Abstract
Background and Aims
Whether patients with cirrhosis have increased risk of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection and the extent to which infection and cirrhosis increase the risk of adverse patient outcomes remain unclear.
Approach and Results
We identified 88,747 patients tested for SARS‐CoV‐2 between March 1, 2020, and May 14, 2020, in the Veterans Affairs (VA) national health care system, including 75,315 with no cirrhosis–SARS‐CoV‐2‐negative (C0‐S0), 9,826 with no cirrhosis–SARS‐CoV‐2‐positive (C0‐S1), 3,301 with cirrhosis–SARS‐CoV‐2‐negative (C1‐S0), and 305 with cirrhosis–SARS‐CoV‐2‐positive (C1‐S1). Patients were followed through June 22, 2020. Hospitalization, mechanical ventilation, and death were modeled in time‐to‐event analyses using Cox proportional hazards regression. Patients with cirrhosis were less likely to test positive than patients without cirrhosis (8.5% vs. 11.5%; adjusted odds ratio, 0.83; 95% CI, 0.69‐0.99). Thirty‐day mortality and ventilation rates increased progressively from C0‐S0 (2.3% and 1.6%) to C1‐S0 (5.2% and 3.6%) to C0‐S1 (10.6% and 6.5%) and to C1‐S1 (17.1% and 13.0%). Among patients with cirrhosis, those who tested positive for SARS‐CoV‐2 were 4.1 times more likely to undergo mechanical ventilation (adjusted hazard ratio [aHR], 4.12; 95% CI, 2.79‐6.10) and 3.5 times more likely to die (aHR, 3.54; 95% CI, 2.55‐4.90) than those who tested negative. Among patients with SARS‐CoV‐2 infection, those with cirrhosis were more likely to be hospitalized (aHR, 1.37; 95% CI, 1.12‐1.66), undergo ventilation (aHR, 1.61; 95% CI, 1.05‐2.46) or die (aHR, 1.65; 95% CI, 1.18‐2.30) than patients without cirrhosis. Among patients with cirrhosis and SARS‐CoV‐2 infection, the most important predictors of mortality were advanced age, cirrhosis decompensation, and high Model for End‐Stage Liver Disease score.
Conclusions
SARS‐CoV‐2 infection was associated with a 3.5‐fold increase in mortality in patients with cirrhosis. Cirrhosis was associated with a 1.7‐fold increase in mortality in patients with SARS‐CoV‐2 infection.
Abbreviations
- aHR
adjusted hazard ratio
- CCI
Charlson Comorbidity Index
- CDW
Corporate Data Warehouse
- HCV
hepatitis C virus
- ICD‐10
International Classification of Diseases, 10th Revision
- MELD
Model for End‐Stage Liver Disease
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- VA
Department of Veterans Affairs
- VHA
Veterans Health Administration
- VINCI
VA Informatics and Computing Infrastructure
The clinical manifestations of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) range from asymptomatic infection to life‐threatening illness.( 1 , 2 ) It remains unclear why some patients infected with SARS‐CoV‐2 develop severe, life‐threatening COVID‐19, while others have a mild or even asymptomatic clinical course. Multiple risk factors for developing severe COVID‐19 disease have been reported, including sociodemographic factors and comorbid conditions.( 1 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 ) Registry and case–control studies from selected tertiary referral centers suggested that patients with cirrhosis infected with SARS‐CoV‐2 have very high mortality, approaching 30%‐40%.( 26 , 27 ) However, these studies were limited to hospitalized patients and likely included a disproportionate number of patients with severe illness. Surprisingly, a study limited to hospitalized patients reported that those with cirrhosis and SARS‐CoV‐2 infection had similar mortality compared to patients with cirrhosis alone.( 26 ) An analysis using population‐based claims data suggested that chronic liver disease was associated with poor outcomes in SARS‐CoV‐2‐infected patients.( 28 ) However, appropriate controls (i.e., uninfected patients with and without cirrhosis and infected patients without cirrhosis) were lacking for these studies.
We sought to address four related clinically relevant questions. First, is cirrhosis associated with an increased risk of testing positive for SARS‐CoV‐2? Second, to what extent does cirrhosis increase the risk of adverse outcomes (hospitalization, mechanical ventilation, or death) in SARS‐CoV‐2‐infected patients? Third, to what extent does SARS‐CoV‐2 infection increase the risk of adverse outcomes in patients with cirrhosis? Fourth, what are the sociodemographic and clinical predictors of adverse outcomes in SARS‐CoV‐2‐infected patients with cirrhosis? To address these questions, we used national data from the Department of Veterans Affairs (VA) health care system on all veterans who were tested for SARS‐CoV‐2 prior to May 15, 2020, including all patients with and without a diagnosis of cirrhosis. The VA health care system provides care for the largest number of patients with cirrhosis of any integrated health care system in the United States and has been used as the source for multiple clinical epidemiology studies in cirrhosis.( 29 )
Methods
Data Source and Study Population
The VA provides care for more than 6 million veterans annually at 168 medical centers and 1,053 outpatient clinics and uses a single comprehensive electronic health care information network. We derived data from the Veterans Health Administration (VHA) Corporate Data Warehouse (CDW), a data repository of the integrated VA electronic medical records, developed by the VA Informatics and Computing Infrastructure (VINCI), to facilitate research. To support and streamline COVID‐19 research, VINCI has developed the COVID‐19 Shared Data Resource,( 30 ) which includes analytic variables extracted from the CDW for all patients in the VHA who were tested for SARS‐CoV‐2. Using this resource, we identified all patients tested for SARS‐CoV‐2 nucleic acid by PCR in the inpatient or outpatient setting between February 28, 2020 (the date of the earliest VA‐recorded test), and May 14, 2020 (n = 88,747), exclusive of those who were VA employees. Cohort members were followed through June 22, 2020, for study outcomes, such that all patients had a minimum follow‐up time of 39 days. This study was approved by the institutional review board of the Veterans Affairs Puget Sound Health Care System, which waived the requirement for informed consent.
Definition of Positive or Negative SARS‐CoV‐2 Status and Index Date
Patients were defined as being positive for SARS‐CoV‐2 if they had at least one positive PCR test (n = 10,131 or 11.4%) during the ascertainment period and as negative for SARS‐CoV‐2 if all their PCR tests were negative (n = 78,616 or 88.6%). Final adjudication of SARS‐CoV‐2 status was performed by the VA National Surveillance Tool, which is intended to be the single, authoritative data source for determination of positive and negative cases within the VHA. The index date for all analyses was defined as the date of the earliest positive test (for SARS‐CoV‐2‐positive patients) or the date of the earliest negative test (for SARS‐CoV‐2‐negative patients), unless the patient was an inpatient at the time of testing who had been admitted to a VA hospital during the preceding 15 days, in which case the date of admission served as the index date (this occurred in 4.6% of the study population).
Definition of Cirrhosis
The diagnosis of cirrhosis was based on documentation of International Classification of Diseases, 10th Revision (ICD‐10), or ICD‐9 codes for cirrhosis recorded at least twice in any inpatient or outpatient records prior to the index date or a diagnosis of cirrhosis recorded only once together with at least one complication of cirrhosis (Supporting Table S1). Our prior work has demonstrated that this approach to identifying patients with cirrhosis using national VA data has a 97% positive predictive value compared to the gold standard of chart extraction.( 31 ) These ICD‐9/10 codes for cirrhosis extracted from VA electronic medical records have been used in many clinical epidemiology studies. We categorized cirrhosis etiology as “active hepatitis C virus” (HCV) in patients with a positive HCV RNA viral load, “prior HCV” in patients with a prior positive HCV RNA viral load that was eradicated by treatment, or “no HCV” in patients who never tested positive for HCV.
Comparison Groups
Veterans tested for SARS‐CoV‐2 were divided into four groups according to cirrhosis and SARS‐CoV‐2 status:
C0‐S0: no cirrhosis and SARS‐CoV‐2‐negative (n = 75,315)
C0‐S1: no cirrhosis and SARS‐CoV‐2‐positive (n = 9,826)
C1‐S0: cirrhosis and SARS‐CoV‐2‐negative (n = 3,301)
C1‐S1: cirrhosis and SARS‐CoV‐2‐positive (n = 305).
Adverse outcomes: Hospitalization, Mechanical Ventilation, and Mortality
We determined the following three outcomes: (1) hospitalization at the index date or within 15 days of the index date; (2) mechanical ventilation at the index date or within 60 days (3) all‐cause mortality at any time after the index date.
Deaths that occurred both in and out of the VA are comprehensively captured in the CDW through a variety of sources including VA inpatient files, the VA Beneficiary Identification and Records Locator System, Social Security Administration death files, and the Department of Defense.( 32 ) All episodes of mechanical ventilation and hospitalization that occurred within the VA are captured as well as those outside the VA if paid for by the VA under the Community Care program.
Baseline Characteristics Evaluated as Predictors of Adverse Outcomes
We included a range of patient characteristics that had been evaluated in prior studies or that we hypothesized might be associated with adverse outcomes in patients infected with SARS‐CoV‐2. Baseline sociodemographic characteristics included age, sex, race, ethnicity, body mass index, urban versus rural residence (based on ZIP codes), and regional COVID‐19 burden operationalized as the number of COVID‐19‐related deaths per million in each participant’s state of residence as of June 11, 2020,( 33 ) categorized as <130, 130‐350, 350‐700, and >700 deaths per million. Comorbid conditions were extracted by VINCI analysts based on ICD‐10 codes recorded in VA administrative data during the 2‐year period on or before the index date.( 30 ) Individuals were considered to have decompensated cirrhosis if they were diagnosed with any of the following complications: ascites, spontaneous bacterial peritonitis, hepatorenal syndrome, hepatopulmonary syndrome, encephalopathy, or variceal bleeding. We used the Charlson Comorbidity Index (CCI) to estimate each patient’s overall burden of comorbidity.
We also included documented symptoms thought to be related to SARS‐CoV‐2 identified by VINCI analysts based on a combination of natural language processing of text notes in patients’ electronic medical records (i.e., outpatient visit notes, admission notes, progress note, discharge summaries) stored as “text integration utilities” notes in the CDW and searching for relevant ICD‐10 codes( 30 ) occurring on or within 30 days prior to the index date. We do not report associations with loss of smell or taste as these were not widely recognized during the ascertainment period and thus rarely reported.
Finally, for hospitalized patients only, we analyzed 13 routinely available laboratory blood tests and calculated the Model for End‐Stage Liver Disease (MELD) score using values for bilirubin, creatinine, and international normalized ratio. For each test, we extracted the value closest to the index date, on or within 10 days before the index date, or, if absent, within 5 days after the index date (88% were performed within 2 days of the index date).
Statistical Analysis
We used multivariable logistic regression to evaluate whether cirrhosis was independently associated with testing positive for SARS‐CoV‐2, with or without adjusting for sociodemographic characteristics, comorbidities, and presenting symptoms. Using the Kaplan‐Meier method, we calculated 30‐day hospitalization, mechanical ventilation, and mortality rates from the index date through June 22, 2020, for each of the four comparison groups (C0‐S0, C0‐S1, C1‐S0, C1‐S1). Participants who did not experience the outcome of interest were censored at the end of follow‐up. We used Cox proportional hazards models to determine whether SARS‐CoV‐2 infection was independently associated with each adverse outcome (hospitalization, mechanical ventilation, mortality) among patients with cirrhosis, adjusting for cirrhosis etiology, decompensation status, age, sex, race, ethnicity, CCI, and geographical region (with or without serum albumin and MELD score among hospitalized patients). We also used Cox proportional hazards models to determine whether cirrhosis was independently associated with each adverse outcome among patients who tested positive for SARS‐CoV‐2, adjusting for the same covariates as above in addition to specific comorbidities that were more prevalent in individuals with cirrhosis (diabetes, cancer, hypertension, congestive heart failure, dialysis, chronic kidney disease, chronic obstructive pulmonary disease). In secondary analyses we used competing risks analysis for the outcomes of hospitalization or ventilation to account for the competing risk of death. Subgroup analyses were performed limited to patients who were hospitalized.
Results
Baseline Characteristics: Comparison by Cirrhosis and SARS‐CoV‐2 Status
Among patients with cirrhosis, those who tested positive for SARS‐CoV‐2 were more likely to be Black (46.2% vs. 24.8%), live in states with high COVID‐19 burden (>700 deaths/million, 35.4% vs. 10.6%), and report fever (44.6% vs. 27.9%) than those who tested negative (Table 1). However, the distributions of CCI and comorbid conditions (Supporting Table S2) were similar.
TABLE 1.
Characteristics of VA Patients Tested for SARS‐CoV‐2, by Cirrhosis and SARS‐CoV‐2 Status
| No Cirrhosis | Cirrhosis | |||
|---|---|---|---|---|
| SARS‐CoV‐2‐Negative C0‐S0, n = 75,315 | SARS‐CoV‐2‐Positive C0‐S1, n = 9,826 | SARS‐CoV‐2‐Negative C1‐S0, n = 3,301 | SARS‐CoV‐2‐Positive C1‐S1, n = 305 | |
| Cirrhosis etiology (%) | ||||
| No HCV | 99.6 | 99.7 | 59.0 | 52.8 |
| Past HCV | 0.3 | 0.2 | 33.7 | 41.3 |
| Current HCV | 0.1 | 0.1 | 7.2 | 5.9 |
| Cirrhosis decompensation prior to SARS‐CoV‐2 ‡ | N/A | N/A | 35.4 | 30.2 |
| Men | 87.8 | 90.8 | 96.2 | 97 |
| Age (years) (%) | ||||
| 18‐49 | 23.2 | 20.0 | 4.5 | 3.9 |
| 50‐64 | 29.9 | 28.7 | 36 | 32.1 |
| 65‐79 | 35.6 | 36.1 | 54.2 | 56.4 |
| ≥80 | 11.3 | 15.2 | 5.3 | 7.5 |
| Race (%) | ||||
| White | 65.4 | 49.6 | 67.5 | 47.2 |
| Black | 24.6 | 41.5 | 24.8 | 46.2 |
| Other | 2.9 | 2.2 | 2.6 | 1.6 |
| Declined/Missing/Unknown | 7.1 | 6.7 | 5.1 | 4.9 |
| Ethnicity (%) | ||||
| Non‐Hispanic | 89.0 | 87.6 | 89.3 | 88.5 |
| Hispanic | 7.5 | 9.3 | 8.8 | 10.2 |
| Declined/Missing/Unknown | 3.5 | 3.1 | 1.9 | 1.3 |
| Geographical region: COVID‐19‐related deaths per million (%)* | ||||
| <130 | 42.4 | 19.1 | 43.2 | 15.4 |
| 130‐350 | 33.7 | 23.3 | 32.2 | 23.6 |
| 350‐700 | 13.8 | 26.0 | 14 | 25.6 |
| ≥700 | 10.2 | 31.7 | 10.6 | 35.4 |
| Urban vs. rural (%) | ||||
| Rural/highly rural | 38.5 | 24.0 | 36.2 | 17.7 |
| Urban | 61.5 | 76.0 | 63.8 | 82.3 |
| BMI at index date | ||||
| <18.5 (underweight) | 3.1 | 2.7 | 3.4 | 3.6 |
| 18.5‐24.9 (normal) | 22.2 | 18.4 | 27.2 | 25.9 |
| 25.29.9 (overweight) | 31.6 | 31.4 | 32.3 | 28.2 |
| 30‐34.9 (obese I) | 23.2 | 25.6 | 21.6 | 19.3 |
| ≥35 (obese II and III) | 17.2 | 19.3 | 15.3 | 22.3 |
| Missing | 2.7 | 2.5 | 0.2 | 0.7 |
| CCI (%)* | ||||
| 0 | 33.3 | 31.8 | 0.0 | 0.0 |
| 1‐2 | 27.6 | 30.2 | 20.9 | 22.3 |
| 3‐4 | 16.4 | 17.6 | 21.4 | 19.3 |
| ≥ 5 | 22.7 | 20.4 | 57.7 | 58.4 |
| Symptoms (%) | ||||
| Fever | 25.6 | 41.2 | 27.9 | 44.6 |
| Cold | 12.8 | 13.8 | 8.5 | 13.4 |
| Chills | 1.4 | 2.9 | 1.3 | 2.6 |
| Myalgia | 1.3 | 2.0 | 1.0 | 1.3 |
| Cough | 19.2 | 26.1 | 14.1 | 19.3 |
| Dyspnea | 18.3 | 18.7 | 23.1 | 21.3 |
| Sore throat | 1.8 | 1.1 | 0.8 | 1 |
| Nausea | 3.8 | 3.2 | 5.4 | 4.6 |
| Headache | 3.2 | 3.4 | 2.5 | 3 |
| Diarrhea | 4.5 | 5.3 | 5.7 | 7.2 |
| Abdominal pain | 4.6 | 2.7 | 9.2 | 3.6 |
| Fatigue | 7.6 | 8.8 | 11.1 | 12.5 |
| Laboratory tests † (mean ± SD) | ||||
| MELD | N/A | N/A | 15.2 ± 7.1 | 14.0 ± 6.1 |
| Albumin (g/dL) | 3.7 ± 0.7 | 3.5 ± 0.6 | 3.3 ± 0.8 | 3.3 ± 0.8 |
| ALT (U/L) | 38.6 ± 120.8 | 39.4 ± 72.0 | 43.4 ± 86.6 | 42.9 ± 71.9 |
| AST (U/L) | 43.6 ± 164.1 | 50.6 ± 64.3 | 64.2 ± 122.5 | 67.0 ± 89.6 |
| Bilirubin (g/dL) | 0.8 ± 1.3 | 0.7 ± 0.8 | 2.0 ± 3.8 | 1.1 ± 2.4 |
| Creatinine (mg/dL) | 1.5 ± 1.5 | 1.8 ± 1.9 | 1.8 ± 2.0 | 2.2 ± 2.3 |
| Platelet count (k/µL) | 239.1 ± 101.7 | 208.1 ± 87.4 | 169.0 ± 98.9 | 159.7 ± 86.6 |
| Hemoglobin (g/dL) | 12.8 ± 2.5 | 13.1 ± 2.2 | 11.8 ± 2.7 | 12.4 ± 2.7 |
| INR | 1.3 ± 1.1 | 1.3 ± 0.7 | 1.5 ± 1.2 | 1.3 ± 0.6 |
| White blood cell count (/µL) | 9.8 ± 5.0 | 7.1 ± 4.0 | 8.5 ± 5.0 | 6.5 ± 3.8 |
| Neutrophil count (/µL) | 7.3 ± 5.1 | 5.2 ± 3.6 | 6.3 ± 4.7 | 4.7 ± 3.1 |
| Lymphocyte count (/µL) | 2.0 ± 3.4 | 1.6 ± 3.5 | 2.0 ± 4.1 | 2.0 ± 5.7 |
| Neutrophil/lymphocyte ratio | 7.2 ± 12.7 | 6.2 ± 6.3 | 7.0 ± 9.0 | 6.6 ± 8.3 |
Categorized by number of COVID‐19‐related deaths per million on June 11, 2020 (33): <130 (AK, AR, CA, HI, ID, KS, KY, ME, MT, NC, ND, NE, OK, OR, PR, SC, SD, TN, TX, UT, VT, WI, WV, WY), 130‐350 (AL, AZ, CO, FL, GA, IA, MN, MO, MS, NH, NM, NV, OH, VA, WA), 350‐700 (DE, IL, IN, LA, MD, MI, PA), > 700 (CT, MA, NJ, NY, RI).
Measured –10/+5 days from index date in hospitalized patients only.
Defined by diagnosis of ascites, spontaneous bacterial peritonitis, hepatorenal syndrome, hepatopulmonary syndrome, encephalopathy or variceal bleeding prior to SARS‐CoV‐2 infection.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; INR, international normalized ratio; N/A, not available.
Among patients who tested positive for SARS‐CoV‐2, those with cirrhosis were more likely to be male, aged 50‐79 years (rather than ≥80 or <50 years), and have higher CCI and higher prevalence of a number of comorbid conditions (including diabetes, hypertension, coronary artery disease, alcohol dependence, and substance use disorder [Supporting Table S2]) than patients without cirrhosis. The two groups were similar in terms of race/ethnicity, geographical distribution, and reported symptoms.
Patients with cirrhosis who tested positive for SARS‐CoV‐2 (n = 305) frequently had past (41.3%) or active (5.9%) HCV, were overwhelmingly male (97%), and had a mean age of 67.3 (SD 9.3) years. This group was racially (47.2% White and 46.2% Black) and ethnically (10.2% Hispanic) diverse and represented almost every state in the country (Fig. 1A). The most common presenting symptoms were fever (44.6%), dyspnea (21.3%), and cough (19.3%, Table 1).
FIG. 1.
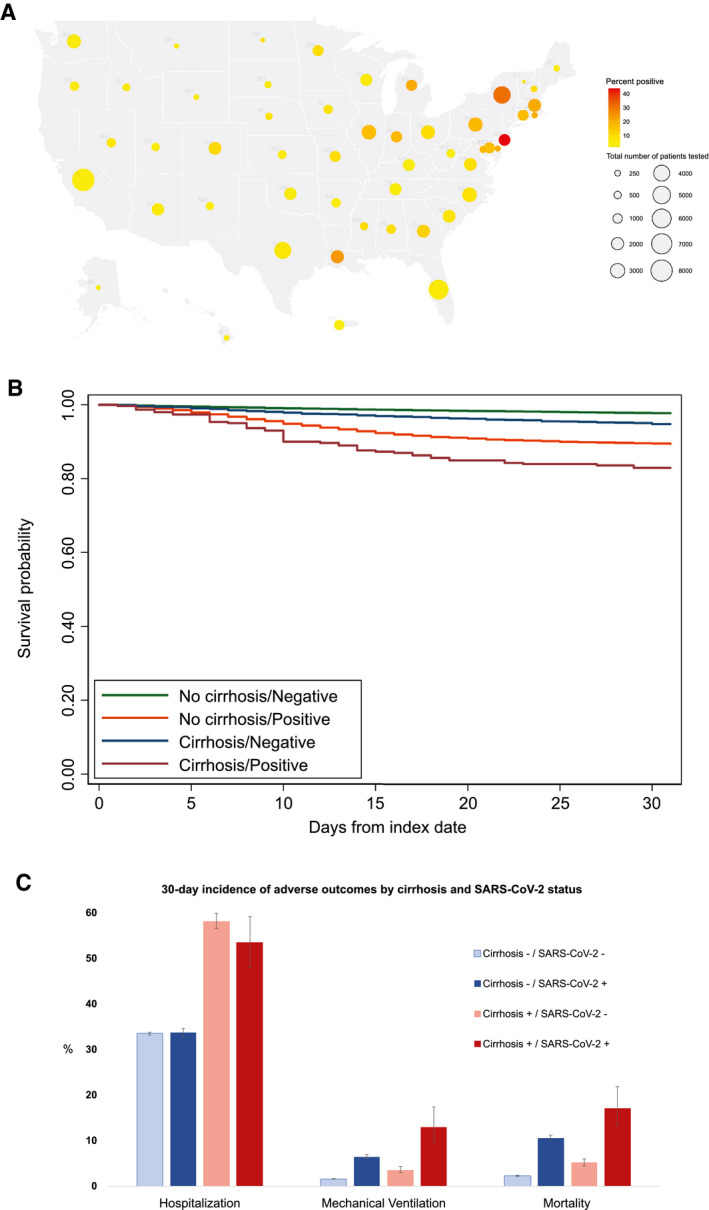
Distribution of VA patients tested for SARS‐CoV‐2 and adverse outcomes according to cirrhosis and SARS‐CoV‐2 status. (A) Number of VA patients tested in each state and percent of tests positive. (B) Kaplan‐Meier curves of 30‐day mortality by cirrhosis and SARS‐CoV‐2 status. (C) Thirty‐day cumulative incidence of hospitalization, mechanical ventilation, and death by cirrhosis and SARS‐CoV‐2 status.
Association Between Cirrhosis and the Likelihood of Testing Positive for SARS‐CoV‐2 in the VA Health Care System
Patients with cirrhosis were less likely to test positive for SARS‐CoV‐2 than patients without cirrhosis (8.5% versus 11.5%), even after adjusting for sociodemographic characteristics and comorbidities (adjusted odds ratio, 0.83; 95% CI, 0.69‐0.99; Table 2). The association was no longer statistically significant after additionally adjusting for presenting symptoms, although the risk estimate only changed minimally (adjusted odds ratio, 0.85; 95% CI, 0.70‐1.01).
TABLE 2.
Association Between Cirrhosis and SARS‐CoV‐2 Test Positivity
| Cirrhosis | SARS‐CoV‐2‐Negative, n = 78,630 (n, %) | SARS‐CoV‐2‐Positive, n = 10,215 (n, %) | Proportion Who Tested Positive (%) | Crude Odds Ratio | Adjusted Odds Ratio* | Adjusted Odds Ratio † |
|---|---|---|---|---|---|---|
| No | 75,315 (95.8) | 9,826 (97.0) | 11.5 | 1 | 1 | 1 |
| Yes | 3,301 (4.2) | 305 (3.0) | 8.5 | 0.71 (0.63‐0.80) | 0.83 (0.69‐0.99) | 0.85 (0.70‐1.01) |
Hospitalization, Mechanical Ventilation, and Mortality Rates by Cirrhosis and SARS‐CoV‐2 Status
Mechanical ventilation rates increased substantially and progressively from C0‐S0 (1.6%) to C1‐S0 (3.6%) to C0‐S1 (6.5%) to C1‐S1 (13.0%; Table 3 and Fig. 1B,C). The same pattern was also seen with mortality, increasing progressively from C0‐S0 (2.3%) to C1‐S0 (5.2%) to C0‐S1 (10.6%) to C1‐S1 (17.1%). On the other hand, hospitalization rates were consistently higher for patients with cirrhosis than those without cirrhosis, regardless of SARS‐CoV‐2 status. Similarly, among patients with cirrhosis, mechanical ventilation and mortality were higher in individuals with SAR‐CoV‐2, whereas hospitalization was higher in those with decompensated cirrhosis (Table 3). There was no evidence of statistically significant interaction between cirrhosis and SARS‐CoV‐2 status with respect to any of these three adverse outcomes. Analyses with death as a competing risk to hospitalization or ventilation yielded almost identical results (Supporting Table S3)
TABLE 3.
Cumulative Incidence of Hospitalization, Mechanical Ventilation, and Mortality by Cirrhosis and SARS‐CoV‐2 Status*
| 30‐Day Cumulative Incidence Rates of | No Cirrhosis | No Cirrhosis | Cirrhosis | Cirrhosis |
|---|---|---|---|---|
| SARS‐CoV‐2‐Negative C0‐S0 | SARS‐CoV‐2‐Positive C0‐S1 | SARS‐CoV‐2‐Negative C1‐S0 | SARS‐CoV‐2‐Positive C1‐S1 | |
| n = 75,315 | n = 9,826 | n = 3,301 | n = 305 | |
| Hospitalization (%) | 33.4 (33.1‐33.7) | 33.7 (32.7‐34.6) | 58.1 (56.4‐59.8) | 53.4 (47.9‐59.1) |
| Hospitalization (%) † | 28.3 (28.0‐28.7) | 29.8 (28.9‐30.8) | 51.9 (50.1‐53.7) | 49.6 (43.9‐55.6) |
| Mechanical ventilation (%) | 1.6 (1.5‐1.7) | 6.5 (6.1‐7.0) | 3.6 (3.0‐4.3) | 13.0 (9.6‐17.4) |
| Mortality (%) | 2.3 (2.1‐2.4) | 10.6 (10.0‐11.2) | 5.2 (4.5‐6.0) | 17.1 (13.3‐21.8) |
| 30‐Day Cumulative Incidence Rates of | Compensated Cirrhosis | Compensated Cirrhosis | Decompensated Cirrhosis | Decompensated Cirrhosis |
| SARS‐CoV‐2‐Negative | SARS‐CoV‐2‐Positive | SARS‐CoV‐2‐Negative | SARS‐CoV‐2‐Positive | |
| n = 2,134 | n = 213 | n = 1,167 | n = 92 | |
| Hospitalization (%) | 52.0 (49.9‐54.1) | 50.5 (44.0‐57.4) | 69.3 (66.6‐71.9) | 60.0 (50.1‐70.0) |
| Hospitalization (%) † | 46.4 (44.2‐48.7) | 47.0 (40.3‐54.2) | 62.8 (59.8‐65.9) | 55.6 (45.3‐66.5) |
| Mechanical ventilation (%) | 2.5 (2.0‐3.3) | 12.5 (8.7‐17.8) | 5.5 (4.3‐7.0) | 14.2 (8.3‐23.7) |
| Mortality (%) | 3.6 (2.9‐4.4) | 12.8 (9.0‐18.1) | 8.2 (6.8‐10.0) | 27.3 (19.2‐37.9) |
Cumulative incidence at 30 days reported as a percentage.
Limited to hospitalization occurring on or after SARS‐CoV‐2 testing date.
Associations Between SARS‐CoV‐2 Infection and Adverse Outcomes in Patients With Cirrhosis
Among patients with cirrhosis, those who tested positive for SARS‐CoV‐2 were 4 times more likely to undergo mechanical ventilation (adjusted hazard ratio [aHR], 4.12; 95% CI, 2.79‐6.10; Table 4) and 3.5 times more likely to die (aHR, 3.54; 95% CI, 2.55‐4.90) than those who tested negative. Rates of hospitalization were not significantly different between patients with cirrhosis who tested positive versus negative for SARS‐CoV‐2. In the subset of patients with cirrhosis who were hospitalized, the magnitude of association between SARS‐CoV‐2 infection and either mechanical ventilation (aHR, 4.43; 95% CI, 2.89‐6.79) or mortality (aHR, 3.23; 95% CI, 2.14‐4.88) was similar.
TABLE 4.
Association Between SARS‐CoV‐2 Infection and Risk of Hospitalization, Mechanical Ventilation, and Mortality in Patients With Cirrhosis
| SARS‐CoV‐2 | n (%) | Event, n (%) | 30‐Day Event Rate (%) | Age aHR | Multivariable aHR* | Multivariable aHR † |
|---|---|---|---|---|---|---|
| Hospitalization | ||||||
| Negative | 3,301 (91.5) | 1,986 (60.2) | 58.1 | 1 | 1 | N/A |
| Positive | 305 (8.5) | 167 (54.8) | 53.4 | 0.89 (0.76‐1.04) | 0.98 (0.83‐1.15) | N/A |
| Hospitalization (occurring on or after the SARS‐CoV‐2 testing date) | ||||||
| Negative | 2,875 (91.1) | 1,560 (54.3) | 51.9 | 1 | 1 | N/A |
| Positive | 282 (8.9) | 144 (51.1) | 49.6 | 0.93 (0.78‐1.10) | 1.03 (0.86‐1.23) | N/A |
| Mechanical ventilation (all patients) | ||||||
| Negative | 3,301 (91.5) | 128 (3.9) | 3.6 | 1 | 1 | N/A |
| Positive | 305 (8.5) | 38 (12.5) | 13.0 | 3.5 (2.4‐5.0) | 4.12 (2.79‐6.10) | N/A |
| Mortality (all patients) | ||||||
| Negative | 3,301 (91.5) | 196 (5.9) | 5.2 | 1 | 1 | N/A |
| Positive | 305 (8.5) | 55 (18.0) | 17.1 | 3.3 (2.4‐4.4) | 3.54 (2.55‐4.90) | N/A |
| Mechanical ventilation (hospitalized patients) | ||||||
| Negative | 1,613 (91.9) | 109 (6.8) | 6.3 | 1 | 1 | 1 |
| Positive | 142 (8.1) | 35 (24.6) | 25.9 | 4.24 (2.89‐6.23) | 4.43 (2.89‐6.79) | 5.09 (3.11‐8.33) |
| Mortality (hospitalized patients) | ||||||
| Negative | 1,613 (91.9) | 144 (8.9) | 8.2 | 1 | 1 | 1 |
| Positive | 142 (8.1) | 35 (24.6) | 24.8 | 3.12 (2.15‐4.52) | 3.23 (2.14‐4.88) | 2.83 (1.78‐4.59) |
Adjusted for cirrhosis etiology, compensated/decompensated cirrhosis, age, sex, race, ethnicity, CCI, and geographical region.
Adjusted for MELD score and serum albumin in addition to the characteristics above.
Associations Between Cirrhosis and Adverse Outcomes in Patients With SARS‐CoV‐2 Infection
Among patients with SARS‐CoV‐2 infection, those who had cirrhosis were more likely than patients without cirrhosis to be hospitalized (aHR, 1.37; 95% CI, 1.12‐1.66), to be mechanically ventilated (aHR, 1.61; 95% CI, 1.05‐2.46), and to die (aHR, 1.65; 95% CI, 1.18‐2.30, Table 5). In the subset of patients with SARS‐CoV‐2 who were hospitalized, no significant association was observed between presence of cirrhosis and either mechanical ventilation (aHR, 1.28; 95% CI, 0.82‐2.01) or mortality (aHR, 1.33; 95% CI, 0.87‐2.01).
TABLE 5.
Association Between Cirrhosis and Risk of Hospitalization, Mechanical Ventilation, and Mortality in Patients With SARS‐CoV‐2 Infection
| Cirrhosis Status | n (%) | Event, n (%) | 30‐Day Event Rate (%) | Age aHR | Multivariable aHR* | Multivariable aHR † |
|---|---|---|---|---|---|---|
| Hospitalization | ||||||
| No cirrhosis | 9,826 (97.0) | 3,340 (34.0) | 33.7 | 1 | 1 | N/A |
| Cirrhosis | 305 (3.0) | 167 (54.8) | 53.4 | 1.55 (1.33‐1.81) | 1.37 (1.12‐1.66) | N/A |
| Hospitalization (occurring on or after the SARS‐CoV‐2 testing date) | ||||||
| No cirrhosis | 9,291 (97.1) | 2,805 (30.2) | 29.8 | 1 | 1 | N/A |
| Cirrhosis | 282 (2.9) | 144 (51.1) | 49.6 | 1.64 (1.38‐1.94) | 1.48 (1.20‐1.84) | N/A |
| Mechanical ventilation (all patients) | ||||||
| No cirrhosis | 9,826 (97.0) | 638 (6.5) | 6.5 | 1 | 1 | N/A |
| Cirrhosis | 305 (3.0) | 38 (12.5) | 13.0 | 1.65 (1.19‐2.30) | 1.61 (1.05‐2.46) | N/A |
| Mortality (all patients) | ||||||
| No cirrhosis | 9,826 (97.0) | 1,043 (10.6) | 10.6 | 1 | 1 | N/A |
| Cirrhosis | 305 (3.0) | 55 (18.0) | 17.1 | 1.70 (1.30‐2.24) | 1.65 (1.18‐2.30) | N/A |
| Mechanical ventilation (hospitalized patients) | ||||||
| No cirrhosis | 2,763 (95.1) | 572 (20.7) | 20.9 | 1 | 1 | 1 |
| Cirrhosis | 142 (4.9) | 35 (24.6) | 25.9 | 1.15 (0.81‐1.61) | 1.28 (0.82‐2.01) | 1.27 (0.79‐2.05) |
| Mortality (hospitalized patients) | ||||||
| No cirrhosis | 2,763 (95.1) | 582 (21.1) | 21.1 | 1 | 1 | 1 |
| Cirrhosis | 142 (4.9) | 35 (24.6) | 24.8 | 1.27 (0.90‐1.79) | 1.33 (0.87‐2.01) | 1.28 (0.81‐2.01) |
Adjusted for cirrhosis etiology, age, sex, race, ethnicity, CCI, diabetes, cancer, hypertension, congestive heart failure, dialysis, chronic kidney disease, chronic obstructive pulmonary disease, and geographical region.
Adjusted for MELD score and serum albumin in addition to the characteristics above.
Predictors of Mortality in Cirrhosis Patients With SARS‐CoV‐2 Infection
Among 305 patients with cirrhosis and SARS‐CoV‐2 infection, 55 (18%) died within 30 days and 142 were hospitalized. Independent predictors of mortality included decompensated cirrhosis, female sex, older age, and, among hospitalized patients, MELD score ≥ 12 (Table 6).
TABLE 6.
Predictors of Mortality Among Patients With Cirrhosis and SARS‐CoV‐2 Infection
| n (%) | 30‐Day Mortality (%) | Hazard Ratio | aHR* | |
|---|---|---|---|---|
| Cirrhosis etiology | ||||
| No HCV | 161 (52.8) | 20.4 | 1 | 1 |
| Past HCV | 126 (41.3) | 15.3 | 0.71 (0.41‐1.24) | 0.79 (0.41‐1.52) |
| Current HCV | 18 (5.9) | 0 | N/A | N/A |
| Cirrhosis decompensation prior to SARS‐CoV‐2 ‡ | ||||
| No | 213 (69.8) | 12.8 | 1 | 1 |
| Yes | 92 (30.2) | 27.3 | 2.01 (1.18‐3.42) | 2.02 (1.11‐3.69) |
| Sex | ||||
| Men | 296 (97.0) | 16.5 | 1 | 1 |
| Women | 9 (3.0) | 37.5 | 2.10 (0.66‐6.73) | 4.42 (1.21‐16.14) |
| Age (years) | ||||
| 18‐49 | 12 (3.9) | 0 | N/A | N/A |
| 50‐64 | 98 (32.1) | 11.3 | 1 | 1 |
| 65‐79 | 172 (56.4) | 19.2 | 1.76 (0.91‐3.41) | 1.97 (0.98‐3.95) |
| ≥80 | 23 (7.5) | 34.8 | 3.69 (1.56‐8.77) | 3.22 (1.29‐8.05) |
| Race | ||||
| White | 144 (47.2) | 15.4 | 1 | 1 |
| Black | 141 (46.2) | 18.9 | 1.18 (0.67‐2.06) | 1.36 (0.73‐2.55) |
| Other | 5 (1.6) | 20 | 1.22 (0.16‐9.00) | 1.13 (0.14‐8.94) |
| Missing/unknown | 15 (4.9) | 15.4 | 2.24 (0.85‐5.89) | 3.16 (0.94‐10.69) |
| Ethnicity | ||||
| Non‐Hispanic | 270 (88.5) | 16.6 | 1 | 1 |
| Hispanic | 31 (10.2) | 23.3 | 1.31 (0.59‐2.90) | 1.20 (0.46‐3.09) |
| Missing/unknown | 4 (1.3) | 0 | 1.34 (0.18‐9.71) | 0.63 (0.06‐6.49) |
| Geographical region: COVID‐19‐related deaths per million | ||||
| <130 | 47 (15.4) | 11.6 | 1 | 1 |
| 130‐350 | 72 (23.6) | 22.5 | 2.04 (0.75‐5.57) | 2.13 (0.76‐5.97) |
| 350‐700 | 78 (25.6) | 14.1 | 1.43 (0.51‐4.02) | 1.59 (0.54‐4.67) |
| ≥700 | 108 (35.4) | 17.8 | 1.74 (0.66‐4.61) | 1.95 (0.70‐5.42) |
| BMI at index date | ||||
| <18.5 (underweight) | 11 (3.6) | 10 | 0.36 (0.05‐2.70) | 0.33 (0.04‐2.63) |
| 18.5‐24.9 (normal) | 79 (25.9) | 20.9 | 1 | 1 |
| 25.29.9 (overweight) | 86 (28.2) | 14.2 | 0.57 (0.28‐1.18) | 0.64 (0.30‐1.38) |
| 30‐34.9 (obese I) | 59 (19.3) | 17.2 | 0.70 (0.32‐1.50) | 0.74 (0.32‐1.68) |
| ≥35 (obese II and III) | 68 (22.3) | 17.9 | 0.79 (0.39‐1.61) | 0.75 (0.36‐1.59) |
| Missing | 2 (0.7) | 0 | N/A | N/A |
| CCI | ||||
| 1‐2 | 68 (22.3) | 11.8 | 1 | 1 |
| 3‐4 | 59 (19.3) | 15.5 | 1.30 (0.53‐3.19) | 1.02 (0.40‐2.58) |
| ≥5 | 178 (58.4) | 19.7 | 1.64 (0.79‐3.40) | 1.07 (0.49‐2.32) |
| MELD score † | ||||
| <12 | 42 (29.6) | 14.3 | 1 | 1 |
| ≥12 | 61 (43.0) | 34.4 | 2.45 (1.04‐5.76) | 2.82 (1.09‐7.31) |
| Missing | 39 (27.5) | 20.3 | 1.38 (0.48‐3.93) | 1.63 (0.52‐5.14) |
| Albumin (g/dL) † | ||||
| >3.3 | 64 (45.1) | 17.2 | 1 | 1 |
| ≤3.3 | 63 (44.4) | 34.9 | 2.45 (1.21‐4.96) | 1.95 (0.87‐4.38) |
| Missing | 15 (10.6) | 8.3 | 0.58 (0.08‐4.47) | 0.43 (0.05‐3.78) |
Adjusted for cirrhosis etiology, compensated/decompensated cirrhosis, age, sex, race, ethnicity, Charlson comorbidity index, geographical region.
Analysis of MELD score and albumin was limited to hospitalized patients.
Defined by diagnosis of ascites, spontaneous bacterial peritonitis, hepatorenal syndrome, hepatopulmonary syndrome, encephalopathy or variceal bleeding prior to SARS‐CoV‐2 infection.
Abbreviations: BMI, body mass index; N/A, not available.
Discussion
In this cohort of 88,747 US veterans (including 3,606 with cirrhosis) who were tested for SARS‐CoV‐2, patients with cirrhosis were less likely to test positive for SARS‐CoV‐2 than those without cirrhosis. Mortality and mechanical ventilation rates increased progressively from those without cirrhosis or SARS‐CoV‐2 to those with cirrhosis alone to those with SARS‐CoV‐2 alone to those with both conditions. Among patients with cirrhosis, those who tested positive for SARS‐CoV‐2 were 4 times more likely to undergo mechanical ventilation and 3.5 times more likely to die than those who tested negative. Among patients with SARS‐CoV‐2 infection, those with cirrhosis were more likely to be hospitalized, to be mechanically ventilated, and to die than patients without cirrhosis. In patients with cirrhosis and SARS‐CoV‐2 infection, the most important predictors of mortality were advanced age, baseline decompensated cirrhosis, and a high MELD score.
An international, voluntary registry study reported even higher mortality rates in SARS‐CoV‐2‐infected patients with cirrhosis, ranging from 24% for Child‐Pugh class A to 63% for class C.( 27 ) However, the lack of a control group as well as reporting and selection bias limit the interpretability of this study. Our results differ substantially from a recent study which reported that, among hospitalized patients with cirrhosis, those with SARS‐CoV‐2 infection did not have significantly higher mortality that those without infection.( 26 ) That study, which included a smaller number of patients with cirrhosis and SARS‐CoV‐2 (n = 37) at seven tertiary referral centers and may have been underpowered, reported a much higher mortality rate for both the cirrhosis‐only (20%) and the cirrhosis and SARS‐CoV‐2 (30%) groups than what we observed. The study likely attenuated the difference between infected and uninfected individuals by limiting the analysis to hospitalized patients. Our analysis of a much larger cohort within a national health care system found 3.5‐fold higher mortality in patients with cirrhosis who tested positive (17.1%) versus negative (5.2%) for SARS‐CoV‐2 after adjusting for potential confounders (aHR, 3.54; 95% CI, 2.55‐4.90). The association between SARS‐CoV‐2 infection and mortality among patients with cirrhosis was only slightly attenuated in the subset of hospitalized patients (aHR, 3.23; 95% CI, 2.14‐4.88). Even after additional adjustment for MELD score and serum albumin, which reflect liver dysfunction, SARS‐CoV‐2 infection was associated with significantly higher mortality in patients with cirrhosis (aHR, 2.83; 95% CI, 1.78‐4.59). The positive association between SARS‐CoV‐2 infection and mechanical ventilation (aHR, 4.12; 95% CI, 2.79‐6.10) in patients with cirrhosis suggests that severe pneumonia, acute respiratory distress syndrome, and respiratory failure were the causes of excess mortality. These results demonstrate the grave, additional risks of SARS‐CoV‐2 infection in patients with cirrhosis.
We found that among all patients who tested positive for SARS‐CoV‐2, the presence of cirrhosis was an independent, significant risk factor for hospitalization (aHR, 1.37; 95% CI, 1.12‐1.66), mechanical ventilation (aHR, 1.61; 95% CI, 1.05‐2.46), and mortality (aHR, 1.65; 95% CI, 1.18‐2.30). However, among the subset of SARS‐CoV‐2‐positive patients who were sick enough to require hospitalization, the presence of cirrhosis was not associated with significantly higher mortality or risk of mechanical ventilation. This suggests that when SARS‐CoV‐2 infection causes severe enough disease to require hospitalization, factors other than cirrhosis are the main drivers of mortality.
Among 305 patients with cirrhosis and SARS‐CoV‐2 infection, 30‐day mortality was 17.1% (95% CI, 13.3‐21.8). Our results suggest that the main risk factors for mortality in these patients are advanced age and liver dysfunction (i.e., decompensation and high MELD). In fact, patients with decompensated cirrhosis had twice the risk of mortality compared to those with compensated cirrhosis (aHR, 2.02; 95% CI, 1.11‐3.69). Therefore, clinicians should be especially vigilant when caring for patients with cirrhosis who are elderly or have a history of decompensated cirrhosis because they have the highest risk for mortality. Although we found that female sex was significantly associated with mortality among patients with cirrhosis and SARS‐CoV‐2, meaningful interpretation is precluded by the small sample size of women. Other factors such as comorbidity burden, obesity, race, ethnicity, and etiology of cirrhosis (HCV versus other) did not appear to be associated with mortality. While it is reassuring that Black and Hispanic patients were not at increased risk of mortality in our study, we did observe that Black patients with cirrhosis were more likely to test positive for SARS‐CoV‐2. This is in agreement with our recent publication that identified Black race as a strong, independent risk factor for testing positive for SARS‐CoV‐2.( 34 )
We also found that patients with cirrhosis were less likely to test positive for SARS‐CoV‐2 infection. Because there is no plausible biological mechanism to suggest that cirrhosis protects against SARS‐CoV‐2, this likely reflects differences in health behavior. As individuals with a chronic illness, patients with cirrhosis may be more concerned about SARS‐CoV‐2 and consequently more likely to practice preventive measures such as physical distancing, handwashing, and self‐quarantine. It is also possible that asymptomatic patients with cirrhosis were being routinely tested prior to medical procedures, which would effectively decrease the test positivity rate. However, when we adjusted for symptoms in addition to sociodemographic characteristics and comorbidities to account for this potential bias, the association between cirrhosis and test positivity was only minimally attenuated.
The strengths of our study include its national scope, large number of patients, relatively long follow‐up for the three most clinically relevant outcomes, and analysis of a variety of potential risk factors. However, several limitations should be acknowledged. Findings from our predominantly male veteran population may not be generalizable to other populations and groups, especially women. Our results are also limited by the use of ICD‐10 codes for the determination of comorbid conditions. However, the majority of these ICD‐10‐based definitions have been widely used and validated in VA studies. Natural language processing plus ICD‐10 codes were used for the definition of SARS‐CoV‐2 symptoms, although the performance characteristics of these definitions is not known yet. Our data capture an early period of the pandemic, and with improvements in clinical management the overall mortality rates and magnitude of association with risk factors may have changed. We captured deaths that occurred both within and outside the VA; however, hospitalizations or mechanical ventilations that occurred outside the VA and were not paid for by the VA were not captured. Finally, our results may reflect institutional policies and practices related to testing that might not be generalizable to other health systems.
In conclusion, our national VA study found that among patients with cirrhosis, SARS‐CoV‐2 infection was associated with a 3.5‐fold increased risk of 30‐day mortality. Among patients with SARS‐CoV‐2, cirrhosis was associated with a 1.7‐fold increased risk of mortality. Patients with cirrhosis should strictly adhere to recommended preventive measures against SARS‐CoV‐2 infection. Clinicians should carefully weigh the risks and benefits of in‐person encounters or procedures in this high‐risk group, and they should recognize that cirrhosis is a negative prognostic factor in SARS‐CoV‐2 infection.
Author Contributions
G.N.I. is the guarantor of this paper. All authors approved the final version of the manuscript. G.N.I. and K.B. were responsible for study concept and design, analysis of data, interpretation of results, drafting of manuscript, critical revision of manuscript. P.S.L. was responsible for study concept and design, interpretation of results, drafting of manuscript, critical revision of manuscript. E.L., A.M.O., J.A.S., K.C., M.C.E., and V.S.F. were responsible for study concept and design, critical revision of manuscript. P.G. was responsible for extraction of data, creation of analytic variables, analysis of data, interpretation of results. J.A.D. was responsible for study concept and design, interpretation of results, critical revision of manuscript.
Supporting information
Supplementary Material
Acknowledgment
We acknowledge the VINCI group who worked tirelessly to create the COVID 19 Shared Data Resource.
Supported in part by Veterans Affairs Clinical Science Research and Development (COVID19‐8900‐11, to G.N.I.).
Potential conflict of interest: Nothing to report.
The contents do not represent the views of the US Department of Veterans Affairs or the US government.
References
- 1. Garg S, Kim L, Whitaker M, O'Halloran A, Cummings C, Holstein R, et al. Hospitalization rates and characteristics of patients hospitalized with laboratory‐confirmed coronavirus disease 2019—COVID‐NET, 14 states, March 1‐30, 2020. MMWR Morb Mortal Wkly Rep 2020;69:458‐464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. CDC COVID‐19 Response Team . Severe outcomes among patients with coronavirus disease 2019 (COVID‐19)—United States, February 12‐March 16, 2020. MMWR Morb Mortal Wkly Rep 2019;69:343‐346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med 2020;180:934‐943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tobias H III, Jurinovic V, Arnreich C, Hellmuth JC, Bergwelt‐Baildon M, Klein M, et al. MerdRxiv perprint: level of IL‐6 predicts respiratory failure in hospitalized symptomatic COVID‐19 patients. https://www.medrxiv.org/content/10.1101/2020.04.01.20047381v1. Accessed June 4, 2020. [Google Scholar]
- 6. Liang W, Guan W, Chen R, Wang W, Li J, Xu K, et al. Cancer patients in SARS‐CoV‐2 infection: a nationwide analysis in China. Lancet Oncol 2020;21:335‐337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Simonnet A, Chetboun M, Poissy J, Raverdy V, Noulette J, Duhamel A, et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) requiring invasive mechanical ventilation. Obesity (Silver Spring) 2020;28:1195‐1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cai Q, Chen F, Wang T, Luo F, Liu X, Wu Q, et al. Obesity and COVID‐19 severity in a designated hospital in Shenzhen, China. Diabetes Care 2020;43:1392‐1398. [DOI] [PubMed] [Google Scholar]
- 9. Cummings MJ, Baldwin MR, Abrams D, Jacobson SD, Meyer BJ, Balough EM, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID‐19 in New York City: a prospective cohort study. Lancet 2020;395:1763‐1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Docherty AB, Harrison EM, Green CA, Hardwick HE, Pius R, Norman L, et al. Features of 20 133 UK patients in hospital with COVID‐19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ 2020;369:m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Iaccarino G, Grassi G, Borghi C, Ferri C, Salvetti M, Volpe M, et al. Age and multimorbidity predict death among COVID‐19 patients: results of the SARS‐RAS study of the Italian Society of Hypertension. Hypertension 2020;76:366‐372. [DOI] [PubMed] [Google Scholar]
- 12. Imam Z, Odish F, Gill I, O'Connor D, Armstrong J, Vanood A, et al. Older age and comorbidity are independent mortality predictors in a large cohort of 1305 COVID‐19 patients in Michigan, United States. J Intern Med 2020;288:469‐476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jain V, Yuan JM. Predictive symptoms and comorbidities for severe COVID‐19 and intensive care unit admission: a systematic review and meta‐analysis. Int J Public Health 2020;65:533‐546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kalligeros M, Shehadeh F, Mylona EK, Benitez G, Beckwith CG, Chan PA, et al. Association of obesity with disease severity among patients with coronavirus disease 2019. Obesity (Silver Spring) 2020;28:1200‐1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Klang E, Kassim G, Soffer S, Freeman R, Levin MA, Reich DL. Morbid obesity as an independent risk factor for COVID‐19 mortality in hospitalized patients younger than 50. Obesity (Silver Spring) 2020;28:1595‐1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kuderer NM, Choueiri TK, Shah DP, Shyr Y, Rubinstein SM, Rivera DR, et al. Clinical impact of COVID‐19 on patients with cancer (CCC19): a cohort study. Lancet 2020;395:1907‐1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee N, McGeer A. The starting line for COVID‐19 vaccine development. Lancet 2020;395:1815‐1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liang W, Liang H, Ou L, Chen B, Chen A, Li C, et al. Development and validation of a clinical risk score to predict the occurrence of critical illness in hospitalized patients with COVID‐19. JAMA Intern Med 2020;180:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Petrilli CM, Jones SA, Yang J, Rajagopalan H, O'Donnell L, Chernyak Y, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ 2020;369:m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Price‐Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among black patients and white patients with Covid‐19. N Engl J Med 2020;382:2534‐2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rentsch CT, Kidwai‐Khan F, Tate JP, Park LS, King JT, Skanderson M, et al. Covid‐19 testing, hospital admission, and intensive care among 2,026,227 United States veterans aged 54‐75 years. medRxiv 2020;2020.04.09.20059964. [Google Scholar]
- 22. Rentsch CT, Kidwai‐Khan F, Tate JP, Park LS, King JT, Skanderson M, et al. Covid‐19 by race and ethnicity: a national cohort study of 6 million United States veterans. medRxiv 2020;2020.05.12.20099135. [Google Scholar]
- 23. Tian J, Yuan X, Xiao J, Zhong Q, Yang C, Liu B, et al. Clinical characteristics and risk factors associated with COVID‐19 disease severity in patients with cancer in Wuhan, China: a multicentre, retrospective, cohort study. Lancet Oncol 2020;21:893‐903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cariou B, Hadjadj S, Wargny M, Pichelin M, Al‐Salameh A, Allix I, et al. Phenotypic characteristics and prognosis of inpatients with COVID‐19 and diabetes: the CORONADO study. Diabetologia 2020;63:1500‐1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ioannou GN, Locke E, Green P, Berry K, O'Hare AM, Shah JA, et al. Risk factors for hospitalization, mechanical ventilation, or death among 10131 US veterans with SARS‐CoV‐2 infection. JAMA Netw Open 2020;3:e2022310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bajaj JS, Garcia‐Tsao G, Biggins S, Kamath PS, Wong F, McGeorge S, et al. Comparison of mortality risk in patients with cirrhosis and COVID‐19 compared with patients with cirrhosis alone and COVID‐19 alone: multicentre matched cohort. Gut. In press. 10.1136/gutjnl-2020-322118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moon AM, Webb GJ, Aloman C, Armstrong MJ, Cargill T, Dhanasekaran R, et al. High mortality rates for SARS‐CoV‐2 infection in patients with pre‐existing chronic liver disease and cirrhosis: preliminary results from an international registry. J Hepatol 2020;73:705‐708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Singh S, Khan A. Clinical characteristics and outcomes of coronavirus disease 2019 among patients with preexisting liver disease in the United States: a multicenter research network study. Gastroenterology 2020;159:768‐771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Beste LA, Leipertz SL, Green PK, Dominitz JA, Ross D, Ioannou GN. Trends in burden of cirrhosis and hepatocellular carcinoma by underlying liver disease in US Veterans, 2001‐2013. Gastroenterology 2015;149:1471‐1482.e1475. [DOI] [PubMed] [Google Scholar]
- 30. Department of Veterans Affairs, Office of Research and Development . COVID‐19 shared data resource. https://vhacdwdwhweb100.vha.med.va.gov/phenotype/index.php/COVID‐19:Shared_Data_Resource. Accessed December 14, 2020. [Google Scholar]
- 31. Ioannou GN, Green P, Lowy E, Mun EJ, Berry K. Differences in hepatocellular carcinoma risk, predictors and trends over time according to etiology of cirrhosis. PLoS One 2018;13:e0204412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sohn MW, Arnold N, Maynard C, Hynes DM. Accuracy and completeness of mortality data in the Department of Veterans Affairs. Popul Health Metr 2006;4:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Worldometer . Coronavirus; 2020. https://www.worldometers.info/coronavirus/. Accessed December 14, 2020. [Google Scholar]
- 34. Fan VS, Dominitz JA, Eastment MC, Locke E, Green P, Berry K, et al. Risk factors for testing positive for SARS‐CoV‐2 in a national US healthcare system. Clin Infect Dis 2020;ciaa1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


