Abstract
Background
COVID‐19 pandemic causes high global morbidity and mortality and better medical treatments to reduce mortality are needed.
Objective
To determine the added benefit of cyclosporine A (CsA), to low‐dose steroid treatment, in patients with COVID‐19.
Methods
Open‐label, non randomized pilot study of patients with confirmed infection of SARS‐CoV‐2 hospitalized from April to May 2020 at a single centre in Puebla, Mexico. Patients were assigned to receive either steroids or CsA plus steroids. Pneumonia severity was assessed by clinical, laboratory, and lung tomography. The death rate was evaluated at 28 days.
Results
A total of 209 adult patients were studied, 105 received CsA plus steroids (age 55.3 ± 13.3; 69% men), and 104 steroids alone (age 54.06 ± 13.8; 61% men). All patients received clarithromycin, enoxaparin and methylprednisolone or prednisone up to 10 days. Patient's death was associated with hypertension (RR = 3.5) and diabetes (RR = 2.3). Mortality was 22 and 35% for CsA and control groups (P = 0.02), respectively, for all patients, and 24 and 48.5% for patients with moderate to severe disease (P = 0.001). Higher cumulative clinical improvement was seen for the CsA group (Nelson Aalen curve, P = 0.001, log‐rank test) in moderate to severe patients. The Cox proportional hazard analysis showed the highest HR improvement value of 2.15 (1.39–3.34, 95%CI, P = 0.0005) for CsA treatment in moderate to severe patients, and HR = 1.95 (1.35–2.83, 95%CI, P = 0.0003) for all patients.
Conclusion
CsA used as an adjuvant to steroid treatment for COVID‐19 patients showed to improve outcomes and reduce mortality, mainly in those with moderate to severe disease. Further investigation through controlled clinical trials is warranted.
Keywords: COVID‐19, cyclosporine A, improvement, mortality, pneumonia, steroids
Introduction
The pandemic caused by the severe acute respiratory syndrome coronavirus 2 or SARS‐CoV‐2, responsible for the clinical entity known as coronavirus disease 19 or COVID‐19, has caused remarkable morbidity and mortality (https://coronavirus.jhu.edu/map.html) and has overloaded healthcare systems worldwide. Clinical symptoms range from asymptomatic to those of severe pneumonia [1]. Whilst some coronavirus such as 229E or NL63 infect only the upper respiratory airways [2], SARS‐CoV, MERS‐CoV (Middle East respiratory syndrome coronavirus) and SARS‐CoV‐2 can infect the lower airways leading to pneumonia and, in some cases, ARDS (Acute Respiratory Distress Syndrome) and death. SARS‐CoV‐2 infection can be eliminated in the upper respiratory tract by mechanisms of innate immunity, with activation of cells producing type I IFN, TNF‐α, IL‐1β, IL‐6, IL‐18, IP10, MCP‐1, M‐CSF and G‐CSF [3]. However, if SARS‐CoV‐2 escapes this innate immune response and reaches the lower respiratory airways, it triggers an enormous accumulation of macrophages, neutrophils and lymphocytes that leads to an uncontrolled immune inflammatory response with a cytokine storm that contributes to ARDS and respiratory failure that can cause the death [4, 5]. The strategy of promoting or inhibiting the cytokine production during the medical treatment of COVID‐19 must take into account the stage of the infection, the course of the disease, and the host’s immune response ([6], reviewed in [7]).
Patients with COVID‐19 who are admitted to hospitals all around the world are being treated following the guidelines for acute hypoxic failure and ARDS, which include the use of supplemental oxygen therapy, assisted mechanical ventilation and antibiotics. Drugs that have been used for COVID‐19 patients have included antimalarials (chloroquine / hydroxychloroquine), protease inhibitors (lopinavir/ritonavir, camostat mesylate), steroids (mainly methylprednisolone), RNA‐dependent RNA polymerase inhibitors (remdesivir, favipiravir, ribavirin) and antibodies (convalescent plasma, polyclonal immunoglobulin or monoclonal antibodies), producing diverse results [8]. The use of corticosteroids is controversial, but some studies suggest that administration at low doses in patients with pneumonia and ARDS may prevent cytokine storm and accelerate lung recovery [9, 10, 11, 12, 13]. A recent placebo‐controlled study in the United Kingdom found that the use of dexamethasone at a low dose of 6 mg daily for 10 days reduced the 28‐day mortality rate in patients who required supplemental oxygen therapy or ventilatory support [14].
Cyclosporine A (CsA) is a calcineurin inhibitor that was initially discovered as an antibiotic (isolated from the fungi Tolypocladium inflatum and Cylandrocarpon lucidum). Due to its property of inhibiting IL‐2 production and suppressing T‐cell responses, it has been used as immunosuppressor in recipients of kidney transplants and patients with autoimmune diseases such as rheumatoid arthritis and systemic erythematosus lupus [15, 16]. Interestingly, CsA is also an immunomodulator of innate immune response, inhibiting pro‐inflammatory cytokines IL‐1β, IL‐12 and TNF‐α whilst promoting anti‐inflammatory IL‐10 [17]. Moreover, CsA has also shown antiviral activity, inhibiting in vitro the replication of several coronavirus, including SARS‐CoV and MERS‐CoV [17, 18, 19, 20]. Therefore, based on the anti‐inflammatory and antiviral properties of CsA, we conducted a pilot study to test the added benefit of CsA to steroids in the treatment of a group of hospitalized patients with COVID‐19.
Methods
Study design and participants
This is a pilot study conducted at a single centre (ISSSTE Regional Hospital, Puebla, Mexico) who provides medical care to government employees. Consecutive adult patients attending the hospital between 15 April and 31 May 2020, with symptoms compatible with COVID‐19 pneumonia, according to WHO guidelines, were invited to participate, and all the patients gave their written informed consent. The inclusion criteria were as follows: patients of 18 to 85 years old (women and men), with oxygen saturation to environment less than 90%, respiratory frequency (FR) equal or higher than 30 per minute, Pa02/FiO2 less than 400, LDH ≥ 250 IU/L, CRP ≥ 10mg/L, D‐Dimer ≥ 500 ng/dL or Ferritin ≥ 500 ng/dL. Exclusion criteria were as follows: clinical and laboratory evidence of influenza infection and bacterial pneumonia. Patients were assigned, in an unblinded and non‐randomized fashion, to receive either steroids plus CsA (intervention group) or steroids only (standard of treatment in this hospital, control group), as per individual clinical judgement. The primary outcome was the number of days to clinical improvement until hospital discharge or death. The secondary outcome was the improvement of patients, defined by the following parameters: lower oxygen requirements (2 litres per minute, or less), absence of fever by three consecutive days, RF < 22, a decrease of 50% or more in the C‐reactive protein on admission, and length of hospital stay.
Forty‐nine per cent of the enrolled patients were healthcare workers, (26% medical doctors, 15% nurses and 8 % administrative personnel of general clinics), who were more likely to have contracted the disease as an occupational infection. The remaining 51% were housewives, students, teachers and administrative employees. SARS‐CoV‐2 viral infection for all patients was confirmed by PCR analysis of nasopharyngeal swabs. Pneumonia was diagnosed on clinical grounds and confirmed by computed tomography (CT) imaging. Upon admission, patients were evaluated for respiratory function and classified according to their PaO2/FiO2 ratio (PaFi) as having mild (>300), moderate (200–300) or severe (<200) pneumonia.
The investigation was conducted according to the Revised Helsinki Declaration and under the approval and supervision of the Institutional Research and Bioethics Committees, according to applicable laws and regulations (Protocol 135.2020).
Pulmonary damage evaluation and criteria of disease progression
Lung damage was evaluated by CT according to the radiological pattern (ground glass, crazy paving, or consolidation), or following the standard criteria of the severity index [21]. The pulmonary damage was considered mild (1 to 5 points), moderate (6 to 14 points) or severe (15–25 points). We also assessed other clinical parameters well known as individual indicators related to lung damage (PaFi, CRP, LDH, fibrinogen, D‐dimer, Ferritin, Troponin, Neutrophils), and the CURB‐65 score (which considers the parameters Confusion, Urea, Respiratory rate, Blood pressure and age >65 years old [22]), to compare the values of CT score with the actual lung damage.
To evaluate the risk of disease progression, we used the recently proposed CALL Score that considers the variables Comorbidities, Age, Lymphocyte and Lactate Dehydrogenase, LDH [23]. Using this score, the risk is considered low (10%) for patients with 1–6 points, middle (10–40%) for those with 7–9 points and high (>50%) for those with 10 to 13 points.
Intervention
All patients were treated with clarithromycin, 500 mg PO twice a day for 14 days, enoxaparin (0.5 mg/ kg/ day for 14 days), and methylprednisolone (0.5 mg/kg IV QD) or prednisone (25 mg PO QD) for 7 days, as per the standard of treatment at the hospital. All patients received usual support treatment. Additionally, patients in the intervention group were treated with oral CsA at a dose of 1–2 mg/kg/day divided into two doses, for 7 days, upon admission. The CsA dose of 1 mg/kg was given only to patients older than 70 years old. Patients with admission creatinine values >2 mg/dL or with uncontrolled hypertension were excluded to receive CsA. Treatment with steroids or CsA + steroids was extended up to 10 days, at the discretion of the treating physician. Patients who finished their steroids or CsA + steroids treatment and stayed in hospital received two daily doses of 100 micrograms of inhaled fluticasone up to 30 days.
Broad‐spectrum antibiotics were used at the discretion of the treating physician when superimposed bacterial infections were suspected.
Statistical analysis
First, the groups of CsA or control patients were compared by their demographic and clinical characteristics employing absolute and relative frequencies (categorical variables), means and standard deviations (numerical variables with Gaussian distributions) or medians and interquartile ranges (numerical variables with non‐Gaussian distributions). A Nelson–Aalen curve was used to summarize time to clinical improvement for both groups (log‐rank test was used to estimate whether the curves are identical).
Second, the multivariable analysis was performed either by logistic regression (mortality) or by Cox proportional hazard regression (time to clinical improvement).
In a separate analysis, we tested the association of the lung damage severity (index of lung injury/CT scan), or the probability of progression (CALL Score), with mortality and improvement.
Data were analysed using R programming language for statistical computing, version 3.6 (R core team). A P cut‐off value of 0.05 was used to deem statistical significance in all analyses.
Results
Study population, clinical characteristics of the groups and outcome
A total of 209 adult patients were enrolled in this study. Of the 209 patients, 105 patients were assigned to the CsA group and 104 patients to the control group. All patients received medical care until recovery or death, and data were analysed a posteriori.
The follow‐up period was 28 days for this protocol, and around 50% of the recovered patients have continued to be surveilled beyond this period through out‐patient services. In total, 149 patients (71.3%) were discharged from the hospital and 60 (28.7%) died. Of the 149 (100%) patients discharged from the hospital, 82 (55 %) received CsA plus steroids, and 67 (45%) steroids alone. Regarding the 60 (100%) of deceased patients, 23 (38.3%) received CsA plus steroids and 37 (61.7%) steroids alone.
A summary of the demographic and clinical data of the 209 patients enrolled in the study is presented in Table 1. Representative imaging patterns of lung damage found by CT are shown in Fig. 1. Both groups of patients received an equivalent daily dose (32.5 ± 11.7 and 31.6 ± 12.3 mg CsA and control, P = 0.8) and an accumulated dose (231 ± 98.6 and 208.3 ± 102 mg, CsA and control, P = 0.6) of steroids. The proportions of COPD (chronic obstructive pulmonary disease), diabetes, hypertension and obesity were similar in both groups. During the hospitalization period, the frequency of complications was similar for patients included in the CsA and control groups: thrombosis 8 (7.6%) vs. 12 (11.5%), P = 0.33; cardiac failure 7(6.7%) vs. 5(4.8%), P = 0.5, respectively. Renal failure was observed in 13 (12%) and 28 (27%) patients (P = 0.0089) in CsA and control groups, likely due to initial exclusion criteria for patients receiving CsA. Similarly, the cause of death was equivalent between CsA and control groups: respiratory insufficiency 19(82%) vs. 32(86%), P = 0.7; thromboembolism 3(13%) vs. 4(10.8%), P = 0.8; septic shock 0(0%) vs. 1(2.7%), P = 0.4; and multiple organic failure 1(4%) vs. 0(0%), P = 0.2, respectively. Interestingly, the plain numbers on mortality at 28 days were 23 (22%) in the CsA group vs. 37 (35.5%) in the control group, P = 0.02. However, at admission, patients in the CsA group had, in average, a more severe COVID‐19 pneumonia than those in the control group, as indicated by a CT severity index of 14.5 vs. 11.54 (P = 0.001), a PaFi of 254.9 vs. 309.5 (P = 0.001) and a C‐reactive protein of 151.9 vs. 108.7 mg/dL, P = 0.004, respectively (Table 1). To account for this difference at baseline, we further re‐analysed the data.
Table 1.
Demographic and clinical characteristics of patients allocated to cyclosporine and control groups
|
Cyclosporine group n = 105 |
Control group n = 104 |
P | |
|---|---|---|---|
| Man No. (%) | 73 (69.5) | 65 (61.1) | 0.28 |
| Woman No. (%) | 32 (30.5) | 39 (37.5) | 0.28 |
|
Age, years ± SD (95%CI) IQR |
55.36 ± 13.37 (52.8–57.91) Q1 = 45; Q3 = 66 IQR = 21 |
55.06 ± 13.86 (52.40–57.73) Q1 = 45.7; Q3 = 64 IQR = 18.3 |
0.87 |
|
Admission CALL Score a ± SD (95%CI) |
8.4 ± 2.40 (7.90–8.80) |
8.4 ± 2.71 (7.90–8.94) |
0.94 |
| Admission Lung CT severity score, ± SD (95%CI) b |
14.50 ± 5.80 (13.37–15.60) |
11.54 ± 7.16 (10.17–12.95) |
0.001 |
| Evolution time before admission, days ± SD (95%CI) c |
10.80 ± 3.66 (10.10–11.51) |
9.45 ± 3.28 (8.82–10.08) |
0.005 |
| Diabetes No. (%) | 32 (30) | 33 (31) | 0.85 |
| Hypertension No. (%) | 31 (29.5) | 37 (35) | 0.35 |
| COPD No. (%) | 7 (6) | 5 (4) | 0.35 |
| Obesity No. (%) | 44 (42) | 43 (41) | 0.12 |
| Recovery time in days ± SD (95%CI) |
6 ± 3.50 (5.33–6.68) |
6.12 ± 5.40 (5.0–7.16) |
0.85 |
| Bacterial superinfection No. (%) | 16 (15.2) | 31 (32) | 0.001 |
|
Leucocytes at admission x 103 ± SD (95%CI) IQR |
9.44 ± 6.0 (8.29–10.6) Q1 = 5.42; Q3 = 11.53 IQR = 6.11 |
8.42 ± 4.88 (7.48–9.36) Q1 = 4.57; Q3 = 10.61 IQR = 6.04 |
0.17 |
|
Neutrophils at admission x 103 ± SD (95%CI) IQR |
7.5 ± 9.01 (5.78–9.22) Q1 = 3.5; Q3 = 8.8 IQR = 5.3 |
6.168 ± 4.29 (5.34–6.99) Q1 = 2.97; Q3 = 8.125 IQR = 5.155 |
0.17 |
| Admission lymphocytes (cells x 103/dL) ± SD (95% CI) |
1.01 ± 0.49 (0.91–1.10) |
1.21 ± 1.19 (0.98–1.45) |
0.11 |
|
CURB‐65 Mild No. (%) Moderate No. (%) Severe No. (%) |
65 (62) 25 (24) 15 (14) |
67 (64.5) 22(21) 15(14.5) |
0.70 0.64 0.97 |
|
PaFi ± SD (95%CI) IQR |
254.90 ± 109.50 (234.01–275.80) Q1 = 150; Q3 = 340 IQR = 190 |
309.50 ± 128.40 (284.80–334.20) Q1 = 200; Q3 = 400 IQR = 200 |
0.001 |
|
CRP at admission mg/L ± SD (95%CI) IQR |
151.9 ± 100.01 (132.71–171) Q1 = 62; Q3 = 215 IQR = 153 |
108.7 ± 115.70 (86.4–130.90) Q1 = 15.9; Q3 = 194.5 IQR = 178.6 |
0.004 |
|
LDH at admission IU/L ± SD (95%CI) IQR |
440 ± 248.90 (392–487.90) Q1 = 273; Q3 = 528 IQR = 255 |
524.7 ± 731.01 (384.20–665.20) Q1 = 224.7; Q3 = 600 IQR = 375.3 |
0.26 |
|
Fibrinogen mg/dL ± SD (95%CI) IQR |
745 ± 272.20 (693.80–797.90) Q1 = 600; Q3 = 900 IQR = 300 |
640.2 ± 353.60 (572.30–708.20) Q1 = 300; Q3 = 900 IQR = 600 |
0.01 |
|
Dimer D mg/L ± SD (95%CI) IQR |
2.22 ± 4.33 (1.40–3.01) Q1=.4; Q3 = 1.5 IQR = 1.1 |
2.19 ± 5.44 (1.14–3.23) Q1=.33; Q3 = 2 IQR = 1.67 |
0.95 |
|
Ferritin at admission ng/L ± SD (95%CI) IQR |
844.4 ± 971.50 (658.60–1030.20) Q1 = 312.5; Q3 = 850 IQR = 537.5 |
645.8 ± 6.44.20 (521.90–769.60) Q1 = 200; Q3 = 900 IQR = 700 |
0.08 |
| Troponin – I ng/L | 0.6 ± 2.8 | 0.8 ± 7.8 | 0.7 |
|
CPK at admission U/L ± SD (95%CI) IQR |
99.7 ± 113.60 (78–121.50) Q1 = 50; Q3 = 104 IQR = 54 |
142.5 ± 256.80 (93–191.9) Q1 = 50; Q3 = 126 IQR = 76 |
0.12 |
| Mortality No. (%) | 23 (22) | 37 (35.5) | 0.02 |
COPD, chronic obstructive pulmonary disease; CRP, C‐reactive Protein; CURB‐65 Confusión, Urea, Respiratory rate, Blood pressure and age >65; CPK, Creatine Phospho Kinase; LDH, Lactate Dehydrogenase; PaFi, PaO2 / FiO2 ratio.
CALL Score (comorbidities, age over 60 years, lactate dehydrogenase and lymphopenia): 1 to 6 points (low risk), 7 to 9 points (10 to 40% probability of progression) and 10 to 13 points (> 50% probability of progression) (Ji Dong, 2020).
Lung CT severity score: 1 to 5 points (mild damage), 6 to 14 points (moderate damage) and 15 to 25 points (severe damage) (WangY, 2020).
Time in days between first clinical symptoms of disease and hospital admission.
Fig. 1.
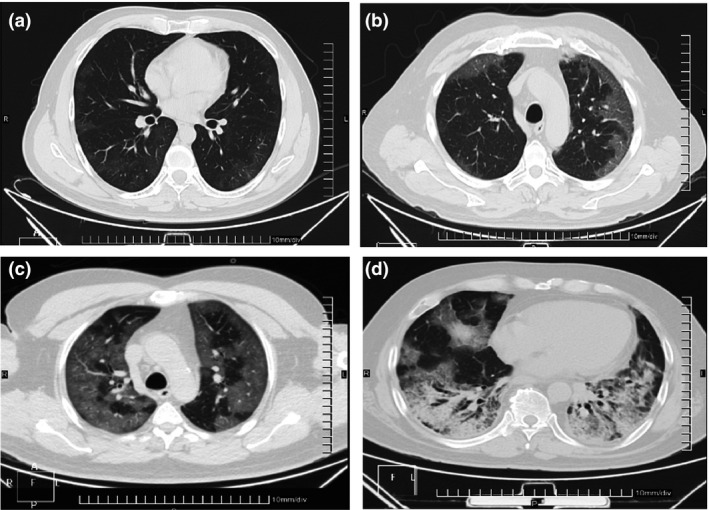
Representative CT images of lung lesions from the studied patients, with distinct damage by SARS‐CoV‐2 pneumonia. CT images were classified according to a CT score for lung damage. (a) Middle pneumonia, CT Score = 5, pattern in ground glass. (b) moderate pneumonia, CT Score = 14, pattern crazy paving. (c) severe pneumonia, CT Score = 23, pattern crazy paving. (d) severe pneumonia, CT Score = 20, pattern consolidation.
We analysed the association of comorbidities (diabetes, hypertension, COPD and obesity) with mortality in 60 deceased patients. A significant association was found for hypertension (RR = 3.5, 95%CI 1.9–6.7, P = 0.0001), obesity (RR = 1.9, 95%CI 1.02–3.5, P = 0.05) and diabetes (RR = 2.3, 95%CI 1.3–4.4, P = 0.006).
Relation of mortality with treatment according to the index of severity of lung damage by CT and probability of progression
We analysed the outcome for the 209 patients in both groups, considering improvement conducting to discharge, or death, at the end of the follow‐up period (Table 2). This analysis was done according to their lung damage score (by CT score and by the specific pattern of lung damage) and by the probability of disease progression by the CALL Score. The analysis of distinct clinical and laboratory parameters – related to the evaluation of lung damage – for all the 209 patients showed a correlation of the CT score with the following parameters: Fibrinogen > CRP > PaFi > Ferritin > LDH, whilst the CALL Score correlated with these parameters: CRP > CT Score > PaFi > Fibrinogen > Ferritin > D‐dimer (Tables S1 and S2 and Fig. S1). We calculated the improvement or death rate for patients in the CsA and control groups with mild, moderate and severe CT lung damage by dividing the number of the improved or deceased patients by the total patients in the corresponding category of disease severity (v.g., deceased / deceased + improved). Following this analysis, we found a statistically significant difference in the improvement rate in patients with severe lung damage by CT: 66% (32/48) in the CsA group vs. 28% (11/39) in the control group (P = 0.001) as shown in Table 2. In addition, in patients with severe lung damage by CT the death rate was 34% (16/48) for the CSA group, and 72% (28/39) for the control group (P = 0.0001). This represented a reduction in mortality of 38 percentual points (or 53% less, comparative to the control) in the CsA group, respect to the control group, at 28 days.
Table 2.
Improvement or death outcome in the patients grouped in the intervention and control subsets. The patients were classified according to the index of severity of lung damage by CT, CT category and probability of progression
|
Cyclosporine group n = 105 |
Control group n = 104 |
P* | |||
|---|---|---|---|---|---|
| Improvement | Death | Improvement | Death | ||
|
Mild CT: No. (%) a 43 (100) |
9 (20) α = 1 – |
0 (0) – β = 0 |
31 (72) α = 0.91 – |
3(8) – β = 0.09 |
0.82 0.17 |
|
Moderate CT: No. (%) 79 (100) |
41 (52) α = 0.84 – |
7 (8.8) – β = 0.16 |
25 (32) α = 0.89 – |
6 (7.2) – β = 0.1 |
0.71 0.28 |
|
Severe CT: No. (%) 87 (100) |
32 (37) α = 0.66 – |
16 (18) – β = 0.34 |
11 (12.6) α = 0.28 – |
28 (32.4) – β = 0.72 |
0.001 0.0001 |
|
Ground glass CT category: No. (%) b 52 (100) |
16 (30.5) α = 0.94 – |
1 (2) – β = 0.06 |
33 (63.5) α = 0.94 – |
2(4) – β = 0.06 |
0.49 0.5 |
|
Crazy paving CT category: No. (%) 118 (100) |
52 (44) α = 0.78 – |
15(13) – β = 0.22 |
26 (22) α = 0.51 – |
25 (21) – β = 0.49 |
0.001 0.001 |
|
Consolidation CT category: No. (%) 39 (100) |
14 (36) α = 0.66 – |
7 (18) – β = 0.33 |
8 (20.5) α = 0.45 – |
10 (25.5) – β = 0.55 |
0. 08 0. 08 |
|
CALL Score < 6 c No. (%) 43 (100) |
19 (44) α = 0.95 – |
1 (2.5) – β = 0.05 |
22 (51) α = 0.96 – |
1 (2.5) – β = 0.04 |
0.45 0.54 |
|
CALL Score 7–9: No. (%) 92 (100) |
42 (45.6) α = 0.8 – |
10 (10.8) – β = 0.2 |
29 (31.5) α = 0.725 – |
11 (11.8) – β = 0.275 |
0. 82 0.17 |
|
CALL Score 10–13: No. (%) 74 (100) |
21 (28.3) α = 0.636 – |
12 (16.2) – β = 0.364 |
16 (21.6) α = 0.39 – |
25 (33.9) – β = 0.61 |
0.01 0.01 |
α = improvement probability, β = death probability (* statistical significance was considered P < 0.05 for the difference between the experimental group and the control group).
Lung CT severity score: 1 to 5 points (mild damage), 6 to 14 points (moderate damage) and 15 to 25 points (severe damage).
CT category: ground glass (initial phase), crazy paving (disease progression – inflammatory phase), and consolidation (advanced disease‐extensive inflammatory infiltrate of the lung parenchyma).
CALL Score (comorbidities, age over 60 years, lactate dehydrogenase and lymphopenia): 1 to 6 points (low risk), 7 to 9 points (10 to 40% probability of progression) and 10 to 13 points (> 50% probability of progression).
Analysing all patients with a radiological pattern of lung damage pattern, we observed that, amongst those presenting a crazy paving CT image in the progression phase of the disease, 52 (44%) patients treated with CsA showed improvement compared to only 26 (22%) in the control group (P = 0.001) (Table 2).
Similarly, the analysis by the CALL Score criterion showed that in the subgroup with 10 to13 points (patients with high probability or risk of progression), there was a 64% probability of improvement for the CsA group vs. a 39% probability of improvement for the control group (P = 0.01). Furthermore, the probability of death was 36% in the CsA group compared to 61% in the control group (P = 0.01) (Table 2).
Therefore, the data analysis of all the patients included in our study showed that patients in the CsA group had a better outcome for those with pneumonia in the progression phase, and a lower mortality and better outcome for those with severe COVID‐19 pneumonia, compared to the control group.
Improvement assessment in the studied patients by Nelson–Aalen estimator
The improvement assessment in patients was based in clinical criteria, throughout a period of 28 days. The cumulative incidence of clinical improvement in the two groups was calculated by using the Nelson–Aalen estimator [24, 25]. We used the log‐rank test to prove the hypothesis contrast between the two group curves. In our study, we determined that this cumulative incidence of improvement at day 10 for patients treated with CsA was 1.55, and for the control group was 1.15. This difference was conserved during the following up period, P = 0.23, log‐rank test (Fig. 2A). We also performed the same analysis for patients with moderate to severe pneumonia, and at day 10, the incidence of improvement was 1.51 for the CsA group, compared to 0.87 for the control group, P = 0.001, log‐rank test (Fig. 2B).
Fig. 2.
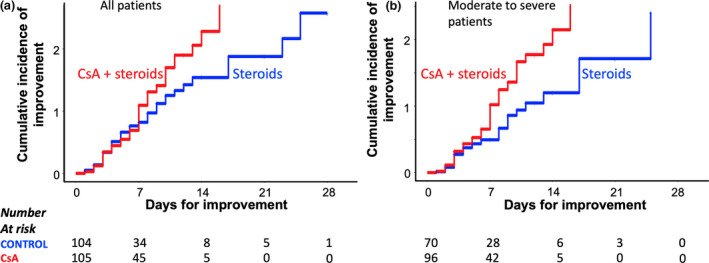
COVID‐19 pneumonia treatment, comparison of Nelson–Aalen cumulative incidence of improvement curves between CsA and control group treatment. (a) Comparison of treatments for all the 209 studied patients, P = 0.23, log‐rank test. (b) Comparison of response to treatments only for patients with moderate to severe disease, P = 0.001, log‐rank test.
Thus, the data analysis for the total of the 209 studied patients indicated that as a group, the patients receiving the combined treatment of CsA plus steroids started to improve more than subjects in the control group, at day 10, and the improvement was more significant in patients with moderate to severe pneumonia.
Mortality in patients with moderate to severe COVID‐19 pneumonia was lower in patients treated with CsA respect patients from control group
The protocol was originally planned as a randomized study, using a random number list for the allocation of patients to the groups. However, patients with creatinine level >2 mg/dL or uncontrolled hypertension could not be allocated to the CsA group, because of a risk to cause renal damage and the operation of this exclusion criterion disturbed the randomization process. In addition, the adaptation process to convert a section of our third‐level General Hospital to an only‐COVID‐dedicated area of the Hospital, caused stress and also COVID‐19 disease, among part of the primary care staff. This process affected doctors, nurses and other support personnel working to give of medical care to the patients attending to our wards, so that the randomization process could not be strictly followed. The involuntary consequence derived of these situations produced uneven groups in our study, with more severe pneumonia patients in the CsA group. Nevertheless, the mortality in the CsA group was 22% compared to 35% in the control group (P = 0.02) (Table 1).
Aware of the potential bias of our first analysis, we performed a sub‐analysis of data considering only participants with moderate to severe COVID‐19 pneumonia (lung CT severity score from 6 to 25) which were 96 in the CsA group and 70 in the control group. In this subset, most of the demographic variables and comorbidities were similar between both subgroups. Following this analysis, there was a significant reduction of 24.5 percentage points in the mortality of the CsA group (or 51.5 % less mortality relative to control) compared to the control group (24% in the CsA group vs. 48.5 % in the control group, P = 0.0004) Table 3.
Table 3.
Clinical characteristics of patients treated with cyclosporine and control group restricted only to patients with moderate to severe COVID‐19 pneumonia
|
Cyclosporine group n = 96 |
Control group n = 70 |
P | |
|---|---|---|---|
| Man No. (%) 127(100) | 67 (69.8) | 50 (71.5) | 0.40 |
| Woman No. (%) | 29 (30.2) | 20 (28.5) | 0.40 |
|
Age, years ± SD (95%CI) IQR |
55.63 ± 13.37 (52.59–58.37) Q1 = 45.75; Q3 = 66.5 IQR = 20.75 |
58.11 ± 12.29 (55.23–60.99) Q1 = 50.25; Q3 = 66.75 IQR = 16.5 |
0.21 |
| Admission lymphocytes (cells x 103/dL) ± SD (CI 95%) |
1.00 ± 0.48 (0.90–1.10) |
1.05 ± 0.54 (0.92–1.17) |
0.55 |
| Admission CALL Score ± SD (95%CI) a |
8.43 ± 2.46 (7.95–8.92) |
9.07 ± 2.55 (8.47–9.67) |
0.94 |
| Admission Lung CT severity score ± SD (95%CI) b |
15.36 ± 5.28 (14.30–16.42) |
15.15 ± 5.90 (13.77–16.54) |
0.81 |
| Evolution time before admission, days ± SD (95%CI) c |
10.70 ± 3.60 (9.98–11.42) |
10.24 ± 3.00 (9.53–10.94) |
0.36 |
| Diabetes No. (%) | 31 (32.3) | 27 (38.5) | 0.40 |
| Hypertension No. (%) | 27 (28.1) | 31 (44.2) | 0.01 |
| COPD No. (%) | 7 (7.2) | 4 (5.7%) | 0.34 |
| Obesity No. (%) | 42 (43.7) | 36 (51.4) | 0.16 |
|
PaFi ± SD (95%CI) IQR |
250.7 ± 108.0 (229.1–272.3) Q1 = 150; Q3 = 300 IQR = 150 |
279.5 ± 135.0 (247.8–311.0) Q1 = 152.5; Q3 = 400 IQR = 247.5 |
0.14 |
|
CRP at admission mg/L ± SD (95%CI) IQR |
156.10 ± 100.8 (135.93–176.27) Q1 = 62; Q3 = 215 IQR = 153 |
133.92 ± 121.58 (105.44–162.41) Q1 = 20.75; Q3 = 200 IQR = 178.6 |
0.21 |
|
LDH at admission IU/L ± SD (95%CI) IQR |
453.77 ± 254.83 (402.79–504.74) Q1 = 275.25; Q3 = 539 IQR = 263.75 |
652.82 ± 861.28 (451.05–854.59) Q1 = 280.25; Q3 = 751.75 IQR = 471.5 |
0.06 |
|
Dimer D mg/L ± SD (95%CI) IQR |
2.31 ± 4.49 (1.41–3.21) Q1=.4; Q3 = 1.5 IQR = 1.1 |
2.04 ± 2.9 (1.36–2.72) Q1=.5; Q3 = 2.10 IQR = 1.6 |
0.64 |
|
Ferritin at admission mg/L ± SD (95%CI) IQR |
889.33 ± 1004.5 (689.01–1089.6) Q1 = 400; Q3 = 888.5 IQR = 488.5 |
774.27 ± 710.45 (607.84–940.71) Q1 = 300; Q3 = 964.7 IQR = 664.7 |
0.38 |
|
Cumulative Steroids Dose ± SD (95%CI) IQR |
228.12 ± 92.55 (209.60–246.64) Q1 = 150; Q3 = 300 IQR = 150 |
216.50 ± 116.93 (189.10–243.89) Q1 = 140; Q3 = 280; IQR = 140 |
0.49 |
| Mortality No. (%) | 23 (24) | 34 (48.5) | 0.0004 |
Moderate to severe COVID‐19 pneumonia cases were obtained from the lung CT severity score (moderate damage = 6 to 14 points; severe damage 15 to 25 points).
COPD, Chronic Obstructive Pulmonary Disease; CRP, C‐reactive Protein; LDH, Lactate Dehydrogenase; PaFi, PaO2/FiO2 ratio.
CALL Score (comorbidities, age over 60 years, lactate dehydrogenase and lymphopenia): 1 to 6 points (low risk), 7 to 9 points (10 to 40% probability of progression) and 10 to 13 points (> 50% probability of progression) (Ji Dong, 2020).
Lung CT severity score: 1 to 5 points (mild damage), 6 to 14 points (moderate damage) and 15 to 25 points (severe damage) (WangY, 2020).
Time in days between first clinical symptoms of disease and Hospital admission.
Predictive variables for improvement of patients with COVID‐19 pneumonia
After we adjusted our data to analyse only the patients with moderate to severe COVID‐19 pneumonia, we assayed 26 variables to test their suitability to predict improvement in patients with COVID‐19. The data were analysed with the Cox proportional hazard regression (HR) model [26], and we identified and chose a subset of six variables with best fit, as potential predictors of improvement or not. For this model, we used the log‐rank, likelihood ratio and Wald tests to statistically compare these six variables (P < 0.05). Thus, applying this analysis, the variables CURB‐65 Score [HR 0.69 (0.44–1.0), 95%CI, P = 0.011)] and CT Score [HR 0.54 (0.51–0.92), 95%CI, P = 0.05)] were the ones that negatively influenced hospital discharge , whilst the variable CsA treatment was associated with a positive influence to predict the discharge of hospital with an HR 2.15 (1.39–3.34, 95%CI, P = 0.0005) (Table 4). The variable with the highest HR for all patients, regardless of disease severity, was also treatment with CsA (HR 1.95 (1.35–2.83), 95%CI, P = 0.0005) (data not included in table).
Table 4.
Predictive variables for improvement restricted only to patients with moderate to severe COVID‐19 pneumonia
| Predictor | coef | HR | 95% CI | P |
|---|---|---|---|---|
| Cyclosporine | 0.76 | 2.15 | (1.394–3.34) | 0.0005 |
| CURB‐65 | −0.37 | 0.69 | (0.44–1.0) | 0.011 |
| Admission Lung CT severity score | −0.61 | 0.54 | (0.5183–0.920) | 0.05 |
| CR Protein | −0.004 | 0.995 | (0.993–0.997) | 0.0001 |
| LDH | −0.001 | 0.998 | (0.997−0.997) | 0.01 |
| Ferritin | −0.003 | 0.999 | (0.999−1.000) | 0.038 |
Moderate to severe COVID‐19 pneumonia cases were obtained from the Lung CT severity score (moderate damage = 6 to 14 points; severe damage 15 to 25 points) n = 166.
Cox regression analysis (Wald test, Likelihood test and Log‐rank test, P < 0.0001).
coef, regression coefficient; HR, Hazard ratio.
Therefore, data analysis from this pilot study indicates that a treatment with CsA plus steroids in patients with COVID‐19 with moderate to severe pneumonia is associated with two times or around 200% higher probability of improvement and survival, compared to steroids alone.
Discussion
In this pilot study, we tested the treatment of COVID‐19 pneumonia with steroids plus CsA, compared to steroids alone, in a group of hospitalized patients in a third‐level hospital in Puebla, Mexico. To the best of our knowledge, this is the first reported series of patients treated with CsA plus steroids for COVID‐19. Our results showed significantly lower mortality (35% vs. 22%) with the addition of CsA in all patients, which was more remarkable in those with moderate to severe pneumonia (48.5% to 24%). Besides, the analysis of our data showed a higher cumulative incidence of clinical improvement in the CsA intervention group with moderate to severe disease, and a trend of higher improvement for all patients; the clinical improvement was computed by the Kaplan–Meier method and comparison between groups with the two‐sample log‐rank test (Nelson–Aalen curve). Furthermore, the Cox hazard regression model showed an HR of 1.95 for CsA treatment to predict improvement in all patients, whilst the highest HR value of 2.15 was observed in those with moderate to severe pneumonia. Therefore, we believe that these first auspicious results, although obtained from a small number of patients, should encourage further investigation of the therapeutic value of CsA in COVID‐19.
Since the beginning of the pandemic, a high diversity of therapeutic approaches has been proposed and studied, aimed to reach distinct targets and stages of the disease. In addition to antiviral drugs or biologicals such as remdesivir, favipiravir or convalescent plasma, several drugs had been used to modulate the uncontrolled immune response, including steroids, anti‐IL‐6 antibodies such as tocilizumab or sarilumab, and tyrosine kinase inhibitors such as imatinib or ruxolitinib (Reviewed in [27]).
The use of corticosteroids in hyperinflammatory states as those observed in sepsis and septic shock, is still controversial as distinct studies have reported contrasting results [11, 28, 29]. In regards to COVID‐19, the use of corticosteroids is also a matter of debate [9, 13, 30]. Some authors suggest that even when corticosteroids reduce the lung damage caused by an amplified immune response, their immunosuppressive effect can also cause a rebound of the viraemia [31, 32], as shown in a study with 151 patients with MERS treated with high dose of corticosteroids [33]. In contrast, other studies have shown that, when administered in early stages of the disease, can improve the clinical conditions of patients with ARDS [34, 35, 36]. The use of steroids may be more appropriate by the eighth day of the disease or when there is evidence of the initiation of a hyperinflammatory response [37]. In support of this notion, the use of dexamethasone in a pilot study with a series of 21 patients was associated with an improvement in hospitalized patients, shortening their stay in hospital [38]. More recently, the efficacy of low‐dose dexamethasone in patients with severe COVID‐19 pneumonia was demonstrated in a large clinical trial in the United Kingdom [14].
In our study, treatment of hospitalized patients with COVID‐19 included either prednisone or methylprednisolone, at low doses of 0.5–1.0 mg/kg per day for 7 and up to 10 days, following the recommendations of the consensus of the Chinese Thoracic Society [13]; this regimen is roughly equivalent to the low dose of dexamethasone recently tested in the RECOVERY trial [14, 39]. Our results suggest that the addition of CsA to a standard treatment with steroids may provide additional benefits on improving outcomes and reducing mortality. We observed a remarkable difference in mortality in the subset of patients with moderate to severe disease, in a similar fashion as in the dexamethasone trial. Furthermore, we also observed a significant difference when all patients were analysed regardless of disease severity, and the use of CsA at early stage of the disease deserves more studies. Nowadays, the first‐line treatment for COVID‐19 in our hospital includes CsA plus dexamethasone.
Although the use of CsA for COVID‐19 treatment had be envisioned [40], we are not aware of any published report of a series of patients treated with this drug. We hypothesized that using a low dose of corticosteroids plus CsA could act synergistically to reduce both the inflammation and the viraemia, helping patients to recover. The observed reduction in mortality in our study supports our hypothesis. We observed an equivalent number of secondary bacterial infections in the intervention and control groups, indicating that there was not an important immunosuppressor effect of CsA inciding on infections. The use of antibiotics in the patients of our study was likely related to long stay in hospital and to endotracheal intubation and no to corticosteroids or CsA administration. The low‐dose and short‐term schedule of CsA used in our study is unlikely to cause serious secondary effects on kidney, liver or immunity, as shown in several clinical trials using low dose and short course of CsA, which have not reported serious adverse events [41, 42, 43], compared to the nephrotoxicity observed in transplanted patients with a prolonged use of CsA (reviewed in [44]).
We speculate that the beneficial effects of CsA in patients with COVID‐19 may be the result of a decrease in the activity of self‐reactive innate cells and therefore in less tissue damage [17, 45]. CsA has shown to inhibit IL‐12 and TNF‐α production by dendritic cells [46] as well as ROS (reactive oxygen species) [47, 48] and NET (neutrophil extracellular traps) [49] by neutrophils. Therefore, CsA contributes to down‐regulate the inflammatory response in patients with COVID‐19. Diffuse alveolar damage with activated type II pneumocytes, but also granulocyte‐dominated bronchopneumonia, has been observed in necropsies of COVID‐19 patients [50], which is consistent with NET as an important driver of tissue damage [51, 52]. Since CsA can inhibit the NET mechanism, it could be helpful to prevent this damage and preserve normal lung function.
Finally, CsA is a potent inhibitor of cyclophilins, which are essential enzymes in the life cycle of coronavirus, conferring antiviral properties against several coronavirus [18, 19, 20, 53, 54]. More studies are warranted to confirm whether this antiviral property may help in SARS‐CoV‐2 elimination at early and advanced stages. An additional advantage of CsA is its low cost.
There are several limitations of this pilot study: it was not randomized, which led to imbalances in the treatment groups, and the number of patients was relatively low. We did not measure the evolution of general or more specific inflammatory markers such as cytokines IL‐1, IL‐12 or TNF‐α during the disease, and data on viral load were not obtained; these data would help in monitoring the response to treatment and in profiling the inflammatory response against a given viral load. Well‐controlled randomized clinical trials involving more patients that include these measurements are warranted.
Conclusion
In conclusion, in our study with 209 hospitalized patients with COVID‐19, we observed better outcomes in patients treated with CsA plus steroids, compared to patients treated with steroids alone, and a significantly lower death rate across all grades of severity, which was even more marked in those with moderate to severe pneumonia. In the face of a lack of effective treatments for COVID‐19 and the public health crisis driven by the pandemic, we believe these observations justify further investigation through controlled randomized clinical trials to determine the efficacy of adding CsA to the treatment of patients with SARS‐CoV‐2 infection.
Author Contribution
Jose Luis Galvez‐Romero: Conceptualization (lead); Data curation (lead); Formal analysis (equal); Investigation (lead); Methodology (lead); Supervision (lead); Writing‐original draft (lead); Writing‐review & editing (equal). Oscar Palmeros‐Rojas: Conceptualization (supporting); Data curation (supporting); Formal analysis (equal); Investigation (supporting); Software (equal); Writing‐review & editing (supporting). Fernando Antonio Real‐Ramírez: Data curation (supporting); Investigation (supporting); Methodology (supporting); Supervision (supporting). Saúl Sánchez‐Romero: Investigation (supporting); Methodology (supporting); Validation (supporting). Ramiro Tome‐Maxil: Investigation (supporting); Methodology (supporting). María Patricia Ramírez‐Sandoval: Investigation (supporting); Methodology (supporting). Rosaura Olivos‐Rodríguez: Investigation (supporting); Methodology (supporting). Salvador Eduardo Flores‐Encarnación: Investigation (supporting); Methodology (supporting). Ana América Cabrera‐Estrada: Investigation (supporting); Methodology (supporting). José Avila‐Morales: Investigation (supporting); Methodology (supporting). Víctor Cortés‐Sánchez: Investigation (supporting); Methodology (supporting). Gonzalo Sarmiento‐Padilla: Investigation (supporting); Methodology (supporting). Sandra Elizabeth Tezmol‐Ramírez: Investigation (supporting); Methodology (supporting); Validation (supporting). David Aparicio‐Hernández: Data curation (supporting); Investigation (supporting); Methodology (supporting). Mario Ivan Urbina‐Sánchez: Investigation (supporting); Methodology (supporting). Miguel Angel Gómez‐Pluma: Investigation (supporting); Methodology (supporting); Validation (supporting). Dinorah Ivonne Rodríguez‐Rivas: Data curation (equal); Investigation (equal); Methodology (supporting); Supervision (equal); Validation (supporting). Sergio Reyes‐Inurrigarro: Investigation (supporting); Methodology (supporting); Validation (supporting). Surizadith Cisneros‐Méndez: Data curation (supporting); Investigation (supporting); Methodology (supporting); Supervision (supporting). Gilberto Cortés‐Díaz: Investigation (supporting); Methodology (supporting). Carlos Cruz‐Delgado: Investigation (supporting); Methodology (supporting); Validation (supporting). Jaqueline Navarro‐González: Data curation (supporting); Investigation (supporting); Methodology (supporting); Supervision (supporting); Validation (supporting). José Deveaux‐Homs: Investigation (supporting); Resources (equal); Supervision (supporting). Sigifredo Pedraza‐Sanchez: Formal analysis (supporting); Writing‐original draft (equal); Writing‐review & editing (lead).
Conflict of interest
The authors declare they have no conflict of interest.
Financial support
This research did not receive any specific grant from funding agencies in the public, commercial or not‐for‐profit sectors.
Supporting information
Table S1. Simple correlation of the CALL Score for COVID‐19 risk progression, related to distinct parameters, evaluated for the 209 studied patients.
Table S2. Simple correlation of the lung CT Score for lung damage, related to distinct variables, evaluated for the 209 studied patients.
Figure S1. Simple regression analysis for the lung CT score values related to distinct clinical parameters in patients with COVID‐19.
Acknowledgments
We thank our patients for their participation in the study, the hospital staff who served in the COVID‐19 areas and the resident doctors of the Internal Medicine Service. We thank Jorge Velazquez for his help in formatting figures and in the design of the graphical abstract. We are very thankful to Dr. Enrique Camacho for the review of the text in English. We acknowledge the valuable suggestions to the manuscript of Dr. Eric Ochoa‐Hein from Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán in México, and Dr. Beate Kampmann from London School of Hygiene and Tropical Medicine in U.K.
Gálvez‐Romero JL, Palmeros‐Rojas O, Real‐Ramírez FA, Sánchez‐Romero S, Tome‐Maxil R, Ramírez‐Sandoval MP, Olivos‐Rodríguez R, Flores‐Encarnación SE, Cabrera‐Estrada AA, Ávila‐Morales J, Cortés‐Sánchez V, Sarmiento‐Padilla G, Tezmol‐Ramírez SE, Aparicio‐Hernández D, Urbina‐Sánchez MI, Gómez‐Pluma MÁ, Cisneros‐Méndez S, Rodríguez‐Rivas DI, Reyes‐Inurrigarro S, Cortés‐Díaz G, Cruz‐Delgado C, Navarro‐González J, Deveaux‐Homs J, Pedraza‐Sánchez S (ISSSTE, Puebla; Universidad Autónoma Chapingo; ISSSTE, Hospital Regional; ISSSTE, Hospital Regional; ISSSTE, Hospital Regional; ISSSTE, Puebla; Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán (INCMNSZ), Ciudad de Mexico México). Cyclosporine A plus low‐dose steroid treatment in COVID‐19 improves clinical outcomes in patients with moderate to severe disease: A pilot study. J Intern Med 2021;289:906–920. 10.1111/joim.13223
Contributor Information
J. L. Gálvez‐Romero, Email: jose.galvez@issste.gob.mx.
S. Pedraza‐Sánchez, Email: sigifredo.pedrazas@incmnsz.gob.mx.
References
- 1. Lake MA. What we know so far: COVID‐19 current clinical knowledge and research. Clin Med (Lond) 2020; 20: 124–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fehr AR, Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Methods Mol Biol 2015; 1282: 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huang C, Wang Y, Li X et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395: 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen N, Zhou M, Dong X et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020; 395: 507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang D, Hu B, Hu C et al. Clinical characteristics of 138 hospitalized patients with 2019 Novel Coronavirus‐infected pneumonia in Wuhan, China. JAMA 2020; 323: 1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mehta P, McAuley DF, Brown M et al. COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet 2020; 395: 1033–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jamilloux Y, Henry T, Belot A et al. Should we stimulate or suppress immune responses in COVID‐19? Cytokine and anti‐cytokine interventions. Autoimmun Rev 2020; 19: 102567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kupferschimidt KCJ. Race to find COVID‐19 treatments accelerates WHO launches megatrial to test repurposed drugs and experimental drug candidates. Science 2020; 367: 1412–3. [DOI] [PubMed] [Google Scholar]
- 9. Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019‐nCoV lung injury. Lancet 2020; 395: 473–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Villar J, Belda J, Anon JM et al. Evaluating the efficacy of dexamethasone in the treatment of patients with persistent acute respiratory distress syndrome: study protocol for a randomized controlled trial. Trials 2016; 17: 342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lamontagne F, Rochwerg B, Lytvyn L et al. Corticosteroid therapy for sepsis: a clinical practice guideline. BMJ 2018; 362: k3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wu C, Chen X, Cai Y et al. Risk factors associated with acute respiratory distress syndrome and death in patients with Coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med 2020; 180:934–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shang L, Zhao J, Hu Y, Du R, Cao B. On the use of corticosteroids for 2019‐nCoV pneumonia. Lancet 2020; 395: 683–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. RECOVERY Collaborative Group, Horby P, Lim WS et al. Dexamethasone in hospitalized patients with Covid‐19 ‐ preliminary report. N Engl J Med 2020:NEJMoa2021436. 10.1056/NEJMoa2021436. [DOI] [Google Scholar]
- 15. Zeidler HK, Kvien TK, Hannonen P et al. Progression of joint damage in early active severe rheumatoid arthritis during 18 months of treatment: comparison of low‐dose cyclosporin and parenteral gold. Br J Rheumatol 1998; 37: 874–82. [DOI] [PubMed] [Google Scholar]
- 16. Tokuda M, Kurata N, Mizoguchi A et al. Effect of low‐dose cyclosporin A on systemic lupus erythematosus disease activity. Arthritis Rheum 1994; 37: 551–8. [DOI] [PubMed] [Google Scholar]
- 17. Liddicoat AM, Lavelle EC. Modulation of innate immunity by cyclosporine A. Biochem Pharmacol 2019; 163: 472–80. [DOI] [PubMed] [Google Scholar]
- 18. Li HS, Kuok DIT, Cheung MC et al. Effect of interferon alpha and cyclosporine treatment separately and in combination on Middle East Respiratory Syndrome Coronavirus (MERS‐CoV) replication in a human in‐vitro and ex‐vivo culture model. Antiviral Res 2018; 155: 89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tanaka Y, Sato Y, Sasaki T. Suppression of coronavirus replication by cyclophilin inhibitors. Viruses 2013; 5: 1250–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. de Wilde AH, Zevenhoven‐Dobbe JC, van der Meer Y et al. Cyclosporin A inhibits the replication of diverse coronaviruses. J Gen Virol 2011; 92: 2542–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang Y, Dong C, Hu Y et al. Temporal changes of CT findings in 90 patients with COVID‐19 pneumonia: a longitudinal study. Radiology 2020; 200843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lim WS, van der Eerden MM, Laing R et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax 2003; 58: 377–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ji D, Zhang D, Xu J et al. Prediction for progression risk in patients with COVID‐19 pneumonia: the CALL Score. Clin Infect Dis 2020;71: 1393–9. 10.1093/cid/ciaa414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Aalen O. Nonparametric inference for a family of counting processes. Ann Stat 1978; 6: 701–26. [Google Scholar]
- 25. Nelson W. Hazard plotting for incomplete failure data. J Qual Technol 1969; 1: 27–52. [Google Scholar]
- 26. Cox DR. Regression models and life‐tables. J R Stat Soc 1972; 34: 188–220. [Google Scholar]
- 27. Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of Coronavirus Disease 2019 (COVID‐19): a review. JAMA 2020; 32: 782–793. 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 28. Annane D, Renault A, Bellissant E. Glucocorticoids with or without fludrocortisone in septic shock. N Engl J Med 2018; 379: 895–6. [DOI] [PubMed] [Google Scholar]
- 29. Venkatesh B, Finfer S, Cohen J et al. Adjunctive glucocorticoid therapy in patients with septic shock. N Engl J Med 2018; 378: 797–808. [DOI] [PubMed] [Google Scholar]
- 30. Alhazzani W, Moller MH, Arabi YM et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID‐19). Intensive Care Med 2020; 46: 854–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wong SS, Yuen KY. The management of coronavirus infections with particular reference to SARS. J Antimicrob Chemother 2008; 62: 437–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lee N, Allen Chan KC, Hui DS et al. Effects of early corticosteroid treatment on plasma SARS‐associated Coronavirus RNA concentrations in adult patients. J Clin Virol 2004; 31: 304–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Arabi YM, Mandourah Y, Al‐Hameed F et al. Corticosteroid therapy for critically Ill patients with middle east respiratory syndrome. Am J Respir Crit Care Med 2018; 197: 757–67. [DOI] [PubMed] [Google Scholar]
- 34. Russell B, Moss C, George G et al. Associations between immune‐suppressive and stimulating drugs and novel COVID‐19‐a systematic review of current evidence. Ecancermedicalscience 2020; 14: 1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhao Z, Zhang F, Xu M et al. Description and clinical treatment of an early outbreak of severe acute respiratory syndrome (SARS) in Guangzhou. PR China. J Med Microbiol 2003; 52: 715–20. [DOI] [PubMed] [Google Scholar]
- 36. Fadel R, Morrison AR, Vahia A et al. Early Short Course Corticosteroids in Hospitalized Patients with COVID‐19. Clin Infect Dis 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cruz AF, Ruiz‐Antorán B, Múñez Rubio E et al. The right time for steroids in Covid‐19. Clin Infect Dis 2020:ciaa865. 10.1093/cid/ciaa865. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Selvaraj V, Dapaah‐Afriyie K, Finn A, Flanigan TP. Short‐term dexamethasone in Sars‐CoV‐2 patients. R I Med J 2013; 2020: 39–43. [PubMed] [Google Scholar]
- 39. Mager DE, Lin SX, Blum RA, Lates CD, Jusko WJ. Dose equivalency evaluation of major corticosteroids: pharmacokinetics and cell trafficking and cortisol dynamics. J Clin Pharmacol 2003; 43: 1216–27. [DOI] [PubMed] [Google Scholar]
- 40. Cour M, Ovize M, Argaud L. Cyclosporine A: a valid candidate to treat COVID‐19 patients with acute respiratory failure? Crit Care 2020; 24: 276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Argaud L, Cour M, Dubien PY et al. Effect of cyclosporine in nonshockable out‐of‐hospital cardiac arrest: the CYRUS randomized clinical trial. JAMA Cardiol 2016; 1: 557–65. [DOI] [PubMed] [Google Scholar]
- 42. Cung TT, Morel O, Cayla G et al. Cyclosporine before PCI in patients with acute myocardial infarction. N Engl J Med 2015; 373: 1021–31. [DOI] [PubMed] [Google Scholar]
- 43. Kreitmann L, Argaud L, Ovize M, Cour M, Group CS . Cyclosporine A prevents cardiac arrest‐induced acute respiratory failure: a post‐hoc analysis of the CYRUS trial. Intensive Care Med 2020; 46: 1281–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Naesens M, Kuypers DR, Sarwal M. Calcineurin inhibitor nephrotoxicity. Clin J Am Soc Nephrol 2009; 4: 481–508. [DOI] [PubMed] [Google Scholar]
- 45. Sanchez‐Pernaute O, Romero‐Bueno FI, Selva‐O'Callaghan A. Why choose cyclosporin a as first‐line therapy in COVID‐19 pneumonia. Reumatol Clin 2020. 10.1016/j.reuma.2020.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pino‐Lagos K, Michea P, Sauma D et al. Cyclosporin A‐treated dendritic cells may affect the outcome of organ transplantation by decreasing CD4+CD25+ regulatory T cell proliferation. Biol Res 2010; 43: 333–7. [PubMed] [Google Scholar]
- 47. Greenblatt MB, Aliprantis A, Hu B, Glimcher LH. Calcineurin regulates innate antifungal immunity in neutrophils. J Exp Med 2010; 207: 923–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Roilides E, Robinson T, Sein T, Pizzo PA, Walsh TJ. In vitro and ex vivo effects of cyclosporin A on phagocytic host defenses against Aspergillus fumigatus . Antimicrob Agents Chemother 1994; 38: 2883–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gupta AK, Giaglis S, Hasler P, Hahn S. Efficient neutrophil extracellular trap induction requires mobilization of both intracellular and extracellular calcium pools and is modulated by cyclosporine A. PLoS One 2014; 9: e97088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Edler C, Schroder AS, Aepfelbacher M et al. Dying with SARS‐CoV‐2 infection‐an autopsy study of the first consecutive 80 cases in Hamburg, Germany. Int J Legal Med 2020; 134: 1275–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Barnes BJ, Adrover JM, Baxter‐Stoltzfus A et al. Targeting potential drivers of COVID‐19: Neutrophil extracellular traps. J Exp Med 2020; 217: e20200652. 10.1084/jem.20200652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Narasaraju T, Tang BM, Herrmann M, Muller S, Chow VTK, Radic M. Neutrophilia and NETopathy as key pathologic drivers of progressive lung impairment in patients With COVID‐19. Front Pharmacol 2020; 11: 870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. de Wilde AH, Raj VS, Oudshoorn D et al. MERS‐coronavirus replication induces severe in vitro cytopathology and is strongly inhibited by cyclosporin A or interferon‐alpha treatment. J Gen Virol 2013; 94: 1749–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Softic L, Brillet R, Berry F et al. Inhibition of SARS‐CoV‐2 Infection by the Cyclophilin Inhibitor Alisporivir (Debio 025). Antimicrob Agents Chemother 2020; 64: e00876‐20. 10.1128/AAC.00876-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Simple correlation of the CALL Score for COVID‐19 risk progression, related to distinct parameters, evaluated for the 209 studied patients.
Table S2. Simple correlation of the lung CT Score for lung damage, related to distinct variables, evaluated for the 209 studied patients.
Figure S1. Simple regression analysis for the lung CT score values related to distinct clinical parameters in patients with COVID‐19.


