Abstract
Severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2), a novel coronavirus causing coronavirus disease 19 (COVID‐19), with an estimated 22 million people infected worldwide so far although involving primarily the respiratory tract, has a remarkable tropism for the liver and the biliary tract. Patients with SARS‐CoV‐2 infection and no antecedent liver disease may display evidence of cytolytic liver damage, proportional to the severity of COVID‐19 but rarely of clinical significance. The mechanism of hepatocellular injury is unclear and possibly multifactorial. The clinical impact of SARS‐CoV‐2 infection in patients with underlying chronic liver disease, a cohort whose global size is difficult to estimate, has been assessed appropriately only recently and data are still evolving. Patients with cirrhosis are at higher risk of developing severe COVID‐19 and worse liver‐related outcomes as compared to those with non‐cirrhotic liver disease. OLT patients have an intermediate risk. Specific interventions in order to reduce the risk of transmission of infection among this high‐risk population have been outlined by international societies, together with recommendations for modified treatment and follow‐up regimens during the COVID‐19 pandemic. When a vaccine against SARS‐CoV‐2 becomes available, patients with fibrotic liver disease and those with OLT should be considered as prime targets for prophylaxis of COVID‐19, as all other highly susceptible subjects.
Keywords: chronic liver disease, cirrhosis, COVID‐19, prioritization, SARS‐CoV‐2, vaccine
Abbreviations
- AASLD
The American Association for the Study of Liver Diseases
- ACE2
angiotensin‐converting enzyme 2
- ACLF
acute‐on‐chronic liver failure
- AIH
autoimmune hepatitis
- ALP
alkaline phosphatise
- ALT
alanine aminotransferase
- APASL
The Asian Pacific Association for the Study of the Liver
- APCOLIS Study
APASL COVID‑19 Liver Injury Spectrum Study
- AST
aspartate aminotransferase
- BMI
body mass index
- CLD
chronic liver disease
- CLIF‐C
chronic liver failure consortium
- CLIF‐OF
chronic liver failure‐organ failure
- COVID‐19
coronavirus disease 2019
- CSSE
Center for Systems Science and Engineering
- DAAs
direct‐acting antivirals
- EASL
European Association for the Study of the Liver
- ESCMID
European Society of Clinical Microbiology and Infectious Diseases
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HR
hazard ratio
- ICU
intensive care unit
- MELD
model for end‐stage liver disease
- NASH
non‐alcoholic steatohepatitis
- OR
odd ratio
- RBD
receptor‐binding domain
- TBIL
total bilirubin
- TIPS
transjugular intrahepatic portosystemic shunt
- TUNEL
terminal deoxynucleotidyl transferase biotin‐dUTP nick end labelling
- ULN
upper limit of normal
- WHO
World Health Organization
- YNHHS
Yale‐New Haven Health System
Bullet points:
Patients with SARS‐CoV‐2 infection and no previous liver disease may develop mild‐to‐moderate liver injury, proportional to the severity of COVID‐19.
Patient with cirrhosis, and to a lesser degree those with OLT, when infected with SARS‐CoV‐2 have a high risk of death.
The prevalence of chronic liver disease among patients with COVID‐19 is undefined at a global level.
Patients with chronic liver disease should be prioritized for immunization once a SARS‐CoV‐2 vaccine becomes available.
1. INTRODUCTION
Since the first evidence of its transmissibility and infectivity, severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) and coronavirus disease 2019 (COVID‐19) 1 have been an international health concern, so much that the World Health Organization (WHO) declared public health emergency and pandemic status on March 2020. As of 20 August 2020, around 22,500,000 cases worldwide have been reported according to the Center for Systems Science and Engineering (CSSE) at John Hopkins University, mostly in United States, Brazil and Russia, causing totally around 800,000 deaths. 2 In order to track seroprevalence estimates, it has also been developed a custom‐built dashboard, which systematically monitors and synthesizes findings from hundreds of global SARS‐CoV‐2 serological studies. 3 A growing number of patients with COVID‐19 continue to succumb worldwide, mostly due to respiratory complications but also showing impairment of other organ systems, including the liver.
During the early phase of the pandemic, most case series reporting liver damage did not make a clear distinction between subjects infected by SARS‐CoV‐2 and a previously normal liver and those in whom the infection occurred in the setting of a pre‐existing liver disease. Even less information was available on the stage of liver disease (ie non‐fibrotic vs fibrotic: compensated vs decompensated), which may be of major importance since patients with cirrhosis are often fragile and may be immunocompromised. In fact, infections of all types are the major determinant of acute‐on‐chronic liver failure (ACLF), a leading cause of mortality in cirrhosis. 4 When facing patients with COVID‐19 who have liver disease, clinicians may have problems both in terms of choosing the putative treatment for COVID‐19 and of managing the higher risk of hepatic decompensation.
We shall review from a clinical perspective some practice points relevant for the hepatologist caring for COVID‐19 patients: (1) Is the liver merely a bystander to severe COVID‐19? (2) Are patients with liver disease at risk for a severe outcome of COVID‐19? (3) How should we alter the management of liver patients during the pandemic?
2. DOES SARS‐CoV‐2 INFECTION CAUSE DAMAGE TO A HEALTHY LIVER?
SARS‐CoV‐2 infects cells through the angiotensin‐converting enzyme 2 (ACE2) host cell receptor, which is mainly present on the alveolar cells (type 2). 5 Crystallization of the SARS‐CoV‐2 RBD (receptor‐binding domain)–ACE2 complex was achieved as soon as possible to understand the structural basis of ACE2 recognition by SARS‐CoV‐2. The spike (S) protein of coronaviruses facilitates viral entry into target cells and it may also be a target for future therapies. Shang et al illustrated the crystal structure of the RBD of the engineered spike protein of SARS‐CoV‐2 in complex with ACE2. 6 Interestingly, ACE2 expression is abundant in lung, heart, ileum, kidney, bladder, gastrointestinal tract and liver. 7 ACE2 receptors at the enterocyte level (glandular cells of gastric, duodenal and distal enterocytes) may explain gastrointestinal involvement, resulting usually in malabsorption, unbalanced intestinal secretion and activated enteric nervous system. 8 Regarding liver involvement, the level of ACE2 expression in cholangiocytes is high (59.7%) and similar to type 2 alveolar cells, and higher than hepatocytes (2.9%). 9 Even if there is this difference between liver and biliary tract, it does not mean that liver is unaffected by the virus. Recently, Wang et al examined liver material from infected patients by electron microscopy, immunohistochemistry, TUNEL assay and pathological studies, concluding that liver involvement is a crucial cause of hepatic impairment in COVID‐19 patients. Indeed, SARS‐CoV‐2 causes conspicuous hepatic cytopathy, with massive apoptosis and presence of binuclear hepatocytes as predominant histological features. 10
During SARS‐CoV‐2 infection, patients may have gastrointestinal manifestations, as reported from China. 11 In a meta‐analysis of 60 studies comprising around 4000 patients, the pooled prevalence of all gastrointestinal symptoms was 17.6%. Among patients with non‐severe COVID‐19, 11.8% complained of gastrointestinal symptoms, while 17.1% of severe COVID‐19 patients had gastrointestinal manifestations. 12 As mentioned above, liver involvement is documented as well, with hepatic dysfunction in severe cases. Severe acute liver injury has been reported with higher mortality. 13 In fact, liver damage mainly arose with a pattern of elevated serum liver biochemistries in hospitalized patients with COVID‐19 (primarily elevated AST and ALT, and slightly elevated bilirubin), ranging from 14% to 53%. Interestingly, it occurs more commonly in more severe COVID‐19 cases than in mild cases. 14 Recently, a retrospective cohort study of 1827 patients within the Yale‐New Haven Health System (YNHHS) confirmed a higher probability of severe infection in patients with increased age, male gender, higher BMI and abnormal liver tests. Moreover, liver injury was predominantly hepatocellular rather than cholestatic, and these patients had liver abnormalities both at admission (AST 66.9%, ALT 41.6%, ALP 13.5%, TBIL 4.3%) and peak hospitalization (AST 83.4%, ALT 61.6%, ALP 22.7%, TBIL 16.1%). In this report, a multivariate analysis revealed the association between admission and peak hospitalization liver tests and clinical outcomes (ICU admission, mechanical ventilation, and death). According to these data, patients with abnormal AST at admission have a higher risk of mechanical ventilation (OR 3.09, P < 0.001), as are those with abnormal AST, ALT and ALP at peak hospitalization (OR 5.87, 2.70 and 3.76, respectively). A subset of patients experienced liver transaminases > 5× ULN during hospitalization (AST 16.6%, ALT 20.6%). Among them medications used in COVID‐19 treatment (lopinavir/ritonavir, hydroxychloroquine, remdesivir and tocilizumab) were associated with peak hospitalization aminotransferase elevations > 5× ULN. 15
Overall, data support a modest cytopathic acute liver damage by SARS‐CoV‐2 through a direct cytopathic effect, due to infection of hepatocytes mediated by the highly expressed ACE2 receptors. Liver injury may also be caused by an uncontrolled immune response due to inflammatory activity, or as a consequence from therapies, manifesting as drug‐induced liver injury (DILI). 16 Other evidence based on autopsies in patients who succumbed to COVID‐19 supports the theory that macrovascular and microvascular thrombosis may play a predominant role in the pathophysiological pathway, determining hypoxic‐ischaemic hepatic necrosis. 17 Another interesting hypothesis is that high levels of positive final expiratory pressure might cause hepatic congestion and hence abnormal liver blood tests, but data suggest that many hospitalized patients with COVID‐19, even without mechanical ventilation, have similar biochemical abnormalities, dismissing this aetiology. 18 In any case, hepatocellular damage occurring in COVID‐19 patients with an otherwise healthy liver rarely, if ever, causes acute liver failure.
3. SARS‐CoV‐2 AND LIVER INJURY AMONG PATIENTS WITH CHRONIC LIVER DISEASE
COVID‐19 studies initially focused on respiratory tract involvement, ignoring the effects of SARS‐CoV‐2 on organs such as liver or gut which were considered as secondarily impaired. At the beginning, a rapid short‐time increase in publication data was motivated by the lack of knowledge against this sudden viral outbreak. For this reason, data quality was inevitably affected by the pressure to publish. Therefore, effects of COVID‐19 on underlying well‐defined chronic liver disease have been properly analysed only recently. Nevertheless, even current data miss well‐defined characteristics of patients regarding hepatic status and function (ie aetiology, chronic liver disease or cirrhosis, Child‐Pugh/MELD or other scores. There is a lack of data about the interaction between chronic liver disease and COVID‐19, because pre‐existing liver conditions or the exact cause of them have not been extensively studied in most of the studies. Even data regarding hepatotropic viruses are lacking, despite the fact that the outbreak started in regions in which the prevalence of these viruses is high. Recently, Lens et al in a Spanish multicentre study aimed to evaluate the incidence of SARS‐CoV‐2 in patients under ‘active’ antiviral therapy with tenofovir and DAAs, 341 and 1764, respectively. Only 1 patient on DAA therapy and 8 patients under tenofovir antiviral therapy had a confirmed PCR diagnosis of SARS‐CoV‐2. Interestingly, seven among them needed hospitalization but none of them died. 19
Given the global burden of chronic liver disease, it should be evaluated how different underlying liver conditions influence liver injury and the severity of SARS‐CoV‐2 infection. However, the literature has progressively focused on cirrhosis. As far as pre‐existing chronic liver impairment is concerned, it should be remembered that it is a condition which determines immunosuppression. This status may contribute to the significantly increased risk of mortality among those patients (Figure 1), as demonstrated by a large cohort study that included a population of 17,425,445 adults investigating death‐associated factors in hospital among people with confirmed COVID‐19 (HR, 95% CI 2.34, 1.94‐2.83 for liver disease). 20 Similar results were obtained by Docherty et al; they conducted a prospective observational cohort study and found worst outcomes in terms of survival in SARS‐CoV‐2‐affected patients who were older, male sex and with chronic comorbidities including moderate/severe liver disease. 21 Moon et al not only confirmed these findings, but also added that, in their population of SARS‐CoV‐2‐infected patients, those with cirrhosis experienced hepatic decompensation, even in the absence of respiratory symptoms. 22 Few studies specified in detail what kind of chronic liver disease their population was affected from, whether the patients were cirrhotic already, aetiology and viral status. This makes somehow difficult the estimate of the global prevalence of chronic liver disease among COVID‐19 populations and the course of the disease in this specific setting. Table 1 shows a summary of available data. A meta‐analysis of 11 observational studies for a total of 20,134 adult individuals assessed a relatively low overall prevalence of chronic liver disease at baseline, being 3% (95% CI 2%‐4%). 23 Some data on prevalence of liver disease can be extrapolated from a case series of 5,700 patients providing characteristics and early outcomes of patients hospitalized in New York City; 0.4% of the patients were known to be cirrhotic, 0.2% had a viral chronic liver disease, but no information was given about non‐alcoholic steatohepatitis nor non‐alcoholic fatty liver disease. However, data about obesity, diabetes and metabolic disease were collected. It cannot be excluded that a great number of patients with early, ongoing liver involvement were underestimated, thus resulting in underrating of prevalence and outcomes. 24
Figure 1.
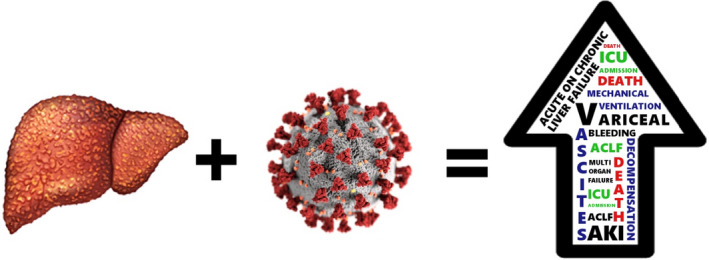
The consequences of SARS‐CoV‐2 infection on a chronic damaged liver: a worsening in clinical outcomes
Table 1.
Studies including COVID‐19 patients with chronic liver disease
| Study | Cirrhotic patients, n (%) | Patients with non‐cirrhotic chronic liver disease, n (%) | Aetiology |
|---|---|---|---|
| Moon et al 22 | 103 | 49 | ‐ |
| Richardson et al 24 | 19 | 11 | HBV, HCV |
| Qi et al 25 | 21 | ‐ | HBV, HCV, alcohol, autoimmune, schistosomiasis, others |
| Iavarone et al 26 | 50 | ‐ | HCV, HBV, alcohol, others |
| Singh et al 27 | 50 | 200 | ‐ |
| SECURE Cirrhosis Registry 32 | 425 | 372 | Alcohol, NASH, HCV, HBV, autoimmune |
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Among studies conducted on cirrhotic patients, Qi et al 25 investigated the clinical course and risk factors for mortality in these affected patients. In fact, they provide a report on the demographic characteristics, comorbidities, laboratory and radiographic findings, and clinical outcomes in twenty‐one SARS‐CoV‐2‐infected patients with pre‐existing cirrhosis. They finally concluded that the cause of death in most cases was due to respiratory failure rather than progression of liver disease such as development of acute‐on‐chronic liver failure. However, a multicentre retrospective study with a cohort of fifty cirrhotic patients gave interesting conclusions. The study aimed to evaluate the impact of COVID‐19 on the clinical outcome of liver disease. It found a significant association between SARS‐CoV‐2 infection and liver decompensation; this being measured as higher scores both in Child‐Pugh and MELD parameters. Consequently, COVID‐19 was associated with elevated mortality, with a thirty‐day rate of 34%, and that was independently predicted by CLIF‐OF, CLIF‐C, MELD and severity of lung failure. 26 Notably, Singh et al 27 obtained similar results among a much wider population of patients with chronic liver disease. They compared outcomes between those with and without pre‐existing liver dysfunction; as expected, the former group experienced higher risk of hospitalizations and mortality, which were confirmed even after propensity score matching. They hypothesized that poorer outcomes in cirrhotic patients may be attributed to many factors, such as an interplay of local liver injury and systemic disturbances, the expression of ACE2 receptors in liver and bile duct cells, medications and hypoxia. Table 2 shows the outcomes of COVID‐19 patients with chronic liver disease. However, outcomes seem not to be excessively modified by current therapies, both in non‐liver‐injured and in liver‐injured patients, probably because current medical management is largely supportive with no targeted therapy available. Several drugs including lopinavir‐ritonavir, remdesivir, hydroxychloroquine and azithromycin have been tested in clinical trials, 28 but none of them have been proven to be a definite therapy yet. Convalescent plasma was routinely used for the treatment of critically ill COVID‐19‐infected patients when available, 29 even if it was shown to have no significant advantage in preventing mortality. 30
Table 2.
Outcomes among patients with cirrhosis
| Study | Study design | Number of patients included | Mean age (years) | Mean follow‐up (months) | Mortality rate, % | Any event of liver decompensation, % |
|---|---|---|---|---|---|---|
| Moon et al 22 | Retrospective | 152 | 61 | 1 | 30.9 | 25.7 |
| Qi et al 25 | Retrospective multicentre | 21 | 68 | ‐ | 23.8 | 52.3 |
| Iavarone et al 26 | Retrospective multicentre | 50 | 67 | 1 | 34 | 48 |
| SECURE Cirrhosis Registry 32 | Multicentre | 957 | 59 | 3 | 32 | 46 |
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
The APCOLIS Study (APASL COVID‑19 Liver Injury Spectrum Study) showed that pre‑existing liver disease is associated with a hepatic injury severe enough to cause liver failure in cirrhosis. These data were taken from 13 Asian countries and 62 investigators, with 228 CLD patients included 43 (18.9%) with cirrhosis (including 18 decompensated cirrhosis) and 185 (81.1%) without cirrhosis. More cirrhotic than non‐cirrhotic patients had acute liver injury at admission (32.6% vs 20%, P < .001) and also developed new‐onset liver injury in hospital (39.5% vs 7%, P < .001). 31
A large‐scale collaborative registry project has been created to collect data on patients with liver disease at any stage or liver transplants who develop laboratory‐confirmed COVID‐19, both for patients who are hospitalized or managed in the community. The aim is to allow for analysis of baseline characteristics associated with specific outcomes in COVID‐19 among those with liver disease. In the latest update, the total cohort include 957 patients, 88% of them being hospitalized and 63% of them being men. The median age was 59 years. Among these patients, 372 have chronic liver disease but not cirrhosis, whether 425 have it. The major aetiology was alcohol (31%), followed by NASH (22%), HCV and HBV infection, and autoimmune hepatitis. 46% of patients experienced an event of decompensation, new ascites or worsening of pre‐existing being the most common; others developed encephalopathy (26%) or had variceal haemorrhage (4%). Finally, 160 affected patients were liver‐transplanted. The rate of hospitalization, intensive care admission and invasive ventilation was similar among non‐cirrhotic, transplanted and cirrhotic patients, but there was a trend of increased risk of death in these categories, being 7%, 19% and 32%, 32 respectively.
4. MANAGEMENT OF PATIENTS WITH CHRONIC LIVER DISEASE IN THE COVID‐19 ERA
It is important for medical staff to get acquainted with how to manage chronic liver disease patients infected by SARS‐CoV‐2 in order to establish prompt interventions to reduce poor outcomes. After the initial outbreak, guidelines and recommendations worldwide have been developed as soon as possible and they have mostly drawn up similar recommendations. AASLD gave some recommendations, which suggest not to stop ongoing antiviral treatment for HBV and HCV, but to consider delaying initiation of HCV therapy; to limit procedures by performing only the urgent or therapeutic ones (liver biopsy, paracentesis, positioning of TIPS, endoscopy for variceal bleeding). 33 A specific field of interest regards autoimmune liver disease. In this setting, Di Giorgio et al 34 investigated the health status of patients with autoimmune liver disease during SARS‐CoV‐2 outbreak in northern Italy, suggesting that, in these patients, tapering or withdrawing immunosuppressive treatment is not required. The same advice is given from Lleo et al 35 adding that a flare of AIH secondary to unnecessary drug reduction or withdrawal would require a higher dose of steroids and thus potentially increased risk of infection.
The Asian Pacific Association for the Study of the Liver (APASL) recommends constant checking of liver function tests in most instances and to screen for hepatitis B surface antigen, as it is do not underestimate drug‐induced liver injury (especially when remdesivir is administered). They focus on considering chronic liver disease, a risk factor for COVID‐19 progression, recommending to limit contact with healthcare workers and encouraging telemedicine. As far as HCC surveillance is concerned, they recommend to continue regular follow‐up in high‐risk subjects and using alpha‐fetoprotein's trend, two‐month delay is considered acceptable in case of ultrasound surveillance. Among already diagnosed, uninfected, HCC patients who are on treatment, HCC treatment should proceed as deemed appropriate, and if already on kinase inhibitors they recommend to continue therapy, whether immunotherapy might be rescheduled every 4‐6 weeks. It was suggested that liver biopsy could be performed in not infected with SARS‐CoV‐2 patients, if histology is expected to have a major impact on management and therapy (suspected autoimmune flares or rejection). A non‐invasive tool from Baveno VI criteria may be used to identify patients with high risk of variceal bleeding, but urgent or emergency endoscopy procedures should be performed anyway, as are other urgent procedures (ie therapeutic paracentesis, transjugular intrahepatic portosystemic shunt, endoscopy for variceal bleeding, band ligation, urgent biliary procedures for cholangitis and sepsis). Orthotopic liver transplantation should be limited to emergency and urgent cases according to local resource limitations and status of the outbreak, and avoided use of deceased or live donors with evidence of COVID‐19. Immunosuppression doses should not be reduced in long‐term liver‐transplanted patients in the absence of SARS‐CoV‐2 infection, but reduction may be considered in patients with moderate COVID‐19 (lymphopenia, fever or worsening pneumonia). Patients with autoimmune liver disease and mild COVID‐19 should continue their usual immunosuppression and other medications, while severe COVID‐19 should be managed according to local protocols, but corticosteroids should not be discontinued. 36
Similar recommendations are given in the EASL‐ESCMID position paper, 37 that distinguishes among patients with compensated chronic liver disease, decompensated chronic liver disease and its complications, and COVID‐19 in chronic liver disease. For the first two groups, the authors conclude having the same approach as suggested by AASLD and APASL. When SARS‐CoV‐2 infects patients with cirrhosis, instead, they underlined that treatment for cirrhosis‐associated complications such as portal hypertension, ascites, hepatic encephalopathy and spontaneous bacterial peritonitis should be continued. In patients with HCC, locoregional therapies should be postponed whenever possible and immune‐checkpoint inhibitor therapy temporarily withdrawn, but the decision on whether to continue kinase inhibitors in non‐severe COVID‐19 should be taken on a case‐by‐case basis. In the delicate condition of patients with COVID‐19 after liver transplantation, dose adjustment of calcineurin and/or mTOR inhibitors might be required depending on the antiviral therapy initiated.
5. SPECIFIC INDICATIONS TO CONTROL VIRUS SPREADING AMONG PATIENTS WITH LIVER DISEASE
After the Chinese outbreak, Italy and Europe were significantly affected between March and May 2020. Italy and European States locked countries down for two months, and currently the Italian status seems to have overcome the first peak of the pandemic with a progressive reduction of both new cases and total positive patients, according to data from the Italian Department of Civil Protection. 38 Taking into account all evidence mentioned so far, the real question is how to deal with the post‐pandemic phase. Tapper et al focalized on the impact of COVID‐19 in cirrhosis care over three waves. The first one concerned the acute phase of the pandemic, resulting in a strong prioritization of delivery of care. The second wave includes the backlog of deferred care and its consequences. The third one, instead, focuses on the concrete steps required to preserve quality of care. This includes an intensification of the preventative care usually provided to patients with cirrhosis, even with proactive chronic disease management, telehealth programmes, and a full reorganization of care delivery. 39 Many interventions aim to reduce chances of infection in this high‐risk population. All over the world social distancing, quarantine and lockdown have been the main strategy and the only effective way so far to mitigate further spread of the virus. Fauver et al demonstrated that domestic spread recently became the main source of new SARS‐CoV‐2 infections in the United States, 40 suggesting how lockdown and more restrictions may have been fundamental to control the outbreak. The next objective should be trying to cohabit with SARS‐CoV‐2, respecting meticulous rules about strict hygiene and sanitary organization, until a vaccine is developed. As far as sanitary organization is concerned, one cannot ignore the huge effort hepatologists have to face, trying to maintain standard care and to restore regular times of follow‐up, avoiding at the same time nosocomial dissemination of the virus to their patients and promoting telemedicine in the outpatient setting. 41
The current consensus is that SARS‐CoV‐2 infection will persist in the general population up to two years from now in the absence of active immunoprophylaxis. Hence, without a specific treatment, a vaccine is urgently required. 42 Because of the lack of aetiological therapies, vaccines are still considered the most promising way to eradicate SARS‐CoV‐2. 43 A relevant question regards vaccine timing of development. In the recent past, vaccine industries urgently tried to respond to epidemics of H1N1 influenza, Ebola, Zika, and now SARS‐CoV‐2. A H1N1 influenza vaccine was developed relatively rapidly, while vaccines for SARS‐CoV, Ebola and Zika did not follow similar performances. Moreover, the SARS‐CoV and Zika epidemics ended before conclusion of vaccine development. Nowadays, multiple platforms are under development. 44 Safety, immunogen's high efficacy, stability, few doses for potency and easier large‐scale production of engineered mRNA make mRNA vaccines more suitable for a rapid response to the COVID‐19 pandemic and to prevent a second wave. 45 , 46 Anyway, several technical issues still affect vaccine development. 47 In addition, although the safety of live attenuated or killed vaccines has been extensively studied, the potential of inducing infection still exists. 48 Currently, no clinically applicable vaccine against COVID‐19 is available. At the moment, various SARS‐CoV‐2 vaccines are in development, and a constantly updated list is available from the WHO, showing 18 candidate vaccines in clinical trials and 129 candidate vaccines in preclinical evaluation. 49 Nevertheless, the mRNA‐1273 vaccine is undergoing a phase I safety and immunogenicity trial, 50 and published positive results support advancement to later‐stage clinical trials. 51 A phase 2 trial of mRNA‐1273 in 600 healthy adults, evaluating doses of 50 and 100 μg, is ongoing (ClinicalTrials.gov number, NCT04405076). When a vaccine becomes available, a vaccination schedule with prioritization of high‐risk patients, including cirrhotic patients, should be organized, in order to reduce poor outcomes.
In conclusion, patients affected by cirrhosis should be considered as increased risk patients. Liver dysfunction that characterizes cirrhosis modifies the pathophysiology in many systems of the body; the management of these dysfunctions has been diligently improved over the years, but no effective recommendations have been given about clinical and logistical management of these patients during a pandemic. The outcomes in patients with cirrhosis with SARS‐CoV‐2 infection are strongly influenced by the susceptibility of these patients. Nevertheless, nobody has thought before this pandemic about how to manage access to clinical facilities in case of rigid restrictions, like those applied during lockdown. Therefore, this lack of adequate emergency programmes created a gap in the follow‐up of chronic and susceptible patients in every medical field, as in hepatology. The delay in screening programmes of cancers and pathology complications may produce an increase in long‐term mortality 52 because of many factors: loss in follow‐up of low‐compliance patients; saturation of clinical capacity to reschedule examinations in short‐term; and difficulty to increase number of daily examinations due to COVID‐regulations regarding distance and sanitization. Therefore, we strongly recommend stratifying patients when rescheduling screening examinations or monitoring suspicious complications in high‐risk patients. Moreover, when the vaccine becomes available, we strongly suggest considering cirrhotic patients among those who need to be vaccinated first. National Health Organizations should generate protocols not only for the ongoing pandemic, but mainly for a second wave which may be even more severe than the first one, especially where the first wave is gone and SARS‐CoV‐2 is spreading at a slower rate. In that case, we recommend protecting cirrhotic patients considering them at high risk of death or complications, allowing them to access vaccination and care programmes as a priority.
REFERENCES
- 1. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727‐733. 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Data from Center for System Science and Engineering (CSSE) at Johns Hopkins University. Available online at https://coronavirus.jhu.edu/map.html
- 3. Dong E, Du H, Gardner L. An interactive web‐based dashboard to track COVID‐19 in real time [published correction appears in Lancet Infect Dis. 2020 Jun 12;:]. Lancet Infect Dis. 2020;20(5):533‐534. 10.1016/S1473-3099(20)30120-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bajaj JS, Moreau R, Kamath PS, et al. Acute‐on‐chronic liver failure: getting ready for prime time? Hepatology. 2018;68(4):1621‐1632. 10.1002/hep.30056 [DOI] [PubMed] [Google Scholar]
- 5. Hoffmann M, Kleine‐Weber H, Schroeder S, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271‐280.e8. 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shang J, Ye G, Shi K, et al. Structural basis of receptor recognition by SARS‐CoV‐2. Nature. 2020;581(7807):221‐224. 10.1038/s41586-020-2179-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zou X, Chen K, Zou J, Han P, Hao J, Han Z. Single‐cell RNA‐seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019‐nCoV infection. Front Med. 2020;14(2):185‐192. 10.1007/s11684-020-0754-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liang W, Feng Z, Rao S, et al. Diarrhoea may be underestimated: a missing link in 2019 novel coronavirus. Gut. 2020;69(6):1141‐1143. 10.1136/gutjnl-2020-320832 [DOI] [PubMed] [Google Scholar]
- 9. Jothimani D, Venugopal R, Abedin MF, et al. COVID‐19 and the liver. Journal of Hepatology. 2020;73(5):1231–1240. 10.1016/j.jhep.2020.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang Y, Liu S, Liu H, et al. SARS‐CoV‐2 infection of the liver directly contributes to hepatic impairment in patients with COVID‐19. Journal of Hepatology. 2020;73(4):807–816. 10.1016/j.jhep.2020.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee N, Hui D, Wu A, et al. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348(20):1986‐1994. 10.1056/NEJMoa030685 [DOI] [PubMed] [Google Scholar]
- 12. Cheung KS, Hung IFN, Chan PPY, et al. Gastrointestinal manifestations of SARS‐CoV‐2 infection and virus load in fecal samples from a Hong Kong cohort: systematic review and meta‐analysis [published online ahead of print, 2020 Apr 3]. Gastroenterology. 2020;159(1):81‐95. 10.1053/j.gastro.2020.03.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bertolini A, Peppel IP, Bodewes FAJA, et al. Abnormal Liver Function Tests in Patients With COVID‐19: Relevance and Potential Pathogenesis. Hepatology. 2020;72(5):1864–1872. 10.1002/hep.31480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang C, Shi L, Wang FS. Liver injury in COVID‐19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5(5):428‐430. 10.1016/S2468-1253(20)30057-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hundt MA, Deng Y, Ciarleglio MM, et al. Abnormal liver tests in COVID‐19: a retrospective observational cohort study of 1827 patients in a major U.S. hospital network. Hepatology. 2020;72(4):1169‐1176. 10.1002/HEP.31487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Feng G, Zheng KI, Yan Q, et al. COVID‐19 and liver dysfunction: current insights and emergent therapeutic strategies. J Clin Transl Hepatol. 2020;8:18‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rapkiewicz A, Carsons S, Pittaluga S, et al. Megakaryocytes and platelet‐fibrin thrombi characterize multi‐organ thrombosis at autopsy in COVID‐19: A case series. EClinicalMedicine. 2020;2:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bangash MN, Patel J, Parekh D. COVID‐19 and the liver: little cause for concern. Lancet Gastroenterol Hepatol. 2020;5(6):529‐530. 10.1016/S2468-1253(20)30084-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lens S, Miquel M, Mateos‐Muñoz B, et al. SARS‐CoV‐2 in patients on antiviral HBV and HCV therapy in Spain. Journal of Hepatology. 2020;73(5):1262–1263. 10.1016/j.jhep.2020.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Williamson E, Walker AJ, Bhaskaran K, et al.OpenSAFELY: factors associated with COVID‐19‐related hospital death in the linked electronic health records of 17 million adult NHS patients. 10.1101/2020.05.06.20092999 [DOI]
- 21. Docherty AB, Harrison EM, Green CA, et al. Features of 20 133 UK patients in hospital with covid‐19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Moon AM, Webb GJ, et al. High mortality rates for SARS‐CoV‐2 infection in patients with pre‐existing chronic liver disease and cirrhosis: Preliminary results from an international registry. Journal of Hepatology. 2020;73(3):705–708. 10.1016/j.jhep.2020.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mantovani A, Beatrice G, Dalbeni A. Coronavirus disease 2019 and prevalence of chronic liver disease: A meta‐analysis. Liver Int. 2020;40:1316‐1320. [DOI] [PubMed] [Google Scholar]
- 24. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the New York City area. JAMA. 2020;323(20):2052‐2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Qi X, Liu Y, Wang J, et al. Clinical course and risk factors for mortality of COVID‐19 patients with pre‐existing cirrhosis: a multicentre cohort study. Gut. 2020;Published Online First: 20 May 2020 10.1136/gutjnl-2020-321666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Iavarone M, D'Ambrosio R, Soria A, et al. High rates of 30‐day mortality in patients with cirrhosis and COVID‐19. Journal of Hepatology. 2020;73(5):1063–1071. 10.1016/j.jhep.2020.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Singh S, Khan A. Clinical characteristics and outcomes of coronavirus disease 2019 among patients with preexisting liver disease in the United States: a multicenter research network study. Gastroenterology. 2020;159(2):768‐771.e3. 10.1053/j.gastro.2020.04.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fragkou PC, Belhadi D, Peiffer‐Smadja N, et al. Review of trials currently testing treatment and prevention of COVID‐19. Clin Microbiol Infect. 2020;26(8):988‐998. 10.1016/j.cmi.2020.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang L, Liu Y. Potential interventions for novel coronavirus in China: A systematic review. J Med Virol. 2020;92(5):479‐490. 10.1002/jmv.25707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li L, Zhang W, Hu Y, et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life‐threatening COVID‐19. [published online ahead of print, 2020 Jun 3]. JAMA. 2020;324(5):460‐ 10.1001/jama.2020.10044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sarin SK, Choudhury A, Lau GK, et al. Pre‐existing liver disease is associated with poor outcome in patients with SARS CoV2 infection; The APCOLIS Study (APASL COVID‐19 Liver Injury Spectrum Study) [published online ahead of print, 2020 Jul 4]. Hepatol Int. 2020;14(5):690‐700. 10.1007/s12072-020-10072-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. SECURE CIRRHOSIS REGISTRY . https://covidcirrhosis.web.unc.edu/updates‐and‐data/. Accessed August 5, 2020
- 33. Fix OK, Hameed B, Fontana RJ, et al. Clinical best practice advice for hepatology and liver transplant providers during the COVID‐19 pandemic: AASLD expert panel consensus statement. Hepatology. 2020;72(1):287‐304. 10.1002/hep.31281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Di Giorgio A, Nicastro E, Speziani C. Health status of patients with autoimmune liver disease during SARS‐CoV‐2 outbreak in northern Italy. J Hepatol. 2020;73(3):702‐705. 10.1016/j.jhep.2020.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lleo A, Invernizzi P, Lohse AW, Aghemo A, Carbone M. Management of patients with autoimmune liver disease during COVID‐19 pandemic. J Hepatol. 2020;73(2):453‐455. 10.1016/j.jhep.2020.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lau G, Sharma M. Clinical practice guidance for hepatology and liver transplant providers during the COVID‐19 pandemic: APASL expert panel consensus recommendations. Hepatol Int. 2020;14(4):415‐428. 10.1007/s12072-020-10054-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Boettler T, Newsome PN, Mondelli MU, et al. Care of patients with liver disease during the COVID‐19 pandemic: EASL‐ESCMID position paper. JHEP Reports. 2020;2(3):100113 10.1016/j.jhepr.2020.100113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Updated data from Italian Department of Civil Protection ‐ Presidency of the Council of Ministers. http://opendatadpc.maps.arcgis.com/apps/opsdashboard/index.html#/b0c68bce2cce478eaac82fe38d4138b1. Accessed August 25, 2020
- 39. Tapper EB, Asrani SK. The COVID‐19 pandemic will have a long‐lasting impact on the quality of cirrhosis care. J Hepatol. 2020;73(2):441‐445. 10.1016/j.jhep.2020.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fauver JR, Petrone ME, Hodcroft EB, et al. Coast‐to‐coast spread of SARS‐CoV‐2 during the early epidemic in the United States. Cell. 2020;181(5):990‐996.e5. 10.1016/j.cell.2020.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Boettler T, Newsome PN, Mondelli MU, et al. Care of patients with liver disease during the COVID‐19 pandemic: EASL‐ESCMID position paper. JHEP Rep. 2020;2:100113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. BASE Medicine Task Force . COVID‐19: Facts and recommendations from A to Z. Sci Insights. 2020;33(1):138‐158. 10.15354/si.20.re061 [DOI] [Google Scholar]
- 43. Prompetchara E, Ketloy C, Palaga T. Immune responses in COVID‐19 and potential vaccines: Lessons learned from SARS and MERS epidemic. Asian Pac J Allergy Immunol. 2020;38(1):1‐9. 10.12932/AP-200220-0772 [DOI] [PubMed] [Google Scholar]
- 44. Lurie N, Saville M, Hatchett R, Halton J. Developing Covid‐19 vaccines at pandemic speed. N Engl J Med. 2020;382(21):1969‐1973. 10.1056/NEJMp2005630 [DOI] [PubMed] [Google Scholar]
- 45. Pardi N, Weissman D. Nucleoside modified mRNA vaccines for infectious diseases. Methods Mol Biol. 2017;1499:109‐121. 10.1007/978-1-4939-6481-9_6 [DOI] [PubMed] [Google Scholar]
- 46. Schlake T, Thess A, Thran M, Jordan I. mRNA as novel technology for passive immunotherapy. Cell Mol Life Sci. 2019;76(2):301‐328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wang F, Kream RM, Stefano GB. An Evidence based perspective on mRNA‐SARS‐CoV‐2 vaccine development. Med Sci Monit. 2020;26:e924700 10.12659/MSM.924700. Published 2020 May 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kamboj M, Sepkowitz KA. Risk of transmission associated with live attenuated vaccines given to healthy persons caring for or residing with an immunocompromised patient. Infect Control Hosp Epidemiol. 2007;28(6):702‐707. [DOI] [PubMed] [Google Scholar]
- 49. World Health Organization . Draft landscape of Covid‐19 candidate vaccines. 2 July, 2020. (https://www.who.int/publications/m/item/draft‐landscape‐of‐covid‐19‐candidate‐vaccines)
- 50. Safety and immunogenicity study of 2019‐nCoV Vaccine (mRNA‐1273) to prevent SARS‐CoV‐2 Infection. ClinicalTrials.gov Identifier: NCT04283461. https://clinicaltrials.gov/ct2/show/NCT04283461
- 51. Jackson LA, Anderson EJ, Rouphael NG, et al. An mRNA Vaccine against SARS‐CoV‐2 — preliminary report [published online ahead of print, 2020 Jul 14]. N Engl J Med. 2020;383(20):1920‐1931. 10.1056/NEJMoa2022483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Maida M. Screening of gastrointestinal cancers during COVID‐19: a new emergency [published correction appears in Lancet Oncol. 2020 Aug; 21(8):e373]. Lancet Oncol. 2020;21(7):e338 10.1016/S1470-2045(20)30341-7 [DOI] [PMC free article] [PubMed] [Google Scholar]


