Abstract
The major epidemic and pandemic diseases that have bothered humans since the Neolithic Age and Bronze Age are surveyed. Many of these pandemics are zoonotic infections, and the mathematical modeling of such infections is illustrated. Plague, cholera, syphilis, influenza, SARS, MERS, COVID‐19, and new potential epidemic and pandemic infections and their consequences are described and the background for the spread of acute and chronic infections and the transition to endemic infections is discussed. The way we can prevent and fight pandemics is illustrated from the old and new well‐known pandemics. Surprisingly, the political reactions through different periods have not changed much during the centuries.
Keywords: Pandemics, epidemics, plague, cholera, influenza, SARS, MERS, COVID‐19
Introduction
Infectious chronic diseases such as osteomyelitis, leprosy, and tuberculosis occurred in humans in ancient time, but spread of infections as epidemics and pandemics was not possible because of the lifestyle of humans in ancient time as described by A. Morabia 2009 (1). The groups of humans were simply too small to sustain acute communicable infectious diseases because the sick patients either died or became immune and thereby further spread of the infections stopped (Fig. 1A–D).
Fig. 1.
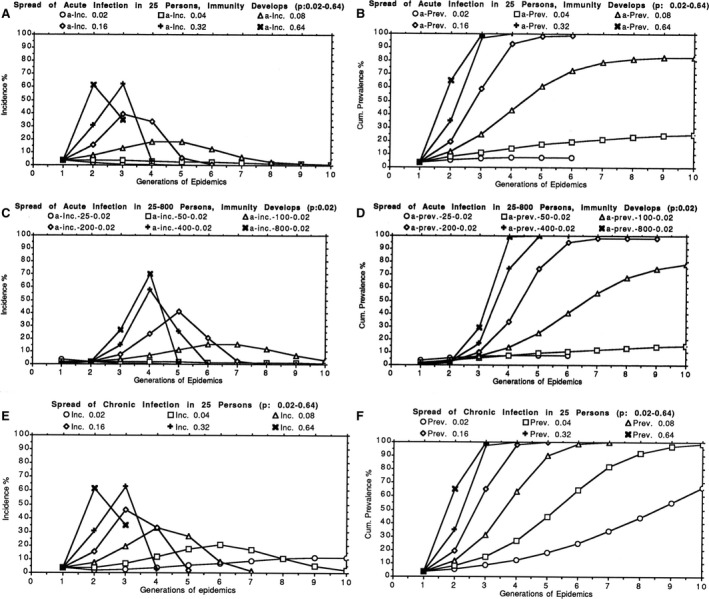
(A and B) Calculated epidemic curves starting with one person being infected in a population of 25 susceptible individuals, assuming effective contact rates (p) for transmission of the infection of 0.02, 0.04, 0.08, 0.16, 0.32, or 0.64 and development of immunity so that the infection is cleared. The abscissas show the generation of the epidemic, for example, 1st generation: one patient infected who subsequently infects 2 new individuals in the 2nd generation of the epidemic, etc. (reproduction number R0 = 2, the epidemic develops faster if R0 increases, but decreases if R0 is below 1). (A) ordinate: incidence (%) of new infected patients. (B) ordinate: accumulated prevalence (%) of infected and recovered (immunity develops) patients. The higher the contact rate, the faster the epidemic develops and terminates due to immunity. The lowest contact rate (0.02) leads to termination of the epidemic, when very few individuals have become infected. The incidence and accumulated prevalence are highest when 50% of the population is infected and 50% are susceptible. Eventually, the infection disappears due to immunity and cannot be introduced again unless the immunity decreases to non‐protective level or if new non‐immune members of the group appear, for example, new children are born. (C and D) Calculated epidemic curves starting with one person being infected in a population of 25, 50, 100, 200, 400, or 800 susceptible individuals, assuming an effective contact rate (p) for transmission of the infection of 0.02 and development of immunity so the infection is cleared (reproduction number R0 = 2). Abscissas and ordinates as in A, B. The incidence and accumulated prevalence increase rapidly with increasing size of the population. (E and F) Calculated epidemic curves starting with one person being infected in a population of 25 susceptible individuals, assuming effective contact rates (p) for transmission of the infection of 0.02, 0.04, 0.08, 0.16, 0.32, or 0.64 and no development of immunity so the infection becomes chronic and continues to be infectious. (reproduction number R0 = 2). Abscissas as in A, B. E: ordinate: incidence (%) of new chronically infected patients. F: ordinate: accumulated prevalence (%) of chronically infected and therefore still infectious patients. The higher the contact rate the faster the epidemic develops and terminates due to immunity. Even the lowest contact rate (0.02) leads to slow spread of the epidemic to the whole population if no precautions (e.g., isolation) are taken and the infection therefore becomes endemic and can continue to infect new members of the group, for example, when new children are born. Calculations are done according to the Reed‐Frost stochastic compartment model, formula: It+1 = (1 − (1−p)I t)St where It is the number of infectious persons in the preceding generation, St is the number of susceptible individuals and p is the effective contact rate between infectious and susceptible individuals (44, 45).
Epidemics were, therefore, not a problem for human beings when they lived in small groups or clans of maybe 25–40 individuals. They lived nomadic as hunters, gatherers, and fishers before the Neolithic revolution (1). The small groups of people seldom met other groups (1). When people about 10 000 years B.C. began to domesticate animals, grow cereal, store food, and establish farms and cities, they gradually became sedentary as agrarians and started to trade and communicate with each other. The changed living conditions paved the way for epidemics and pandemics which were facilitated by long‐distance journeys, trade, and migration by ships or by horses. At the same time, animal domestication created favorable conditions for animal microbes to adapt to humans such as measles, tuberculosis, and smallpox from cattle, influenza from pigs and ducks, and pertussis from pigs and dogs (1). The growing population density in cities was likewise favorable for emergence of epidemics.
An example is the morbilli (measles) epidemic (incubation time 10–11 days) on the 18 North Atlantic Faeroe Islands from April 4–5 to September 17, 1846, which was described by the Danish physician and later professor of physiology Peter Ludvig Panum (1820–1885). Panum together with a colleague was sent to the islands to help the two local physicians to control the epidemic. The old persons, who experienced the last morbilli epidemic in 1781, were immune and did not become sick during the 1846 epidemic whereas most of the 7782 inhabitants became sick except those 1500 persons who were placed in quarantine by Panum. The epidemic stopped because it could not be sustained in such a small population (2). If populations are large enough, however, enough new susceptible children are born, and under such conditions, the spread of the infection does not stop completely but continues at a low level and becomes endemic giving rise to local epidemics, for example, every 2–3 years. This was the case with morbilli before universal vaccination of children was introduced in the 1980s that provides lifelong immunity. Even in such cases, outbreaks are observed if less than 95% of the children are vaccinated and that has unfortunately been the case in, for example, Europe and the United States (3). In case of pertussis (whooping cough), where the vaccine does not provide long‐lasting immunity in spite of re‐vaccination, outbreaks continue to occur, for example, among schoolchildren and adolescents but old people may also become infected (Fig. 2) (4).
Fig. 2.
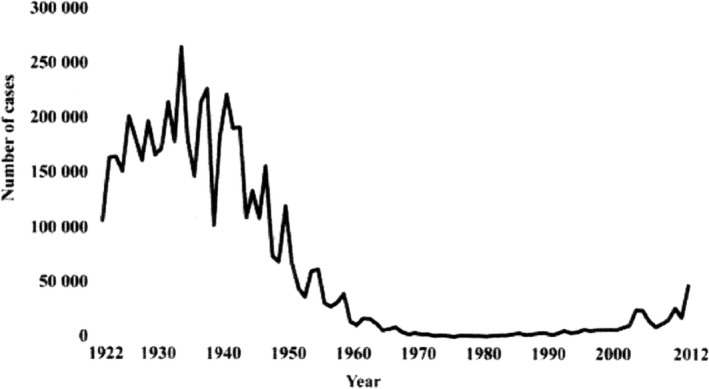
Pertussis cases by year in the United States 1922–2012. A whole‐cell pertussis vaccine trial documented its effectiveness in 1940 and shortly after it became available and recommended by the American Academy of Pediatrics. The whole‐cell vaccines were gradually replaced by acellular vaccines 1992–97 and thereafter small epidemic started to occur among vaccinated adolescents and school‐aged children (4).
The stop of epidemics due to immunity is, however, not working when an infection such as tuberculosis of the lungs is chronic and continues to be contagious (Fig. 1E, F). The incubation time of an infection is important for the spread of infections between cities or even countries. If the incubation time is long and many infected persons succumb, the clinically healthy (during incubation time) but infected persons may be horrified and try to escape to other cities and when they then become sick, they will spread the infection in that city. This was the case with the variola (smallpox) epidemic (incubation time 8–17 days) in Central America which was brought to the Indians by Christopher Columbus (1451–1506) in 1492 and by Hernando Cortez (1485–1547) who arrived in Hispaniola (Dominican Republic) in 1504 and conquered the Aztec Empire in Mexico 1521. The result was devastating and reduced the local population dramatically so that they could not be used as workers (slaves) on the plantations by the Spanish conquistadors and, therefore, the Atlantic slave trade between Africa and the Americas began.
Epidemics occurred in ancient Sumer, Babylon, and Egypt around 2000 B.C. (1) whereas epidemics do not seem to have occurred in Central America in the Aztec empire where about 25 million inhabitants lived before the Spanish Conquest and where the capital Tenochtitlan (now Mexico City) was one of the largest cities in the world with about 200 000 inhabitants. The Aztecs, however, had only domesticated dogs and turkeys as food resources. The transatlantic exchange exported epidemics of childhood diseases such as smallpox, and yellow fever, malaria, and other diseases from Europe and Africa to the Americas with catastrophic consequences since the population of Central America decreased from 25 million in 1518 to 700 000 a century later (1). Syphilis, however, was exported from the Indians in America with infected Spanish sailors back to Europe, where it was disseminated with mercenary soldiers and their accompanying prostitutes.
The oldest records of epidemics are Chinese and they cover 488 outbreaks in the entire 2000 years duration of the Chinese Empire from 243 B.C. to 1911 A.C. During that long period, agriculture was the dominating way of living in China with many domesticated animals and birds living close together with the peasants. The Chinese administration’s enumeration of the population and the outbreaks showed a striking correlation between the growing population and the growing frequency of epidemics (Fig. 3) (1). Outbreaks were rare 100 B.C. Between 100 and 1100 A.C., there were about 10 outbreaks per century but thereafter the number increased to 80 outbreaks per century after 1800 A.C. during which period the population increased from about 100 million to more than 300 million inhabitants (1). It is not known which microbes caused these epidemics, but the correlation between the growing size of the population and the increasing frequency of outbreaks is important for understanding the dynamics of the spread of epidemics and pandemics (Fig. 1).
Fig. 3.
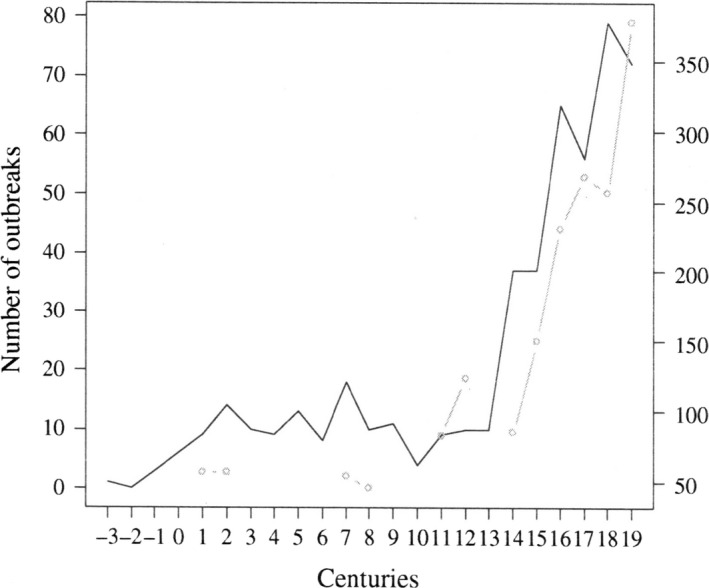
Number of epidemic outbreaks by century reported between 300 B.C. and 1911 A.C. in China (solid line) and population size (millions, gray dotted line)(1).
The etiology of infections and the way they spread was unknown until the work of Louis Pasteur (1822–1895) and Robert Koch (1843–1910) although bacteria had already been observed microscopically by the inventor of the microscope Anthony van Leeuwenhoek (1632–1723) from Delft in the Netherlands and by Otto Friederich Müller from Denmark (1730–1784) the founder of systematic bacteriology who in 1773–74 in his book Vermium terestium et fluviatilium historia and later in Animalcula infusoria (1786) described 6 different species in the genus Vibrio which became the basis for taxonomy the following 100 years. The Danish author H.C. Andersen published a fairy tale ‘The Drop of Water’ in 1847 which described the microscopic behavior of microorganisms in muddy water so bacteria were well known at that time but their importance was unknown. Pasteur and Koch and their assistants described the etiology of many of the major infections in the Golden Period of Microbiology 1874–1906 (5). In ancient time, epidemic infectious diseases like plague were believed to be punishments of God (Fig. 4) (6). In 1828, the German homeopathic physician Samuel Friederich Christian Hahnemann (1755–1843) published his idea of miasma (miasma, foul‐smelling vapor from rotten organic matter) in his book ‘Chronic Diseases, their peculiar nature and their homeopathic cure’(7). Subsequently, there were two different medical explanations of how contagious diseases were spread. One theory was named the contagium vivum theory and the idea was that infections were transmitted by touching the sick patient or his excretions. The other was named the miasma theory and the idea was that infections were transmitted by bad or noxious air. After 1880, both theories were replaced by the modern germ theory of infectious diseases based on Pasteur’s and Koch’s work. In spite of these obsolete old theories, efficient measures such as quarantine and isolation had been used for many years before Pasteur and Koch to stop the spread of epidemics and pandemics as exemplified by Panum’s effort to stop the morbilli epidemic in the Faeroe Islands in 1846.
Fig. 4.
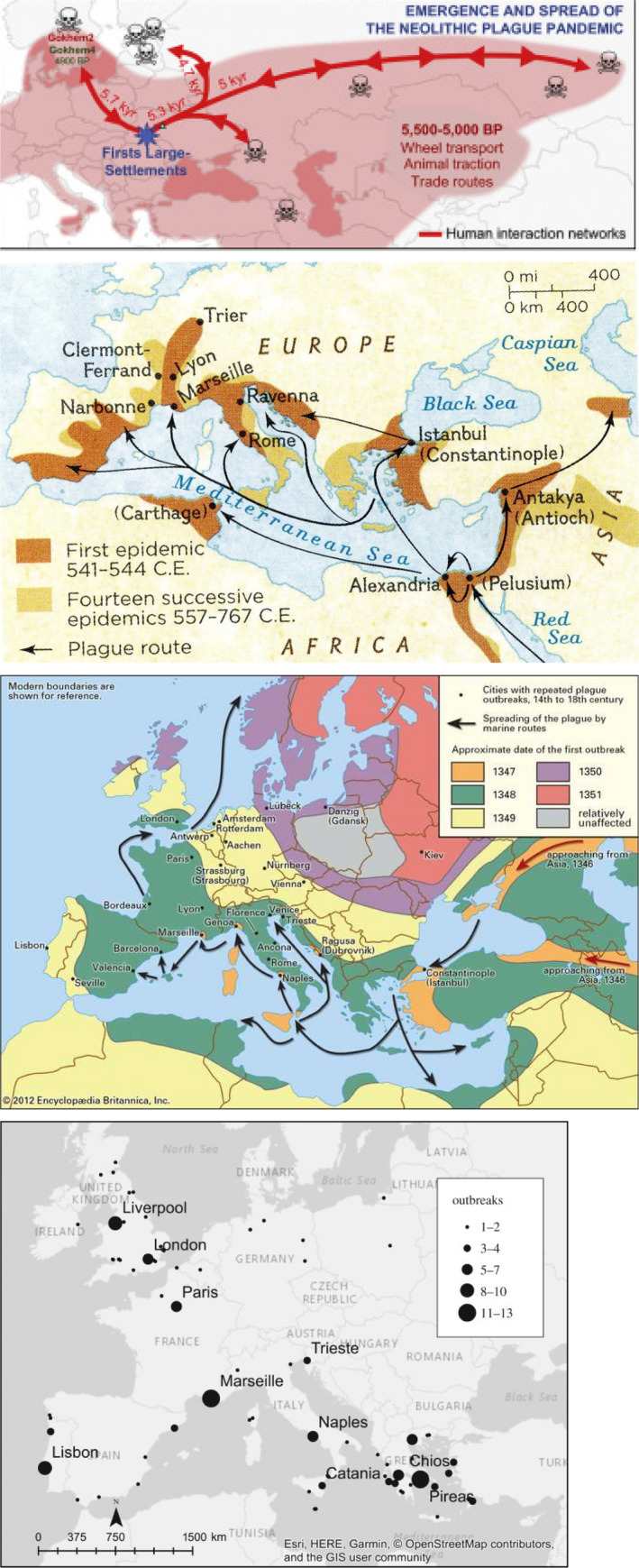
Spread of the plague in Europe. Top: Neolithic time (8). Middle: The first pandemic: The Justinianic Plague in the Roman Empire (9). Below and bottom: The second pandemic: The Black Death in the Middle Ages in Europe (www.britanica.com/event/Black‐Death). Bottom: The third pandemic (46).
Plague Epidemics and Pandemics
The cause of plague, Yersinia pestis, was detected by Alexander Yersin (1863–1943) in 1894 during the deadly 3rd plague pandemic in Hong Kong, and he also described the bacteria in the lymph nodes of dead rats during the pandemic (6). In 1898, Paul‐Louis Simond detected that fleas transmitted the bacteria between rats and from rats to humans so the transmission of Y. pestis was clarified in very few years. When spread by flea bites, the infection disseminates through the lymph and lymph nodes to the blood and cause sepsis. The swollen lymph nodes, buboes, are the reason for the name bubonic plague. If the lungs are involved, the disease is called pneumonic plague, which is very contagious by droplet transmission to other people. Later it was discovered that plague occurs as urban (city‐dwelling rats) plague in cities and as sylvatic (forest‐dwelling animals such as rodents) plague in the countryside and forests. The disease can be prevented and eradicated from cities by eradication of rats but it is very difficult to eradicate from the countryside, where it, therefore, causes sporadic cases in many countries in Asia, Africa, and the Americas. Since such animals often live very close to humans, plague epidemics have been the cause of the deadliest epidemics in human history (6).
Plague spread already with the great migration and settlements in Neolithic time and in Bronze Age from Asia 5500–5000 B.C. causing the neolithic plague pandemic which came from Asia and spread as far as Scandinavia, and it was presumably the reason for the decrease of the population during that period (Fig. 4)(8). The best‐known ancient plague pandemic was The Justinianic Plague 541–543 A.C. which is named after the emperor of the Eastern Roman (Byzantine) Empire Justinian the Great who ruled the empire 527–565 A.C. It is considered the first major bubonic plague epidemic and presumably contributed to the fall of the Roman Empire since one‐third to half of the population of the Eastern Roman Empire died (Fig. 4) (9). It spread from Asia and Africa where it hit the Roman port city Pelusium in Egypt 541 A.D. and spread to Alexandria and to Europe with the trade routes over land and sea and caused the death of about 25 million people. The plague reoccurred in Eurasia and North Africa over the next two centuries (10). Probably the most famous and fabled pandemic is the 2nd plague pandemic which was named The Black Death due to the dark cyanosis of lips, skin, and mucosa of the patients. It ravaged Europe from around 1350 A.C. (Figs 4 and 5). It came by ship to the harbor of Messina in Sicily 1347 and spread to Florence and the rest of Italy and killed people within 3 days according to Giovanni Boccacio’s book Decameron (1350). Venice, as a trading center, was especially vulnerable, and it was realized that the only solution was to separate people, to take away the sick people, or suspected sick people who were isolated on two islands of the Venetian lagoon. Lazzaretto Vecchio and Lazzaretto Nuovo became a spot where ships coming from places experiencing the plague, or those with suspected sick passengers or crew, anchored. There, people and goods spent a period of quarantine before being allowed into the heart of the city. We owe the English word ‘quarantine’ to the Italian term for 40 days, quaranta giorni.
Fig. 5.

Perception of the pandemic plague ravage before its etiology was known (top: flagellants in the Netherlands scourging themselves in atonement, believing that the Black Death is a punishment from God for their sins, 1349 (www.britanica.com/event/Black‐Death). Middle left: painting by Arnold Böcklin, 1827–1901) and protective clothing against plague (middle right) (6). Bottom: Doctors wearing gauze masks designed by Dr. Wu Lien‐teh during the Manchurian Plague 1910–11 (University of Hong Kong Libraries).
During the following 100 year, this plague pandemic caused about 25 million death people and reduced the European population with about 25%. Denmark was also reached by the plague pandemic 1349–50 and the following 50 years and during in the 1400s there were 9 outbreaks of plague in Denmark. The Danish King Valdemar (1320–1375) pardoned all death penalties during this period due to the declining population in Denmark caused by plague!
The plague pandemic continued in Europe during the next centuries but not so devastating as before (6). Control of the plague improved when the centralized state powers could legislate. In Denmark, King Christian IV (1588–1648) introduced the first plague legislation in the world in 1625 which determined that every city should have a house for plague patients and a plague master who had the responsibility for isolation (from isola Italian word for an island) treatment and care of such patients. Thereafter, the eternal plague epidemic stopped for a while; however, the Great Nordic War between Sweden and Denmark and most of the North European powers 1700–1721 destroyed all the administrative routines. The last plague epidemic, therefore, came to Elsinore (famous for its Kronborg Castle of Hamlet) in Denmark 1710 and hit Copenhagen (60 000 inhabitants) in 1711. It was a branch of the plague epidemic which 1704–1714 had spread to Russia, Silesia, Prussia, Pomerania, Holstein, Brunswick, Karlskrona, Uppsala, and Stockholm and later to Prague. The outbreak in Denmark started among poor fishermen in Elsinore, and in spite of military fencing, it spread all over North Zealand and further to Copenhagen 1711 where at its peak 2000 patients died every week and all together 20 000 inhabitants succumbed (11). The old plague legislation was re‐activated and new precautions were introduced, for example, ships arriving from harbors in other countries were quarantined for 40 days before they were allowed to enter Danish harbors and neither sailors nor cargo could approach Danish ground before it was proven that they were free of plague and did not come from a harbor where plague was present. If ships arrived from a well‐known plague harbor, they were according to royal resolution 1799 directed from any harbor in Denmark to Kristiansand located at the most southern part of Norway and founded by King Christian IV in 1641 since Denmark and Norway were one double‐kingdom until 1814. A quarantine station and a quarantine hospital with 250 beds were built in Kristiansand in 1804. In 1831, there were 339 ships in that harbor which was in use until 1914. Outside Copenhagen, the Saltholm Island in Øresund was used as quarantine station. The first inspection of an arriving ship was done by the ship pilot arriving from the harbor of Kristiansand. There was death penalty for the captain of a ship if he tried to hide a sick person and if a person tried to escape a quarantined ship. If the ship pilot suspected infectious diseases, he hoisted a yellow flag and a Health Commission including a physician arrived to the ship and quarantined the sick patients and the ship pilot and cleaned the ship and dripped it with vinegar.
The 3rd bubonic plague pandemic started in Yunnan, China, 1855, hit Hong Kong 1894, Europe, Africa, and South America and San Francisco in California, USA, 1900–1904 (6), and finally Cuba in 1912. Big business in San Francisco influenced Governor Gage and the newspapers so the plague in the city was denied. The plague in the city came from its Chinatown but the inhabitants would not collaborate with the health authorities. A smear campaign was organized against the health authorities especially the quarantine officer of the naval base Dr. Joseph J. Kinyon who was an educated bacteriologist. He had cultured the plague bacteria in a lymph node of a patient and passed it to experimental animals in his laboratory. He ordered anti‐plague serum from Washington for treatment of the patients. Antiserum from vaccinated animals was developed by Yersin at the Pasteur Institute and he used it to treat plague patients in Hong Kong 1896 with very good results. The health authorities quarantined Chinatown and ordered spray disinfection of the city’s public means of transportation which were not allowed to stop at Chinatown. The quarantine was canceled after 60 h and a judge decided that US federal president William McKinley’s (1843–1901) order to limit Chinese people’s access to public means of transportation was illegal in California and filed a lawsuit against Dr. Kinyoun! There was no political support for the health authorities’ fight against the plague until a new governor was elected in 1903 and the plague epidemic disappeared thereafter (6).
In what was known as the Great Manchurian plague in China and Mongolia (1910–11), the plague spread by droplets through the air and caused pneumonic plague which caused the death of infected patients within 24–48 h and the mortality was 100%. About 60 000 patients died from plague. The plague came from a tarbagan marmot infected with plague pneumonia. Such animals were hunted in Manchuria for their fur and the gathering of marmot hunters during the winter months and the eventual travel of migrant workers during the Chinese New Year magnified the spread of the plague. The Malaysian (ethnic Chinese) physician Wu Lien‐teh (1879–1960) was educated in Cambridge 1896 and spent his clinical training in London and Liverpool at the time surgical masks were introduced in the operating theaters. He was now living in China and worked for the Chinese government since 1907. He was called by the government to travel to Harbin and lead the fight against the plague in Manchuria. He was the scientist who detected that the plague spreads by air and he then promoted quarantine and invented a protective face mask consisting of 2 layers of gauze enclosing a flat, oblong piece of absorbent cotton measuring 15 × 10 cm to cover the mouth and nose (Fig. 5). He oversaw the mass production of the 60 000 masks for doctors, nurses, patients, contacts, and the population at large to the degree it was possible. The French doctor Gérald Mesny, who arrived to replace Wu, did not believe in the effect of the masks and said ‘What can we expect from a Chinaman?’. Mesny proceeded to work without a mask but contracted the plague himself and died two days later and so did a young Scottish missionary doctor. It was the first time such an epidemic containment measure had been attempted. The impressive results of using face masks and other personal protective equipment to stop the spread of disease and to protect medical workers and people were so convincing that masks were used during the Spanish influenza pandemic 1918–1919 and they were later further developed to the N95 masks (12, 13, 14). Dr. Wu Lien‐teh modernized China’s public healthcare system and became the first director of the National Quarantine Service and also became honorary doctor of Chinese and Japanese universities and he was nominated for the Nobel Prize 1935 for his fight against the plague.
Ultimately about 12 million people died in India and China alone of the 3rd plague pandemic, and it continued to be active until 1960 when the worldwide casualties had dropped below 200 per year.
Cholera Epidemics and Pandemics
Cholera is the other great and fabled pandemic disease which for centuries has horrified humans. The cause of cholera, Vibrio cholerae, was detected and described by Robert Koch in a report from Alexandria 1883, when he was sent out as a leader of a commission to Egypt and India to investigate the cause of cholera in 12 patients admitted to a Greek, a German, and an Egyptian hospital. The patients were children and adult from Germany, Austria, Greece, Nubia, and Turkey. He detected the cholera vibrio by microscopy and culture of rice water‐like stools and intestinal contents of the patients but he found no spread to their blood or organs (15). Vibrio had already been described by microscopy in the intestines of cholera patients in Florence in 1854 by an Italian anatomist Fillippo Pacine (1812–1883) but it was neglected by other scientists who believed in miasma as the cause of cholera and his observations were not published (16). Two biotypes of V. cholerae have caused the pandemics, the classical strain and the El Tor strain, both O1 serotype, but about 1990s a O139 serotype also occurred (Fig. 6). V. cholerae’s habitat is water where it adheres as biofilms to aquatic plants and crustaceans. It may, therefore, be present in contaminated food and water and infect people when they eat and drink such contaminated food or beverages. The classical origin of cholera is Bangladesh where cyclones and floods make hygienic measures difficult. From there, cholera can migrate to other countries with people carrying the bacteria, since healthy carriers far outnumber symptomatic patients.
Fig. 6.
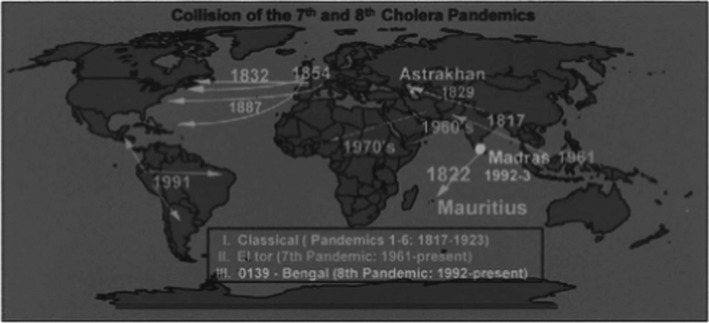
Spread of classical, El Tor, and O139 cholera epidemics (17).
There have been 8 described pandemics of cholera and each of them continued for many years. The classical O1 biotype was responsible for pandemic 1st–6th 1817–1923, the El Tor biotype for the 7th pandemic 1961–present, and the O139 for the 8th pandemic (Bengal) 1992–present (17) (Fig. 6). The Americas had been free of cholera, but was invaded in 1991 where after cholera became endemic.
The first European pandemic took place in 1830–37 and originated in the Ganges delta in 1817, migrated toward west to Africa, Middle East, Europe, and Russia and toward east to China and Japan (6) (17).
When cholera approached Denmark in 1831, military barriers were set at the border between Germany and Denmark and the Copenhagen quarantine station Saltholm was used for 20‐days quarantine of travelers to Zealand and 4 other islands in Denmark (Sprogø, Askø, Brandsø, and Kyholm) served the same purpose. In spite of that, a few cases occurred in Holstein, which was part of Denmark until the war with Germany 1864. When the next outbreak of cholera occurred in Europe 1850, the opinion in Denmark was anti‐contagionist in spite of Panum’s early success with fighting a cholera outbreak in the small port city of Bandholm on the island of Lolland August 1–October 1850 by barrier—and isolation precautions and treatment of patients in a small hospital he constructed for the epidemic. Panum was sent to Bandholm by the government and he became convinced that cholera was contagious (2). However, he did not convince the leading health authorities in Copenhagen who accused him for heretic opinions, so that he had to leave his position at the General Hospital in Copenhagen in spite of that he had received the order of the Knight’s Cross from the Danish King! The leading health authorities had great political influence and all the quarantine provisions were repealed in 1852 and the Danish King had little political influence since the Absolute Monarchy finished June 5, 1849, when the free Danish Constitutional Act was adopted and signed by King Frederik 7th.
The cholera pandemic reached Copenhagen on June 11, 1853, the year after all the quarantine provisions had been repealed (11). Copenhagen had 130 000 inhabitants who lived inside the city walls where there was no sewer but latrine pits under the houses and they were only emptied twice a year. The smelly drinking water came from uncleaned surface water in lakes around Copenhagen and was transported in leaking wood gutters to water posts in the streets. Much like the situation in London during the cholera outbreak 1854 where John Snow (1813–1858) showed that the public water pump on Broad Street was the source of cholera infection of people drinking water from the pump (6, 16). Ten percent of the inhabitants in Copenhagen were poor and supported by the city and the hospitals were overcrowded. The cholera epidemic in Copenhagen lasted until October 1953 and 7219 persons (5.6%) became sick and 4737 (66% of the sick) died and they were mostly poor people, for example, 415 died out of 1200 patients admitted to the Ordinary Hospital (1769–1892) for poor people. The epidemic did not change the perception of the leading miasmatic physicians, actually they thought that it confirmed that cholera was of miasmatic nature. However, the public opinion demanded better living conditions for the poor people and new houses named The Medical Association’s housing was built in 1853–57. A new hospital (Kommunehospitalet) with isolation facilities was built in 1859–63, and a quarantine hospital (Øresundshospitalet) was built in 1875–76, and a modern pavilion hospital for epidemic diseases (Blegdamshospitalet) was built in 1879. A water plant was also built whereas it took 40 years until efficient sewers were constructed in Copenhagen. The cholera epidemic in Copenhagen and the fast development of microbiology including the germ theory had long‐lasting influence on the development of the city.
Today, cholera outbreaks and epidemics are mainly connected to natural disasters, wars, and refugee camps where the hygienic conditions are poor. An example is the El Tor cholera epidemic which hit Haiti 2010–12 after a devastating earthquake January 12, 2010, with a death toll of more than 100 000 from the earthquake and major damages to the infrastructure of the country. The cholera strain was possibly introduced from the Indian subcontinent by UN peacekeepers from Nepal, and the cholera death toll was calculated to be 8534 in 2014 (18). Due to the civil war, a cholera epidemic has been ongoing since October 2016 in Yemen and as of June 28, 2020, according to WHO 1 384 423 cases and 1574 deaths have been reported.
In countries free from war or natural disasters or refugee camps and with high level of hygiene, clean drinking water and well‐sewered houses and cities, cholera epidemics are unlikely to occur, although individual cases are still imported, for example, returning travelers but they have never spread the disease to the society.
Influenza Pandemics
Many influenza A viruses are harmless for swimming birds, wading birds, chicken, other birds, and swine (low pathogenic avian influenza, the LPAI H5/H7 types). Other influenza A types are pathogenic and lethal for birds causing bird influenza (high pathogenic avian influenza, the HPAI H5N1 type) and give rise to large epidemic when they are transmitted over long distances by migratory birds following their major flyways, for example, to Turkey in 2005, where 400 000 chicken were culled to prevent spreading of an outbreak. HPAI can spread to other animals, for example, domestic cats and tigers in zoos who are fed with infected birds and also to humans but that requires close contact such as in families. However, the mortality is high in humans since the virus gives rise to influenza pneumonia and in Turkey 4 out of 21 infected humans died in 2005. Infection is normally acquired from living birds or their excretions. The reason for the low infectiousness of HPAI is that H5N1 influenza virus’ hemagglutinin requires sialic acid as receptor on the respiratory cells (19). The receptor in the airway epithelial cells has a 2–6 or a 2–3 sialic acid linkage which binds sialic acid to integral glycoproteins and glycolipids but also is the binding site for influenza virus. The 2–3 linkage is present in birds, swine, and in the lungs of humans, whereas the 2–6 linkage is also present in swine and in the upper airways of humans. That is the reason why HPAI is not very contagious to humans. Since swine have both receptors, they can be infected with influenza virus from both birds and humans and recombination (reassortment) can take place (antigenic shift). However, only few mutations are necessary in avian influenza virus to change two amino acids (antigenic drift) in H5N1 influenza virus, which can then also bind to human 2–6 receptors in the upper respiratory tract and become much more infectious and at the same time also more pathogenic to humans. Influenza viruses are RNA viruses and that means that mutations occur frequently (20) and that is one of the reasons why influenza vaccines often have to be changed to cover new variants of influenza A. Such antigenic change happened in the H1N1 influenza A virus which caused the Spanish influenza pandemic 1918–19 which was characterized by a very high frequency of influenza pneumonia and therefore high death toll. The reason was most likely an antigenic shift caused by swine being infected with 2 different influenza viruses at the same time (21). Such antigenic drifts or shifts may likely also happen in the future and that is the reason why the influenza situation is followed very closely by many laboratories in the world which report to WHO and also why the spread of the avian influenza in 2005 to many different countries by migratory birds upset the scientific community and the public (Fig. 8). WHO published a protocol in January 2006 for rapid response and containment of pandemic influenza. The high mutagenicity of influenza A viruses also means that resistance to oseltamivir, which is used to treat influenza A, can occur (22, 23). Another anti‐influenza drug amantadine has been used in China to treat birds with avian influenza and resistance against this drug is therefore very common (19). Farmers in China, Indonesia, Vietnam, etc., live close to their swine and ducks and migratory birds are attracted to the lakes where these domesticated birds live when they are resting and eating during their migration, so exchange of avian influenza viruses can easily take place by excretions from the birds. The domesticated birds and swine are brought to marketplaces where the avian influenza viruses may infect humans and that may then be the start of an epidemic or pandemic with a new influenza A virus. This is an integrated part of human culture which is very difficult to change.
Fig. 8.
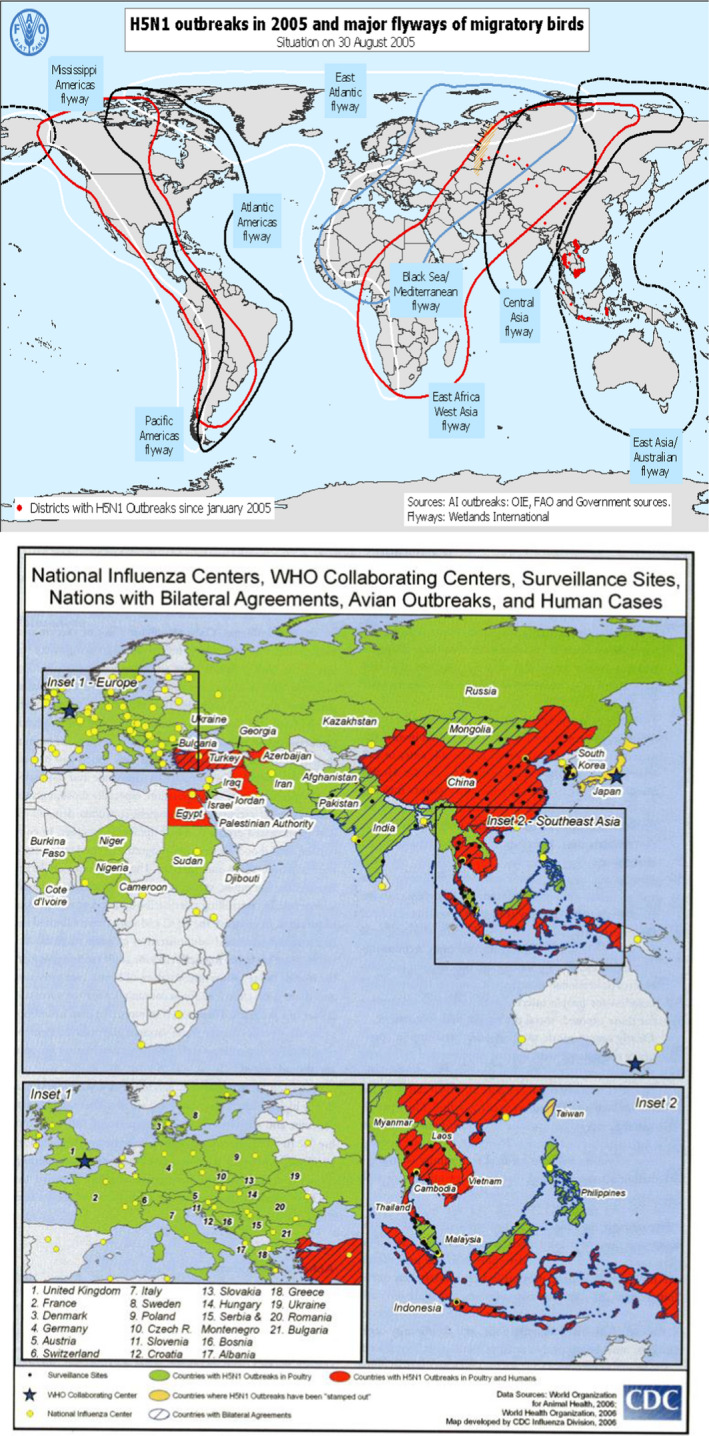
In the spring 2005, an outbreak of HPAI influenza A H5N1 was detected in bar‐headed geese at Qinghai Lake in western China and spreads to many countries with migrating birds. Top: The major flyways of migratory birds. The H5N1 influenza spreads to many different birds in many different countries (FAO). Bottom: Outbreaks in poultries and humans (CDC).
Before the Spanish influenza pandemic 1918–20, there had been 2 influenza epidemics in Denmark and other countries, 1846–48 and 1889–92 (from Russia, probably 1 million death) the year where the German bacteriologist Richard Friederich Johannes Pfeiffer (1858–1945) from Berlin, where he had been one of Koch’s assistants, published that Haemophilus influenzae (Pfeiffer’s bacillus) was the cause of influenza. This was heavily discussed and doubted by other scientists but it was not until 1933 that three British scientists W. Smith, C.H Andrews, and P.P. Laidlaw detected influenza virus as the cause of influenza (24). The etiology of the 2 early influenza pandemics is not certain since the diagnosis relied on clinical symptoms, that is, what is now called influenza‐like illness where today only about 50% are caused by influenza virus. The viral etiology of the Spanish influenza epidemic has, however, been identified (25). The Spanish influenza pandemic 1918–20 started in the United States (26) and spread among soldiers who were sent to Europe to participate in the First World War and from there to all European countries and to Africa, Middle East, Asia, India, Australia, New Zealand, Canada, etc. It caused a death toll of 50–100 million people. At that time, the spread was by ships and soldiers participating in the war from the colonies of the European countries were important for spreading the influenza. Due to censorship in the countries involved in the war, the news about the disease came from the neutral Spain, and therefore, it was named The Spanish Disease or Spanish influenza. It returned in several waves, the first wave came from France and spread in Denmark July–September 1918 and 80 000 people became sick. The second wave was in October 1918–April 1919 and 650 000 people became sick, and third and last wave was in January–April 1920 and 170 000 people became sick (27). The total death toll was 10 650 patients in Denmark and many of those were soldiers in the army and navy who were convened to protect Denmark against invasion during The Great War.
The Asian influenza A (H2N2) pandemic 1957 (1 million persons died) was caused by mutations and reassortment (antigenic shift), and the Hong Kong influenza A (H3N2) pandemic 1968 (750 000 died) was also caused by antigenic shift. The Russian Flu pandemic 1977–78 was a H1N1 strain which has been suspected to originate from the Soviet Union by an accident from vaccine production or vaccine trial or from a laboratory accident (28). In the 2009–10 Swine influenza, which was a H1N1 like the 1918–20 pandemic, it was a unique triple reassortment of bird, swine, and human influenza viruses further combined with a Eurasian or Mexican pig influenza virus (29). It began in North America and its death toll was about 284 000 people which is at the same level as the WHO estimates of 250 000–500 000 people who die of seasonal influenza annually (29). These pandemics were milder than Spanish influenza and the death were mainly infants and old people whereas Spanish influenza also had a high mortality among young people due to influenza pneumonia (Fig. 7) (24). In addition to influenza A virus pneumonia, which was prevalent in the 1918–20 pandemic, there was also bacterial pneumonia as superinfections. In the 1918–20 pandemic, it was Streptococcus pneumoniae, Streptococcus pyogenes group A, and Staphylococcus aureus, in the 1957 pandemic, it was S. aureus, and in the 1968 pandemic, it was S. aureus and S. pneumoniae (30). In the 2009–10 swine H1N1 pandemic, it was again S. pneumoniae, S. aureus, and S. pyogenes group A. In the interpandemic seasonal influenza H1N1 in the 1970s–2012, it was S. aureus and S. pneumoniae. If a new influenza A pandemic occur caused by a mutated or reassorted HPAI strain, it will spread much more rapidly—like the COVID‐19 pandemic—due to the enormous number of national and international travels by aircrafts. The major preventive measure against influenza pandemics is comprehensive yearly vaccination with vaccines consisting of the circulating influenza viruses from last season, but as the COVID‐19 pandemic has demonstrated, it takes many months to develop and produce an efficient vaccine against a completely new virus.
Fig. 7.
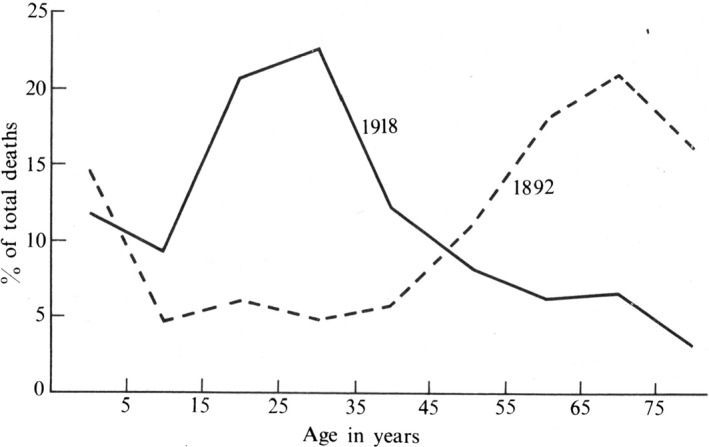
The change in the age distribution of deaths from influenza in the 1918–19 pandemic. The percentages of deaths falling in the successive age groups are shown for the 1892 and 1918 epidemics of influenza. In both pandemics, there were no drugs effective against virus or bacteria. In the 1892 pandemic, the chief incidence of death was on old people. In the 1918–19 pandemic, it was on young adults in each of its three waves and in all the countries struck of the pandemic. This was not the case in the more recent pandemics in 1957 and 1968 and 1977 and 2009, where most of the deaths occurred in old people like in the 1892 pandemic (24).
Recent Pandemics
Severe Acute Respiratory Syndrome (SARS)
SARS is also a zoonotic pandemic, and it originated from the southern Guangdong Province in China in November 2002. It was caused by a coronavirus SARS‐CoV‐1 (RNA virus) that came from cave‐dwelling horseshoe bats in the Yunnan Province and passed to Asian palm civet which then infected humans and spread to seven cities in China and to Hong Kong, Canada, France, Ireland, Italy, Switzerland, Singapore, UK, Taiwan, Thailand, Germany, the United States, and Vietnam (Fig. 9). Approximately 8000 people became infected and about 10% died. No case has been reported since 2004. Like many times before, the political reaction was surprising. In China, many more people contracted the infection than officially reported, and as a consequence, the health minister was dismissed. The prime minister in Canada and the mayor in Toronto denied the severity of SARS and protested against WHO’s warning against traveling to Toronto, where there was a large outbreak of SARS, due to the big economic consequences for Canada (31).SARS had actually substantial economic consequences for Canada and a number of Asian countries and Far Eastern Economic Review settled the loss for especially the aviation companies and hotels to more than 10 billion US $.
Fig. 9.
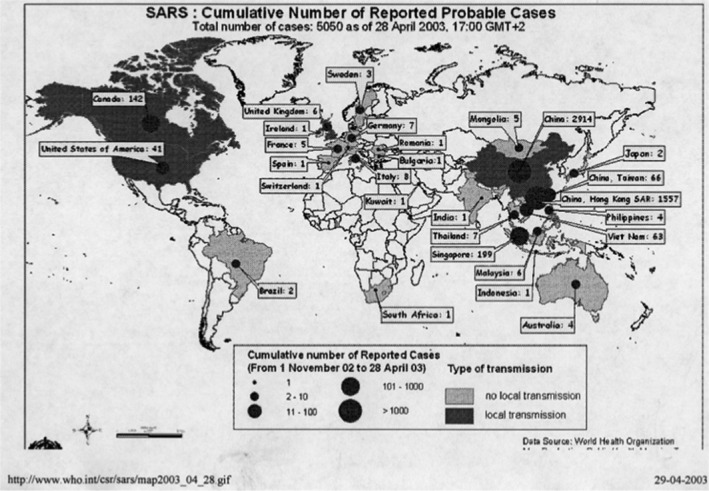
The SARS pandemic in 2003. It started in China 2002 and was recognized by WHO March 2003 but was initially denied by China but it spread worldwide (WHO).
Middle East Respiratory Syndrome (MERS)
MERS was described in 2012, and it is also caused by a zoonotic coronavirus (MERS‐CoV) and gives rise to similar but less severe symptoms as SARS. Its reservoir is probably fruit‐eating bats which have passed it to dromedary camels at some point in the past and camels are the source of human infections in people working with camels. It is primarily found on the Arabic peninsula and Egypt especially in Saudi Arabia but since there are many guest workers in this area, MERS has also been exported to 27 other countries including South Korea where 186 cases have been diagnosed, and to Europe, United States, Iran, and Thailand. Altogether 2494 laboratory‐confirmed cases have been identified (2019). The risk of further spread of MERS is low but 35% of the patients have died (32).
COVID‐19
Also, the third zoonotic coronavirus disease (COVID‐19) came from the cave‐dwelling fruit‐eating horseshoe bats and started in the city of Wuhan in China. Bats are social animals living in large numbers in caves and almost 9% carry at least one of 91 distinct coronaviruses that infect bats (33). The virus causing COVID‐19 is named SARS‐CoV‐2 and is related to SARS‐CoV‐1 and MERS‐CoV. The civet or pangolin may be the hosts which transmitted the virus to humans in the wet seafood market early in December 2019 in Wuhan (12 million inhabitants) the capital of the Hubei Province in central China. A hypothetic accident in high‐security virus laboratory in the Wuhan Institute of Virology has also been suspected to be the cause of the pandemic. It was a completely new infection, so it took some time before its severity and pandemic properties were realized in China, WHO, CDC, and ECDC. The Chinese outbreak was reported to WHO December 31, 2019, and its Emergency Committee declared a Public Health Emergency of International Concern January 30, 2020, and a global pandemic situation March 11, 2020. The international spread of the COVID‐19 pandemic was facilitated by the Chinese New Year celebration which took place January 15 to February 11. During this period, millions of Chinese people travel inside China and from abroad, for example, Europe and North America to China to visit their families and then return after end of the New Year celebration (34). In Wuhan, 5 million out of its 12 million inhabitants travelled to other cities during this period! Many of the Chinese people live and work or study in large European cities. One of these cities is Milan, Italy, which is located south of the Alps (34). In February, schools in Europe closed for one week for winter holiday where many thousands of adults and children travel from all over Europe to the Alps for skiing and North Italy and Austria became the center for further spread of COVID‐19 in Europe. October 27, 2020, COVID‐19 has spread to 189 countries and a total of 67.9 million cases have been diagnosed worldwide and 1 550 579 have died and the economic consequences are enormous. A major problem which interferes with the combat of COVID‐19 is that many asymptomatic patients occur, which are as infective as symptomatic patients. In China, which was the initial epicenter of COVID‐19, the country was closed and internal transportation was restricted on January 23 and drastic non‐pharmacologic measures were imposed (Fig. 10). The results were impressive (35), and the outbreak was stopped within a month or so and the total number of diagnosed cases recorded was 93 770 and 4746 death December 8, 2020, which is less than Sweden’s 297 732 cases and 7200 death (Sweden has 10.4 million inhabitants vs. China’s 1.4 billion inhabitants) (coronavirus.jhu.edu). Even a second wave of 368 cases in Beijing, June–July 2020 was stopped very fast (36). The legacy of Dr. Wu Lien‐teh’s fight against pulmonic plague and the methods he used is still remembered and employed in Asia! The results of the efforts in other Asian countries were, therefore, even better than in China, whereas the situation in Iran and Middle East, Europe, South Africa, the United States, Canada, Latin and South America, Australia, India, and Russia is very serious. Some promising new treatments have, however, been developed and vaccines are also being developed. The future development of COVID‐19 is uncertain. In theory, there are three possibilities. (i) SARS‐CoV‐2 may disappear like SARS‐CoV‐1 since apart from the horseshoe bat SARS‐CoV‐2 has not established other important zoonotic reservoirs. (ii) SARS‐CoV‐2 may continue like MERS where the camels have been established as a zoonotic reservoir that is close to humans. SARS‐CoV‐2 has been able to infect civets and pangolins but also minks in large mink farms in Holland and in Denmark and SARS‐CoV‐2 can also infect pet animals like dogs and cats or even monkeys in animal experiments. (iii) SARS‐CoV‐2 may become endemic in humans like the four common cold coronaviruses OC43, 229E, HKU1, and NL63 which are found in humans worldwide and have no recognized zoonotic reservoir (37).
Fig. 10.
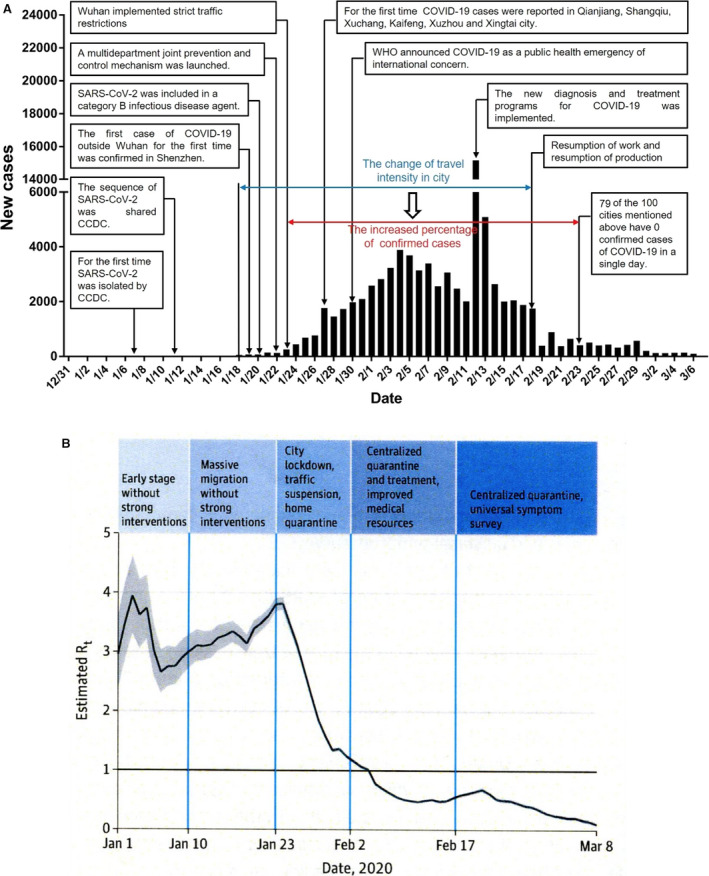
The effect of the Chinese public health interventions to stop the spread of the COVID‐19 epidemic in Wuhan (36). (A) Timeline of key SARS‐CoV‐2 events and new cases by day in China. (B) The effective reproduction number Rt is defined as the mean number of secondary cases generated by a typical primary case at time t in a population calculated for the whole period over a 5‐day moving average given the limited number of diagnosed cases and limited diagnostic capacity in December 2019. The darkened horizontal line indicates Rt = 1, below which sustained transmission is unlikely so long as anti‐transmission measures are sustained, indicating that the outbreak is under control. The 95% credible interval (Cris) is presented as gray shading.
Epidemics of Chronic Infections Where Immunity does not Develop
The epidemiology of chronic infections is quite different from acute infections, since the immune response is neither healing nor protective (Fig. 1E, F). On the contrary, the immune response may increase the inflammatory response and thereby contribute to the pathology of the infections. The patients, therefore, continue to be infectious and if the infection is symptomatic or, if it is subclinical, may become infectious again if it becomes symptomatic. Epidemics and pandemics with chronic infections may therefore change to become endemic infections if isolation, vaccination, or chemotherapy cannot be efficiently employed. This has previously been the case with, for example, tuberculosis, leprosy, AIDS.
Syphilis
Syphilis is an example of a pandemic chronic infection which subsequently became endemic. The name syphilis originates from the Italian physician, poet, astronomer, and geologist Girolarno Fracastoro (1476–1553) who published a poem in 1520 ‘Syphilis, sive morbus Gallicus’ about a young shepherd, Syphilis, who seduces the people to betray the God of Sun who therefore sends a new terrible plague to humans and Francastoro named the plague after the shepherd. Syphilis was according to ‘The Columbian Theory’ introduced from America in 1493 by the crew of Christopher Columbus who rediscovered America (Caribbean) in 1492 and returned to Palos, Andalusia, Spain with the three ships Santa Maria, Nina, and Pinta. They were not quarantined when they returned. Columbus returned to America 3 times 1493, 1498, and 1502 (38, 39). Columbus’ crew became infected with syphilis in Caribbean and the disease spread to other port cities and venereal spread became obvious. Syphilis then spread with the army of King Charles VIII of France (1470–98) who invaded Italy to secure his rights to the Neapolitan throne (1494–98) with a large army of mercenary soldiers to take Naples in 1495. His army was accompanied by a swarm of prostitutes and syphilis spread epidemically among the soldiers in the army and thereafter with the mercenary soldiers to other European countries as a pandemic, and it was therefore called the French or Spanish disease. There was no cure for syphilis at that time (Fig. 11). Syphilis came to Denmark in 1497 with Junker (colonel) Thomas Slentz’s ‘Black Garde’ of 6000 mercenary soldiers who came from the Netherlands and were hired by the Danish King Hans (1455–1513) to fight the war against Sten Sture in Sweden. At that time, the clinical course of syphilis was much more acute than nowadays. It was characterized by large gangrenous chancres, high fever, strong headache, and strong pain in the bones and joints, early skin eruptions, severely affected general condition, and even death. The course of syphilis became more chronic with four clinical stages (the acute primary and secondary stages, the latent stage, and the chronic and destructive tertiary stage) during the following 50 years. Syphilis had a much milder course in the local Indians according to contemporary historians Gonzalo Hernandez de Oviedo (1478–1557) and Bartolome de las Casas (1474–1566) who had visited America. This probably represents an evolution of the syphilis bacteria Treponema pallidum similar to what has been observed with both bacteria such as Pseudomonas aeruginosa in the lungs of cystic fibrosis patients (40) and viruses such as myxoma virus which cause myxomatosis in rabbits (41). Treponema pallidum as the cause of syphilis was discovered by Fritz Schaudin (1871–1906) and Erich Hoffmann (1868–1959) from Germany in 1905. In the beginning of the 20th century, tertiary stage of syphilis was the major cause of neurological, cardiovascular, and psychiatric diseases. In Denmark, the number of patients suffering from syphilis was 18 per 10 000 inhabitants in 1920 and about 150 cases of congenital syphilis were recorded yearly at that time (Fig. 12) (42). With efficient prophylaxis and therapy syphilis nearly disappeared but in Denmark, there were outbreaks again during the German occupation of Denmark 1940‐45 and in homosexual males when the epidemic of AIDS began (42).
Fig. 11.

Treatment of syphilis with mercury ointment which was recommended by the Arabs who occupied the Iberian peninsula 711‐1492 (woodcut from 1493(47)).
Fig. 12.
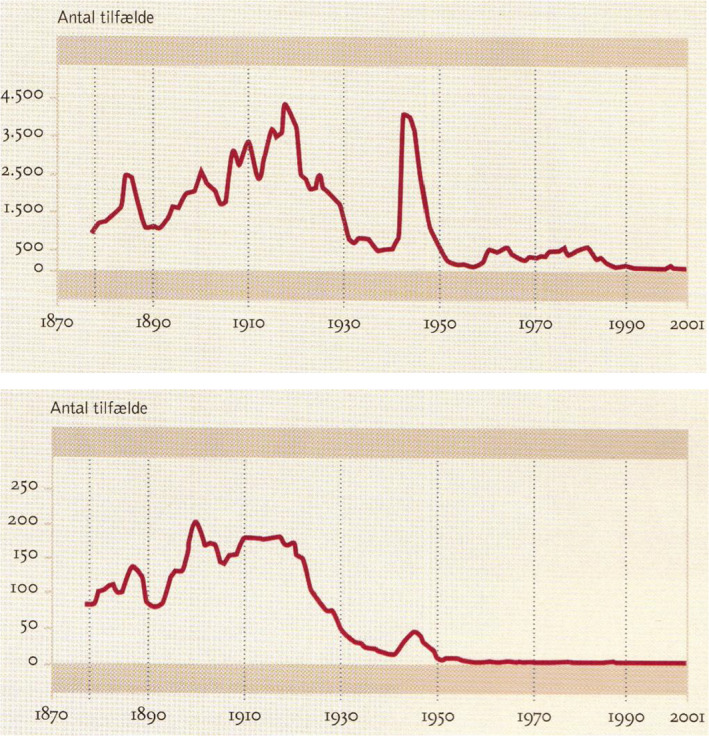
Occurrence of acquired (upper figure) and congenital syphilis (lower figure) in Denmark 1877–200 (43)1. Since 1946, blood from all pregnant women was examined for syphilis by means of the Wassermann antiphospholipid antibody test. Ordinates: Number of cases with syphilis, abscissas: Year. The statistics originate from the medical officers 1877–1918 and from the State Serum Institute since 1919. No other country has such a long period of syphilis statistic which has been crucial for defeating syphilis.
Future Pandemics
The conditions which favor development of epidemics and pandemics are (i) growing populations living in megacities that facilitate rapid spread of infections, (ii) humans living close to domesticated and wild animals which facilitate zoonotic microbes to adapt to humans and become infective and also the intensive husbandry of food animals and food birds which facilitate the spread of zoonotic microbes between the animals and birds and further to the farmers and consumers, (iii) intensive and fast national and international travel and migration which facilitate a local epidemic to become a pandemic, (iv) wars and natural disasters which destroy the infrastructure and hygienic conditions and generate refugees in overpopulated refugee camps, and (v) change of climate which leads to migration of people and intermediate hosts such as mosquitoes and their microbes I have changed the sentence such as e.g. yellow fever and malaria. Some properties of microorganisms and the infections they cause favor epidemics and pandemics are (i) long incubation time, (ii) asymptomatic carriers who are infective, and (iii) the ability of, for example, viruses to mutate or recombine so that the human immune response is evaded.
According to these conditions, it is likely that new pandemics caused by mutated or recombined avian and swine influenza viruses will occur and that new SARS‐CoV‐2 mutants or other zoonotic coronaviruses from bats also will be able to cause pandemics in the future. There are, however, several other infections which have caused smaller and larger epidemics, which until now did not become pandemics.
Nipah virus infection in 1998 in the Malaysian village Nipah, which was excreted in the stools of fruit‐eating bats. Nipah virus infected 582 people and 50–75% died.
West Nile fever was originally detected in Uganda 1937, but in the latest 20 years, it has spread to North America, Africa, Europe, Asia, and Australia. It is transmitted by mosquitoes that become infective by biting birds or people that host the infection. Most infected people do not become sick but about 20% get fever, headache, vomiting, and exanthema and a few get encephalitis and 10% of those patients die.
Zika virus caused an outbreak in Brazil and other countries in 2014–15. Zika virus has been known since 1947. When zika virus infected pregnant women, the infection could lead to birth defects including microcephaly. Zika virus is transmitted by bites of mosquitoes and is primarily transmitted among monkeys. For unknown reason, the zika virus infection has decreased in Brazil since 2017.
Smallpox (variola major virus) was eradicated from all countries in 1980 according to WHO. However, the virus is still kept in freezers in special laboratories in the United States and Russia and vaccines are again produced in order to be able to vaccinate against biological terrorism or biological war. However, monkeypoxvirus gives rise to a similar but much milder disease in man. It was first detected in laboratory monkeys in 1958 and in a child in DR Congo in 1970. Monkeypoxvirus occurs in Central and West Africa and circulates with certain rodents. An outbreak of monkeypox with more than 400 cases took place in DR Congo 1996–97, and since then, there have been more outbreaks in DR Congo but also in Sudan and there have been sporadic cases in several countries in West Africa 1970–78 and a larger outbreak in 17 states in Nigeria with 122 cases with 6% mortality. Most cases came from animals but human‐to‐human transmission was also observed. In the United States, rodents imported from Gambia and sold in a pet shop caused 47 cases of monkeypox in 2003. Since no people were vaccinated against smallpox, and it took some time until it was confirmed that the disease was not smallpox, it was unfortunately difficult to get healthcare persons to treat and care for the patients because they were concerned that they would be infected by the patients (43).
Hemorrhagic fever viruses are RNA viruses and comprise Lassa fever virus (arenavirus), from rodents and Ebola fever virus (filovirus) from fruit‐eating bats. They cause viral hemorrhagic fever in humans. Lassa fever causes minor outbreaks in West Africa in December–April but is difficult to control since 80% of the infected humans are asymptomatic. Ebola virus that also can infect great apes caused in 2013–15 a major outbreak in West Africa with 28 616 cases and 11 310 died and a new epidemic started in DR Congo with 3481 cases and 2299 death and 1162 survivors. The epidemic has now stopped (WHO June 2020). However, since these diseases require close contact to the sick and their excretions, there is at the present time no major concern for risk of a pandemic. Since they are RNA viruses, mutations may occur which change the possibility of transmission and thereby their pandemic potentials.
Conclusion
Development of epidemics and pandemics is depending on human interaction with animals, birds, and other humans, with human population density, with traveling, trade, and migration both of humans but also of animals and birds who carry and spread the microbes. These conditions have always been the cause of epidemics in the past and present and will also be the cause in the future. Since the population density increases, the harvesting of forests continues and thereby leads to closer exposure of human to animals and the national and international traveling increases and become more rapid it is certain that epidemics and pandemics will occur in the future with well‐known microbes and with new microbes. However, the prevention, and fight against epidemics and pandemics will probably be the same: clinical diagnosis, identification, and testing for the offending microbes, detection of asymptomatic carriers and contacts, isolation, quarantine, personal protective equipment, vaccines, and antimicrobial drugs. All these efforts rely heavily on preparedness and international collaboration.
Høiby N. Pandemics: past, present, future. APMIS.2021; 129: 352–371.
References
- 1. Morabia A. Epidemic and population pattern in the Chinese Empire (243 B.C.E. to 1911 C.E.): quantitative analysis of a unique but neglected epidemic catalogue. Epidemiol Infect 2009;137:1361–8. [DOI] [PubMed] [Google Scholar]
- 2. Gjedde A. Peter Ludvig Panums videnskabelige indsats. Copenhagen, Denmark: Costers Bogtrykkeri, 1971: 256. [Google Scholar]
- 3. Muscat M, Bang H, Wohlfahrt J, Gliesmann S, Mølbak K. Measles in Europe: an epidemiological assessment. Lancet 2009;373:383–9. [DOI] [PubMed] [Google Scholar]
- 4. Clark T. Changing pertussis epidemiology: everything old is new again. J Infect Dis 2014;209:978–81. [DOI] [PubMed] [Google Scholar]
- 5. Nørskov‐Lauritsen N. Et paradigmeskifte. Bibl Læg 2016;208:304–23. [Google Scholar]
- 6. Marks G, Beatty W. Epidemics. New York, NY: Charles Scribner’s Sons, 1976. [Google Scholar]
- 7. Wikipedia . Samuel Hahnemann. en.m.wikipedia.org, 2020.
- 8. Rascovan N, Sjögren K, Kristiansen K, Willerslev E, Desnues C, Rasmussen S. Emergence and spread of basal lineages of Yersinia pestis during the Neolithic decline. Cell 2019;176:295–305. [DOI] [PubMed] [Google Scholar]
- 9. Atlas of the Ancient World . Exploring Great Civilizations. National Geographic Society, Washington, DC, USA, 2018. [Google Scholar]
- 10. Mordechai L, Eisenberg M, Newfield T, Izdebski A, Kay J, Poinar H. The Justinianic plague: an inconsequential pandemic? PNAS 2019;116:25546–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hertel K. Tre store københavnske epidemier. Copenhagen: FADLs Forlag, 1980. [Google Scholar]
- 12. Strasser BJ, Schlich T. A history of the medical mask and the rise of throwaway culture. Lancet 2020;396:19–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wikipedia . Wu Lien‐teh. en.wikipedia.orxg, 2020.
- 14. Lynteris C. Plague masks: the visual emergence of anti‐epidemic personal protection equipment. Med Anthropol 2018;37:442–57. [DOI] [PubMed] [Google Scholar]
- 15. Koch R. Berichte über die Tätigheit der zur Erforschung der Cholera in Jahre 1883 nac Êgypten und Indien entsandten Kommission an S. Exzellenz den Staatssekretär des Innern Herrn Staatsminister von Bötticher. Gesundheitsamte Bd. III. Berlin, Germany, 1887. [Google Scholar]
- 16. Lippi D, Gotuzzo E. The greatest steps towards the discovery of Vibrio cholerae. Clin Microbiol Infect 2014;20:191–5. [DOI] [PubMed] [Google Scholar]
- 17. Guerrant R, Cameiro‐Filho B, Dillingham R. Cholera, and oral rehydration therapy: triumph and indictment. Clin Infect Dis 2003;37:398–2003. [DOI] [PubMed] [Google Scholar]
- 18. Orata F, Keim P, Boucher Y. The 2010 cholera outbreak in Haiti: how science solved a controversy. PLoS Pathog 2014;10:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. van Riel D, Munster V, de Witt E, Rimmelzwaan G, Fouchier R, Osterhaus A, et al. H5N1 virus attachment to lower respiratory tract. Science 2006;312:399. [DOI] [PubMed] [Google Scholar]
- 20. Lavenu A, Leruez‐Ville M, Chaix M, Boelle P, Rogez S, Freymuth F, et al. Detailed analysis of the genetic evolution of influenza virus during the course of an epidemic. Epidemiol Infect 2006;134:514–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tumpey T, Maines T, van Hoeven N, Glaser L, Solorzano A, Pappas C, et al. A two‐amino acid change in the hemagglutinin of the 1918 influenza virus abolish transmission. Science 2007;315:655–9. [DOI] [PubMed] [Google Scholar]
- 22. Hayden FG. Antiviral resistance in influenza viruses – implications for management and pandemic response. N Engl J Med 2006;354:785–8. [DOI] [PubMed] [Google Scholar]
- 23. Moscana A. Oseltamivir resistance – disabling our influenza defenses. N Engl J Med 2005;353:2633–6. [DOI] [PubMed] [Google Scholar]
- 24. Burnet M, White D. The Natural History of Infectious Disease, 4th ed. London, UK: Cambridge University Press, 1972. [Google Scholar]
- 25. Tumpey T, Basler C, Aguilar P, Zeng H, Solorzano A, Swayne D, et al. Characterization of the reconstructed 1918 Spanish influenza pandemic virus. Science 2005;310:77–80. [DOI] [PubMed] [Google Scholar]
- 26. Barry J. The Great Influenza. The Epic Story of the Deadliest Plaque in History. London, UK: Penguin Books, 2005. [Google Scholar]
- 27. Trier H. Angst og Engle. Den spanske syge i Danmark. Copenhagen: Gads Forlag, 2018. [Google Scholar]
- 28. Rozo M, Gronvall GK. The reemergent 1977 H1N1 strain and the gain‐of‐function debate. MBio 2015;6:1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wikipedia . 2009 swine flu pandemic. en.wikepedia.org, 2020.
- 30. McCullers J. Do specific virus‐bacteria pairings drive the clinical outcome of pneumonia? Clin Microbiol Infect 2013;19:113–8. [DOI] [PubMed] [Google Scholar]
- 31. Krauss C. The SARS epidemic: Canada; Toronto mayor calls for understanding from businesses and consumers. New York Times, April 25, 2003.
- 32. Organization WH . Middle East respiratory syndrome coronavirus (MERS‐CoV), 2020.
- 33. Burki T. The origin of SARS‐CoV‐2. Lancet Infect Dis 2020;20:1018–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Haider N, Yavlinsky A, Simons D, Osman AY, Ntoumi F, Zumla A, et al. Passenger’s destinations from China: low risk of novel coronavirus (2019‐nCoV) transmission into Africa and South America. Epidemiol Infect 2020;148:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pan AS, Liu L, Wang C, Guo H, Hao X, Wang Q, et al. Association of public health interventions with the epidemiology of the COVID‐19 outbreak in Wuhan, China. JAMA 2020;323:1915–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wu Z, Wang Q, Zhao J, Yang P, McGoogan J, Feng Z, et al. Time course of a second outbreak of COVID‐19 in Beijing, China, June‐July 2020. JAMA 2020;324:1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Peters A, Vetter P, Guitart C, Lotfinejad N, Pittet D. Understanding the merging coronavirus: what it means for health security and infection prevention. J Hospital Infection 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Weismann K, Søndergaard J. Syfilis møder AIDS. Ugeskr Læg 1993;155:947–51. [PubMed] [Google Scholar]
- 39. Weismann K. Da syfilis kom tilverden. Berlingske Tidende, 2002.
- 40. Folkesson A, Jelsbak L, Yang L, Johansen H, Ciofu O, Høiby N, et al. Adaptation of Pseudomonas aeruginosa to the airways of cystic fibrosis patients. Nat Rev 2012;10:841–51. [DOI] [PubMed] [Google Scholar]
- 41. Fenner F, White D. Medical Virology, 2nd ed. New York, NY: Academic Press, 1976. [Google Scholar]
- 42. Jensen K. Bekæmpelse af infektionssygdomme. Statens Seruminstitut 1902–2002. Copenhagen, Denmark: Nyt Nordisk Forlag Arnold Busk, 2002. [Google Scholar]
- 43. Anderson MG, Frenkel LD, Homann S, Guffey J. A case of severe monkeypox virus disease in an American child: emerging infections and changing professional values. J Ped Infect Dis 2003;22:1093–6. [DOI] [PubMed] [Google Scholar]
- 44. Black FL, Singer B. Elaboration versus simplification in the refining mathematical models of infectious disease. Annu Rev Microbiol 1987;41:677–701. [DOI] [PubMed] [Google Scholar]
- 45. Høiby N, Pedersen SS. Estimated risk of cross‐infection with Pseudomonas aeruginosa in Danish cystic fibrosis patients. Acta Pædiat Scand 1989;78:395–404. [DOI] [PubMed] [Google Scholar]
- 46. Bramanti B, Dean KR, Walløe L, Stenseth NC. The third plaque pandemic in Europe. Proc Biol Sci 2019;286:E11790–E11797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Godtfredsen G. Medicinens Historie. Copenhagen, Denmark: Nyts Nordisk Forlag Arnold Busk, 1964. [Google Scholar]


