Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) emerged in late 2019 and has since caused a global pandemic. Experimental studies and sporadic reports have confirmed susceptibility of dogs and cats to SARS‐CoV‐2 infection. However, the importance of pet animals in the epidemiology of this infection is unclear. This study reports on a first large‐scale serosurvey of SARS‐CoV‐2 infections in dogs and cats in Europe. From 26 February 2020, just one day after the first confirmed human case of SARS‐CoV‐2 infection in Croatia, to 15 June 2020, dog and cat serum samples were collected from animals admitted to three veterinary facilities in Croatia. Additionally, on 25 May 2020, a total of 122 serum samples from employees of the Faculty of Veterinary Medicine University of Zagreb were collected. Total of 656 dogs and 131 cat serum samples were tested using an in‐house microneutralisation test (MNT). Human serum samples, as well as 172 randomly selected, dog sera were tested using enzyme‐linked immunosorbent assay (ELISA). ELISA‐positive human sera were subsequently tested using MNT. Neutralising antibodies were confirmed in 0.76% cats and 0.31% dogs. ELISA reactivity was recorded in 7.56% tested dog sera. On the other hand, 5.19% of administrative, basic and pre‐clinical sciences department personnel and 5.13% of animal health service providers and laboratory personnel tested ELISA positive. Neutralising antibodies were not confirmed in any of the human samples. In conclusion, seropositivity among pet animals in Croatia is low, especially when compared to results from China. A small number of seropositive animals with a low titre of neutralising antibodies suggest infections are rare and are following infections in the human population. Additionally, contact with animals does not seem to be an occupational risk for veterinary practitioners.
Keywords: antibodies, cats, COVID‐19, dogs, neutralizing, public health, seroepidemiologic studies
1. INTRODUCTION
In late 2019, a new virus, named severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), emerged in Wuhan Province in China and spread around the globe (Li et al., 2020; Zhou et al., 2020). In the field conditions, there have been sporadic cases of pet animals that have tested positive for SARS‐CoV‐2 (Animal & Plant Health Inspection Service, 2020; Sit et al., 2020). Almost all positive pet or captured animals shared households or have been in close contact with infected individuals (Oreshkova et al., 2020; SciCom, 2020). As a result, there is a major concern about making a diagnosis based only on quantitative reverse transcription polymerase chain reaction (qRT‐PCR) results. There is a high possibility of pets being passive vehicles of the virus and positive results do not confirm an infection. Serosurveys could provide better insight into the prevalence of SARS‐CoV‐2 infection in pet animals, but until now only one larger‐scale study has been conducted among cats in Wuhan, China (Zhang, Zhang, et al., 2020).
The goal of this study was to assess SARS‐CoV‐2 seroprevalence in pet animals during the coronavirus disease (COVID‐19) pandemic in Croatia. To evaluate a potential public health impact, we have conducted additional serosurvey among animal health workers as the group at higher risk for SARS‐CoV‐2 infection from infected pet animals.
2. MATERIALS AND METHODS
2.1. Sample collection
From 26 February to 15 June 2020, remaining sera samples were collected from animals admitted to three veterinary units: Veterinary Teaching Hospital (VTH) in Zagreb, and two private clinics from Zagreb and Split. Zagreb and Split were selected as the two cities with the most reported human COVID 19 cases in Croatia. Out of 787 samples, 131 were samples originating from cats and 656 from dogs. Microneutralisation test (MNT) has proven to be highly specific (Perera et al., 2020; Zhang, Zhang, et al., 2020) so all animal sera samples were tested for neutralisation activity first. Since neutralising antibody titre in infected dogs is proven to be low (Bosco‐Lauth et al., 2020) to assess the level of exposure to SARS‐CoV‐2, we additionally tested 172 randomly selected dog sera using enzyme‐linked immunosorbent assay (ELISA).
Besides, on 25 May 2020, a total of 122 serum samples from employees of the Faculty of Veterinary Medicine University of Zagreb were collected. All participants included in the study filled out a questionnaire regarding their demographic information, clinical symptoms, and possible exposure to COVID‐19. Participants were divided into groups based on the risk of contact with infected animals. The first group were animal health service providers (veterinary doctors, veterinary nurses, veterinary technicians and associates) who may not have provided direct care to the animals but have contact with the patient's body fluids, potentially contaminated materials or devices and equipment linked to the patient). The second group were laboratory personnel who have been in contact with animal clinical samples. The final group were administrative and basic and pre‐clinical sciences departments personnel who had no known contact with animals admitted to VTH. Human sera samples were tested using ELISA and MNT.
2.2. Serology testing
2.2.1. Microneutralisation test (MNT)
An in‐house microneutralisation test (MNT) was developed. The stock virus used was the third passage of the SARS‐CoV‐2 strain isolated in E6 Vero cells (ATCC CRL‐1586) from a Croatian COVID‐19 patient. Virus titre (TCID50) was calculated using the Reed and Muench formula (Reed & Muench, 1938). An equal volume (25 µl) of serial twofold dilutions of heat‐inactivated sera (56°C, 30 min) and 100 TCID50 of SARS‐CoV‐2 were mixed and incubated at 37°C with CO2 for an hour. Finally, 50 µl of 2 × 105 E6 Vero cells/ml were added to each well. To ensure optimal testing results, virus antigen used in each run was back titrated and positive human sample with known titre and negative control sera were included in each plate. The plates were incubated at 37°C with CO2 and starting from the third day the plates were checked for cytopathic effect. Antibody titre was defined as the reciprocal of the highest dilution of the serum that showed at least 50% neutralisation. MNT procedure was performed in the facility with biosecurity level 3.
2.2.2. Enzyme‐linked immunosorbent assay (ELISA)
Serum samples were tested by indirect ELISA for SARS‐CoV‐2 using the spike (S) and nucleocapsid (N) protein antigens. Human serum samples were tested according to the manufacturer protocol (Vircell, Granada, Spain). We have additionally tested 172 randomly selected dog sera. Antigen‐coated plates provided in the commercial ELISA test used for human sera testing (Vircell, Granada, Spain) were used to test dog serum samples. Serum samples were added at dilution 1:100 to the wells of the ELISA plate. As the secondary antibodies, 1:20,000 diluted rabbit anti‐dog IgG Fc (Abcam, Cambridge, UK) in a volume of 100 µl were used. After incubation for 30 min at 37°C and five washes, 100 µl of TMB substrate (Vircell, Granada, Spain) was added and incubated for an additional 20 min. The reaction was ended by adding 50 µl of the stop solution, and optical density (OD) was measured at 450 nm. Dog serum samples positive for neutralisation antibodies were used as a positive control. A cut‐off value was calculated as the mean of 42 control samples collected during 2018 and 2019 plus three standard deviations. To evaluate possible cross‐reactions, the above‐mentioned panel of 42 sera included 17 samples previously established as positive for Canine respiratory coronavirus (CRCoV) antibodies and 11 samples that have tested positive for Canine coronavirus (CCoV) antibodies.
2.3. Statistical analysis
For the statistical analysis of results, Fisher's exact test was used (Fisher, 1992).
3. RESULTS AND DISCUSSION
The conducted literature review showed that this is the first larger‐scale serosurvey in cats outside of China and first at all seroprevalence study in dogs. Neutralising antibodies were confirmed in 0.76% cats and 0.31% dogs (Table 1.). The observed prevalence in cats in Croatia is in sharp contrast with 10.8% MNT‐positive cats in Wuhan (Zhang, Zhang, et al., 2020) (Fisher's exact test p = .0006). We have tested cat sera using only MNT. Still, our results are comparable with the results from France, where only one of 11 cats tested ELISA positive for SARS‐CoV‐2‐specific antibodies even though all animals originated from infected households (Sailleau et al., 2020).
Table 1.
Serum samples used in this study and the results of serological testing
| Population | Sample | Method | Number of samples tested | Positive | Seroprevalence (%) |
|---|---|---|---|---|---|
| Pet animals | Cats | MNT | 131 | 1 | 0.76 |
| Dogs | MNT | 654 | 2 | 0.31 | |
| ELISA | 172 | 13 | 7.56 | ||
| Veterinary faculty employees | AHSP a | ELISA | 29 | 1 | 3.45 |
| MNT | 29 | 0 | 0.00 | ||
| Laboratory personnel | ELISA | 12 | 1 | 8.33 | |
| MNT | 12 | 0 | 0.00 | ||
| Others b | ELISA | 81 | 4 | 4.94 | |
| MNT | 81 | 0 | 0.00 | ||
| Total | ELISA | 122 | 6 | 4.92 | |
| MNT | 122 | 0 | 0.00 |
Abbreviations: ELISA, Enzyme‐linked immunosorbent assay; MNT, Microneutralisation test.
Animal health service providers: veterinary doctors, veterinary nurses, veterinary technicians and associates who may have not provided direct care to the animals but have contact with the patient's body fluids, potentially contaminated materials or devices and equipment linked to the patient;
Administrative and basic and pre‐clinical sciences departments personnel who had no known contact with animals admitted to Veterinary teaching hospital.
The seropositive cat in this study was traced back to COVID‐19‐infected household, but, even so, MNT titre in the cat was low (1:2), again in contrast with higher titres reported in China (Zhang, Zhang, et al., 2020). As it seems, under natural conditions, cat infections in Europe are sporadic. Low susceptibility of pet animals in natural conditions was also confirmed in France by Temmam et al. (2020) when 21 pet animals tested negative despite the high risk of exposure to SARS‐CoV‐2. Wuhan was the epicentre of the disease with a highly contaminated environment which can explain much higher seropositivity in cats.
In experimental conditions, cats are more susceptible to SARS‐CoV‐2 infection (Shi et al., 2020) and develop much higher neutralising antibody titre than dogs (Bosco‐Lauth et al., 2020). Despite these data, no significant difference in the prevalence of neutralising antibodies between cat and dog population was recorded in this study (Fisher's exact test p = .42). In our opinion, the main reason is the difference in the way cats and dogs are kept in Croatia and most of Europe. A large portion of cats is kept outdoor or outdoor–indoor, making dogs more exposed to SARS‐CoV‐2 in infected households.
To analyse the level of exposure to SARS‐CoV‐2, we additionally tested dog serum samples using ELISA. To our knowledge currently, there are two SARS‐CoV‐2 ELISA protocols used for the testing of animal sera. One is detecting the presence of antibodies against S protein and the other against N antigen (Bosco‐Lauth et al., 2020; Sailleau et al., 2020). To increase the sensitivity of ELISA testing, we used plates coated with both antigens (S and N). The mean optical density of 42 negative control sera was 0.384 with a standard deviation of 0.117, giving a cut‐off value of 0.734. Of the 172 randomly selected dog sera, 13 (7.56%) tested positive, including two sera samples with neutralising activity (Table 2.). Due to a different level of conservation of immunogenic proteins between coronaviruses, there was a concern of cross‐reaction of CCoV and CRCoV antibody‐positive dog sera with SARS‐CoV‐2 S and N protein used in ELISA. The N protein, as a major immunogenic protein, was widely used in the serological assays for severe acute respiratory syndrome (SARS) diagnostics (Meyer et al., 2014). Different studies have shown that cross‐reactivity with other human alpha and betacoronaviruses occurs when using N protein‐based assays (Che et al., 2005; Woo et al., 2004). In this study, there were no signs of cross‐reactivity with CCoV.
Table 2.
SARS‐CoV‐2 ELISA‐positive dogs
| Breed | Age (years) | Collection date | Location | ELISA (OD) | MNT (titre) |
|---|---|---|---|---|---|
| American Staffordshire terrier | 8 | 17 Apr | Zagreb | 1.792 | 1:16 |
| Mixed breed | 3 | 21 Apr | Zagreb | 1.15 | Negative |
| Maltese | 9 | 24 Apr | Zagreb | 1.858 | Negative |
| Belgian shepherd | 13 | 27 Apr | Zagreb | 0.957 | Negative |
| Mixed breed | 12 | 7 May | Zagreb | 0.897 | Negative |
| Belgian shepherd | 2 | 11 May | Zagreb | 0.787 | Negative |
| Medium poodle | 12 | 12 May | Zagreb | 1.023 | Negative |
| Mixed breed | 1 | 12 May | Zagreb | 1.24 | Negative |
| Mixed breed | 13 | 19 May | Zagreb | 0.824 | Negative |
| Mixed breed | 9 | 20 May | Zagreb | 0.783 | Negative |
| NA | NA | 22 May | Zagreb | 0.802 | Negative |
| NA | NA | NA | Split | 1.641 | 1:4 |
| Yorkshire terrier | 12 | 27 May | Zagreb | 1.421 | Negative |
Abbreviations: ELISA, enzyme‐linked immunosorbent assay; MNT, Microneutralisation test; NA, not available; OD, optical density.
Even after thorough research, we haven't been able to find any literature data regarding cross‐reactivity of CCoV‐positive samples. We have noticed that our data are in line with already published findings on feline infectious peritonitis virus (FIPV), another closely related alphacoronavirus of pet animals. Those studies have not reported cross‐reactivity of FIPV hyperimmune serum with SARS‐CoV‐2 S or N ELISA (Sailleau et al., 2020; Zhang, Zhang, et al., 2020).
New canine coronavirus, CRCoV, was first detected in 2003 and is widespread in Europe (Day et al., 2020; Erles et al., 2003). Like SARS‐CoV‐2, CRCoV is a member of the Betacoronavirus genus, and one of our primary concerns was to establish if serum samples positive for CRCoV antibodies can interfere with SARS‐CoV‐2 ELISA results. None of the CRCoV‐positive samples collected before the pandemic gave positive reactions.
With MNT used as a gold standard, ELISA specificity was 93.53% and sensitivity 100%. There is a considerably higher number of dog samples that have tested ELISA than MNT positive. It is possible that a portion of dogs which tested ELISA positive were sampled early or late in the course of the infection when antibody titre is low. However, it is also possible that the number of dogs in field conditions develops only mild infections resulting only in ELISA reactivity of their serum samples with no measurable neutralising antibodies. Same was recorded in human cases (Okba et al., 2020; Zhang, Zhou, et al., 2020).
First ELISA‐positive dog serum was collected on 17 April in the city of Zagreb, approximately seven weeks after the first qRT‐PCR‐positive human case in the same area, and the highest number of positive dog samples were collected six weeks after the peak in the number of human cases (Figure 1.). Since this is a serology testing, the lag period was expected, and these results suggest the prevalence of ELISA‐positive dogs is following the number of infected people with no evidence of an outbreak among the dog population (Figure 2.).
Figure 1.
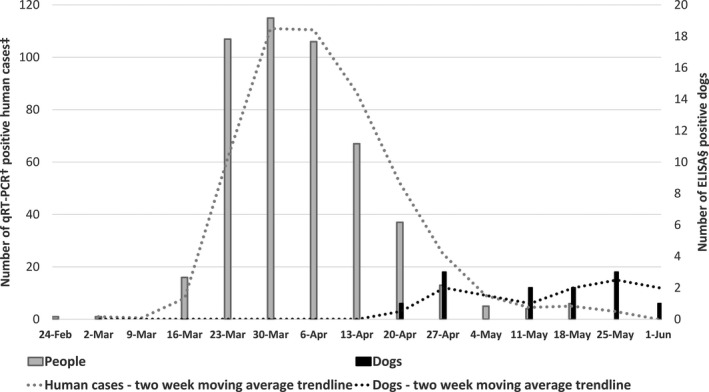
The weekly number of qRT‐PCR‐positive human samples and ELISA‐positive dogs in the city of Zagreb and Zagreb County from 23 February to 1 June 2020. †qRT‐PCR—quantitative reverse transcription polymerase chain reaction, ‡Number of new qRT‐PCR‐positive human cases is publicly available at the official website of the Government of the Republic of Croatia (https://www.koronavirus.hr), §ELISA—enzyme‐linked immunosorbent assay
Figure 2.
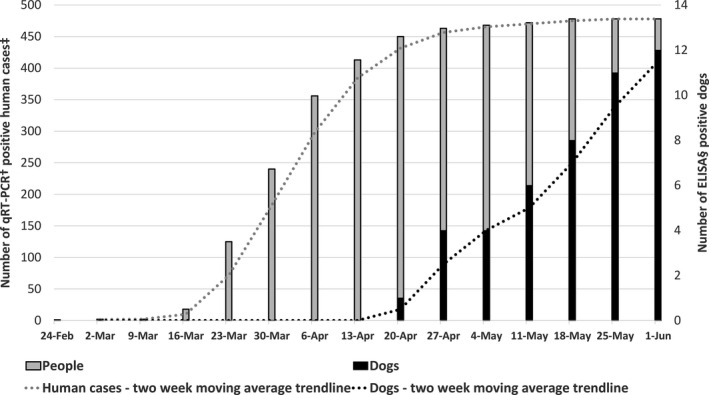
The weekly cumulative number of qRT‐PCR‐positive human samples and ELISA‐positive dogs in the city of Zagreb and Zagreb County from 23 February to 1 June 2020. †qRT‐PCR—real‐time quantitative reverse transcription polymerase chain reaction, ‡Number of new qRT‐PCR‐positive human cases is publicly available at the official website of the Government of the Republic of Croatia (https://www.koronavirus.hr), §ELISA, enzyme‐linked immunosorbent assay
To further address pets as a possible source of infection for humans, we have tested employees of the Veterinary Faculty University of Zagreb. Out of 122 human samples, six tested ELISA positive (4.92%), but none had neutralising activity (Table 1.). Half of the participants who had tested ELISA positive had no history of symptoms that could be related to COVID‐19 (fever ≥ 38.0°C, chills, fatigue, myalgia, sore throat, cough, rhinorrhea, shortness of breath). The other three had some combination of the symptoms mentioned above, but they were mild, and none of them reported fever. None of the participants had reported contact with a confirmed COVID‐19 case; none had been qRT‐PCR tested nor had been in self‐isolation. It is possible that due to the mild symptoms, or asymptomatic course of the infection, serum samples were ELISA positive but MNT negative (Okba et al., 2020; Zhang, Zhou, et al., 2020).
There was no difference in seroreactivity of administrative and basic and pre‐clinical sciences departments personnel compared to animal health service providers and laboratory personnel (Fisher's exact test p = 1). Taking into account the limited size of the cohort studied, it seems that contact with pet animals is not a risk factor for SARS‐CoV‐2 infection. At the time of sampling for this study, human serum samples were collected in the general population. Using ELISA, IgG antibodies were detected in 2.7% of participants (Vilibic‐Cavlek et al., 2020). Once again, there was no significant difference in seropositivity between animal health service providers and the general population (Fisher's exact test p = .13).
4. FINAL REMARKS
In conclusion, seropositivity among pet animals in Croatia is low, especially when compared to results from China. A small number of seropositive animals with a low titre of neutralising antibodies suggest infections are rare and are following infections in the human population. Even though animal to animal transmission has been described (Halfmann et al., 2020; Shi et al., 2020), as well as a possible animal to human transmission (ProMed‐mail, 2020), we believe those are exceptions that are limited to experimental conditions or to highly contaminated environments, such as fur farms. This study gives no evidence that dogs and cats are significant sources of infection for people. Still, serosurveys in pet animals could be an additional tool for surveillance of SARS‐CoV‐2 in the human population.
ETHICAL APPROVAL
The authors confirm that the ethical policies of the journal, as noted on the journal's author guidelines page, have been adhered to. The study was approved by the Ethics Committee of the Croatian Institute of Public Health (Decision number: 030‐02/20‐05/1).
CONFLICT OF INTEREST
The authors declare that there they have no conflict of interests.
AUTHORS’ CONTRIBUTIONS
V. Stevanovic, T. Vilibic‐Cavlek, I. Tabain and Lj. Barbic conceptualised and designed the study. V. Stevanovic, I. Tabain, S. Kovac and Z. Hruskar developed and carried out microneutralisation test. V. Stevanovic, I. Benvin, S. Kovac collected animal serum samples, designed and carried out ELISA for dog serum samples. T. Vilibic‐Cavlek, Lj. Milasincic and Lj. Antolasic collected and carried out ELISA testing of human sera samples. M. Mauric statistically analysed collected data. V. Stevanovic and V. Staresina wrote the manuscript. All authors read and approved the final version of the manuscript.
ACKNOWLEDGEMENTS
We gratefully acknowledge Ana Miljan and Martina Gancevic for collecting the clinical specimens and data. We are grateful to Josko Mise for reviewing the final manuscript and Ljiljana Zmak for all the help during the work in biosecurity level 3 laboratory.
Stevanovic V, Vilibic‐Cavlek T, Tabain I, et al. Seroprevalence of SARS‐CoV‐2 infection among pet animals in Croatia and potential public health impact. Transbound Emerg Dis.2021;68:1767–1773. 10.1111/tbed.13924
Vladimir Stevanovic and Tatjana Vilibic‐Cavlek should be considered the joint first author.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Animal and Plant Health Inspection Service . (2020). USDA Statement on the Confirmation of COVID‐19 in a Tiger in New York. United States Department of Agriculture. https://www.aphis.usda.gov/aphis/newsroom/news/sa_by_date/sa‐2020/ny‐zoo‐covid‐19 [Google Scholar]
- Bosco‐Lauth, A. , Hartwig, A. E. , Porter, S. , Gordy, P. , Nehring, M. , Byas, A. , Bowen, R. A. (2020). Pathogenesis, transmission and response to re‐exposure of SARS‐CoV‐2 in domestic cats. bioRxiv. 10.1101/2020.05.28.120998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Che, X.‐Y. , Qiu, L.‐W. , Liao, Z.‐Y. , Wang, Y.‐D. , Wen, K. , Pan, Y.‐X. , Hao, W. , Mei, Y.‐B. , Cheng, V. C. C. , & Yuen, K.‐Y. (2005). Antigenic cross‐reactivity between severe acuterespiratory syndrome‐associated coronavirus and human coronaviruses 229Eand OC43. The Journal of Infectious Diseases, 191(12), 2033–2037. 10.1086/430355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day, M. J. , Carey, S. , Clercx, C. , Kohn, B. , MarsilIo, F. , Thiry, E. , Freyburger, L. , Schulz, B. , & Walker, D. J. (2020). Aetiology of canine infectious respiratory disease complex and prevalence of its pathogens in Europe. Journal of Comparative Pathology, 176, 86–108. 10.1016/j.jcpa.2020.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erles, K. , Toomey, C. , Brooks, H. W. , & Brownlie, J. (2003). Detection of a group 2 coronavirus in dogs with canine infectious respiratory disease. Virology, 310(2), 216–223. 10.1016/s0042-6822(03)00160-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher, R. A. (1992). Statistical methods for research workers. In Kotz S., & Johnson N. L. (Eds.), Breakthroughs in statistics (pp. 66–70). Springer. [Google Scholar]
- Halfmann, P. J. , Hatta, M. , Chiba, S. , Maemura, T. , Fan, S. , Takeda, M. , Kinoshita, N. , Hattori, S.‐I. , Sakai‐Tagawa, Y. , Iwatsuki‐Horimoto, K. , Imai, M. , & Kawaoka, Y. (2020). Transmission of SARS‐CoV‐2 in domestic cats. The New England Journal of Medicine, 383(6), 592–594. 10.1056/NEJMc2013400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Q. , Guan, X. , Wu, P. , Wang, X. , Zhou, L. , Tong, Y. , Ren, R. , Leung, K. S. M. , Lau, E. H. Y. , Wong, J. Y. , Xing, X. , Xiang, N. , Wu, Y. , Li, C. , Chen, Q. I. , Li, D. , Liu, T. , Zhao, J. , Liu, M. , … Feng, Z. (2020). Early transmission dynamics in wuhan, china, of novel coronavirus‐infected pneumonia. The New England Journal of Medicine, 382(13), 1199–1207. 10.1056/nejmoa2001316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer, B. , Drosten, C. , & Müller, M. A. (2014). Serological assays for emerging coronaviruses: Challenges and pitfalls. Virus Research, 194, 175–183. 10.1016/j.virusres.2014.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okba, N. M. A. , Müller, M. A. , Li, W. , Wang, C. , GeurtsvanKessel, C. H. , Corman, V. M. , & Haagmans, B. L. (2020). Severe acute respiratory syndrome coronavirus 2‐specific antibody responses in coronavirus disease 2019 patients. Emerging Infectious Diseases, 26(7), 1478–1488. 10.3201/eid2607.200841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oreshkova, N. , Molenaar, R. J. , Vreman, S. , Harders, F. , Oude Munnink, B. B. , Hakze‐van der Honing, R. W. , Gerhards, N. , Tolsma, P. , Bouwstra, R. , Sikkema, R. S. , Tacken, M. G. J. , de Rooij, M. M. T. , Weesendorp, E. , Engelsma, M. Y. , Bruschke, C. J. M. , Smit, L. A. M. , Koopmans, M. , van der Poel, W. H. M. , & Stegeman, A. (2020). SARS‐CoV‐2 infection in farmed minks, the Netherlands, April and May 2020. Eurosurveillance, 25(23), 2001005. 10.2807/1560-7917.ES.2020.25.23.2001005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera, R. A. , Mok, C. K. , Tsang, O. T. , Lv, H. , Ko, R. L. , Wu, N. C. , & Peiris, M. (2020). Serological assays for severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). Eurosurveillance, 25(16), 2000421. 10.2807/1560-7917.ES.2020.25.16.2000421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ProMed‐mail . (2020). Coronavirus disease 2019 update (209): Netherlands (North Brabant), farmed mink, animal‐to‐human, cat, epidemiology. International Society for Infectious Diseases. https://promedmail.org/promed‐post/?id=20200525.7375359 [Google Scholar]
- Reed, L. J. , & Muench, A. (1938). Simple method of estimating fifty per cent endpoints. American Journal of Epidemiology., 27(3), 493–497. 10.1093/oxfordjournals.aje.a118408 [DOI] [Google Scholar]
- Sailleau, C. , Dumarest, M. , Vanhomwegen, J. , Delaplace, M. , Caro, V. , Kwasiborski, A. , Hourdel, V. , Chevaillier, P. , Barbarino, A. , Comtet, L. , Pourquier, P. , Klonjkowski, B. , Manuguerra, J.‐C. , Zientara, S. , & Le Poder, S. (2020). First detection and genome sequencing of SARS‐CoV‐2 in an infected cat in France. Transboundary and Emerging Diseases, 10.1111/tbed.13659 00, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SciCom . (2020). Risque zoonotique du SARS‐CoV2 (Covid‐19) associé aux animaux de compagnie: infection de l’animal vers l’homme et de l’homme vers l’animal. 2020. The Federal Agency for the Safety of the Food Chain. http://www.afsca.be/comitescientifique/avis/2020/_documents/Conseilurgentprovisoire04‐2020_SciCom2020‐07_Covid‐19petitsanimauxdomestiques_27‐03‐20_001.pdf [Google Scholar]
- Shi, J. , Wen, Z. , Zhong, G. , Yang, H. , Wang, C. , Huang, B. , Liu, R. , He, X. , Shuai, L. , Sun, Z. , Zhao, Y. , Liu, P. , Liang, L. , Cui, P. , Wang, J. , Zhang, X. , Guan, Y. , Tan, W. , Wu, G. , … Bu, Z. (2020). Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS–coronavirus 2. Science, 368(6494), 1016–1020. 10.1126/science.abb7015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sit, T. H. C. , Brackman, C. J. , Ip, S. M. , Tam, K. W. S. , Law, P. Y. T. , To, E. M. W. , & Peiris, M. (2020). Infection of dogs with SARS‐CoV‐2. Nature, 586(7831), 776–778. 10.1038/s41586-020-2334-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temmam, S. , Barbarino, A. , Maso, D. , Behillil, S. , Enouf, V. , Huon, C. , Eloit, M. (2020). Absence of SARS‐CoV‐2 infection in cats and dogs in close contact with a cluster of COVID‐19 patients in a veterinary campus. bioRxiv. 10.1101/2020.04.07.029090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilibic‐Cavlek, T. , Stevanovic, V. , Tabain, I. , Betica‐Radic, L. J. , Sabadi, D. , Peric, L. J. , & Barbic, L. J. (2020). SARS‐CoV‐2 seroprevalence among personnel in healthcare facilities in Croatia. Revista Da Sociedade Brasileira De Medicina Tropical, 53, e20200458. 10.1590/0037-8682-0458-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo, P. C. Y. , Lau, S. K. P. , Wong, B. H. L. , Chan, K.‐H. , Hui, W.‐T. , Kwan, G. S. W. , Peiris, J. S. M. , Couch, R. B. , & Yuen, K.‐Y. (2004). False‐positive results in a recombinant severeacute respiratory syndrome‐associated coronavirus (SARS‐CoV) nucleocapsidenzyme‐linked immunosorbent assay due to HCoV‐OC43 and HCoV‐229E recti‐fied by Western blotting with recombinant SARS‐CoV spike polypeptide. Journal of Clinical Microbiology, 42(12), 5885–5888. 10.1128/JCM.42.12.5885-5888.2004 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zhang, B. , Zhou, X. , Zhu, C. , Feng, F. , Qiu, Y. , Feng, J. , & Wamg, J. (2020). Immune phenotyping based on neutrophil‐to‐lymphocyte ratio and IgG predicts disease severity and outcome for patients with COVID‐19. Frontiers in Molecular Biosciences, 7, 157. 10.3389/fmolb.2020.00157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Q. , Zhang, H. , Huang, K. , Yang, Y. , Hui, X. , Gao, J. , Jin, M. (2020). SARS‐CoV‐2 neutralizing serum antibodies in cats: A serological investigation. bioRxiv. 10.1101/2020.04.01.021196 [DOI] [Google Scholar]
- Zhou, P. , Yang, X.‐L. , Wang, X.‐G. , Hu, B. , Zhang, L. , Zhang, W. , Si, H.‐R. , Zhu, Y. , Li, B. , Huang, C.‐L. , Chen, H.‐D. , Chen, J. , Luo, Y. , Guo, H. , Jiang, R.‐D. , Liu, M.‐Q. , Chen, Y. , Shen, X.‐R. , Wang, X. I. , … Shi, Z.‐L. (2020). A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature, 579(7798), 270–273. 10.1038/s41586-020-2012-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


