Abstract
Background
Patients with cancer are considered at high risk for the novel respiratory illness coronavirus disease 2019 (COVID‐19). General measures to keep COVID‐19–free cancer divisions have been adopted worldwide. The objective of this study was to evaluate the efficacy of triage to identify COVID‐19 among patients with cancer.
Methods
From March 20 to April 17, 2020, data were collected from patients who were treated or followed at the authors' institution in a prospective clinical trial. The primary endpoint was to estimate the cumulative incidence of COVID‐19–positive patients who were identified using a triage process through the aid of medical and patient questionnaires. Based on a diagnostic algorithm, patients with suspect symptoms underwent an infectious disease specialist's evaluation and a COVID‐19 swab. Serologic tests were proposed for patients who had symptoms or altered laboratory tests that did not fall into the diagnostic algorithm but were suspicious for COVID‐19.
Results
Overall, 562 patients were enrolled. Six patients (1%) were diagnosed with COVID‐19, of whom 4 (67%) had the disease detected through telehealth triage, and 2 patients (33%) without suspect symptoms at triage had the disease detected later. Seventy‐one patients (13%) had suspect symptoms and/or altered laboratory tests that were not included in the diagnostic algorithm and, of these, 47 patients (73%) underwent testing for severe acute respiratory syndrome coronavirus 2 antibody: 6 (13%) were positive for IgG (n = 5) or for both IgM and IgG (n = 1), and antibody tests were negative in the remaining 41 patients.
Conclusions
The triage process had a positive effect on the detection of COVID‐19 in patients with cancer. Telehealth triage was helpful in detecting suspect patients and to keep a COVID‐19–free cancer center. The overall incidence of COVID‐19 diagnosis (1%) and antibody positivity (13%) in patients with suspect symptoms was similar to that observed in the general population.
Keywords: coronavirus disease 2019 (COVID‐19), COVID‐19 antibody, COVID‐19 infection, triage, severe acute respiratory syndrome coronavirus 2 (SARS–CoV‐2)
Short abstract
The adoption of a structured triage process can be useful for the detection of coronavirus disease 2019 symptoms and signs. Oncology activities can be safely carried out during the pandemic with the aid of a triage process and containment measures.
Introduction
Worldwide, health services are facing the challenge of the severe acute respiratory syndrome coronavirus 2 (SARS–CoV‐2) pandemic. 1 The most common symptoms of the novel respiratory illness coronavirus disease 2019 (COVID‐19) are fever, cough, and dyspnea; but myalgias, diarrhea, headache, cutaneous manifestations, sore throat, ageusia, and anosmia have also been reported. Although most patients present with mild symptoms, in a subset of infected individuals, patients worsen to pneumonia, multiorgan failure, and eventually die. As of June 2020, COVID‐19 has spread to >200 countries worldwide, with >9.2 million affected individuals; the number of confirmed deaths is >475,000. 2 After China, Italy was the second country to experience the rapid spread of COVID‐19. In Italy, the pandemic initially affected the Lombardy region, especially Milan, where our hospital is located; it had higher lethality here than in China and worldwide. 3
Patients with immunocompromised status, like those who have cancer, are at high risk of infection. Cancer develops in an immunocompromised field, and oncologic treatments (eg, chemotherapy, radiotherapy) can increase the risk of infection. 4 , 5 Medical oncologists have changed their daily clinical practice in view of the current emergency 6 with the aid of rapidly drafted guidelines for patient management during the pandemic. 7 However, the limited data have not led to evidence‐based recommendations. Evidence suggests that COVID‐19 diffusion in patients with cancer is not as prominent as expected; however, mortality seems to be higher than that reported for the general population and correlates with general risk factors as well as those unique to patients with cancer. 8 , 9 , 10 , 11 , 12 Indeed, other comorbidities (eg, cardiovascular disease, diabetes, chronic obstructive pulmonary disease) correlate with a higher risk of infection and severe events. 13
Most treatment procedures in oncology cannot be delayed without compromising the efficacy of treatment itself. To guarantee the provision of oncology services, general measures to keep cancer centers COVID‐free have been adopted, for example, the use of protective devices, patient triage with on‐site temperature evaluation, and questions regarding contact and travel history in high‐risk areas before accessing the hospital. 14 Telehealth has replaced follow‐up for nonurgent visits. Telehealth has also been used to avoid allowing patients with fever and/or recent‐onset respiratory symptoms access to oncology services, with redirection to general practitioners for home care or, whenever required, to hospitalization. 14
However, fever and/or respiratory symptoms can be very common findings in patients with cancer, and the interpretation of such symptoms in these patients can be challenging because they are potentially attributed to the seasonal flu, COVID‐19, or may simply relate to the underlying disease and/or ongoing systemic treatment. In light of the need for appropriate resource allocation, the objective of our current study was to evaluate the frequency of COVID‐19–like symptoms in patients with cancer and to identify high‐risk patients to be evaluated for COVID‐19 infection by using a diagnostic and decisional algorithm.
Materials and Methods
This study was approved by our Institutional Review Board. The primary endpoint was to estimate the cumulative incidence of COVID‐19–positive patients identified by triage. The exploratory endpoint was to create a diagnostic and decisional algorithm for patients with suspected COVID‐19.
We prospectively collected data from all patients who were treated or followed at our institution from March 20 to April 17, 2020. Institutional guidelines, supported by the Italian Association of Medical Oncology (Associazione Italiana di Oncologia Medica), indicated rescheduling nonurgent visits. Telehealth visits replaced in‐person visits. All patients accessing the hospital gave their written informed consent for the use of clinical data for scientific purposes, whereas those undergoing telehealth visits gave their verbal agreement to participate, and informed consent was signed during their earliest in‐person visit.
Patients' baseline characteristics and data regarding cancer treatment were collected. Patients were triaged using 2 paper questionnaires. Questionnaire 1 was completed by physicians in direct patient interviews and assessed the following items: performance status (according to the Eastern Cooperative Oncology Group scale), fever and/or COVID‐19–like respiratory and nonrespiratory symptoms in the previous 30 days, thoracic examination, and previous or ongoing thoracic radiotherapy. A full version of the questionnaire is provided in Supporting Table 1.
Questionnaire 2 was completed by patients at the hospital and assessed the following items: smoking history; concomitant receipt of corticosteroids, acetaminophen, and antibiotics plus insulin and oxygen therapy and a possible recent increase in daily need of these treatments; respiratory illnesses (asthma, chronic obstructive pulmonary disease, emphysema); a history of pleuritis and/or pneumonitis; recent vaccinations (seasonal flu, pneumococcal); fever and/or respiratory symptoms in patients and close contacts (household, friends); and travel to high‐risk areas within the previous 15 days. A full version of the patient questionnaire is provided in Supporting Table 2.
Vital signs (blood pressure, heart rate, blood oxygen saturation, and respiratory rate) were recorded by nurses before treatment administration; body temperature was measured with a digital, contactless, infrared thermometer. Pretreatment blood tests included complete blood count, lactate dehydrogenase, and C‐reactive protein (CRP).
The diagnostic algorithm for patients with suspected COVID‐19 came from recommendations of national guidelines and data from the literature available at the beginning of the pandemic. 15 , 16 , 17 An infectious disease specialist's evaluation was promptly performed when the triage process and the medical questionnaire detected fever and new‐onset respiratory symptoms; chest imaging and a SARS–CoV‐2 swab were performed if clinically indicated (Fig. 1). Through the subsequent analysis of the questionnaires, patients were followed for symptom evolution. SARS–CoV‐2 rapid tests (Beijing LEPU Medical Technology Company, Ltd) were offered to patients who reported suspect signs or symptoms that did not fall into the diagnostic algorithm (SARS–CoV‐2 IgM and IgG: sensitivity, 88.7%; specificity, 90.6%). 18 Patients who were positive for IgM and/or IgG on rapid tests underwent chemiluminescence immunoassays (CLIAs) (LIAISON [Diasorin SpA] and MAGLUMI [Snibe Diagnostics, Shenzhen New Industries Biomedical Engineering Company, Ltd]. LIAISON is an indirect test detecting IgG against the subunits S1 and S2 of the spike viral proteins (specificity, approximately 98%; sensitivity, 25% at <5 days, 90.4% at 5‐15 days, and 97.4% at >15 days from the onset of symptoms). A negative result is defined by an IgG level <15 AU/mL, whereas a positive result is defined as an IgG level ≥15 AU/mL. 19 , 20 , 21 MAGLUMI is a CLIA for IgM and IgG against SARS–CoV‐2 S‐antigen and N‐protein (IgM and IgG sensitivity, 78% and 91%, respectively; IgM and IgG specificity, 97%; combined IgM and IgG sensitivity, 89.9%‐95.6%; IgM and IgG specificity, 96.5%‐96.0%). The IgM and IgG cutoff levels for positive tests were 1.0 and 1.1 AU/mL, respectively. 22 , 23 , 24 Patients with positive CLIA serology underwent rhinopharyngeal swabs for SARS–CoV‐2.
Figure 1.
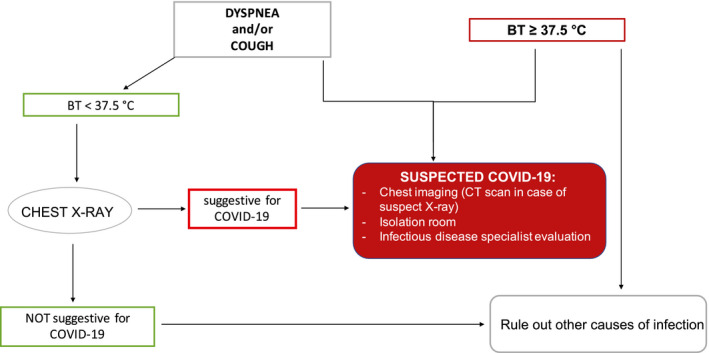
This is the algorithm used for the management of patients with cancer who presented with signs and symptoms suspicious for coronavirus disease 2019 (COVID‐19). BT indicates body temperature; CT, computed tomography.
To evaluate the role of symptoms and to identify the incidence of SARS–CoV‐2 positivity in asymptomatic patients, we performed a case‐control (1:1) comparison. Serologic tests were performed in patients with known COVID‐19 to verify the serology in the case of documented infection.
Patients who were included in the current study were followed for COVID‐19 onset. Data regarding specific characteristics of the infection were prospectively collected in a dedicated database in the case of documented SARS–CoV‐2 infection.
Statistical Analysis
Basic descriptive statistics were used to describe the population. Differences in categorical variables were analyzed using the Fisher exact test. The chi‐square test was used to analyze proportions when appropriate (comparing 3 groups). Odds ratios and 95% CIs were calculated for each comparison. The t test and the Mann‐Whitney test were used to compare continuous variables, as appropriate. P values <.05 were considered statistically significant. Statistical analysis was performed using GraphPad Prism version 6.0 (GraphPad Software Inc) and IBM‐Microsoft SPSS version 20.0 (SPSS Statistics, IBM Inc) for Mac (Apple Inc).
Results
Baseline Patient Characteristics
Overall, 675 patients were eligible for the study, 562 patients were enrolled, and 113 patients were excluded. Figure 2 illustrates the flow of patients through the study period. Group A had 193 (34%) telehealth visits, group B had 108 (19%) outpatient ambulatory visits, and group C had 261 (47%) day hospital visits (including 68 outpatient on‐treatment visits).
Figure 2.
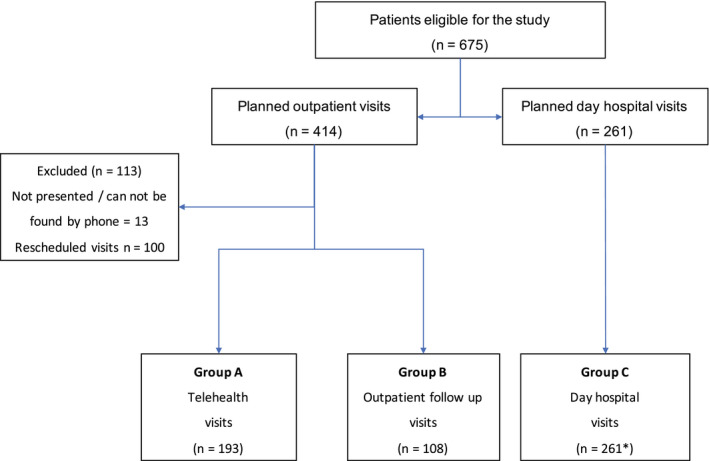
This is a flow diagram of the enrolled patients. *In group C, 68 outpatient on‐treatment visits were included (for complete details, refer to the text).
Table 1 lists the baseline characteristics of the study population. The median age of patients was 69 years (range, 26‐94 years), with a 1:1.8 male‐to‐female ratio. The most common cancer diagnosis was breast cancer (37%), followed by thoracic cancer (25%), and gastrointestinal tumors (18%). In total, 344 patients (61%) were receiving active anticancer treatment, including approximately 30% of patients in groups A and B and all patients in group C (P < .001). Most patients were receiving chemotherapy (37%), followed by immunotherapy (13%), chemotherapy plus monoclonal antibodies (11%), or chemotherapy plus immunotherapy (4%); and 122 patients (35%) were receiving various anticancer agents (see Table 1). Most patients in groups A and B were receiving oral therapies (83% and 80%), and a minority of patients who were receiving intravenous treatment did not access the day hospital during the study period. Conversely, 117 patients (45%) in group C were receiving intravenous chemotherapy, 44 (17%) were receiving immunotherapy, 35 (13%) were receiving chemotherapy plus biologic agents, 12 (5%) were receiving chemotherapy plus immunotherapy, and 52 (20%) were receiving oral (17%) and intramuscular/subcutaneous therapy (3%). In group A, 90% of patients were receiving neoadjuvant/adjuvant treatment compared with 61% in group B and 19% in group C (P < .001). A higher proportion of patients in group C were receiving >2 lines of treatment (n = 92 [35%]; P < .001).
TABLE 1.
General Characteristics of the Study Population
| Characteristic | No. (%) | |||
|---|---|---|---|---|
| All Patients, N = 562 | Group A: Telehealth Visits, n = 193 | Group B: Outpatient Visits, n = 108 | Group C: Day Hospital Visits, n = 261 | |
| Age: Median [range], y | 69 [26‐94] | 69 [36‐94] | 66 [31‐88] | 69 [26‐91] |
| City of residence | ||||
| Milan | 375 (67) | 140 (73) | 72 (67) | 163 (62) |
| Milan district | 106 (19) | 27 (14) | 19 (17) | 60 (23) |
| Other a | 81(14) | 26 (13) | 17 (16) | 38 (15) |
| Sex | ||||
| Men | 196 (35) | 58 (30) | 43 (40) | 95 (36) |
| Women | 366 (65) | 135 (70) | 65 (60) | 166 (64) |
| Ethnicity | ||||
| Caucasian | 527 (94) | 187 (97) | 99 (92) | 241 (92) |
| Asiatic | 19 (3) | 3 (2) | 5 (4) | 11 (4) |
| Hispanic | 9 (2) | 1 (<1) | 4 (4) | 4 (2) |
| African | 7 (1) | 2 (1) | — | 5 (2) |
| Cancer diagnosis b | ||||
| Thoracic | 138 (25) | 25 (13) | 23 (21) | 90 (35) |
| Breast | 207 (37) | 96 (50) | 28 (26) | 83 (32) |
| GI tract | 118 (21) | 48 (24) | 26 (24) | 44 (17) |
| Head and neck | 11 (2) | — | 3 (3) | 8 (3) |
| Pancreas and biliary tract | 33 (6) | 9 (5) | 8 (7) | 16 (6) |
| Skin: Melanoma, nonmelanoma | 18 (3) | 5 (3) | 7 (7) | 6 (2) |
| Genitourinary tract | 23 (4) | 5 (3) | 8 (7) | 10 (4) |
| Other | 14 (2) | 5 (2) | 5 (5) | 4 (1) |
| Active treatment | ||||
| Yes | 344 (61) | 58 (30) | 25 (23) | 261 (100) |
| No, follow‐up | 218 (39) | 135 (70) | 83 (77) | — |
| Type of treatment | ||||
| Chemo | 126 (37) | 3 (5) | 5 (20) | 118 (45) |
| Immunotherapy | 46 (13) | 1 (2) | 1 (4) | 44 (17) |
| Chemo and immunotherapy | 12 (4) | — | — | 12 (5) |
| Chemo and biologic agents c | 38 (11) | 2 (3) | 1 (4) | 35 (13) |
| Other d | 122 (35) | 52 (90) | 18 (72) | 52 (20) |
| Treatment administration | ||||
| Intravenous | 185 (53) | 5 (8) | 4 (16) | 176 (67) |
| Oral | 112 (32) | 48 (83) | 20 (80) | 44 (17) |
| Intravenous and oral | 21 (6) | — | 1 (4) | 20 (8) |
| Intramuscular and oral | 18 (5) | 4 (7) | — | 14 (5) |
| Intramuscular/subcutaneous | 8 (2) | 1 (2) | — | 7 (3) |
| Line of treatment | ||||
| Adjuvant/neoadjuvant | 119 (35) | 52 (90) | 17 (68) | 50 (19) |
| 1 | 129 (37) | 5 (8) | 5 (20) | 119 (46) |
| 2 | 57 (17) | 1 (2) | 1 (4) | 55 (21) |
| ≥3 | 39 (11) | — | 2 (8) | 37 (14) |
Abbreviations: Chemo, chemotherapy; GI, gastrointestinal.
Other indicates the remaining geographic areas outside of the Milan district.
Six patients (1%) had second primary cancers.
These include the following monoclonal antibodies: antivascular endothelial growth factor, antiepidermal growth factor receptor, and antihuman epidermal growth factor receptor 2.
These include the following treatments: cyclin‐dependent kinase 4 (CDK4)/CDK6 inhibitors, hormonal treatment, oral targeted agents (eg, tyrosine kinase inhibitors, mitogen‐activated pathway kinase inhibitors), and somatostatin analogues.
Results of the Triage Process
The items assessed in questionnaire 1 are displayed in Table 2. Overall, 138 patients (25%) reported at least 1 symptom. Of these, 30 patients (22%) reported COVID‐19–like respiratory symptoms, 68 (49%) reported COVID‐19–like nonrespiratory symptoms, and 40 (29%) reported both respiratory and nonrespiratory symptoms. After excluding patients with preexisting symptoms, 68 (49%) had new‐onset or worsening COVID‐19–like respiratory symptoms (n = 17; 25%), or nonrespiratory symptoms (n = 23; 34%), or both (n = 28; 41%). Twelve patients (2%) had increased CRP levels (ie, >10 times the upper limit of normal), either alone (n = 6) or with COVID‐19–like symptoms (n = 6).
TABLE 2.
Items Assessed in Questionnaire 1 (Completed by Medical Oncologists)
| Item | No. (%) | |||
|---|---|---|---|---|
| All Patients, N = 562 | Group A: Telehealth Visits, n = 193 | Group B: Outpatient Visits, n = 108 | Group C: Day Hospital Visits, n = 261 | |
| ECOG PS | ||||
| 0 | 350 (62) | 150 (78) | 63 (58) | 137 (53) |
| 1 | 168 (30) | 33 (17) | 35 (33) | 100 (38) |
| ≥2 | 44 (8) | 10 (5) | 10 (9) | 24 (9) |
| History of thoracic RT | ||||
| Yes | 75 (13) | 27 (14) | 24 (22) | 24 (9) |
| No | 487 (87) | 166 (86) | 84 (78) | 237 (91) |
| Fever | ||||
| Yes | 22 (4) | 6 (3) | 4 (4) | 12 (5) |
| No | 540 (96) | 187 (97) | 104 (96) | 249 (95) |
| Symptoms | ||||
| Yes | 138 (25) | 12 (6) | 20 (18) | 106 (41) |
| No | 424 (75) | 181 (94) | 88 (82) | 155 (59) |
| COVID‐19–like respiratory symptoms | 30 (22) | — | 4 (20) | 26 (24) |
| Dyspnea | 17 (57) | — | 3 (75) | 14 (54) |
| Stable | 10 (59) | — | 3 (100) | 9 (64) |
| Worsened | 7 (41) | — | — | 5 (36) |
| Cough | 9 (30) | — | — | 9 (35) |
| Stable | 3 (33) | — | — | 6 (67) |
| Worsened | 6 (67) | — | — | 3 (33) |
| Dyspnea and cough | 4 (13) | — | 1 (25) | 3 (11) |
| Stable | 4 (100) | — | 1 (100) | 3 (100) |
| Worsened | — | — | — | — |
| COVID‐19–like nonrespiratory symptoms a | 68 (49) | 6 (50) | 8 (40) | 54 (51) |
| Fatigue | 47 (69) | 1 (17) | 4 (50) | 42 (78) |
| Stable | 42 (89) | 1 (100) | 4 (100) | 37 (88) |
| Worsened | 5 (11) | — | — | 5 (12) |
| Myalgias | 7 (10) | — | — | 7 (13) |
| Stable | 2 (29) | — | — | 5 (71) |
| Worsened | 5 (71) | — | — | 2 (29) |
| Conjunctivitis | 8 (12) | — | 1 (12) | 7 (13) |
| Stable | 4 (50) | — | — | 4 (57) |
| Worsened | 4 (50) | — | 1 (100) | 3 (43) |
| Rhinitis/pharyngodinia | 6 (9) | 2 (33) | 2 (25) | 2 (4) |
| Stable | 2 (33) | 1 (50) | 1 (50) | 1 (50) |
| Worsened | 4 (67) | 1 (50) | 1 (50) | 1 (50) |
| GI symptoms b | 7 (10) | 1 (17) | 1 (12) | 5 (9) |
| Stable | 5 (71) | 1 (100) | — | 4 (80) |
| Worsened | 2 (29) | — | 1 (100) | 1 (20) |
| Other c | 3 (4) | 2 (33) | 1 (12) | 2 (4) |
| COVID‐19–like respiratory and nonrespiratory symptoms | 40 (29) | 6 (50) | 8 (42) | 26 (24) |
| Dyspnea and other symptoms | 18 (45) | 3 (50) | 4 (50) | 11 (42) |
| Cough and other symptoms | 14 (35) | 3 (50) | 2 (25) | 9 (35) |
| Dyspnea, cough, and other symptoms c | 8 (20) | — | 2 (25) | 6 (23) |
Abbreviations: COVID‐19, coronavirus disease 2019; ECOG, Eastern Cooperative Oncology Group; GI, gastrointestinal; PS, performance status; RT, radiotherapy.
These included patients with ≥1 symptom(s).
GI symptoms included nausea (n = 5), vomiting (n = 2), diarrhea (n = 2), and decreased food intake (n = 1).
These included the following symptoms: headache (n = 4), chills (n = 3), anorexia (n = 1), cutaneous rash (n = 1), noncardiac chest pain (n = 1), anxiety (n = 1), malaise (n = 1), and ageusia (n = 1).
Table 3 displays the answers to questionnaire 2. The overall completion rate was 89%. In group B, 48% of patients had no comorbidities; whereas 23%, 16%, and 13% had 1, 2, and ≥3 comorbidities, respectively. In group C, 47% of patients had no comorbidities; whereas 28%, 15%, and 10% had 1, 2, and ≥3 comorbidities, respectively. Forty‐eight percent of patients in group B and 31% of those in group C received seasonal flu vaccination (P < .001). Most patients were former smokers (33% in both groups) or never smokers (38% in group B, 49% in group C); and 11% and 13% of patients were smokers in group B and C, respectively (P = .586). Compared with group B, a higher proportion of patients in group C were receiving ongoing corticosteroid therapy (26% vs 5%; P < .001), and a slightly (not significant) higher proportion were receiving ongoing acetaminophen therapy (23% vs 16%; P = .119). A minority of patients were receiving ongoing antibiotic therapy (4% vs 5%; P = .701). Overall, 94% of patients in group B and 88% in group C did not report high‐risk contacts (P = .063). Fever was an uncommon finding: 96% of patients in group B and 95% in group C did not report fever within 30 days before completing the questionnaires. Similarly, the proportion of patients without suspect symptoms was 83% in group B and 61% in group C (P < .001).
TABLE 3.
Items Assessed in Questionnaire 2 (Completed by Patients)
| Item | No. (%) | ||
|---|---|---|---|
| All Patients: Group B and C, N = 369 | Group B: Outpatient Visits, n = 108 | Group C: Day Hospital Visits, n = 261 | |
| Comorbidity(ies) a | |||
| None | 176 (48) | 52 (48) | 124 (47) |
| 1 | 97 (26) | 25 (23) | 72 (28) |
| 2 | 57 (15) | 17 (16) | 40 (15) |
| ≥3 | 39 (11) | 14 (13) | 25 (10) |
| Oxygen therapy | |||
| Yes | 16 (4) | 7 (6) | 9 (3) |
| No | 353 (96) | 101 (94) | 252 (97) |
| Seasonal flu vaccination | |||
| Yes | 132 (36) | 52 (48) | 81 (31) |
| No | 172 (47) | 27 (25) | 145 (56) |
| Missing data | 65 (17) | 29 (27) | 35 (13) |
| Other vaccination(s) | |||
| Yes, antipneumococcal | 5 (1) | 3 (2) | 2 (<1) |
| No | 297 (81) | 75 (69) | 223 (85) |
| Not assessed | 67 (18) | 30 (28) | 36 (14) |
| Smoking history | |||
| Smoker | 46 (13) | 12 (11) | 34 (13) |
| Former smoker b | 122 (33) | 36 (33) | 86 (33) |
| Never smoked | 170 (46) | 41 (38) | 129 (49) |
| Unknown | 31 (8) | 19 (18) | 12 (5) |
| Concomitant therapy(ies) | |||
| Corticosteroids | 75 (20) | 6 (5) | 69 (26) |
| Acetaminophen c | 77 (21) | 17 (16) | 60 (23) |
| Antibiotics | 16 (4) | 4 (4) | 12 (5) |
| High‐risk contacts | |||
| Yes | 6 (1) | 4 (4) | 2 (1) |
| No | 335 (91) | 104 (96) | 233 (89) |
| Unknown | 28 (8) | — | 26 (10) |
| Fever | |||
| Yes | 16 (4) | 4 (4) | 12 (5) |
| No | 353 (96) | 104 (96) | 249 (95) |
| Symptoms | |||
| Yes | 120 (32) | 18 (17) | 102 (39) |
| No | 249 (68) | 90 (83) | 159 (61) |
Relevant comorbidities included the following: cardiovascular disease (ischemic heart disease, heart failure), hypertension, respiratory disease (asthma, pulmonary emphysema, chronic obstructive pulmonary disease), diabetes, chronic infections (hepatitis B virus, hepatitis C virus, HIV infections), autoimmune conditions (including rheumatologic diseases), hematologic disease (chronic lymphocytic leukemia, multiple myeloma), and neurologic conditions (cerebrovascular disease).
Patients were considered former smokers if smoking cessation happened ≥1 year ago.
These included patients receiving acetaminophen as needed.
Blood tests showed a neutrophil‐to‐lymphocyte ratio ≥5 in 25 patients (10%; mean ± SD, 2.8 ± 3.3); and CRP and lactate dehydrogenase levels were within the reference interval in 171 (66%) and 185 (71%) of patients, respectively. All patients had a body temperature <37 °C (mean ± SD, 36 °C ± 0.35 ° C) and had adequate blood oxygen saturation (mean ± SD, 97% ± 1.8%). The mean ± SD respiratory rate was 16 ± 5 breaths per minute, and a higher rate was observed in patients who had thoracic primary or secondary tumors and was not further investigated. No medical intervention was required based on vital signs. The results of laboratory analyses and vital signs are detailed in Table 4.
TABLE 4.
Laboratory Analysis and Vital Signs
| Variable | Group C: Day Hospital Visits, n = 261 |
|---|---|
| CRP, mg/dL | |
| Mean ± SD | 1.02 ± 1.9 |
| Median [range] | 0.28 [0.01‐11.51] |
| No. (%) | |
| <ULN | 171 (66) |
| ≥ULN to ≤ULN × 3 | 43 (16) |
| >3 × ULN to ≤5 × ULN | 16 (6) |
| >5 × ULN to ≤10 × ULN | 19 (7) |
| >10 × ULN | 12 (5) |
| NLR ratio | |
| Mean ± SD | 2.8 ± 3.3 |
| Median [range] | 2.1 [0.4‐41.5] |
| No. (%) | |
| <5 | 236 (90) |
| ≥5 | 25 (10) |
| LDH, no. (%) | |
| <ULN | 185 (71) |
| ≥ULN | 76 (29) |
| Body temperature, °C | |
| Mean ± SD | 36 ± 0.35 |
| Median [range] | 36 [34.6‐36.2] |
| SpO2, % | |
| Mean ± SD | 97 ± 1.8 |
| Median [range] | 98 [95‐100] |
| Respiration rate, bpm | |
| Mean ± SD | 16 ± 5 |
| Median [range] | 15 [10‐25] |
Abbreviations: bpm, breaths per minute; CRP, C‐reactive protein; LDH, lactate dehydrogenase; NLR, neutrophil‐to‐lymphocyte ratio; SpO2, blood oxygen saturation; ULN, upper limit of normal.
Based on the medical questionnaires, 4 patients (<1%) met the algorithm criteria (3 telehealth triages and 1 ambulatory visit), and all had a COVID‐19 diagnosis. Two patients (<1%) had a negative triage (1 in group B, 1 in group C) and developed fever and respiratory symptoms after 20 and 30 days from triage evaluation, respectively. These 2 patients subsequently were diagnosed with COVID‐19. Based on patients' questionnaires, no patients met the algorithm criteria to be tested for COVID‐19. Patients who reported at least 1 symptom at the questionnaire and/or altered laboratory tests were followed and selected for serologic tests.
Results of Serologic Tests
Figure 3 illustrates the flow of patients selected for serologic tests. Of the 74 patients who had new‐onset symptoms and/or altered laboratory tests, 47 (63%) underwent rapid SARS–CoV‐2 IgM/IgG tests. The remaining patients (n = 27; 37%) were not tested based on the patient's refusal (n = 12), poor performance status (n = 7), hospitalization (n = 3), or death (n = 5).
Figure 3.
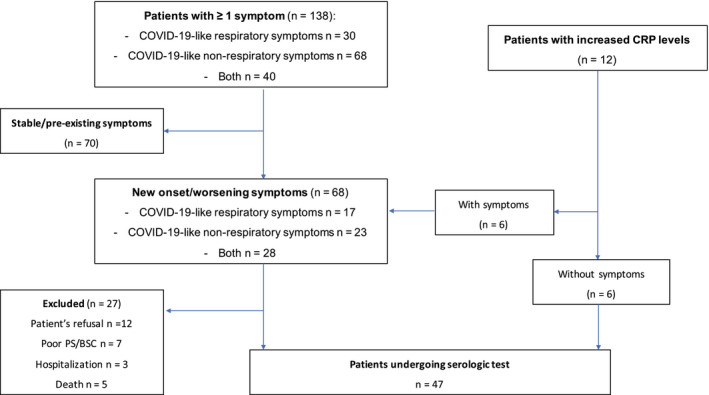
This is a flow diagram of symptomatic patients or patients with altered laboratory tests who were selected for coronavirus disease 2019 (COVID‐19) serologic tests. CRP indicates C‐reactive protein; PS, performance status; BSC, best supportive care.
The control group included 47 asymptomatic patients. Table 5 displays the characteristics of patients undergoing serologic tests. Among the patients who were screened with the LEPU test, 6 (13%) were positive, including 4 who were IgG‐positive, 1 who was IgM‐positive, and 1 who was both IgM‐positive and IgG‐positive. IgG positivity was confirmed in 4 of 5 patients who were screened with the LIAISON test and in 2 of 5 patients who were screened with the MAGLUMI test (2 patients had borderline positive values); all MAGLUMI tests were negative for IgM. In the control group, 5 patients (11%) had a positive LEPU test, including 2 who were IgM‐positive (weak), 1 who was IgG‐positive, 1 who was IgG‐positive (weak), and 1 who was both IgM‐positive and IgG‐positive. LIAISON and MAGLUMI tests confirmed IgG positivity in 1 IgG‐positive patient and in 1 IgM‐positive/IgG‐positive patient; all MAGLUMI tests were negative for IgM. The prevalence of an IgM‐positive/IgG‐positive result using the LEPU test was similar between patients with symptoms and the control group (13% vs 11%; P = .748).
TABLE 5.
Baseline Characteristics and Serologic Results of Positive Patients Among the Group of Patients With Symptoms (POLI‐001–POLI‐049) and in the Control Group (C01‐C27)
| ID No. | Sex | Age, y | ECOG PS | Diagnosis | Systemic Treatment, Line | Reason(s) for Test | Comorbidity | Colloidal Gold Immunochromatography | Liaison CLIA Test | MAGLUMI CLIA Test | Swab | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IgM | IgG | IgG, AU/mL | IgM, AU/mL | IgG, AU/mL | |||||||||
| POLI‐001 | Man | 69 | 1 | Pleural mesothelioma | CDDP and pemetrexed, 1 line | PCR increased | CHD | Neg | Pos | <3.8 | 0.39 | 0.18 | Neg |
| POLI‐002 | Man | 44 | 0 | Melanoma | Nivolumab, adjuvant | Fever, cough | None | Pos | Pos | 155 | 0.798 | 0.902 a | Neg |
| POLI‐026 | Woman | 78 | 0 | Pleural mesothelioma | CBDCA and pemetrexed, 1 line | Fever, high risk contact | Hypertension | Neg | Pos | 66.4 | 0.53 | 14.87 | Neg |
| POLI‐030 | Woman | 67 | 1 | Breast cancer | Docetaxel pertuzumab and trastuzumab, 1 line | Fever, cough | Arrhythmia | Pos | Neg | <3.8 | 0.44 | 0.23 | Neg |
| POLI‐034 | Woman | 85 | 1 | Breast cancer | Capecitabine and vinorelbine, 2 lines | Fever, cough, asthenia, pneumonitis | None | Neg | Pos | 69.5 | 0.57 | 0.9 a | Neg |
| POLI‐049 | Woman | 53 | 0 | Breast cancer | Pegylated liposomal and doxorubicin, 3 lines | Fever | None | Neg | Pos | 3.424 | 0.318 | 29 | Neg |
| C01 | Woman | 73 | 0 | NSCLC (ADC) | Pembrolizumab, 2 lines | — | Hypertension, obesity | Pos (weak) | Neg | <3.8 | 0.57 | 0.18 | ND |
| C03 | Man | 76 | 1 | SCLC | Topotecan, 3 lines | — | None | Pos (weak) | Neg | <3.8 | 0.5 | 0.16 | ND |
| C12 | Woman | 68 | 0 | NSCLC (ADC) | Atezolizumab, 2 lines | — | None | Neg | Pos | 93.5 | 0.58 | 2.02 | Pos |
| C20 | Woman | 74 | 1 | NSCLC (ADC) | Osimertinib,1 line | — | None | Neg | Pos (weak) | 4.6 | 0.53 | 0.17 | ND |
| C27 | Man | 84 | 1 | NSCLC (ADC) | Nivolumab, 3 lines | — | Hypertension, diabetes, TIA | Pos | Pos | 43.3 | 0.53 | 16.23 | Neg |
Abbreviations: ADC, adenocarcinoma; CBDCA, carboplatin; CDDP, cisplatin; CHD, coronary heart disease, CLIA, chemiluminescence immunoassay; ECOG, Eastern Cooperative Oncology Group; ID, identification; ND, not done; Neg, negative; NSCLC, nonsmall cell lung cancer; PCR, polymerase chain reaction; Pos, positive; PS, performance status; SCLC, small cell lung cancer; TIA, transient ischemic attack.
This value indicates borderline positive results.
All patients who had positive LEPU tests and/or CLIAs underwent rhinopharyngeal swabs at a median of 40 days (range, 35‐45 days) after questionnaire completion, and all were negative except for 1 patient in the control group.
Discussion
The ONCOVID study is the first prospective study collecting data from patients with cancer at the beginning of the COVID‐19 outbreak using a structured triage process. From the time of study conception throughout the period of data collection, the number of COVID‐19 cases increased from 22,000 to 63,000 in Lombardy and from 1550 to 6300 in the city of Milan. 6 , 25 , 26 Therefore, the need for a triage process for oncology facilities rapidly became essential for our clinical practice. The results of our study suggest that triage can be useful for detecting COVID‐19 symptoms and signs without being associated with false‐positive or false‐negative results. Telehealth triage seems to help with detecting suspect patients and keeping a COVID‐19–free cancer center.
Data on COVID‐19 and cancer are growing rapidly. Most prospective studies, however, have focused on patients who have cancer with a confirmed COVID‐19 diagnosis (either clinical, radiologic, or performed with serologic tests/rhinopharyngeal swabs). 27 , 28 , 29 Overall, there does not appear to be a higher incidence of COVID‐19 in patients with cancer, and the risk does not seem to be increased in those receiving anticancer treatment. Still, patients with cancer seem to be at higher risk of severe COVID‐19 and reduced intensive care unit access, leading to higher mortality rates. In a recent report from the National Cancer Institute of Milan, a triage process, consisting of high‐resolution, low‐dose computed tomography thoracic scans followed by a rhinopharyngeal swab, was used to detect SARS–CoV‐2 positivity in hospital patients. 30 However, the routine use of radiologic and serologic examinations may be less applicable in clinical practice. The implementation of triage could be an easy method to monitor patients in this setting of care.
The ONCOVID study provides several important findings. First, our data confirm the relatively low incidence of COVID‐19 in patients with cancer. From the beginning of the pandemic over a 5‐month study period—including patients who were not enrolled in the study—in total, 16 patients at our institution had a documented COVID‐19 diagnosis, and 2 had a strong clinical suspicion of COVID‐19. Another important finding is that the overall incidences of COVID‐19 diagnosis in our study population (1%) and antibody positivity (13%) in patients with suspect symptoms were similar to those observed in patients who had cancer without suspect symptoms (11%). Therefore, data from our study suggest that most oncology activities can be safely performed during the pandemic. Simple containment measures to reduce hospital access can be pursued without compromising cancer care delivery.
Our study has some intrinsic limitations. The relatively low rate of COVID‐19 diagnosis in our population might have been caused by the protective measures carried out by patients rather than a reduced risk of COVID‐19. Because of the inability to perform serologic tests on all patients, the incidence of COVID‐19 is not supported by secure numerators and denominators of infection for this calculation. Nevertheless, the number of positive cases seems low, suggesting that containment measures and rapid detection of symptoms with triage might help physicians to safely provide oncology care. Because of the low observed incidence of COVID‐19, an excessively high number of patients should have been evaluated to confirm statistically significant results. However, the results of case‐control comparisons suggest there is a superimposable incidence of antibody positivity among the 2 groups. Finally, the low number of confirmed COVID‐19 cases does not allow us to derive considerations on prognostic factors for infection and outcomes. The identification of patients who are at higher risk for COVID‐19 is currently under study through the validation of a risk‐assessment score in the study population (the Milano Policlinico ONCOVID score). 31
Conclusions
In our experience, the triage process had a positive impact on the detection of COVID‐19 in patients with cancer. The overall incidence of COVID‐19 diagnosis and antibody positivity in patients with suspect symptoms was similar to that observed in asymptomatic patients. The identification of risk factors for COVID‐19 in patients with cancer is needed to customize treatment and provide the best care in the current situation.
Funding Support
This work was supported in part by the Italian fiscal contribution “5 × 1000” 2016 devolved to the Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico.
Conflict of Interest Disclosures
The authors made no disclosures.
Author Contributions
Alice Indini: Conceptualization, data curation, formal analysis, investigation, methodology, supervision, visualization, writing–original draft, and writing–review and editing. Monica Cattaneo: Data curation, formal analysis, investigation, methodology, visualization, and writing–original draft. Michele Ghidini: Data curation, investigation, visualization, and writing–original draft. Erika Rijavec: Data curation, investigation, visualization, and writing–original draft. Claudia Bareggi: Data curation, investigation, visualization, and writing–original draft. Barbara Galassi: Data curation, investigation, visualization, and writing–original draft. Donatella Gambini: Data curation, investigation, visualization, and writing–original draft. Rosalia Ceriani: Data curation, investigation, visualization, and writing–original draft. Ferruccio Ceriotti: Methodology, project administration, supervision, and writing–review and editing. Emilio Berti: Funding acquisition, methodology, project administration, resources, supervision, and writing–review and editing. Francesco Grossi: Conceptualization, funding acquisition, methodology, project administration, resources, supervision, and writing–review and editing.
Supporting information
Supplementary Material
Supplementary Material
Indini A, Cattaneo M, Ghidini M, Rijavec E, Bareggi C, Galassi B, Gambini D, Ceriani R, Ceriotti F, Berti E, Grossi F. Triage process for the assessment of coronavirus disease 2019‐positive patients with cancer: The ONCOVID prospective study. Cancer.2021. 10.1002/cncr.33366
The first 2 authors contributed equally to this article.
We thank all of the patients, family members, and staff who participated in the study.
References
- 1. The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team . Vital surveillances: the epidemiological characteristics of an outbreak of 2019 novel coronavirus disease (COVID‐19)—China, 2020. China CDC Weekly. 2020;2:113‐122. [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization (WHO) . Coronavirus disease (COVID‐19) pandemic, update: June 25, 2020. WHO; 2020. Accessed August 27, 2020. https://www.who.int/emergencies/diseases/novel‐coronavirus‐2019 [Google Scholar]
- 3. Lazzerini M, Putoto G. COVID‐19 in Italy: momentous decisions and many uncertainties. Lancet Glob Health. 2020;8:e641‐e642. doi: 10.1016/S2214-109X(20)30110-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Menges P, Menges P, Kessler W, et al. Surgical trauma and postoperative immune dysfunction. Eur Surg Res. 2012;48:180‐186. doi: 10.1159/000338196 [DOI] [PubMed] [Google Scholar]
- 5. Kaltsas A, Sepkovitz K. Community acquired respiratory and gastrointestinal viral infections: challenges in the immunocompromised host. Curr Opin Infect Dis. 2012;25:423‐430. doi: 10.1097/QCO.0b013e328355660b [DOI] [PubMed] [Google Scholar]
- 6. Indini A, Aschele C, Daniele B, et al. Reorganization of medical oncology departments during the novel coronavirus disease‐19 pandemic: a nationwide Italian survey. Eur J Cancer. 2020;132:17‐23. doi: 10.1016/j.ejca.2020.03.024 [DOI] [PubMed] [Google Scholar]
- 7. Burki TK. Cancer guidelines during the COVID‐19 pandemic. Lancet Oncol. 2020;21:629‐630. doi: 10.1016/S1470-2045(20)30217-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liang W, Guan W, Chen R, et al. Cancer patients in SARS‐CoV‐2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335‐337. doi: 10.1016/S1470-2045(20)30096-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang L, Zhu F, Xie L, et al. Clinical characteristics of COVID‐19‐infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann Oncol. 2020;31:894‐901. doi: 10.1016/j.annonc.2020.03.296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yu J, Ouyang W, Chua MLK, Xie C. SARS‐CoV‐2 transmission in patients with cancer at a tertiary care hospital in Wuhan, China. JAMA Oncol. 2020;6:1108‐1110. doi: 10.1001/jamaoncol.2020.0980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Horn L, Whisenant JG, Torri V, et al. Thoracic Cancers International COVID‐19 Collaboration (TERAVOLT): impact of type of cancer therapy and COVID therapy on survival [abstract]. J Clin Oncol. 2020;38(18 suppl):LBA111. doi: 10.1200/JCO.2020.38.18_suppl.LBA111 [DOI] [Google Scholar]
- 12. Warner JL, Rubinstein S, Grivas P, et al. Clinical impact of COVID‐19 on patients with cancer: data from the COVID‐19 and Cancer Consortium (CCC19) [abstract]. J Clin Oncol. 2020;38(18 suppl):LBA110. doi: 10.1200/JCO.2020.38.18_suppl.LBA110 [DOI] [Google Scholar]
- 13. Wang B, Li R, Lu Z, Huang Y. Does comorbidity increase the risk of patients with COVID‐19: evidence from meta‐analysis. Aging (Albany NY). 2020;12:6049‐6057. doi: 10.18632/aging.103000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang Z, Wang J, He J. Active and effective measures for the care of patients with cancer during the COVID‐19 spread in China. JAMA Oncol. 2020;6:631‐632. doi: 10.1001/jamaoncol.2020.1198 [DOI] [PubMed] [Google Scholar]
- 15. Spina S, Marrazzo F, Migliari M, Stucchi R, Sforza A, Fumagalli R. The response of Milan's emergency medical system to the COVID‐19 outbreak in Italy. Lancet. 2020;395:e49‐e50. doi: 10.1016/S0140-6736(20)30493-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nicastri E, Petrosillo N, Ascoli Bartoli T, et al. National Institute for the Infectious Diseases “L. Spallanzani,” IRCCS. Recommendations for COVID‐19 clinical management. Infect Dis Rep. 2020;12:8543. doi: 10.4081/idr.2020.8543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507‐513. doi: 10.1016/S0140-6736(20)30211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li Z, Yi Y, Luo X, et al. Development and clinical application of a rapid IgM‐IgG combined antibody test for SARS‐CoV‐2 infection diagnosis. J Med Virol. 2020;92:1518‐1524. doi: 10.1002/jmv.25727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. DiaSorin . DiaSorin's LIAISON® SARS‐CoV‐2 S1/S2 IgG. Accessed August 27, 2020. www.diasorin.com/covid19CE
- 20. Morrison A, Li Y, Loshak H. Serological Tests for COVID‐19 (CADTH Horizon Scan; No. 188). Canadian Agency for Drugs and Technologies in Health; 2020. [Google Scholar]
- 21. Tre‐Hardy M, Wilmet A, Beukinga I, Dogne JM, Douxfils J, Blairon L. Validation of a chemiluminescent assay for specific SARS‐CoV‐2 antibody. Clin Chem Lab Med. 2020;58:1357‐1364. doi: 10.1515/cclm-2020-0594 [DOI] [PubMed] [Google Scholar]
- 22. Snibe Diagnostics, Shenzhen New Industries Biomedical Engineering Company, Ltd . M5001E01‐MAGLUMI® 2019‐nCoV IgG‐IgM CLIA assay. Accessed August 27, 2020. http://www.snibe.com/zh_en/en_newsView.aspx?id=576
- 23. Lippi G, Salvano GL, Pegoraro M, et al. Assessment of immune response to SARS‐CoV‐2 with fully automated MAGLUMI 2019‐nCoV IgG and IgM chemiluminescence immunoassays. Clin Chem Lab Med. 2020;58:1156‐1159. doi: 10.1515/cclm-2020-0473 [DOI] [PubMed] [Google Scholar]
- 24. Montesinos I, Gruson D, Kabamba B, et al. Evaluation of two automated and three rapid lateral flow immunoassays for the detection of anti‐SARS‐CoV‐2 antibodies. J Clin Virol. 2020;128:104413. doi: 10.1016/j.jcv.2020.104413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bogani G, Ditto A, Bosio S, Brusadelli C, Raspagliesi F. Cancer patients affected by COVID‐19: experience from Milan, Lombardy. Gynecol Oncol. 2020;158:262‐265. doi: 10.1016/j.ygyno.2020.06.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fondato da Enrico Mentana . Coronavirus, ancora numeri drammatici in Lombardia: 381 morti in un giorno, i nuovi contagi sono 2,171. Fondato da Enrico Mentana; 2020. Accessed April 17, 2020. https://www.open.online/2020/03/20/coronavirus‐bollettino‐regione‐lombardia [Google Scholar]
- 27. Lee LY, Cazier JB, Angelis V, et al. COVID‐19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020;395:1919‐1926. doi: 10.1016/S0140-6736(20)31173-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kuderer NM, Choueiri TK, Shah DP, et al. Clinical impact of COVID‐19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395:1907‐1918. doi: 10.1016/S0140-6736(20)31187-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Garassino MC, Whisenant JG, Huang LC, et al. COVID‐19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry‐based, cohort study. Lancet Oncol. 2020;21:914‐922. doi: 10.1016/S1470-2045(20)30314-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Valenza F, Papagni G, Marchiano A, et al. Response of a comprehensive cancer center to the COVID‐19 pandemic: the experience of the Fondazione IRCCS‐Istituto Nazionale dei Tumori di Milano. Tumori. 2020;106:193‐202. doi: 10.1177/0300891620923790 [DOI] [PubMed] [Google Scholar]
- 31. Indini A, Rijavec E, Ghidini M, Cattaneo M, Grossi F. Developing a risk assessment score for patients with cancer during the coronavirus disease 19 pandemic. Eur J Cancer. 2020;135:47‐50. doi: 10.1016/j.ejca.2020.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Supplementary Material


