Abstract
Coronavirus disease 2019 (COVID‐19) has caused a significant impact on all aspects of life, with the number of death cases still increasing. Therefore, identification of potential treatment for reducing the severity of the disease is important. Currently, the data regarding the effectiveness of tocilizumab as treatment agents for COVID‐19 infection is still conflicting. This study aims to give clear evidence regarding the potential benefit of tocilizumab in reducing the biomarkers of COVID‐19 infection. We systematically searched the PubMed Central database using specific keywords related to our aims until July 24th, 2020. All articles published on COVID‐19 and tocilizumab were retrieved. A total of 9 studies with a total of 577 patients were included in our analysis. Our meta‐analysis showed that tocilizumab treatment is associated with reduction of C‐reactive protein (mean difference [MD]: −106.69 mg/L [95% confidence interval [CI]: −146.90, −66.49 mg/L], p < .00001; I 2 = 98%, random‐effect modeling), d‐dimer (MD: −3.06 mg/L [95% CI: −5.81, −0.31 mg/L], p = .03; I 2 = 98%, random‐effect modeling), Ferritin (MD: −532.80 ng/ml [95% CI: −810.93, −254.67 ng/ml], p = .0002; I 2 = 25%, random‐effect modeling), procalcitonin (MD: −0.67 ng/ml [95% CI: −1.13, −0.22 ng/ml], p = .004; I 2 = 92%, random‐effect modeling], and increment in the levels of lymphocyte count (MD: 0.36 × 103/μl [95% CI: 0.18, 0.54 × 103/μl], p < .0001; I 2 = 88%, random‐effect modeling). Administration of tocilizumab is effective in reducing the biomarkers of the COVID‐19 infection.
Keywords: coronavirus disease 2019, COVID‐19, immunomodulator, interleukin‐6, tocilizumab
Highlights
There are no widely accepted drugs for the management of COVID‐19 patients.
The benefit of tocilizumab administration in reducing the biomarkers of COVID‐19, but not on the mortality outcome.
Physicians may hence consider adding tocilizumab as a treatment for patients with COVID‐19, especially in patients with severe disease.
1. INTRODUCTION
Four months have passed since coronavirus disease 2019 (COVID‐19) was declared as a pandemic disease by the World Health Organization, but the number of positive and death cases from COVID‐19 is still increasing. This disease has caused a significant burden on all aspects of life across the world. Several comorbidities have been demonstrated to be associated with the development of severe outcomes from COVID‐19 infection, such as hypertension, diabetes, cardiovascular disease, dyslipidemia, thyroid disease, and pulmonary disease. 1 , 2 , 3 The pathogenesis of severe COVID‐19 is related to the development of cytokine storm caused by robust activation of the immune system and hyperinflammation that will result in the increment of the inflammatory markers, such as C‐reactive protein (CRP), procalcitonin (PCT), and also reduction of lymphocyte count. Meta‐analysis has shown that several markers, such as CRP, d ‐dimer, ferritin, PCT, and lymphocyte count, can be used to indicate the severe outcome of COVID‐19. 4 The presence of these markers can be helpful for early detection of the severe outcome of the disease. Unfortunately, until now, there are no widely accepted drugs for the management of COVID‐19 patients. Several potential agents have been proposed to help in achieving faster recovery time, preventing the severe outcome of the disease, and reducing the mortality rate in COVID‐19 patients, and one of the agents is tocilizumab, an interleukin‐6 (IL‐6) inhibitor. Tocilizumab has been approved for the treatment of rheumatoid arthritis, juvenile idiopathic arthritis, and giant cell arteritis. 5 Recently, tocilizumab has been offered to help in reducing the proinflammatory cytokines in COVID‐19 and preventing the cytokine storm syndrome that could contribute to the development of the severe outcome. However, the evidence regarding the potential benefit of tocilizumab in COVID‐19 patients is still conflicting. A meta‐analysis is therefore required to aid in solving this problem. This article aims to explore the potential benefit of tocilizumab in reducing the biomarkers of COVID‐19 infection.
2. MATERIALS AND METHODS
A search of the literature was conducted on PubMed Central using the keywords “tocilizumab” AND “coronavirus disease 2019” OR “COVID‐19,” between 2019 and the present time (July 24th, 2020) with language restricted to English only. The title, abstract, and full text of all articles identified that matched the search criteria were assessed, and those reporting the comparison of pre‐ and post‐tocilizumab treatment with the data regarding laboratory parameters were included in this meta‐analysis. The references of all identified studies were also analyzed (forward and backward citation tracking) to identify other potentially eligible articles.
A meta‐analysis was performed using Review Manager 5.4 (Cochrane Collaboration) software. Continuous variables were calculated using the Inverse‐Variance formula with random‐effects models. We used the I 2 statistic to assess the heterogeneity, value of less than 25%, 26%–50%, and greater thn 50% considered as low, moderate, and high degrees of heterogeneity, respectively. The effect estimate was reported as mean difference (MD) along with its 95% confidence intervals (CIs) for continuous variables, respectively. p Value was two‐tailed, and the statistical significance was set at ≤.05. When data were reported as medians and interquartile ranges, we would convert them to means and standard deviations for meta‐analytical pooling using the formula by Wan et al. 6
3. RESULTS
A total of 1,574 records were obtained through systematic electronic searches and other ways. After screening titles, abstracts, and full texts, nine studies 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 with a total of 577 COVID‐19 patients were included in the meta‐analysis. The essential characteristics of included studies are summarized in Table 1, while Figure 1 showed the individual and pooled MDs for the difference between pre‐ and post‐administration of tocilizumab and the levels of CRP (Figure 1A), d ‐dimer (Figure 1B), ferritin (Figure 1C), PCT (Figure 1D), and lymphocyte (Figure 1E) levels in COVID‐19 infection. Our pooled analysis showed that administration of tocilizumab treatment is associated with a reduction in the levels of CRP (MD: −106.69 mg/L [95% CI: −146.90, −66.49 mg/L], p < .00001; I 2 = 98%, random‐effect modeling), d ‐dimer (MD: −3.06 mg/L [95% CI: −5.81, −0.31 mg/L], p = .03; I 2 = 98%, random‐effect modeling), ferritin (MD: −532.80 ng/mL [95% CI: −810.93, −254.67 ng/ml], p = .0002; I 2 = 25%, random‐effect modeling), PCT (MD: −0.67 ng/ml [95% CI: −1.13, −0.22 ng/ml], p = .004; I 2 = 92%, random‐effect modeling), and increment in the levels of lymphocyte count (MD 0.36 × 103/μl [95% CI: 0.18, 0.54 × 103/μl], p < .0001; I 2 = 88%, random‐effect modeling). However, only the differences in CRP and ferritin were large enough to be clinically important, while the other laboratory parameters ( d ‐dimer, PCT, and lymphocyte) only show a small difference between pre‐ and post‐tocilizumab administration, which results in a reduction of their importance clinically.
Table 1.
Characteristics of included studies
| Study | Sample size | Design | Age (years) | Tocilizumab dose | Tocilizumab administration | Laboratory follow‐up | Laboratory outcomes |
|---|---|---|---|---|---|---|---|
| Alattar et al. 4 | 25 | Retrospective cohort | 57 ± 9.6 | 8 mg/kg IV, once | Day 7 (4–16) after the onset of symptoms | Day 14 after treatment | CRP, PCT, lymphocytes |
| Conrozier et al. 5 | 40 | Retrospective cohort | 75.9 ± 17.7 | 8 mg/kg IV, twice | Day 7–17 after the onset of symptoms | Day 8 after treatment | CRP, d ‐dimer, lymphocytes, ferritin |
| De Rossi et al. 6 | 90 | Retrospective cohort | 62.9 ± 12.5 | 400 mg IV, once | Day 1 – 2 after hospital admission | Day 5 after treatment | CRP, PCT, lymphocytes |
| Issa et al. 7 | 10 | Case‐series | N/A | 8 mg/kg IV, once | Day 10 after the onset of symptoms | Day 3 after treatment | CRP, PCT, d ‐dimer, ferritin |
| Mastroianni et al. 8 | 12 | Retrospective cohort | 59.3 ± 17.7 | 162 mg SC, twice | N/A | Day 7 after treatment | CRP, PCT, d‐dimer, fFerritin |
| Potere et al. 9 | 40 | Case‐control | 59.8 ± 16.9 | 162 mg SC, twice | N/A | Day 3 after treatment | CRP, lymphocytes |
| Price et al. 10 | 239 | Retrospective cohort | 61.6 ± 11 | 8 mg/kg IV, once | Day 5–10 after the onset of symptoms | Day 14 after treatment | CRP, d‐dimer, lymphocytes |
| Toniati et al. 11 | 100 | Retrospective cohort | 63.3 ± 10.3 | 8 mg/kg IV, twice | Day 12 (9–14) after the onset of symptoms | Day 10 after treatment | CRP, d‐dimer, lymphocytes, ferritin |
| Xu et al. 12 | 21 | Case‐series | 56.8 ± 16.5 | 400 mg IV, once | N/A | Day 5 after treatment | CRP, PCT, lymphocytes |
Abbreviations: CI, confidence interval; CRP, C‐reactive protein; PCT, procalcitonin.
Figure 1.
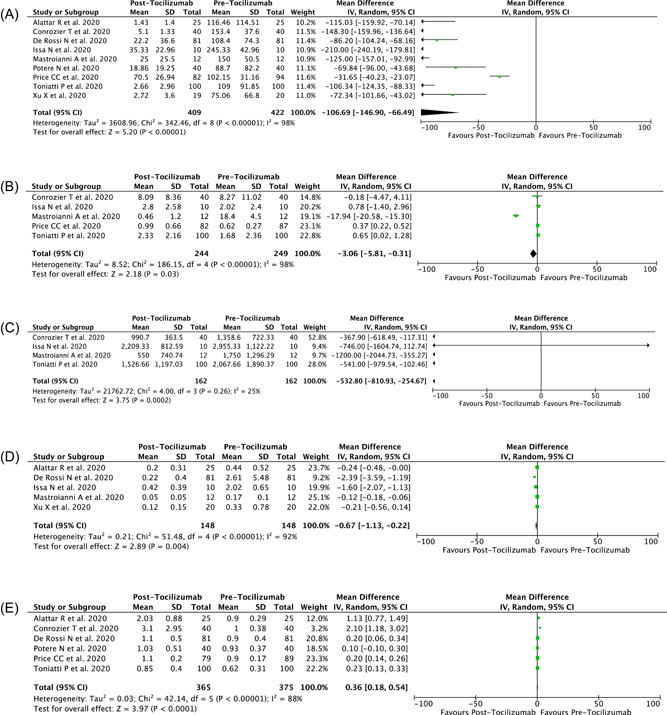
Forest plot that demonstrates the association of administration of tocilizumab with the levels of (A) CRP, (B) d ‐dimer, (C) ferritin, (D) procalcitonin, and (E) lymphocyte levels in COVID‐19 infection. COVID‐19, coronavirus disease 2019; CRP, C‐reactive protein
4. DISCUSSION
On the basis of a contrite meta‐analysis of available data, tocilizumab seems to be beneficial in reducing the biomarkers of COVID‐19 infection. Several reasons can be proposed to explain this result. First, similar to the inflammatory cytokines in severe acute respiratory syndrome (SARS) and middle East respiratory syndrome, patients with COVID‐19 also have increased plasma concentrations of inflammatory cytokines, such as tumor necrosis factor‐α, interleukins (IL) 2, 6, 7, and 10, granulocyte‐colony stimulating factor, monocyte chemoattractant protein 1, macrophage inflammatory protein 1 alpha, and interferon‐γ‐inducible protein 10, especially in intensive care unit patients, which implied a cytokine storm occurred. 16 Cytokine storm refers to an excessive inflammatory response flaring out of control and the immune system gone awry, which can be caused by infection, some drugs, and other factors. SARS cornavirus 2 binds to alveolar epithelial cells and then activates the innate and adaptive immune systems, resulting in the release of a large number of cytokines, including IL‐6. Besides, vascular permeability is increased by these proinflammatory factors, resulting in a large amount of fluid and blood cells entering the alveoli, resulting in dyspnea and even respiratory failure. 5 IL‐6 itself plays a central role in the cytokine storm, which is frequently observed in patients with severe COVID‐19 infection and higher levels of IL‐6 have been observed in severe COVID‐19 disease compared with the nonsevere disease. 5 Induction of cytokine storm by IL‐6 will result in a robust inflammatory response as evidenced by an increment in CRP and PCT levels while reduction in Lymphocyte counts, activation of coagulation or clotting pathway as evidenced by high levels of d ‐dimer, hypoxia, and also shock. 17 IL‐6 itself is also a strong inducer of acute‐phase reactive proteins. It can induce hepatocytes to synthesize acute‐phase reactive protein at the gene transcription level, especially serum amyloid A and CRP, which can make the level of CRP in severe COVID‐19 patients become significantly increased. 18 , 19 Moreover, IL‐6 can also induce the release of hepcidin from the liver that blocks the iron‐exporter (ferroportin), and eventually results in hyperferritinemia. Hyperferritinemia will give rise to ferroptosis, with high oxidative stress and lipoperoxidation that can precipitate the inflammatory or immune overresponse (cytokine storm). 20 All of these combinations will result in the development of severe outcomes of the disease. Tocilizumab specifically binds soluble and membrane‐bound IL‐6 receptors (sIL‐6R and mIL‐6R) and inhibits sIL‐6R‐ and mIL‐6R‐mediated signal transduction. Therefore, by inhibiting IL‐6 through tocilizumab administration, we can reduce the incidence of cytokine storm, preventing the overt inflammatory and coagulation cascade, also preventing the ferroptosis from happening. All of those associated markers (CRP, d ‐dimer, ferritin, PCT, lymphocyte count) will then follow to show improvement (by either reduction or increment).
The strength of this study is the meta‐analysis design of this study itself, which can give clear evidence and help in resolving the conflicting data regarding the benefit of tocilizumab treatment. Moreover, most of the included studies use the same dosage of tocilizumab treatment, which can reduce the heterogeneity of this meta‐analysis. However, this study has several limitations. First, most of the included studies that analyze the benefit of tocilizumab treatment is an observational study or case‐series studies, while none of them was a randomized controlled trial study. The weakness of an observational study is the presence of several confounding factors that can affect the outcomes of tocilizumab treatment, such as the age, pre‐existing comorbid conditions of patients, and bias from the researcher itself (e.g., selection bias). Second, the route of administration, days from symptoms onset to tocilizumab administration, and duration of laboratory follow‐up are different among the included studies, which can increase the heterogeneity of this meta‐analysis and potentially affect the difference in the treatment outcomes. Finally, this study only analyze the benefit of tocilizumab administration in reducing the biomarkers of COVID‐19, but not on the mortality outcome, although these two parameters may be connected to each other.
5. CONCLUSION
Administration of tocilizumab is effective in reducing the biomarkers of the COVID‐19 infection, especially CRP and ferritin levels. Physicians may hence consider adding tocilizumab as a treatment for patients with COVID‐19, especially in patients with severe disease, to help in reducing the severity of COVID‐19. A more randomized clinical trial of tocilizumab is still needed to give a better assessment of tocilizumab efficacy. Finally, tocilizumab shall be regarded as an important agent in future treatment models for COVID‐19.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Timotius Ivan Hariyanto: Conceptualization, data curation, methodology, investigation, validation, visualization, writing–original draft, review, and editing. Andree Kurniawan: Conceptualization, alidation, resources, writing–original draft, review and editing, and supervision.
Hariyanto TI, Kurniawan A. Tocilizumab administration is associated with the reduction in biomarkers of coronavirus disease 2019 infection. J Med Virol. 2021;93:1832–1836. 10.1002/jmv.26698
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in PubMed at http://pubmed.ncbi.nlm.nih.gov/, reference number 4–12.
REFERENCES
- 1. Hariyanto TI, Kurniawan A. Anemia is associated with severe coronavirus disease 2019 (COVID‐19) infection. Transfus Apher Sci. 2020:102926. 10.1016/j.transci.2020.102926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hariyanto TI, Kurniawan A. Thyroid disease is associated with severe coronavirus disease 2019 (COVID‐19) infection. Diabetes Metab Syndr. 2020;14(5):1429‐1430. 10.1016/j.dsx.2020.07.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hariyanto TI, Kurniawan A. Dyslipidemia is associated with severe coronavirus disease 2019 (COVID‐19) infection. Diabetes Metab Syndr. 2020;14:1463‐1465. 10.1016/j.dsx.2020.07.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Henry BM, de Oliveira MHS, Benoit S, Plebani M, Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID‐19): a meta‐analysis. Clin Chem Lab Med. 2020;58(7):1021‐1028. 10.1515/cclm-2020-0369 [DOI] [PubMed] [Google Scholar]
- 5. Zhang C, Wu Z, Li JW, Zhao H, Wang GQ. Cytokine release syndrome in severe COVID‐19: interleukin‐6 receptor antagonist tocilizumab may be the key to reduce mortality. Int J Antimicrob Agents. 2020;55(5):105954. 10.1016/j.ijantimicag.2020.105954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. 10.1186/1471-2288-14-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alattar R, Ibrahim TBH, Shaar SH, et al. Tocilizumab for the treatment of severe coronavirus disease 2019. J Med Virol. 2020;92:2042‐2049. 10.1002/jmv.25964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Conrozier T, Lohse A, Balblanc JC, et al. Biomarker variation in patients successfully treated with tocilizumab for severe coronavirus disease 2019 (COVID‐19): results of a multidisciplinary collaboration. Clin Exp Rheumatol. 2020;38(4):742‐747. https://www.clinexprheumatol.org/abstract.asp?a=15887 [PubMed] [Google Scholar]
- 9. De Rossi N, Scarpazza C, Filippini C, et al. Early use of low dose tocilizumab in patients with COVID‐19: A retrospective cohort study with a complete follow‐up. EClin Med. 2020;25:100459. 10.1016/j.eclinm.2020.100459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Issa N, Dumery M, Guisset O, Mourissoux G, Bonnet F, Camou F. Feasibility of tocilizumab in ICU patients with COVID‐19. J Med Virol. 2020. 10.1002/jmv.26110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mastroianni A, Greco S, Apuzzo G, et al. Subcutaneous tocilizumab treatment in patients with severe COVID‐19‐related cytokine release syndrome: an observational cohort study. EClin Med. 2020;24:100410. 10.1016/j.eclinm.2020.100410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Potere N, Di Nisio M, Cibelli D, et al. Interleukin‐6 receptor blockade with subcutaneous tocilizumab in severe COVID‐19 pneumonia and hyperinflammation: a case‐control study. Ann Rheum Dis. 2020:annrheumdis‐2020‐218243. 10.1136/annrheumdis-2020-218243 [DOI] [PubMed] [Google Scholar]
- 13. Price CC, Altice FL, Shyr Y, et al. Tocilizumab treatment for cytokine release syndrome in hospitalized COVID‐19 patients: survival and clinical outcomes. Chest. 2020;158:1397‐1408. 10.1016/j.chest.2020.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Toniati P, Piva S, Cattalini M, et al. Tocilizumab for the treatment of severe COVID‐19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: a single center study of 100 patients in Brescia, Italy. Autoimmun Rev. 2020;19(7):102568. 10.1016/j.autrev.2020.102568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xu X, Han M, Li T, et al. Effective treatment of severe COVID‐19 patients with tocilizumab. Proc Natl Acad Sci USA. 2020;117(20):10970‐10975. 10.1073/pnas.2005615117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fu B, Xu X, Wei H. Why tocilizumab could be an effective treatment for severe COVID‐19? J Transl Med. 2020;18(1):164. 10.1186/s12967-020-02339-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang S, Li L, Shen A, Chen Y, Qi Z. Rational use of tocilizumab in the treatment of novel coronavirus pneumonia. Clin Drug Investig. 2020;40(6):511‐518. 10.1007/s40261-020-00917-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schmidt‐Arras D, Rose‐John S. IL‐6 pathway in the liver: from physiopathology to therapy. J Hepatol. 2016;64(6):1403‐1415. 10.1016/j.jhep.2016.02.004 [DOI] [PubMed] [Google Scholar]
- 19. Fraunberger P, Wang Y, Holler E, et al. Prognostic value of interleukin 6, procalcitonin, and C‐reactive protein levels in intensive care unit patients during first increase of fever. Shock. 2006;26(1):10‐12. 10.1097/01.shk.0000215319.06866.bd [DOI] [PubMed] [Google Scholar]
- 20. Cavezzi A, Troiani E, Corrao S. COVID‐19: hemoglobin, iron, and hypoxia beyond inflammation. A narrative review. Clin Pract. 2020;10(2):1271. 10.4081/cp.2020.1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are openly available in PubMed at http://pubmed.ncbi.nlm.nih.gov/, reference number 4–12.


