Abstract
In December 2019, a new type of coronavirus was detected for the first time in Wuhan, Hubei Province, China. According to the reported data, the emerging coronavirus has spread worldwide, infecting more than fifty‐seven million individuals, leading to more than one million deaths. The current study aimed to review and discuss the hematological findings of COVID‐19. Laboratory changes and hematologic abnormalities have been reported repeatedly in COVID‐19 patients. WBC count and peripheral blood lymphocytes are normal or slightly reduced while these indicators may change with the progression of the disease. In addition, several studies demonstrated that decreased hemoglobin levels in COVID‐19 patients were associated with the severity of the disease. Moreover, thrombocytopenia, which is reported in 5%‐40% of patients, is known to be associated with poor prognosis of the disease. COVID‐19 can present with various hematologic manifestations. In this regard, accurate evaluation of laboratory indicators at the beginning and during COVID‐19 can help physicians to adjust appropriate treatment and provide special and prompt care for those in need.
Keywords: COVID‐19, hematology, laboratory findings, SARS‐CoV‐2
1. INTRODUCTION
In late 2019, a new coronavirus was isolated from pneumonia patients with unknown etiology for the first time in Wuhan, China. 1 , 2 According to the latest reported data, the emerging coronavirus has crossed international borders, infecting more than thirty‐seven million individuals, leading to more than million deaths. 3 The new coronavirus, known as Severe Acute Respiratory Syndrome Coronavirus 2 (SARS‐CoV‐2), belongs to the beta group of Coronaviridae family and it is the third notorious zoonotic disease of coronaviruses after Severe Acute Respiratory Syndrome (SARS) and Middle East Respiratory Syndrome (MERS). SARS‐CoV‐2 is responsible for a multi‐systemic disease, called COVID‐19 with an incubation period of one to 14 days. 4 , 5 Moreover, COVID‐19 presents with a wide spectrum of clinical signs and symptoms, varying from asymptomatic infection to acute respiratory distress syndrome, multifunctional organ dysfunction, and death. Clinically diagnosis of COVID‐19 may be difficult since the clinical manifestations of COVID‐19 are very similar to that of various viral infections. 6 In addition, studies have reported laboratory features of COVID‐19 patients. 7 However, unfortunately, these studies are limited to one or few blood indicators. As the COVID‐19 pandemic grows, it is crucial to raise the physicians’ awareness about COVID‐19 hematologic features. In this regard, the current study aimed to review and discuss the hematological findings of COVID‐19 derived from automated hematology and clinical immunology/ flow cytometry.
2. MECHANISMS OF SARS‐COV‐2 INVASION TO HOST CELLS
Zhao et al observed that SARS‐CoV‐2, similar to SARS‐CoV, enters the host cells through angiotensin‐converting enzyme 2 (ACE2) receptors. 8 , 9 Studies showed that ACE2 is expressed in the alveolar epithelial cells type 2 and 1, myocytes, vascular endothelial cells, and some other cells, including hematopoietic stem cells and progenitors. 10 The bind of SARS‐CoV‐2 to ACE2 receptors increases the expression of this receptor, leading to further damage of alveolar cells, which may in turn cause a series of systemic reactions leading to death. After attaching to the ACE2 receptors, the spike proteins of SARS‐CoV‐2 are broken down through acid‐dependent proteolysis by cathepsin, Transmembrane protease‐serine 2 (TMPRSS2), or furin protease, and consequently, the SARS‐CoV‐2 merges with the cell membrane. Compared to the other coronavirus proteins, spike protein has the most variable sequence of amino acids among all coronavirus genes, which provides a strong option for coronavirus to adapt to its hosts. 11 , 12 Recent studies suggested that SARS‐CoV‐2 may attack host cells via the CD147‐spike protein (SP) pathway. In this regard, SP facilitates the virus invasion by connecting to CD147. The CD147, also known as BSG (Basigin) and extracellular matrix metalloproteinase inducer (EMMPRIN), is a plasma membrane protein from the immunoglobulin family, and it is encoded by the BSG gene in humans. 10 , 13 This protein is expressed to a varying extent in the hematopoietic cells, mesenchymal stem cells, leukocytes, epithelial and endothelial cells, and has a wide range of physiological and pathological activities. Studies showed that CD147 expression on the red blood cells (as an adhesion molecule) plays a crucial role in the circulation of mature red blood cells from the spleen to the bloodstream. 13 , 14
Figure 1 Possible invasion mechanism of SARS‐CoV‐2, CD147, and ACE2 acts as receptors of host cells for the invasion of SARS‐CoV‐2.
Figure 1.
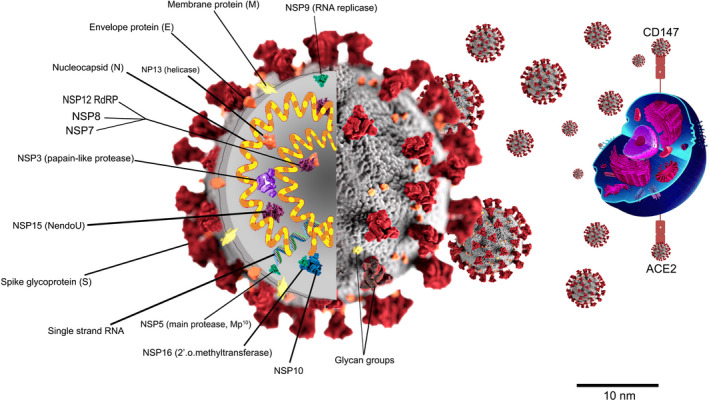
Possible invasion mechanism of SARS‐CoV‐2, CD147, and ACE2 acts as receptors of host cells for the invasion of SARS‐CoV‐2
3. HEMATOLOGY FINDINGS OF COVID‐19 AND ITS RELATIONSHIP WITH DISEASE SEVERITY
Laboratory changes and hematologic abnormalities have been reported repeatedly in COVID‐19 patients, which will be discussed in the following (Table 1).
Table 1.
Laboratory finding studies in COVID‐19 patients
| Subject of study | Region study | Sample size | Main findings | References |
|---|---|---|---|---|
| Laboratory data analysis of novel coronavirus (COVID‐19) screening in 2510 patients | Clinic of Xiangya Second Hospital of Central South University | 2510 | The count of monocyte increases in COVID‐19 patients compared to influenza patients. | [9] |
| Viral load dynamics and disease severity in patients infected with SARS‐CoV‐2 in Zhejiang province, China, January‐March 2020: retrospective cohort study | Designated hospital for patients with COVID‐19 in Zhejiang province, China | 96 | Observed no significant quantitative difference in monocyte level between the COVID‐19 patients and healthy individuals | [39] |
| Pathogenic T cells and inflammatory monocytes incite inflammatory storms in severe COVID‐19 patients | Review | observed that GM‐CSF + Th1 cells and CD14+ CD16+ monocytes accelerated the immune response and exacerbated the COIVID‐19 disease through over expression of IL‐6. | [40] | |
| Abnormalities of peripheral blood system in patients with COVID‐19 in Wenzhou, China | Wenzhou | 116 | Observed that there is significant difference between COVID‐19 patients and the control group in terms of monocyte count, the severity of the disease, and ICU admission requirement. | [41] |
| COVID‐19 infection induces readily detectable morphological and inflammation‐related phenotypic changes in peripheral blood monocytes, the severity of which correlate with patient outcome | Multicenter Study | 28 | Stated that the sequential detection and monitoring of this subset of inflammatory monocytes using flow cytometry can help determine the prognosis of COVID‐19 patients | [27] |
| Morphological anomalies of circulating blood cells in COVID‐19 | Parallel COVID‐19 hospital at Fondazione Policlinico A. Gemelli of Rome, a | 40 | They observed that the absolute neutrophil count mostly increased in the first few days of hospitalization, while decreased slightly before or immediately after the treatment | [42] |
| Hematological findings and complications of COVID‐19 | Review Article | They found that neutrophilic leukocytosis was associated with an increased risk of acute respiratory syndrome, risk of death, and elevated troponin level. | [29] | |
| Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID‐19): a meta‐analysis | Meta‐analysis | They confirmed that neutrophil count was significantly higher in non‐survivors COVID‐19 patients compared to survivors. | [46] | |
| Patients of COVID‐19 may benefit from sustained Lopinavir‐combined regimen and the increase of Eosinophil may predict the outcome of COVID‐19 progression | Xixi hospital in Hangzhou, China | 10 | Increasing eosinophils may be an indicator of COVID‐19 improvement. | [49] |
| Evaluation of Hepatic Enzymes Changes and Association with Prognosis in COVID‐19 Patients | Mazandaran University of Medical Sciences’ hospitals | 93 | Hemoglobin levels were lower in COVID‐19 patients than in the control group, although the difference was not statistically significant | [52] |
| Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID‐19) | Seventh Hospital of Wuhan City, China | 187 | observed that hemoglobin levels were not significantly different in COVID‐19 patients with a history of myocardial injury and elevated TnT levels compared with those with normal TnT levels | [53] |
| Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID‐19) infections: A meta‐analysis | Meta‐analysis | A relationship between platelet count at the time of hospitalization and the severity of the disease was observed | [58] | |
| Association between platelet parameters and mortality in coronavirus disease 2019: Retrospective cohort study | Central Hospital of Wuhan | 383 | Baseline platelet levels and changes were associated with subsequent mortality. Monitoring platelets during hospitalization may be important in the prognosis of patients with coronavirus disease in 2019. | [57] |
4. WHITE BLOOD CELLS
Studies showed that in the early stages of COVID‐19 disease when patients have no exclusive symptoms, White blood cells (WBC) count and peripheral blood lymphocytes are normal or slightly reduced while these indicators may change with the progression of the disease. 15 Zhang et al, 16 in a study on 140 hospitalized patients, who were diagnosed with COVID‐19 based on the computed tomography (CT) scan findings, showed that the leukocyte count was within normal ranges in 68.1% of patients, increased in 12.3% of patients, and decreased in 19.6% of patients. Also, leukopenia has been reported in other studies to be between 28.1% and 68.1% depending on the severity of the disease and the underlying conditions, suggesting a possible association between the severity of leukopenia and the severity of the COVID‐19. 17 , 18 , 19 , 20 In a retrospective study in Wuhan, China, it was reported that COVID‐19 patients with a history of myocardial infarction (MI) had a higher leukocyte count than other patients. In another study, it was found to be an association between troponin levels and leukocyte counts, and patients with higher troponin had higher leukocyte counts, indicating a link between underlying disease and the leukocyte count in COVID‐19 patients. 21 In a study by Mardani et al, 22 they observed that absolute WBC count was lower in the COVID‐19 patients with positive RT‐PCR test than in patients with negative RT‐PCR test. Javanian et al, in a study indicated that WBC count is higher in non‐survivor patients than those who survive. They concluded that lymphopenia and increased WBC count may be associated with increased C‐reactive protein (CRP) and mortality. 23 According to the results of a meta‐analysis conducted by Henry et al, the WBC count was significantly higher in non‐survivors COVID‐19 patients in comparison with survivors. Their meta‐analysis also showed that the WBC count increases mildly in people with severe COVID‐19, while a significant increase in WBC count may predict a poor clinical prognosis. 24 , 25 , 26 All the current evidences highly suggest that although WBC count can be used as a predictor factor for severer COVID‐19 condition, factors such as the onset of the disease, underlying diseases, severity of the disease, and RT‐PCR results should be taken into account.
5. LYMPHOCYTE
Similar to other coronaviruses and viral infections, a common feature of COVID‐19 patients is lymphopenia. In these patients, the total number of lymphocytes, TCD4+, TCD8+, B cells, and Natural killer cells (NK cells) decreases, which the reduction of CD8+ T cells is more significant. 27 Several studies reported the prevalence of lymphopenia to be between 40% and 91.6% in COVID‐19 patients and suggested that lymphopenia can be used as a prognostic prediction factor for COVID‐19. 8 In a study conducted by BE FAN and colleagues, in addition to observing lymphopenia in 69 patients with COVID‐19, they demonstrated that 69% of patients with lymphopenia had reactive lymphocytes (Lymphoplasmacytoid). 15 The study also found that intensive care unit (ICU) admitted patients had lower levels of CD45+, CD19+, CD8+, CD4+, and CD16/56+ than non‐ICU patients. 15 However, unlike other viral infections such as human immunodeficiency virus (HIV) and cytomegalovirus (CMV), the CD4+/8+ ratio was not reversed in any patients. Similarly, in a study by Chan et al, 28 on 75 COVID‐19 patients, they observed that the level of total lymphocytes, T cells, B cells, and NK cells were meaningfully decreased in COVID‐19 patients in comparison with healthy individuals, indicating a possible relation between lymphocyte subset alteration and the pathogenesis of SARS‐CoV‐2. Moreover, the mentioned study demonstrated that COVID‐19 ICU patients had considerably lower level of total lymphocytes, CD4+ T cells, CD8+ T cells, and B cells. 28 Several studies have investigated the association between lymphopenia and the severity of the disease, acute respiratory distress syndrome, ICU admissions, and mortality. Their studies suggested that there might be a significant relationship between the severity of lymphopenia and the severity of the COVID‐19, the need for ICU admissions. 8 , 29 , 30 , 31 , 32 , 33 Also, those with severe lymphopenia are at a higher risk of death during hospitalization. It is also stated that there might be a strong association between a decreased in Lymphocyte/WBC ratio at the admission, or during hospitalization with the severity of COVID‐19. Also, Zhao et al 8 in a meta‐analysis study demonstrated that lymphopenia increases the risk of severe COVID‐19 by about threefold.
Moreover, a study by Qin et al showed that both T helper and suppressor T cells were decreased in COVID‐19 patients. They also observed that the decreased levels of T helper are associated with the severity of COVID‐19. 34 They stated that the percentage of naive T helper cells was increased in COVID‐19 patients, and also memory T helper cells were decreased in severe cases as well. In addition, reduced levels of regulatory T cells were more significant in COVID‐19 patients. 34 Although the lymphopenia associated with COVID‐19 may be simply justified with the depletion of circulating T cells during the inflammatory responses or administration of steroids, other hypotheses have been suggested. Since lymphocytes express ACE2 and CD147 on their membrane, it is hypothesized that SARS‐CoV‐2 may directly invade lymphocytes, leading to lysis of lymphocytes and lymphopenia. 14 Also, it is observed that 7‐14 days from the onset of the initial symptoms, new sets of clinical symptoms appear along with elevated levels of inflammatory mediators. 35 , 36 Therefore, another hypothesis that can be proposed is that the significant activity of cytokines first leads to atrophy of secondary lymphatic organs, including spleen and disrupt the turn‐over of lymphocytes, and secondly increases the expression of death receptor FAS and Apoptosis leading to lymphopenia. It should be noted that there is a negative relationship between serum levels of interleukin 6 (IL‐6), interleukin 10 (IL‐10), and Tumor Necrosis Factor‐Alpha (TNF‐α) with the number of lymphocytes. 27 , 37 In contrast to other studies, Hong‐Yi Zheng et al 21 reported that they did not observe any lymphopenia in their studied population, and the absolute count of leukocyte in both mild and severe COVID‐19 patients has been remained within the normal range. The study also suggested that SARS‐CoV‐2 and the resulting cytokine storm may disrupt the function of TCD4+ and T regulatory cells by exceeding activation of T cytotoxic, and activation‐induced cell death, leading to TCD4+ and T regulatory cells apoptosis and exacerbation of the disease. Another study suggested that the coexisting lactic acid acidosis (especially in cancer patients) may be responsible for COVID‐19 associated lymphopenia. Current evidences suggest that due to the high prevalence of lymphopenia in patients with COVID‐19 and its strong association with the severity of the disease, lymphocytes count, especially levels of CD4+, can be used as a predictive biomarker for the severity of the disease.
6. MONOCYTES AND MACROPHAGES
Monocytes trigger inflammation through the production of cytokines and the activation of lymphocytes. Studies showed that the acute pulmonary injury and Acute respiratory distress syndrome (ARDS) in COVID‐19 patients are consistent with over activation of monocytes/macrophages and cytokine storms. 15 , 37 , 38 , 39 Yun et al demonstrated that the count of monocyte increases in COVID‐19 patients compared to influenza patients. 9 However, some studies indicated a decreased count of monocytes, especially in ICU‐admitted patients. Interestingly, Zhang et al 27 in a quantitative and qualitative study of monocytes in COVID‐19 patients reported no significant quantitative difference considering monocyte level between the COVID‐19 patients and healthy individuals. However, they observed significant differences in morphology and function of monocyte between COVID‐19 patients and healthy individuals. They found that patients with COVID‐19 have larger monocytes, along with the CD11b+, CD14+, CD16+, CD68+, CD80+, CD163+, CD206+, and the ability to secrete IL‐6, IL‐10, and TNF‐α, which are compatible with an inflammatory phenotype. 27 Individuals with these kinds of monocytes required long‐term hospitalization and ICU admission. Also, in a study by Zeng et al, 25 they observed that monocyte distribution width (MDW) was significantly increased in COVID‐19 patients than the control group. In addition, Zhou et al observed that Granulocyte‐macrophage colony‐stimulating factor (GM‐CSF) + Th1 cells and CD14+ CD16+ monocytes accelerated the immune response and exacerbated the COVID‐19 disease through overexpression of IL‐6. 40 Moreover, a study by Sun et al 41 showed that there is a significant difference between COVID‐19 patients and the control group in terms of monocyte count, the severity of the disease, and the requirement of ICU admission. Also, they demonstrated that the monocyte‐lymphocyte ratio (MLR) was higher in COVID‐19 patients than in the control group, especially in those with severe COVID‐19. 41 The study by Zhang et al 27 suggested that the sequential detection and monitoring of this subset of inflammatory monocytes using flow cytometry can help determine the prognosis of COVID‐19 patients. However, due to the lack of access to flow cytometry in most laboratories and the contradictory evidence, further studies are required to consider monocytes as prognostic biomarkers in COVID‐19 patients.
7. GRANULOCYTES
Blood neutrophil levels usually increase in the face of infectious agents and tissue damage. In a study by Zini et al, 42 on 40 COVID‐19 patients, they observed that the absolute neutrophil count mostly increased in the first few days of hospitalization, while decreased slightly before or immediately after the treatment. Also, they indicated that in the COVID‐19 patients, the neutrophils were morphologically abnormal with the cytoplasm containing dark‐like toxic granules with peripheral light blue agranular areas. In this regard, hypogranular neutrophils were abundant in a small percentage of patients. Besides granular changes, neutrophils were severely impaired in nuclear lobulation, and Pseudo Pelger‐Huet was observed numerously in peripheral blood smears. 42 Immature granulocytes, especially small myelocytes, and metamyelocytes were demonstrated in COVID‐19 patients at the time of hospitalization and before treatment. Moreover, in two studied patients, neutrophil peroxidase activity was severely decreased. After 5‐7 days of treatment with antiviral and anti‐inflammatory drugs and re‐examination of a peripheral blood smear, all the above changes were completely abolished. Also, high levels of apoptotic cells were observed in peripheral blood smear of COVID‐19 patients. 42 In a study by Sadigh et al 43 on 78 peripheral blood smears of COVID‐19 patients, they observed that smudged neutrophils were significantly increased in COVID‐19 patients in comparison with the control group. In summary, granulocytic reaction with immaturity, dysmorphism, and apoptotic‐degenerative morphology was observed at the time of hospitalization in patients with COVID‐19. In a review study by Terpos, it was found that neutrophilic leukocytosis was associated with an increased risk of acute respiratory syndrome, risk of death, and elevated troponin levels. 29 Another study indicated that the neutrophil‐to‐lymphocyte ratio (NLR ratio) was higher in severe COVID‐19 patients. 44 NLR, which is easily calculated by dividing the absolute number of neutrophils by the absolute number of lymphocytes, is of great importance in expressing the general inflammatory condition of the patient. 45 The aforementioned study suggested that the calculation of NLR during and after hospitalization can be a predictor of mortality risk in hospitalized COVID‐19 patients, although further studies are suggested. 33 , 45 A meta‐analysis conducted by Henry et al confirmed that neutrophil count was significantly higher in non‐survivors COVID‐19 patients compared to survivors. 46 Similarly, another meta‐analysis by Shi et al revealed that non‐survival COVID‐19 patients had higher neutrophil counts in comparison with the survival COVID‐19 patients. 47 Sun et al, also reported that ICU admitted COVID‐19 patients had a higher neutrophil count and NLR compared to the control group. 41 In addition, an autopsy study of COVID‐19 patients reported neutrophil infiltration into the pulmonary capillaries, extravasation of neutrophils into the alveolar space, and neutrophilic mucositis. 48 According to the current evidences, the neutrophil count not only determines the prognosis of COVID‐19 but also plays an important role in the immunopathology of severe COVID‐19. Moreover, increased neutrophil counts (by cytokines such as Granulocyte colony‐stimulating factor [G‐CSF]), the recall of neutrophils, and their inflammatory activities (production) as well as (Neutrophil Extracellular Traps) NETs from overactive neutrophils, may be responsible for severe complications of COVID‐19, including ARDS. This raises the question of whether the neutrophil microparticles are involved in COVID‐19‐associated complications. Microparticles, also known as extracellular vesicles, are particles with a 30‐1000 nm diameter, which are released from a variety of cells in physiological and pathological conditions. These particles play an important role in the development and progression of various diseases. In addition to neutrophilia, in some studies, eosinopenia is reported in COVID‐19 patients. In a study, Zhang et al 16 observed an association between eosinopenia and lymphopenia in patients with severe COVID‐19. In addition, the study by Liu et al 49 demonstrated eosinophilia in COVID‐19 patients at the time of hospitalization. They reported that the use of sustained Lopinavir‐combined regimen could help increasing eosinophils, and eosinophil count increased after 7‐9 days of treatment. Finally, they suggested that increased eosinophil count could be a sign of COVID‐19 recovery. 49 In addition, Sun et al suggested that a sequential assessment of peripheral blood, especially eosinophil count, could help predict severe cases of COVID‐19. Similar to neutrophils and lymphocytes, eosinophil levels can be considered as a prognostic biomarker in predicting severe cases of COVID‐19. Basophils are another group of peripheral blood granulocytes that can be involved in allergic diseases and play a role in the release of histamine and many other biologically active molecules. Studies have reported the reduced basophil count in patients with COVID‐19. 34
8. RED BLOOD CELLS AND HEMOGLOBIN
ARDS is a common manifestation of various respiratory diseases with no exclusive therapy. Until recently, research on the pathophysiology of ARDS was focused on the interaction between white blood cells, platelets, pulmonary endothelium and epithelium, and red blood cells were considered as a neutral observer in the pathogenesis of ARDS. 50 Nevertheless, the role of Red blood cell (RBC) as a pathological mediator in ARDS has gained attention with new insights into RBC membrane changes during critical illness, and the role of cell‐free hemoglobin in oxidative stress and endothelial damage. 51 So far, studies of COVID‐19 did not address the role of RBCs in the pathogenesis of the diseases, and only a few limited studies were conducted considering hemoglobin levels (the main constituent of RBCs). Huang et al 31 demonstrated that in COVID‐19 patients, the increased level of inhibitory cytokines such as interleukin 4 (IL‐4) and IL‐10 was responsible for the inhibition of erythropoiesis and lymphopenia. In another study by Omrani‐Nava et al, 52 they reported that hemoglobin levels were lower in COVID‐19 patients than in the control group, although the difference was not statistically significant. In addition, Guo et al observed that hemoglobin levels were not significantly different in COVID‐19 patients with a history of myocardial injury and elevated Cardiac troponin T (TnT) levels compared with those with normal TnT levels. 53 Moreover, Yun et al observed that red cell distribution width (RDW) increases in COVID‐19 patients compared to patients with influenza. 9 In contrast to the mentioned study, Pan and colleagues observed a decrease in RDW and an increase in hemoglobin and hematocrit levels in COVID‐19 patients. 54 These studies showed that decreased hemoglobin levels in COVID‐19 patients were associated with the severity of the disease. Therefore, in addition to a broader quantitative and qualitative study of erythroid cells and their role in the pathogenesis of COVID‐19, further studies are suggested to assess whether blood transfusions in these clinical conditions can help prevent COVID‐19 progression and complications.
9. PLATELET
Platelets not only play a crucial role in homeostasis but also are responsible for inflammatory and defense mechanisms. Thrombocytopenia is one of the most common clinical manifestations of COVID‐19, reported in 5%‐40% of patients, and it is known to be associated with poor prognosis of the disease. 29 , 55 , 56 , 57 In a meta‐analysis by Lippi et al, 58 a relationship between platelet count at the time of hospitalization and the severity of the disease was observed, this was confirmed by several other studies. Furthermore, Yang and his colleagues indicated that thrombocytopenia is a common finding in patients with COVID‐19 and it is associated with an increased risk of mortality. 59 In a recent study by Liu et al on 383 confirmed hospitalized COVID‐19 patients, they observed that patients with thrombocytopenia are older than those without thrombocytopenia. These patients had higher levels of Acute Physiology And Chronic Health Evaluation II (APACHE II), procalcitonin levels, CRP, total bilirubin, aspartate aminotransferase, blood urea nitrogen, creatinine, and D‐dimer. 57 While partial pressure of oxygen (PaO2), fraction of inspired oxygen (FiO2), WBC count, neutrophils, and lymphocytes were lower in these patients. Evaluation of platelet parameters revealed that patients with thrombocytopenia have higher levels of Mean platelet volume (MPV) and lower levels of Platelet‐large cell ratio (P‐LCR). The study also found that platelet count and plateletcrit (PCT) are independent risk factors of mortality in COVID‐19 patients. 57 In addition, a study by Chen et al observed a delayed antibody thrombocytopenia in COVID‐19 patients. 60 Several mechanisms have been proposed for the development of thrombocytopenia in COVID‐19 patients. One of these mechanisms is the direct and indirect effect of SARS‐CoV‐2 on the hematopoietic cells and endothelial cells, which can be associated with impaired megakaryocyte maturation, increased platelet aggregation, platelet activation and, consequently, platelet consumption in the microcirculation of damaged lung tissue. Platelets can be linked directly to viruses via toll‐like receptors, P‐selectin, and surface integrins. Therefore, it is assumed that SARS‐CoV‐2 can bind to specific receptors, inhibits erythropoiesis in the bone marrow and result in thrombocytopenia by disrupting the platelet production. Recent studies have shown that CD147, expressed at the level of hematopoietic cells (mesenchymal stem cells, leukocytes, epithelial and endothelial cells), can cause abnormal erythropoiesis. 13 , 14 In addition, activation of platelets in pulmonary microcirculation not only contributes to procoagulant activity but also exacerbates pulmonary damage by triggering respiratory distress and the requirement of mechanical ventilation. The combination of viral infection and mechanical ventilation, in turn, causes endothelial cell damage, platelet activation, platelet aggregation, and thrombosis in the lungs, leading to high platelet consumption. Furthermore, since the lung can release mature platelets from megakaryocytes, a decrease or morphological changes in the pulmonary capillary bed can lead to deranged platelet defragmentation. Disseminated intravascular coagulation (DIC) is known to lessen the thrombocytopenia of COVID‐19 patients. Afterwards, the cytokine storms and inflammation, which is the prominent feature of COVID‐19, should not be neglected. Inflammatory cytokines can cause thrombocytopenia by destroying progenitors in the bone marrow and reducing platelet production. Finally, thrombocytopenia may be due to the presence of autoantibodies, and the destruction of platelets. Due to the relationship between platelet count and increased risk of COVID‐19 mortality, the platelet count can be used as a prognostic marker during hospitalization. 26
10. CONCLUSION
COVID‐19 can present with various hematologic manifestations. In this regard, accurate evaluation of laboratory indicators at the beginning and during the course of COVID‐19 can help physicians to adjust appropriate treatment and provide special and prompt care for those in need.
CONFLICT OF INTEREST
Authors declare that they have no conflict of interests.
AUTHORS CONTRIBUTIONS
MK and FR designed the study and performed the research. HRN wrote the draft and MK and FR revised the draft.
ETHICAL APPROVAL
This article does not contain any studies with human participants or animals performed by any of the authors.
Karimi Shahri M, Niazkar HR, Rad F. COVID‐19 and hematology findings based on the current evidences: A puzzle with many missing pieces. Int J Lab Hematol.2021;43:160–168. 10.1111/ijlh.13412
DATA AVAILABILITY STATEMENT
No datasets were generated or analyzed during the current study.
REFERENCES
- 1. Niazkar HR, Niazkar M. COVID‐19 international outbreak and the need for a suitable estimation model: a second‐order polynomial equation with constant coefficients based on imported infected cases seems inadequate. Asian Pac J Trop Biomed. 2020;13:185‐186. Wolters Kluwer Medknow Publications. [Google Scholar]
- 2. Niazkar HR, Niazkar M. Application of artificial neural networks to predict the COVID‐19 outbreak. Glob Health Res Policy. 2020;5(1). 10.1186/s41256-020-00175-y [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Coronavirus Disease (COVID‐19) Situation Reports [Internet]. [cited Jun 24, 2020]. Available from: https://www.who.int/emergencies/diseases/novel‐coronavirus‐2019/situation‐reports. Accessed November 25, 2020.
- 4. Niazkar M, Niazkar HR. COVID‐19 outbreak: application of multi‐gene genetic programming to country‐based prediction models. Electron J Gen Med. 2020;17(5):em247. 10.29333/ejgm/8232 [DOI] [Google Scholar]
- 5. Niazkar M, Eryılmaz Türkkan G, Niazkar HR, Türkkan YA. Assessment of Three Mathematical Prediction Models for Forecasting the COVID‐19 Outbreak in Iran and Turkey. Comput Math Methods Med. 2020;2020(7056285):1‐13. https://www.hindawi.com/journals/cmmm/2020/7056285/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Niazkar HR, Zibaee B, Nasimi A, Bahri N. The neurological manifestations of COVID‐19: a review article. Neurol Sci. 2020;41(7):1667‐1671. 10.1007/s10072-020-04486-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dorgalaleh A, Dabbagh A, Tabibian S, et al., Patients with congenital bleeding disorders appear to be less severely affected by SARS‐CoV‐2: is inherited hypocoagulability overcoming acquired hypercoagulability of coronavirus disease 2019 (COVID‐19)? Semin Thromb Hemost. 2020;46(7):853‐855 Thieme Medical Publishers. 10.1055/s-0040-1713435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhao Q, Meng M, Kumar R, et al. Lymphopenia is associated with severe coronavirus disease 2019 (COVID‐19) infections: a systemic review and meta‐analysis. Int J Infect Dis. 2020;96:131‐135 10.1016/j.ijid.2020.04.086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yun H, Sun Z, Wu J, Tang A, Hu M, Xiang Z. Laboratory data analysis of novel coronavirus (COVID‐19) screening in 2510 patients. Clin Chim Acta. 2020;507:94‐97. 10.1016/j.cca.2020.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Amiral J, Vissac AM, Seghatchian J. Covid‐19, induced activation of hemostasis, and immune reactions: can an auto‐immune reaction contribute to the delayed severe complications observed in some patients? Transfus Apher Sci. 2020;59:102804. 10.1016/j.transci.2020.102804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wrapp D, Wang N, Corbett KS, et al. Cryo‐EM structure of the 2019‐nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260‐1263. 10.1126/science.abb2507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mitra A, Dwyre DM, Schivo M, et al. Leukoerythroblastic reaction in a patient with COVID‐19 infection. Am J Hematol. 2020;95(8):999‐1000. 10.1002/ajh.25793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ulrich H, Pillat MM. CD147 as a target for COVID‐19 treatment: suggested effects of azithromycin and stem cell engagement. Stem Cell Rev and Rep. 2020;16:434‐440. 10.1007/s12015-020-09976-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Watanabe A, Yoneda M, Ikeda F, Terao‐Muto Y, Sato H, Kai C. CD147/EMMPRIN acts as a functional entry receptor for measles virus on epithelial cells. J Virol. 2010;84(9):4183‐4193. 10.1128/JVI.02168-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fan BE, Chong VCL, Chan SSW, et al. Hematologic parameters in patients with COVID‐19 infection. Am J Hematol. 2020;95(6):E131‐E134. 10.1002/ajh.25847 [DOI] [PubMed] [Google Scholar]
- 16. Zhang J‐J, Dong X, Cao Y‐Y, et al. Clinical characteristics of 140 patients infected with SARS‐CoV‐2 in Wuhan, China. Allergy. 2020;75:1730‐1741. 10.1111/all.14238 [DOI] [PubMed] [Google Scholar]
- 17. Wang LS, Wang YR, Ye DW, Liu QQ. A review of the 2019 Novel Coronavirus (COVID‐19) based on current evidence. Int J Antimicrob Agents. 2020;19:105948. 10.1016/j.ijantimicag.2020.105948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Abdo‐Cuza A, Castellanos‐Gutiérrez R, Treto‐Ramirez J, et al. Safety and efficacy of intranasal recombinant human interferon alfa 2b as prophylaxis for COVID‐19 in patients on a hemodialysis program. J Ren Endocrinol. 2020;7(1):e05. [Google Scholar]
- 19. Khaled SA, Hafez AA. Aplastic anemia and COVID‐19: how to break the vicious circuit? Am J Blood Res. 2020;10(4):60. [PMC free article] [PubMed] [Google Scholar]
- 20. Radisic MV, Piro MA, Mori I, Rotryng F, Santamarina JF. SARS‐CoV‐2 and dengue virus co‐infection. A case report. Hemoglobin. 2020;16(16.8):15–5. [PubMed] [Google Scholar]
- 21. Zheng HY, Zhang M, Yang CX, et al. Elevated exhaustion levels and reduced functional diversity of T cells in peripheral blood may predict severe progression in COVID‐19 patients. Cell Mol Immunol. 2020;17(5):541‐543. 10.1038/s41423-020-0401-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mardani R, Ahmadi Vasmehjani A, Zali F, et al. Laboratory parameters in detection of COVID‐19 patients with positive RT‐PCR; a diagnostic accuracy study. Arch Acad EmergMed. 2020;8(1):e43. [PMC free article] [PubMed] [Google Scholar]
- 23. Javanian M, Bayani M, Shokri M, et al. Clinical and laboratory findings from patients with COVID‐19 pneumonia in Babol North of Iran: a retrospective cohort study. Rom J Intern Med. 2020;58(3):161‐167. 10.2478/rjim-2020-0013 [DOI] [PubMed] [Google Scholar]
- 24. Henry BM. COVID‐19, ECMO, and lymphopenia: a word of caution. Lancet Respir Med. 2020;8(4):e24. 10.1016/S2213-2600(20)30119-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zeng X, Xing H, Wei Y, et al. Monocyte volumetric parameters and lymph index are increased in SARS‐CoV‐2 infection. Int J Lab Hematol. 2020;42(6):e266‐e269. 10.1111/ijlh.13323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Demeester S, Demuyser T, Fauconnier C, et al. Routine haematology parameters in COVID‐19 patients and clinical outcome: a Belgian single‐centre study. Int J Lab Hematol. 2020;42(6):e252‐e255. 10.1111/ijlh.13313 [DOI] [PubMed] [Google Scholar]
- 27. Zhang D, Guo R, Lei L, et al. COVID‐19 infection induces readily detectable morphological and inflammation‐related phenotypic changes in peripheral blood monocytes. J Leukoc Biol. 2020;1‐10. 10.1002/JLB.4HI0720-470R [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chan SSW, Christopher D, Tan GB, et al. Peripheral lymphocyte subset alterations in COVID‐19 patients. Int J Lab Hem. 2020;42:e199‐e203. 10.1111/ijlh.13276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Terpos E, Ntanasis‐Stathopoulos I, Elalamy I, et al. Hematological findings and complications of COVID‐19. Am J Hematol. 2020;95:834‐847. 10.1002/ajh.25829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lovato A, de Filippis C. Clinical presentation of COVID‐19: a systematic review focusing on upper airway symptoms. Ear, Nose & Throat J. 2020;99(9):569–576. 10.1177/0145561320920762 [DOI] [PubMed] [Google Scholar]
- 31. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yang MO, Ng MHL, Li CK. Thrombocytopenia in patients with severe acute respiratory syndrome (review). Hematology. 2005;10(2):101‐105. 10.1080/10245330400026170 [DOI] [PubMed] [Google Scholar]
- 33. Zhang H, Cao X, Kong M, et al. Clinical and hematological characteristics of 88 patients with COVID‐19. Int J Lab Hematol. 2020;42(6):780‐787. 10.1111/ijlh.13291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Qin C, Ziwei MP, Tao SY, Ke PC, Shang MM.Dysregulation of immune response in patients with COVID‐19 in Wuhan, China; Clinical Infectious Diseases; Oxford Academic. Clinical Infectious Diseases.
- 35. Liu J, Li S, Liu J, et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS‐CoV‐2 infected patients. EBioMedicine. 2020;55(102763):1‐9. 10.1016/j.ebiom.2020.102763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhu Z, Cai T, Fan L, et al. Clinical value of immune‐inflammatory parameters to assess the severity of coronavirus disease 2019. Int J Infect Dis. 2020;95:332‐339. 10.1016/j.ijid.2020.04.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Giamarellos‐Bourboulis EJ, Netea MG, Rovina N, et al. Complex immune dysregulation in COVID‐19 patients with severe respiratory failure. Cell Host Microbe. 2020;27(6):992‐1000.e3. 10.1016/j.chom.2020.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Merad M, Martin JC. Pathological inflammation in patients with COVID‐19: a key role for monocytes and macrophages. Nat Rev Immunol. 2020;20:355‐362. 10.1038/s41577-020-0331-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zheng S, Fan J, Yu F, et al. Viral load dynamics and disease severity in patients infected with SARS‐CoV‐2 in Zhejiang province, China, January‐March 2020: retrospective cohort study. BMJ. 2020;369:m1443. 10.1136/bmj.m1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhou Y, Fu B, Zheng X, et al. Pathogenic T‐cells and inflammatory monocytes incite inflammatory storms in severe COVID‐19 patients. Natl Sci Rev. 2020;7(6):998‐1002. 10.1093/nsr/nwaa041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sun S, Cai X, Wang H, et al. Clinica Chimica Acta Abnormalities of peripheral blood system in patients with COVID‐19 in. Clin Chim Acta. 2020;507:174‐180. 10.1016/j.cca.2020.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zini G, Bellesi S, Ramundo F, d'Onofrio G. Morphological anomalies of circulating blood cells in COVID‐19. Am J Hematol. 2020;95:870‐872. 10.1002/ajh.25824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sadigh S, Massoth LR, Christensen BB, Stefely JA, Keefe J, Sohani AR. Peripheral blood morphologic findings in patients with COVID‐19. Int J Lab Hematol. 2020;42(6):e248‐e251. 10.1111/ijlh.13300 [DOI] [PubMed] [Google Scholar]
- 44. Kong J, Wang T, Di Z, et al. Analysis of hematological indexes of COVID‐19 patients from fever clinics in Suzhou, China. Int J Lab Hem. 2020;42:e204‐e206. 10.1111/ijlh.13290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Liu Y, Du X, Chen J, et al. Neutrophil‐to‐lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID‐19. J Infect. 2020;81(1):e6‐e12. 10.1016/j.jinf.2020.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Henry B, de Oliveira M, Benoit S, Plebani M, Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID‐19): a meta‐analysis. Clin Chem Lab Med. 2020;58(7):1021‐1028. 10.1515/cclm-2020-0369 [DOI] [PubMed] [Google Scholar]
- 47. Shi L, Wang Y, Liang X, et al. Is neutrophilia associated with mortality in COVID‐19 patients? A meta‐analysis and meta‐regression: International Journal of Laboratory Hematology. 2020;42(6):e244‐e247. 10.1111/ijlh.13298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Barnes BJ, Adrover JM, Baxter‐Stoltzfus A, et al. Targeting potential drivers of COVID‐19: neutrophil extracellular traps. J Exp Med. 2020;217(6):1‐7. 10.1084/jem.20200652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Liu F, Xu A, Zhang Y, et al. Patients of COVID‐19 may benefit from sustained Lopinavir‐combined regimen and the increase of Eosinophil may predict the outcome of COVID‐19 progression. Int J Infect Dis. 2020;95:183‐191. 10.1016/j.ijid.2020.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA ‐ J Am Med Assoc. 2020;323(11):1061‐1069. 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Janz DR, Ware LB. The role of red blood cells and cell‐free hemoglobin in the pathogenesis of ARDS. J Intensive Care. 2015;3:20. 10.1186/s40560-015-0086-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Omrani‐Nava V, Maleki I, Ahmadi A, et al. Evaluation of hepatic enzymes changes and association with prognosis in COVID‐19 patients. Hepat Mon. 2020;20(4):e103179. 10.5812/hepatmon.103179 [DOI] [Google Scholar]
- 53. Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID‐19). JAMA Cardiol. 2019;5(7):811. 10.1001/jamacardio.2020.1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pan Y, Ye G, Zeng X, et al. Can routine laboratory tests discriminate SARS‐CoV‐2‐infected pneumonia from other causes of community‐acquired pneumonia? Clin Transl Med. 2020;10:161‐168. 10.1002/ctm2.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Amgalan A, Othman M. Hemostatic laboratory derangements in COVID‐19 with a focus on platelet count. Platelets. 2020;29:1‐6. 10.1080/09537104.2020.1768523 [DOI] [PubMed] [Google Scholar]
- 56. Giannis D, Ziogas IA, Gianni P. Coagulation disorders in coronavirus infected patients: COVID‐19, SARS‐CoV‐1, MERS‐CoV and lessons from the past. J Clin Virol. 2020;127:104362. 10.1016/j.jcv.2020.104362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Liu Y, Sun W, Guo Y, et al. Association between platelet parameters and mortality in coronavirus disease 2019: retrospective cohort study. Platelets. 2020;31(4):490‐496. 10.1080/09537104.2020.1754383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lippi G, Plebani M, Henry BM. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID‐19) infections: a meta‐analysis. Clin Chim Acta. 2020;506:145‐148. 10.1016/j.cca.2020.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yang X, Yang Q, Wang Y, et al. Thrombocytopenia and its association with mortality in patients with COVID‐19. J Thromb Haemost. 2020;18:1469‐1472. 10.1111/jth.14848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chen W, Li Z, Yang B, et al. Delayed‐phase thrombocytopenia in patients with coronavirus disease 2019 (COVID‐19). Br J Haematol. 2020;190:179‐184. 10.1111/bjh.16885 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analyzed during the current study.


