 (1.42–9.91) | 0.005 |
| Age‐adjusted Charlson Comorbidity Index >4 | 2.10 (0.99–4.46) | 0.051 |
| D‐dimer ≥0.5 μg/mL | 2.06 (0.97–4.40) | 0.057 |
| Lymphocyte count <1.5 × 109/L | 1.67 (0.58–4.81) | 0.340 |
Patients aged 70–80 years were assigned an odds ratio of 1.
CI, confidence interval; OR, odds ratio; SpO2, oxygen saturation.
Table 4.
Risk factors for mortality in COVID‐19 patients aged >70 years by logistic regression multivariate analysis
| Multivariate analysis | ||
|---|---|---|
| Risk factors | OR (95% CI) | P‐value |
| Age >80 years † | 1.99 (0.75–5.30) | 0.167 |
| Confusion | 1.66 (0.60–4.66) | 0.332 |
| SpO2 ≤93% on room air | 11.65 (3.26–41.66) | <0.001 |
| Moderate or severe dependency | 1.82 (0.52–6.36) | 0.349 |
| Age‐adjusted Charlson Comorbidity Index >4 | 0.89 (0.34–2.36) | 0.815 |
| D‐dimer ≥0.5 μg/mL | 1.38 (0.57–3.34) | 0.470 |
Patients aged 70–80 years were assigned an odds ratio of 1.
CI, confidence interval; OR, odds ratio; SpO2, oxygen saturation.
Discussion
In the present study, the clinical presentation in older adults with COVID‐19 was different from younger adults. These patients showed more atypical presentations of COVID‐19, such as confusion, in contrast with younger adults who had more typical presentations, such as fever, cough or myalgia. As expected, the prognosis was worse for older adults, who had more complications and higher mortality. SpO2 ≤93% on room air on admission was an independent risk factor for mortality. It is worth mentioning that the proportion of men was significantly higher in all the age groups, although at older ages, women were more predominant in the general population. 16 The higher proportion of male COVID‐19 patients has already been described. 3
We found important differences in clinical manifestations between the different age groups. First, confusion on admission was more prevalent among older adults. Second, the commonly reported symptoms of COVID‐19, such as fever, cough or myalgia, as well as the characteristic symptom of hyposmia, 17 were more common in the younger adult groups. It is well known that age can make the diagnosis more complex, as older adults with infections frequently have atypical manifestations. 5 The clinical presentation of pneumonia in older patients might be different from that of the general adult population; in general, the more common symptoms associated with pneumonia in older persons are falls and altered mental status. 18 We have to be cautious when dealing with older patients to avoid misdiagnosis of COVID‐19.
In addition, in the present study, older adults had more severe signs on admission, such as respiratory rate >24 breaths/min (from 28% in the 18–49 years age group to 69.6% in the >80 years age group; P < 0.001). The percentage of patients with SpO2 ≤93% on room air on admission progressively increased as the age of the groups increased (from 19.2% in the 18–49 years age group to 54.2% in the >80 years age group; P < 0.001). These results are similar to findings of recent studies with a smaller number of patients showing that older patients have more severe presentations of COVID‐19. 6 , 8
The most common laboratory abnormalities observed in the present study were lymphopenia and elevated LDH, findings already described in previous studies. 19 No differences were found between patients with lymphopenia among the different age groups, but the percentage of patients with LDH ≥250 U/L was higher in the older age groups.
Among older adults, the proportion of patients with neutrophilia, D‐dimer ≥0.5 μg/mL and prothrombin time ≥16 s was significantly higher. Neutrophilia has been related to cytokine storm induced by virus invasion, and coagulation activation related to sustained inflammatory response. 19 The proportion of patients with creatinine ≥1.5 mg/dL was higher among older adults, which could be a manifestation of more common chronic kidney failure in the older adult groups.
The proportion of underlying chronic diseases was much higher in older adults than among the non‐older patients, especially dementia, chronic obstructive pulmonary disease, heart failure and chronic kidney disease. A total of 61 (39.5%) older adults had an age‐adjusted CCI >4 (vs 4.3% in non‐older patients; P < 0.001). These findings are similar to those in the main epidemiological studies 3 , 20 and other studies comparing older patients with younger patients. 7 , 8 , 9 , 10 This is not surprising given the positive association between age and comorbidity. Other authors have reported that COVID‐19 patients were more likely to have specific comorbidities, and this might suggest that SARS‐CoV2 is more likely to infect people with underlying chronic diseases. 21 For treatment of COVID‐19 in older patients, we should take into account not only the manifestations due to COVID‐19, but also their underlying chronic diseases and baseline situation, after a comprehensive evaluation. Among the various chronic diseases, 4.3% of patients aged 70–79 years and 16.9% of patients aged >80 years had dementia. This is worth mentioning, because it might explain the high percentage of patients with atypical presentation, such as confusion. People with underlying health conditions appear to be at higher risk for severe COVID‐19‐associated disease than those without these conditions, 22 and calls for increased emphasis on future interventions to better protect people with chronic diseases from infection with SARS‐CoV‐2. 23
The most frequent complications found were ARDS and AKI. ARDS was associated with age, as previously described. 9 The higher risk of ARDS among older adults has been associated with the cytokine release syndrome or cytokine storm, the main pathogenic mechanism of COVID‐19. 24 In older adults, the process of inflamm‐aging,25 together with immunosuppression or immunosenescence, creates the perfect situation to increase the risk of poor evolution, and might be responsible for the phenomenon of COVID‐19 age‐related mortality and disease severity. In the present study, older adults with ARDS had higher percentages of LDH ≥250 U/L (P = 0.018), neutrophilia (P = 0.047) and D‐dimer ≥0.5 μg/mL (P = 0.013) on admission in comparison with older adults without ARDS. These laboratory parameters have been previously associated with ARDS in patients of all ages with COVD‐19. 19 , 26
The incidence of AKI was significantly higher in older adults aged ≥70 years (11.8% vs 5.3%). Previous studies have found that the incidence of AKI in COVID‐19 patients varies from 3% in all hospitalized patients to 19% in ICU patients. 27 The possible mechanisms interacting in renal disturbances by COVID‐19 comprise sepsis by COVID‐19, cytokine storm syndrome, direct virus invasion to the renal cells and diabetes or hypertension that can be associated with a pre‐existing chronic decline of kidney function. 28 All these features might explain the higher percentage of AKI in older adults. In this series, there was no association with diabetes or previous chronic renal disease, but we cannot exclude a type II error.
In the total population analyzed in the present study, 53 patients (16.2%) died. In the older adults aged ≥70 years, the death rate was 28.9%, whereas in the adults aged <70 years, this rate was 6.5% (P < 0.001). This is in line with other studies where older people had higher mortality. 1 , 20 Previous studies in older adults have identified different risk factors for mortality, such as lymphocyte count, older age or comorbidities. 6 , 29 In the present study, when trying to identify clinical characteristics on admission associated with death in older people, older age (P < 0.001), confusion (P = 0.001), SpO2 ≤93% on room air (P = 0.001) and dependency (P = 0.005) were associated with death. In a multivariate analysis, only impaired oxygenation on admission (tested as SpO2 ≤93% on room air) was associated with mortality. In the present study, in which all patients had pneumonia, it is our view that this is a clinical sign that is closely related to the development of ARDS, which has been related to mortality in previous studies. A recent study described admission SpO2 of <88% together with markers of inflammation were the most strongly associated with critical illness and mortality. 30
The benefit of admitting patients into ICU was carefully assessed, and people in whom a minimum benefit was expected were not admitted, prioritizing those with a longer quality‐adjusted life expectancy. Older patients had comorbidities that limit their quality‐adjusted life years; however, given the exceptional public health situation caused by the COVID‐19 pandemic, we cannot rule out a limitation in ICU admission due to lack of beds.
The present study had several limitations: First, this was a retrospective study with its inherent difficulty in limiting the exposure to bias. Second, this is from a single center, and its findings could be different in other settings. Third, some patients remained in the hospital at the time of data cut‐off and these cases were not included in the death analysis. Fourth, we included patients with a microbiological diagnosis of COVID‐19 made only on the basis of antibody tests. This can skew the patient cohort, because it can detect eligible patients at their later stage of infection. In all, in the present study, patients were consequently recruited and the main characteristics of older adults with COVID‐19 were analyzed.
In conclusion, older adults with COVID‐19 have a different clinical presentation to younger adults, characterized by more atypical and fewer typical symptoms. This should be taken into account to avoid the misdiagnosis of COVID‐19 in this population. The prognosis in older adults is worse, and it is associated with SpO2 ≤93% on room air on admission.
Disclosure statement
The authors declare no conflict of interest.
Acknowledgements
We acknowledge all the investigators who participate in the SEMI‐COVID‐19 Network.
We also thank the SEMI‐COVID‐19 Registry Coordinating Center, S&H Medical Science Service, for their quality control data, logistic and administrative support.
Gómez‐Belda AB, Fernández‐Garcés M, Mateo‐Sanchis E, et al. COVID‐19 in older adults: What are the differences with younger patients? Geriatr. Gerontol. Int. 2021;21:60–65. 10.1111/ggi.14102
References
- 1. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020; 323: 1239–1242. [DOI] [PubMed] [Google Scholar]
- 2. Yang X, Yu Y, Xu J et al. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Respir Med 2020; 8: 475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guan W, Ni Z, Hu Y et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382: 1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Equipo COVID‐19 . RENAVE. CNE. CNM (ISCIII). Situación de COVID‐19 en España a 04 de mayo de 2020. Informes COVID‐19. Informe n° 31 [monograph on the internet]. Ministerio de Sanidad; 2020 [Cited 20 May 2020.] Available from URL: https://www.isciii.es/QueHacemos/Servicios/VigilanciaSaludPublicaRENAVE/EnfermedadesTransmisibles/Paginas/InformesCOVID-19.aspx
- 5. Limpawattana P, Phungoen P, Mitsungnern T, Laosuangkoon W, Tansangworn N. Atypical presentations of older adults at the emergency department and associated factors. Arch Gerontol Geriatr 2016; 62: 97–102. [DOI] [PubMed] [Google Scholar]
- 6. Wang L, He W, Yu X et al. Coronavirus disease 2019 in elderly patients: characteristics and prognostic factors based on 4‐week follow‐up. J Infect 2020; 80: 639–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Niu S, Tian S, Lou J et al. Clinical characteristics of older patients infected with COVID‐19: a descriptive study. Arch Gerontol Geriatr 2020; 89: 104058. 10.1016/j.archger.2020.104058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu K, Chen Y, Lin R, Han K. Clinical feature of COVID‐19 in elderly patients: a comparison with young and middle‐aged patients. J Infect 2020; 80: e14–e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lian J, Jin X, Hao S et al. Analysis of epidemiological and clinical features in older patients with Corona Virus Disease 2019 (COVID‐19) out of Wuhan. Clin Infect Dis 2020; 71: 740–747. 10.1093/cid/ciaa242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen T, Dai Z, Mo P et al. Clinical characteristics and outcomes of older patients with coronavirus disease 2019 (COVID‐19) in Wuhan, China (2019): a single‐centered, retrospective study. J Gerontol A Biol Sci Med Sci 2020; 75: 1788–1795. 10.1093/gerona/glaa089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Covino M, De Matteis G, Santoro M et al. Clinical characteristics and prognostic factors in COVID‐19 patients aged ≥80 years. Geriatr Gerontol Int 2020; 20: 704–708. 10.1111/ggi.13960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aprahamian I, Cesari M. Geriatric syndromes and SARS‐COV‐2: more than just being old. J Frailty Aging 2020; 9: 127–129. 10.14283/jfa.2020.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ranieri VM, Rubenfeld GD, Thompson BT et al. Acute respiratory distress syndrome: the Berlin definition. JAMA 2012; 307: 2526–2533. [DOI] [PubMed] [Google Scholar]
- 14. Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol 1994; 47: 1245–1251. [DOI] [PubMed] [Google Scholar]
- 15. Shankar‐Hari M, Phillips GS, Levy ML et al. Developing a new definition and assessing new clinical criteria for septic shock: for the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis‐3). JAMA 2016; 315: 775–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pérez J, Abellán A, Aceituno P, Ramiro D. Un perfil de las personas mayores en España, 2020. Indicadores estadísticos básicos. Informe n° 25 [monograph on the internet]. Madrid: Informes Envejecimiento en red; 2020 [Cited 30 May 2020.] Available from URL: http://envejecimiento.csic.es/documentos/documentos/enred-indicadoresbasicos2020.pdf
- 17. Lechien JR, Chiesa‐Estomba CM, De Siati DR et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild‐to‐moderate forms of the coronavirus disease (COVID‐19): a multicenter European study. Eur Arch Otorhinolaryngol 2020; 277: 2251–2261. 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cilloniz C, Ceccato A, San Jose A, Torres A. Clinical management of community acquired pneumonia in the elderly patient. Expert Rev Respir Med 2016; 10: 1211–1220. [DOI] [PubMed] [Google Scholar]
- 19. Wang D, Hu B, Hu C et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA 2020; 323: 1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Richardson S, Hirsch JS, Narasimhan M et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the New York City area. JAMA 2020; 323: 2052–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Atkins JL, Masoli JA, Delgado J et al. Preexisting comorbidities predicting severe Covid‐19 in older adults in the UK Biobank Community Cohort. medRxiv 2020; 75: 2224‐2230. 10.1101/2020.05.06.20092700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. CDC COVID‐19 Response Team . Preliminary Estimates of the Prevalence of Selected Underlying Health Conditions Among Patients with Coronavirus Disease 2019 ‐ United States, February 12–March 28, 2020. MMWR Morb Mortal Wkly Rep 2020; 69: 382–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yang J, Zheng Y, Gou X et al. Prevalence of comorbidities and its effects in patients infected with SARS‐CoV‐2: a systematic review and meta‐analysis. Int J Infect Dis 2020; 94: 91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Qin C, Zhou L, Hu Z et al. Dysregulation of immune response in patients with COVID‐19 in Wuhan, China. Clin Infect Dis 2020; 71: 762–768. 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Franceschi C, Bonafè M, Valensin S et al. Inflamm‐aging. An evolutionary perspective on Immunosenescence. Ann N Y Acad Sci 2000; 908: 244–254. [DOI] [PubMed] [Google Scholar]
- 26. Wu C, Chen X, Cai Y et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med 2020; 180: 934–943. 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ng JJ, Luo Y, Phua K, Choong AMTL. Acute kidney injury in hospitalized patients with coronavirus disease 2019 (COVID‐19): a meta‐analysis. J Infect 2020; 81: 647–679. 10.1016/j.jinf.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fanelli V, Fiorentino M, Cantaluppi V et al. Acute kidney injury in SARS‐CoV‐2 infected patients. Crit Care 2020; 24: 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sun H, Ning R, Tao Y et al. Risk factors for mortality in 244 older adults with COVID‐19 in Wuhan, China: a retrospective study. J Am Geriatr Soc 2020; 68: E19–E23. 10.1111/jgs.16533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Petrilli CM, Jones SA, Yang J et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ 2020; 369: m1966. 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]


