COVID‐19 has been initially characterized by respiratory manifestations. Neurologic manifestations are being increasingly recognized as a part of the disease spectrum that affects both the central nervous system as well as the peripheral nervous system. Headache has been reported to be present in many patients of COVID‐19 with or without other neurological symptoms but headache with raised intracranial pressure has not been described with COVID‐19. We present a case of COVID‐19 with headache and raised intracranial pressure as a manifestation of SARS‐CoV‐2 infection.
In February 2020, the World Health Organization designated Coronavirus disease 2019 as COVID‐19. It was declared a pandemic on March 11, 2020. The predominant clinical presentation involves the respiratory system but neurological manifestations are being increasingly recognized. In the recently published literature, various case reports and case series have described the neurological manifestations causing encephalitis, acute disseminated encephalomyelitis, stroke, myelitis, venous sinus thrombosis, anosmia, and Guillain‐Barre syndrome. Available studies do not adequately describe the characteristics and severity of headache in patients of COVID‐19. As headache is the commonest neurologic symptom, it needs to be evaluated in the present pandemic considering the neurotrophic potential of the virus.
We report the case of a 15‐year‐old boy presenting to an emergency room with complaints of headache from 3 days prior to admission. It was abrupt in onset, holocranial, throbbing, coming in paroxysms of 5‐10 minutes, and was associated with vomiting and photophobia. The patient complained that the headache was worse on getting up in the morning and would increase when bending forward. There was no reported retro‐orbital pain, no recent trauma, double vision, and visual obscuration. The patient had a history of low‐grade fever 5 days prior to the onset of headache which resolved with antipyretics. There was no history of cough, sore throat, chest pain, respiratory distress, diarrhea, body ache, ageusia, and anosmia.
On examination, the patient was afebrile but irritable and agitated. He was moving all 4 limbs and intermittently responding to commands. There were no signs of meningeal irritation. He had right conjunctival congestion. Fundus examination was normal. A diagnosis of encephalitis was considered. The brain CT was normal and brain MRI with contrast did not reveal any leptomeningeal enhancement, venous sinus thrombosis, hydrocephalus, and cerebral edema (Fig. 1). A chest X‐ray was also normal. Routine blood investigations revealed normal hemoglobin with blood counts and normal liver function and renal function tests. Tests for malaria, typhoid, and leptospira were negative.
Fig. 1.
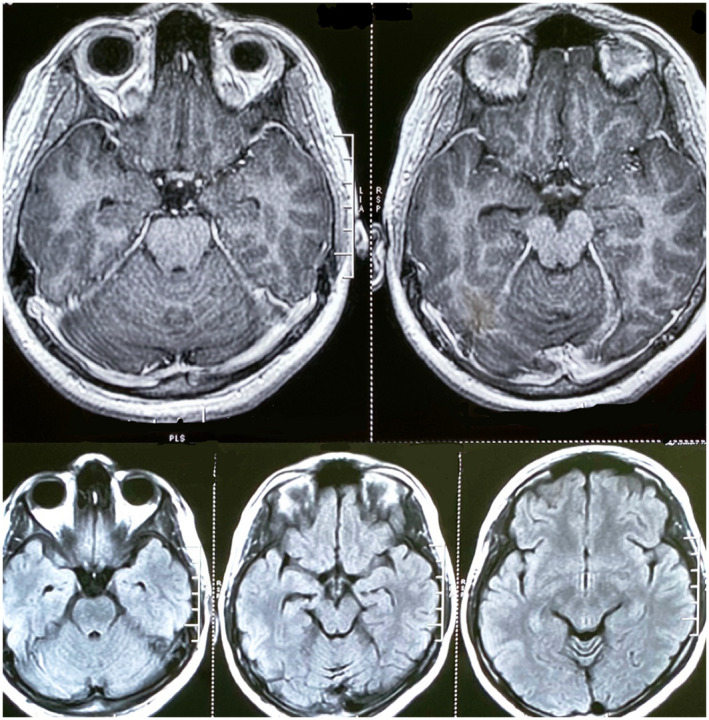
Brain MRI of the patient with contrast (top panel) and T2 FLAIR (bottom panel) showing no abnormality. [Color figure can be viewed at wileyonlinelibrary.com]
Cerebrospinal fluid examination revealed 12 cells with 60% lymphocytes and 40% neutrophils with normal sugar, protein, and chloride levels with markedly increased pressure of 30 cm of water in the left lateral decubitus position. Tests for viral pathogens (herpes simplex virus 1 and 2, mumps virus, varicella zoster virus, enterovirus, parechovirus) in the CSF by real‐time PCR were negative. The tuberculosis bacilli were not detected in the CSF by PCR.
He was empirically started on intravenous acyclovir, intravenous fluids, and paracetamol for headache. On day 5 of treatment, he was conscious and well oriented to time, place, and person. He had no behavioral symptoms but there was no improvement in the headache and it was poorly responsive to analgesics. No organisms were detected on India Ink preparation and CSF cryptococcal antigen was negative.
Considering the ongoing pandemic and persistent headache, patient's nasopharyngeal, and throat swab as well as CSF were sent for SARS‐CoV‐2 testing. The patient's throat swab was positive for SARS‐CoV‐2 by PCR, but PCR of CSF was negative. A repeat cerebrospinal fluid examination was carried out which showed increased pressure of 28 cm of water with 2 cells, all of them being lymphocytes with normal sugar and protein levels. His headache improved drastically with a second lumbar puncture. He was managed with dexamethasone, mannitol, acetazolamide, and topiramate for a duration of 2 weeks during inpatient stay. His headache improved and dexamethasone and mannitol were tapered off and he was discharged on tapering dosages of acetazolamide and topiramate. On follow‐up after 3 weeks, the patient reports to be doing well and is not on any medication.
The available reports on COVID‐19 have not really emphasized the nature of headache. A meta‐analysis of 3598 patients has shown headache to be present in 11‐14% of patients infected with COVID‐19. 1 , 2 In a series of 262 confirmed cases of the COVID‐19 in Beijing, the most common symptoms at the onset of illness were cited as fever, cough, fatigue, and dyspnea followed closely by headache with a rate of 6.5%. 3 Similarly, a study from Wuhan reported headache to be a less common symptom, being present in 8% of cases. 4 In a study by Mannan et al among 50 pediatric patients, headache was present in only 3 cases. Most of them had encephalopathy, brainstem, and cerebellar signs and muscle weakness. 5
Various mechanisms for headache in COVID‐19 have been described. The first possibility could be a direct invasion of trigeminal nerve endings in the nasal cavity by the SARS‐CoV‐2. 6 Angiotensin‐converting enzyme 2 (ACE2), a transmembrane metalloproteinase has been identified as a host receptor for SARS‐CoV‐2 for its entry into the cells. In the brain, ACE2 expression is detected mainly in neurons and glial tissues and motor cortex, raphe nucleus, solitary tractus, and nucleus ambiguus. 6 This expression of ACE2 in endothelial cells could play a role in trigeminovascular activation and headache. Wettervik et al. reported a case of acute encephalitis following COVID‐19 with intracranial pressure monitoring. Intracranial pressure data exhibited a high incidence of plateau waves with intracranial pressure insults above 40 mm Hg that required cerebrospinal fluid drainage. The combination of low intracranial compliance and intact pressure autoregulation could explain the high degree of plateau waves. 7
Our patient had headache as a manifestation of SARS‐CoV2 infection, which was disabling and did not respond to analgesics. The most striking finding was the elevated CSF pressure and relief of headache upon second lumbar puncture. To the best of our knowledge, there is, presently, just an isolated case report of COVID‐19 and idiopathic intracranial hypertension (IIH). 8 However, this patient had no risk factors or any imaging findings consistent with IIH. A raised opening pressure has been described in a patient of COVID‐19 related encephalitis by Moriguchi et al. 9 They described a young male with fever, sore throat, and seizures with altered sensorium. The opening pressure was reported to be high and patient required intubation and ventilation as per the case report. Our patient had the only headache with raised opening pressure and abnormal behavior with a positive RT PCR for COVID‐19 in nasopharyngeal swab suggestive of probable COVID‐19 encephalitis. 10
We already know that SARS‐CoV‐2 has neurotrophic potential, so in the current pandemic headache as a complaint should not be ignored especially any new‐onset headache. Any change in the character of chronic headache should prompt testing for COVID‐19. Raised intracranial pressure was observed in our case. It would be interesting to see in the future whether COVID‐19 meningoencephalitis is more broadly associated with raised intracranial pressure as we see in bacterial or tubercular etiology, and if untreated, whether it leads to complications like hydrocephalus. There is no definite defined mechanism to explain the association of raised intracranial pressure and SARS‐CoV‐2 infection. We suggest a systemic immune response to SARS‐CoV‐2 infection alters the dynamics of CSF production and resorption leading to elevated intracranial hypertension. This can lead to cerebral edema due to the effect of cytokines or direct viral invasion on endothelium. Further studies are needed in patients of COVID‐19 for studying and substantiating the characteristic pattern and severity of headache and its possible complications. The need for intracranial pressure monitoring in patients developing acute encephalopathy following COVID‐19 also needs to be explored with further research.
Conflict of Interest: None
Written informed consent was obtained from the patient's father for the publication of this case report, including any identifiable data or images that might reveal patient's identity.
REFERENCES
- 1. Borges do Nascimento IJ, Cacic N, Abdulazeem HM, et al. Coronavirus infection (COVID‐19) in humans: A scoping review and meta‐analysis. J Clin Med. 2020;9:941. doi: 10.3390/jcm9040941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhang X, Cai H, Hu J, et al. Epidemiological, clinical characteristics of cases of SARS‐CoV‐2 infection with abnormal imaging findings. Int J Infect Dis. 2020;94:81‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tian S, Hu N, Lou J, et al. Characteristics of COVID‐19 infection in Beijing. J Infect. 2020;80:401‐406. [Epub 2020 Feb 27]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;15:497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Abdel‐Mannan O, Eyre M, Löbel U, et al. Neurologic and radiographic findings associated with COVID‐19 infection in children. JAMA Neurol. Published online July 01, 2020;e202687 10.1001/jamaneurol.2020.2687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Doobay MF, Talman LS, Obr TD, Tian X, Davisson RL, Lazartigues E. Differential expression of neuronal ACE2 in transgenic mice with overexpression of the brain renin‐angiotensin system. Am J Physiol Regul Integr Comp Physiol. 2007;292:R373‐R381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wettervik TS, Kumlien E, Rostami E, et al. Intracranial pressure dynamics and cerebral vasomotor reactivity in coronavirus disease 2019 patient with acute encephalitis. Crit Care Explor. 2020;2:e0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Noro F, Cardoso FM, Marchiori E. COVID‐19 and benign intracranial hypertension: A case report. Rev Soc Bras Med Trop. 2020;53:e20200325. doi: 10.1590/0037-8682-0325-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Moriguchi T, Harii N, Goto J, et al. A first case of meningitis/encephalitis associated with SARS‐coronavirus‐2. Int J Infect Dis. 2020;94:55‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ellul M, Benjamin L, Singh B, et al. Neurological associations of COVID‐19. Lancet Neurol. 2020;19:767‐783. [DOI] [PMC free article] [PubMed] [Google Scholar]


