Abstract
Objectives
To report changes in practice brought about by COVID‐19 and the implementation of new guidelines for the management of tonsillitis and peritonsillar abscess (PTA), and to explore factors relating to unscheduled re‐presentations for patients discharged from the emergency department (ED).
Design
Prospective multicentre national audit over 12 weeks from 6 April 2020.
Setting
UK secondary care ENT departments.
Participants
Adult patients with acute tonsillitis or PTA.
Main outcome measures
Re‐presentation within 10 days for patients discharged from the ED.
Results
83 centres submitted 765 tonsillitis and 416 PTA cases. 54.4% (n = 410) of tonsillitis and 45.3% (187/413) of PTAs were discharged from ED. 9.6% (39/408) of tonsillitis and 10.3% (19/184) of PTA discharges re‐presented within 10 days, compared to 9.7% (33/341) and 10.6% (24/224) for those admitted from ED. The subsequent admission rate of those initially discharged from ED was 4.7% for tonsillitis and 3.3% for PTAs.
IV steroids and antibiotics increased the percentage of patients able to swallow from 35.8% to 72.5% for tonsillitis (n = 270/754 and 441/608) and from 22.3% to 71.0% for PTA (n = 92/413 and 265/373).
77.2% of PTAs underwent drainage (n = 319/413), with no significant difference in re‐presentations in those drained vs not‐drained (10.6% vs 9.5%, n = 15/142 vs 4/42, P = .846).
Univariable logistic regression showed no significant predictors of re‐presentation within 10 days.
Conclusions
Management of tonsillitis and PTA changed during the initial peak of the pandemic, shifting towards outpatient care. Some patients who may previously have been admitted to hospital may be safely discharged from the ED.
Keywords: ambulatory, discharge, multicentre, observational, Outpatient
Key Points.
Fewer patients presented to the emergency department (ED) with tonsillitis and peritonsillar abscess (PTA) during the COVID‐19 pandemic.
IV antibiotics and steroids given in the ED led to a twofold‐threefold increase in the proportion of patients able to swallow, to over 70%.
Approximately half of tonsillitis and PTA patients were discharged from the ED, without an increase in later admission.
Nearly, a quarter of patients with suspected PTA had no drainage performed, and re‐presentation rates were not higher in this group
There were no significant predictors of re‐presentation in those discharged from ED.
1. INTRODUCTION
The SARS‐CoV‐2 (COVID‐19) pandemic led to necessary changes in the management of common ENT emergency presentations internationally, including tonsillitis and peritonsillar abscess (quinsy, PTA), two of the most common emergency presentations to ENT. 1 It has been demonstrated that COVID‐19 infection leads to high viral titres in the nasal and oral cavities, 2 with virus transmission thought to be predominantly via aerosols or droplet formation.3, 4 Aspects of the management of tonsillitis and PTA, including oral examination, PTA drainage and flexible nasendoscopy, were considered to represent a potential route of transmission of COVID‐19.5, 6
In March 2020, new UK guidelines were issued for the management of tonsillitis and PTA presenting to emergency departments (ED), in light of COVID‐19. 7 There were no accepted national guidelines in the UK for the management of tonsillitis or PTA prior to COVID‐19 for comparison. The major shifts in practice proposed were as follows: the avoidance of oral examination; the routine discharge of patients who could swallow fluids and medications; the routine administration of intravenous (IV) medications to effect this; and the avoidance of PTA drainage if possible. This advice aimed to minimise the personnel involved in managing the acute presentation, reduce oral instrumentation, and to reduce admission rates, thus, preserving hospital bed capacity for the anticipated COVID‐19 demand.
The implementation of these relatively untested guidelines resulted in significant changes to established practices that had been shown to be safe over many years. This article aims to:
Report the findings of a 12‐week prospective audit of acute tonsillitis and PTA care, conducted during the initial peak of COVID‐19 in the UK.
Explore factors relating to unscheduled re‐presentation to hospital in tonsillitis and PTA patients discharged from the ED.
2. METHODS
The protocol for this study was published in advance at https://entintegrate.co.uk. This manuscript has been prepared with reference to the STROBE checklist for cohort studies. 8
2.1. Ethical considerations
The Health Research Authority decision tool determined the study design to fall under the remit of audit, and so no ethical approval was required (available at: http://www.hra‐decisiontools.org.uk/research/).
2.2. Study design and setting
A national prospective audit of the hospital management of tonsillitis and PTA by UK secondary care ENT departments was conducted, in parallel to an audit of acute epistaxis emergency care. 9 The audit was developed and run by INTEGRATE, the UK ENT Trainee Research Network. All UK ENT departments were invited to participate via advertisement, and registration with local audit and Clinical Governance Departments was required. Sites could open at any point during the prospective data collection period.
2.3. Participants
Consecutive patients with tonsillitis and PTA, aged 18 years and older, and referred to ENT secondary care, were eligible for inclusion, whether managed by telephone advice or face‐to‐face review.
2.4. Data collection
Eligible cases were identified over a 12‐week period, between 6 April 2020 and 29 June 2020. Each case was followed‐up for 10 days. A standardised electronic case report form was created using Excel software (Microsoft Corporation, Washington, USA) and made available online (Supplementary material), incorporating data validation to encourage valid data entry and completeness. Data were initially held offline at each centre, and patient identifiable data were removed prior to submission to the project management team. Data were collected on: patient demographics; COVID‐19 status; reviewing clinicians; IV medications and their effect; discharge medications; examinations performed; and PTA drainage.
2.5. Interim reports
The 12‐week audit was divided into three 4‐week periods. Two interim reports were produced (after periods 1 and 2) allowing for rapid feedback of management and preliminary outcomes to the UK ENT community. Both interim reports were disseminated electronically via ENTUK mailouts within 10 days of data submission and hosted online at https://entuk.org and https://entintegrate.co.uk.
2.6. Data analysis
The primary outcome was unscheduled re‐presentation to hospital within 10 days for ED discharges. This intended to assess the safety of the lower rates of admission anticipated.
Univariable binary logistic regression analysis was used to identify significant determinants of the primary outcome measure. The level of significance was set at < 0.05 with Bonferroni corrections applied, where applicable. Analysis was performed using R statistical software (R Foundation, Vienna, Austria).
3. RESULTS
3.1. Centres
Data were submitted by 83/86 UK centres who registered to take part (72 in England, 5 in Scotland, 3 in Wales and 3 in Northern Ireland) covering a total of 905 weeks. 2/83 centres submitted data covering the first period only. The dates centres opened are shown in Figure 1, alongside the median rates of tonsillitis and PTA cases referred per centre per week.
FIGURE 1.
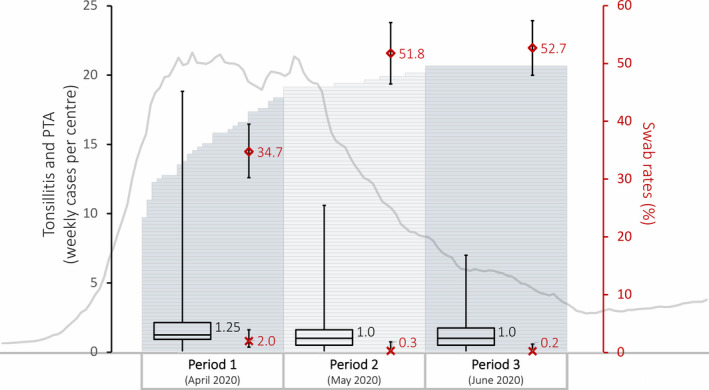
Combination graphic to communicate trends in data over 12‐week audit period: 1) The bar chart forming the background shows sequential opening of centres across the UK as the audit period progressed, 2) The box and whisker plots show the median, range and interquartile range of tonsillitis and quinsy patients presenting per centre per week for each of the three 4‐week audit periods, 3) The scatter plot and error bars are COVID‐19 swab rates (%) (diamonds) and positive swab rates (%) (crosses) with 95% confidence intervals, 4) The grey line chart is the 7‐day rolling average of UK cases from 1 March to 31 July (available at: https://coronavirus.data.gov.uk/cases)
3.2. Submissions
1,181 cases met the prespecified eligibility criteria across the three periods (765 tonsillitis cases [276, 212 and 277 cases, respectively] and 416 PTA cases [131, 129 and 156 cases, respectively]). Characteristics of both populations are shown in Tables 1 and 2. Data completeness was 99.2% (n = 592/597) of cases having data for the primary outcome.
TABLE 1.
Characteristics of tonsillitis population, with proportions admitted/discharged and re‐presentation rates for ED discharges (with univariable regression analysis)
| Variable | Status | ED admissions % (n) | ED discharges % (n) |
Re‐presentation rates of ED discharges % (n) |
Univariable logistic regression p value |
|---|---|---|---|---|---|
| Overall | ‐ | 45.6 (344/754) | 54.4 (410/754) | 9.6 (39/408) | ‐ |
| Sex | Female | 45.3 (196/433) | 54.7 (237/433) | 10.6 (25/236) | .406 |
| Male | 46.1 (148/321) | 53.9 (173/321) | 8.1 (14/172) | ||
| Age in years |
Cohort Median (Range) (Interquartile range) |
26.5 (18 to 86) (21 to 34) |
27 (18 to 86) (21 to 35) |
Re‐presented vs no 23 vs 27 (18 to 68) vs (18 to 86) (20 to 37) vs (21 to 34) |
.791 |
| Previous course of oral antibiotics | No | 48.3 (184/381) | 51.7 (197/381) | 8.1 (16/197) | .342 |
| Yes | 42.9 (160/373) | 57.1 (213/373) | 10.9 (23/211) | ||
| Examinations | None | 23.9 (16/67) | 76.1 (51/67) | 7.8 (4/51) | .679 |
| Yes (any) | 47.8 (324/678) | 52.2 (354/678) | 9.7 (34/352) | ||
| Oral examination only | 46.1 (297/644) | 53.9 (347/644) | 9.6 (33/345) | .694 | |
| FNE only | (0/0) | (0/0) | (0/0) | ‐ | |
| Oral examination & FNE | 79.4 (27/34) | 20.6 (7/34) | 14.3 (1/7) | .575 | |
| Swallowing fluids/medications prior to IV medications? | No | 60.1 (291/484) | 39.9 (193/484) | 8.8 (17/193) | .625 |
| Yes | 19.6 (53/270) | 80.4 (217/270) | 10.2 (22/215) | ||
| Swallowing fluids/medications after IV medications? | No | 97.6 (163/167) | 2.4 (4/167) | 0 (0/4) | .991 |
| Yes | 38.8 (171/441) | 61.2 (270/441) | 7.8 (21/269) | ||
| Statim IV medications | None | 7.6 (11/144) | 92.4 (133/144) | 13.5 (18/133) | .125 |
| Yes (any) | 54.6 (333/610) | 45.4 (277/610) | 7.6 (21/275) | ||
| IV antibiotics only | 60.0 (63/105) | 40.0 (42/105) | 12.7 (30/237) | .146 | |
| IV steroids only | 20.0 (2/10) | 80.0 (8/10) | 11.1 (1/9) | .986 | |
| IV antibiotics & steroids | 54.1 (268/495) | 45.9 (227/495) | 7.1 (3/42) | .126 | |
| Discharge medications | None | ‐ | (16) | 12.5 (2/16) | .652 |
| Yes (any) | ‐ | (374) | 9.1 (34/372) | ||
| Oral antibiotics only | ‐ | (350) | 8.6 (30/348) | .595 | |
| Oral steroids only | ‐ | (2) | 50.0 (1/2) | .225 | |
| Oral antibiotics & steroids | ‐ | (22) | 13.6 (3/22) | .919 |
TABLE 2.
Characteristics of PTA population, with proportions admitted/discharged and re‐presentation rates for ED discharges (with univariable regression analysis)
| Variable | Status | ED admissions % (n) | ED discharges % (n) |
Re‐presentation rates of ED discharges % (n) |
Univariable logistic regression p value |
|---|---|---|---|---|---|
| Overall | ‐ | 54.7 (226/413) | 45.3 (187/413) | 10.3 (19/184) | ‐ |
| Sex | Female | 58.5 (93/159) | 41.5 (66/159) | 6.2 (4/65) | 0.178 |
| Male | 52.4 (133/254) | 47.6 (121/254) | 12.5 (15/120) | ||
| Age in years | Cohort | ‐ | ‐ | Re‐presented vs no | |
| Median | 31 | 31 | 31 vs 32 | ||
| (Range) | (18 to 91) | (18 to 85) | (18 to 76) vs (18 to 85) | ||
| (Interquartile range) | (25 to 42.75) | (23 to 42.5) | (25 to 51.5) vs (23 to 42) | 0.407 | |
| Previous course of oral antibiotics | No | 55.8 (121/217) | 44.2 (96/217) | 9.6 (9/94) | 0.713 |
| Yes | 53.8 (105/195) | 46.2 (90/195) | 11.2 (10/89) | ||
| Examinations | None | 32.4 (11/34) | 67.6 (23/34) | 8.7 (2/23) | 0.770 |
| Yes (any) | 56.9 (214/376) | 43.1 (162/376) | 10.7 (17/159) | ||
| Oral examination only | 55.2 (186/337) | 44.8 (151/337) | 10.1 (15/148) | 0.830 | |
| FNE only | (0/0) | (0/0) | (0/0) | ‐ | |
| Oral examination & FNE | 71.8 (28/39) | 28.2 (11/39) | 18.2 (2/11) | 0.431 | |
|
Swallowing fluids/medications prior to IV medications? |
No | 63.2 (203/321) | 36.8 (118/321) | 11.2 (13/116) | 0.609 |
| Yes | 25.0 (23/92) | 75.0 (69/92) | 8.8 (6/68) | ||
|
Swallowing fluids/medications after IV medications? |
No | 89.8 (97/108) | 10.2 (11/108) | 20 (2/10) | 0.439 |
| Yes | 47.9 (127/265) | 52.1 (138/265) | 11.6 (16/138) | ||
| Statim IV medications | None | 7.5 (3/40) | 92.5 (37/40) | 2.9 (1/35) | 0.140 |
| Yes (any) | 59.8 (223/373) | 40.2 (150/373) | 12.1 (18/149) | ||
| IV antibiotics only | 66.7 (46/69) | 33.3 (23/69) | 4.3 (1/23) | 0.762 | |
| IV steroids only | 0 (0/3) | 100 (3/3) | 0 (0/3) | 0.992 | |
| IV antibiotics & steroids | 58.8 (177/301) | 41.2 (124/301) | 13.8 (17/123) | 0.105 | |
| Discharge medications | None | ‐ | (17) | 12.5 (2/16) | 0.785 |
| Yes (any) | ‐ | (167) | 10.3 (17/165) | ||
| Oral antibiotics only | ‐ | (155) | 1.4 (2/140) | 0.734 | |
| Oral steroids only | ‐ | (0) | (0/0) | ‐ | |
| Oral antibiotics & steroids | ‐ | (12) | 60.0 (15/25) | 0.756 | |
| Drainage | None | 54.3 (51/94) | 45.7 (43/94) | 9.5 (4/42) | 0.846 |
| Yes (any) | 54.9 (175/319) | 45.1 (144/319) | 10.6 (15/142) | ||
| I&D (any) | 61.2 (41/67) | 38.8 (26/67) | 7.7 (2/26) | 0.583 | |
| Needle aspiration (any) | 52.8 (130/246) | 47.2 (116/246) | 11.4 (13/114) | ||
| I&D, no pus | 75.0 (9/12) | 25.0 (3/12) | 0 (0/3) | 0.992 | |
| I&D, Pus | 58.2 (32/55) | 41.8 (23/55) | 8.7 (2/23) | 0.912 | |
| Needle aspiration, no pus | 50.9 (57/112) | 49.1 (55/112) | 16.4 (9/55) | 0.333 | |
| Needle aspiration, Pus | 54.5 (73/134) | 45.5 (61/134) | 6.8 (4/59) | 0.616 | |
| No pus (either method) | 53.2 (66/124) | 46.8 (58/124) | 15.5 (9/58) | 0.130 | |
| Pus (either method) | 55.6 (105/189) | 44.4 (84/189) | 7.3 (6/82) |
3.3. COVID‐19
Figure 1 shows the number of patients with suspected or confirmed COVID‐19, at the time of presentation and following testing, for the three audit periods, alongside the UK incidence of COVID‐19.
3.4. Acute management of ED patients
An oral examination was performed in 91.0% of tonsillitis patients and 91.7% of PTA patients (n = 678/745 and n = 376/410). Flexible nasendoscopy (FNE) was performed in 4.6% of tonsillitis patients and 9.5% of PTA patients (n = 34/745 and n = 39/410).
The rates of use of IV medications are shown in Tables 1 and 2. IV antibiotics were given in 79.6% of tonsillitis patients (n = 600/754) and 89.6% of PTA patients (n = 370/413). IV steroids were given in 67.0% of tonsillitis patients (n = 505/754) and 73.6% of PTA patients (n = 304/413).
Suspected PTA underwent attempted drainage in 77.2% of cases (n = 319/413). Needle aspiration was used in 78.6% of these cases (n = 246/313) and produced pus in 54.4% (n = 134/246). Incision and drainage were used in 21.4% (n = 67/313) and produced pus in 82.1% (n = 55/67). Using either method, pus was obtained in 60.4% (n = 189/313). The reasons for no attempt at drainage were given as follows: 63.8% following guidelines (n = 60); 14.9% patient choice (n = 14); 10.5% not trained (n = 10); 8.5% trismus (n = 8); and 2.1% suspected COVID‐19 (n = 2).
The administration of IV steroids and antibiotics increased the percentage of patients able to swallow from 35.8% to 72.5% for tonsillitis (n = 270/754 and 441/608) and from 22.3% to 71.0% for PTA (n = 92/413 and 265/373).
Telephone advice only was given by way of remote management in 6.6% of tonsillitis cases (n = 50/759) and 3.6% of PTA cases (n = 15/413).
For tonsillitis cases, when seen face to face (n = 709), the majority of patients were reviewed by pre‐specialty grade junior doctors (51.6%, n = 366) followed by specialty grade junior doctors (40.2%, n = 285), consultants (6.1%, n = 43), and nurse practitioners (2.1%, n = 15).
For PTA cases, when seen face to face (n = 398), the majority of patients were reviewed by pre‐specialty grade junior doctors (48.2%, n = 192) followed by specialty grade junior doctors (41.2%, n = 164), consultants (7.8%, n = 31) and nurse practitioners (2.8%, n = 11).
3.5. Admission to hospital from ED
Tables 1 and 2 show the discharge rates for tonsillitis and PTA patients reviewed by ENT after presenting to the ED. The overall discharge rate was 54.4% for tonsillitis (n = 410/754) and 45.3% for PTA (n = 187/413). These data are also visualised in Figure 2.
FIGURE 2.
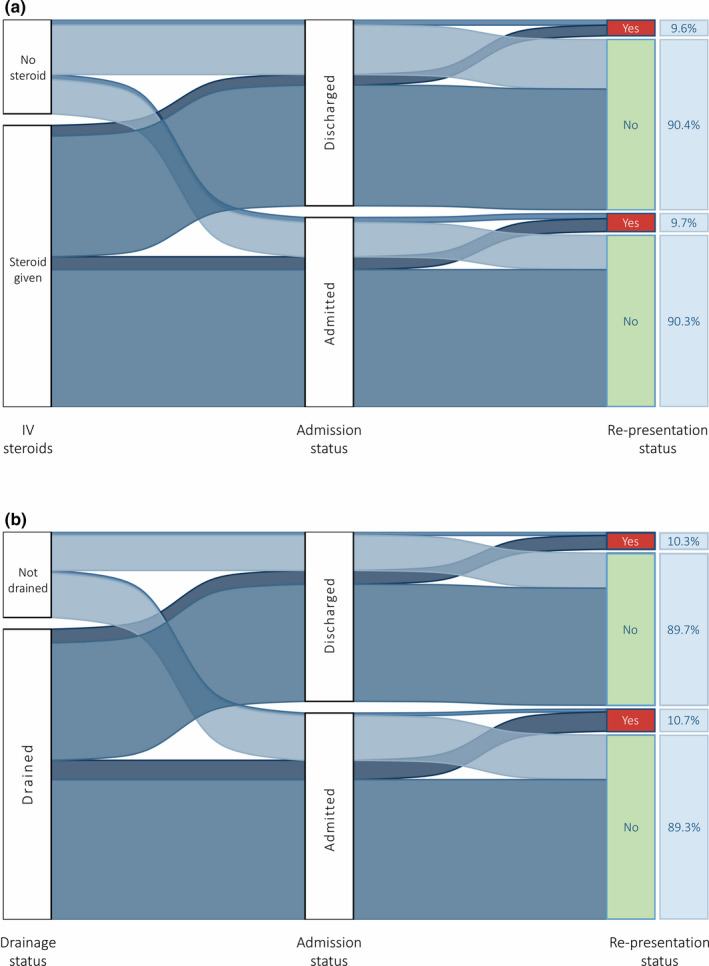
Sankey chart visualising proportions of patients being admitted or discharged from the ED, with rates of subsequent re‐presentation, stratified by A) IV steroid use for tonsillitis patients and B) drainage for PTA patients
Discharge rates from ED before and after statim IV medications were as follows: tonsillitis 17.6% (n = 133/754) and 44.6% (n = 277/754); PTA 9.0% (n = 37/413) and 39.9% (150/376), respectively.
Length of stay data were available for all eligible patients admitted to hospital. Patients staying ≥ 1 day, ≥2 days and ≥ 3 days were as follows: tonsillitis 61.9%, 38.1%, 14.2% (n = 213/344, 131/344 and 49/344) and PTA 61.9%, 38.1%, 12.4% (n = 140/226, 86/226 and 28/226).
3.6. Planned follow‐up for ED discharges
No follow‐up was arranged in 71.7% of tonsillitis patients (n = 294/410) with 5.9% having face‐to‐face (n = 81/410) and 19.8% having telephone appointments scheduled (n = 81/410) (9 reported as “other”, 2 directly listed for tonsillectomy).
No follow‐up was arranged in 50.3% of PTA patients (n = 94/187) with 8.6% having face‐to‐face (n = 16/187) and 38.5% having telephone appointments scheduled (n = 72/187) (5 reported as “other”).
3.7. Unscheduled re‐presentation within 10 days
Re‐presentation rates and outcomes from re‐presentation are shown in Table 3. Further detail for ED discharges, by patient and management factors, is shown in Tables 1 and 2. Figure 2 additionally shows re‐presentation rates for tonsillitis patients being discharged from the ED receiving no IV steroid (10.2%, n = 10/98) and any IV steroid (8.1%, n = 19/234), and tonsillitis patients admitted from the ED receiving no IV steroid (12.9%, n = 9/70) and any IV steroid (8.6%, n23/268).
TABLE 3.
Re‐presentation rates and outcomes from re‐presentation by condition and admission status
| Cohort | Re‐presentation rate% (n) | Drainage attempted after re‐presentation % (n) |
Admission rate after re‐presentation % (n) |
Admission rate after re‐presentation as proportion of initial presentation management % (n) |
|---|---|---|---|---|
| Tonsillitis admissions | 9.7 (33/341) | 24.2 (8/33) | 66.7 (22/33) | 6.5 (22/341) |
| Tonsillitis discharges | 9.6 (39/408) | 7.7 (3/38) | 50.0 (19/38) | 4.7 (19/408) |
| PTA admissions | 10.7 (24/224) | 70.8 (17/24) | 83.3 (20/24) | 8.9 (20/224) |
| PTA discharges | 10.3 (19/184) | 63.2 (12/19) | 31.6 (6/19) | 3.3 (6/184) |
Univariable logistic regression showed no significant predictors of re‐presentation within 10 days (Tables 1 and 2). No deaths were recorded.
4. DISCUSSION
COVID‐19 led to significant disruption of well‐established standards of care, but it is increasingly recognised that these changes may have unveiled positive developments in our management of certain conditions. This discussion focuses on lessons that can be learned from the collective national experience regarding the management of acute tonsillitis and PTA.
4.1. COVID‐19, tonsillitis and peritonsillar abscess
Sore throat and pharyngeal inflammation are recognised manifestations of COVID‐19, 10 but a consistent association with acute tonsillitis has not been described. Acute tonsillitis may have a viral or bacterial aetiology, with acute bacterial tonsillitis often preceded by a viral infection. 11 Coronaviruses are not one of the known viral pathogens frequently contributing to acute tonsillitis. 12 The only comparable pre–COVID‐19 presentation data comes from the UK Multicentre Audit of Quinsies (MAQ), 13 where the mean number of cases of PTA alone was 3.9 per centre per month (325 cases over 2 months in 42 centres). This compares to 2.0 PTA cases per centre per month during the COVID‐19 audit (416 cases over 905 weeks of data across 83 centres). Given seasonal variation has limited effect, 14 this apparent drop in presentations may be due to the reduced spread of common viral infection with COVID‐19 population measures, or a reduced willingness of patients to present to ED. 15
4.2. Changes to care during the COVID‐19 pandemic
Acute management of tonsillitis and PTA in ED aims to reduce the proportion of patients that require admission. This practice has been advocated for several years,16, 17 but COVID‐19 provided further pressure to clinicians to only admit cases where essential.
During the audit, clinicians discharged just over half of tonsillitis patients, and just under half of those with PTA. This represents a significant shift in practice, with historically high levels of admission for tonsillitis presenting to ENT in the UK,16, 18 and only 8% of PTA cases discharged from ED in 2014. 13
The use of IV antibiotics and steroids in ED was common, with the majority of both tonsillitis and PTA patients receiving both. The effect of this treatment, and any concurrent analgesia, was dramatic. This management approach doubled the proportion of patients able to swallow fluids in the subgroup later deemed suitable for discharge, with a large benefit also seen in those later admitted. This finding is in line with previous small studies that suggest IV steroids may reduce pain and trismus, facilitating earlier oral intake.19, 20, 21
Another COVID‐19‐provoked shift in practice has been a move away from PTA drainage (cf MAQ), with the aim of reducing clinician exposure to aerosol and droplets. Nearly, a quarter of patients with suspected PTA had no drainage performed, and this did not appear to influence the decision to admit, nor did the presence or absence of pus on incision or aspiration. There was also no difference in 10‐day re‐presentation rates between those drained or managed conservatively. Inevitably milder or borderline cases are more likely to have been managed conservatively in our dataset, however, a recent meta‐analysis also demonstrated no difference in outcomes between patients initially treated with conservative or surgical interventions for PTA. 22 This audit, combined with ongoing emphasis on reducing clinician exposure to oral secretions, should promote further exploration of the role of conservative management of PTA, and outcomes associated with not draining.
Some aspects of management did not change with the introduction of COVID‐19 guidelines. Avoidance of oral examination was recommended, yet 9 out of 10 patients still underwent an oral assessment. Furthermore, once patients were admitted, the length of stay for both tonsillitis and PTA was between 1 and 2 days, the same as previously found. 13
4.3. Drivers of clinical decision‐making
The ENTUK COVID‐19 guidelines suggested the decision to admit should be primarily based on the patient's ability to swallow fluids and medication, with the initial triage focusing on airway concern and sepsis, similar to other published criteria.16, 17, 18, 23 Unsurprisingly, the inability to swallow fluids and medications, especially after statim IV medications, led to admission in almost all cases. Physiological markers of sepsis were not recorded and so it is uncertain what role these played in the decision to admit.
4.4. Outcomes from outpatient care
While discharging patients from ED can benefit patients and the hospital, it must be balanced against the potential harm of deterioration or complications occurring within the community. 10‐day re‐presentation rates were similar to or below previously reported data,16, 17 and considerably lower than the 30‐day figure from MAQ. 13 For both tonsillitis and PTA cohorts, the readmission rate was low, and higher in the group initially admitted from ED. Even in those patients re‐presenting, many could continue to be managed as an outpatient after further review in ED. Enhanced management within ED and early discharge therefore appears safe.
4.5. Implications for clinical practice
The practice adopted across the UK during the COVID‐19 epidemic has reduced admissions, and to a lesser extent reduced possible clinician exposure to patient secretions. While the decision‐making process currently adopted appears safe, it is difficult from the data to fully characterise the optimal pathway or criteria for admission.
Looking for possible improvements in current management, in this audit, the 101 patients not examined did not demonstrate a higher re‐presentation rate. It could therefore be argued that the continued use of patient examination by an ENT clinician is not justified, given both the risk of COVID‐19 transmission, and the existence of a common management pathway for tonsillitis and PTA, with unclear benefit of PTA drainage.
Given the observational nature of this study, it is not possible to determine exactly which tonsillitis and PTA patients can be routinely managed as outpatients, but the current guidance appears to provide a sound basis for triage, probably incorporating a review of the presence of physiological markers of sepsis. It has, however, been shown that a greater proportion of patients with these conditions can be safely managed at home than has traditionally been the case.
4.6. Strengths and limitations of the study
This large prospective national study gives a comprehensive report of tonsillitis and PTA management and is uniquely placed to learn from the synchronous UK‐wide changes in practice brought about by the initial peak of the COVID‐19 pandemic. Despite disruption to routine care, extremely high levels of data completeness were seen.
To reduce the burden on data collection during a clinically challenging time, data were not collected on physiological parameters, laboratory/imaging results and past medical history. The classification of patients as tonsillitis or PTA was solely based on clinician judgement, and some PTA treated conservatively may have not re‐presented true abscesses. Re‐presentations to community health care will have been missed, although these are unlikely to represent severe complications. Re‐presentations beyond 10 days would also have been missed but this timeframe was chosen as a compromise between identifying the majority of instances of failed acute management and allowing rapid feedback to the ENT community via the interim reports. Finally, patient hospital‐avoidance behaviour at the height of the pandemic likely raised the threshold for re‐attending with milder complications.
5. CONCLUSION
Presentations with tonsillitis and PTA during the initial peak of the COVID‐19 pandemic were below previous rates. The management of these cases was notably different, with high rates of IV steroid use, avoidance of PTA drainage for some patients, and an overall shift towards outpatient care. This national study highlights that many patients who may previously have been admitted to hospital following their acute assessment and management, may be safely discharged from the ED.
6. AUTHORSHIP AND PARTICIPATION
6.1.
Citable collaborators Writing committee: John C Hardman, Chloe Swords, John P J Rocke, Abigail Walker, James E Bryan, Kristijonas Milinis, Rajeev G Mathew, Gareth H Jones, Oliver McLaren, Matthew E Smith* (*corresponding author).
Project management team (alphabetical): James E Bryan, John C Hardman, Kristian Hutson, Gareth H Jones, Rajeev G Mathew, Oliver McLaren, Kristijonas Milinis, John P J Rocke, Anna Slovick, Matthew E Smith, Chloe Swords, Abigail Walker.
Data processing: John C Hardman, James E Bryan, Kristijonas Milinis, Matthew E Smith.
Protocol reviewers: George McNally, Andrea Burgess.
Consultant leads and Site leads at contributing centres: Muhammad Shakeel, Anas Gomati (Aberdeen Royal Infirmary); Manohar Bance, James E Bryan (Addenbrooke's Hospital, Cambridge); Jeffrey Lancaster, Natalie Maple (Aintree University Hospital); Catherine Smyth, Colm Dorris (Altnagelvin Area Hospital); Andrew Kelly, David McCrory (Antrim Area Hospital); Yogesh M Bhatt, Guled M Jama (Barnet Hospital, London); Montio Morgan, Victoria Perkins (Basildon University Hospital); Paul Spraggs, Thomas Geyton (Basingstoke and North Hampshire Hospital); Yohanna Takwoingi, Srinish Gopala‐Krishnan (Birmingham City Hospital); David Strachan, Robert Taylor (Bradford Royal Infirmary (BRI)); Mark Puvanendran, Matthew Egan (Broomfield Hospital, Chelmsford); Catherine Rennie, Nicholas Cereceda‐Monteoliva (Charing Cross Hospital, London); Arun Cardozo, Antonia Tse (Chorley and South Ribble Hospital); Duncan McRae, Omar T Burgan (Colchester General Hospital); Ekambar Reddy, Brendan Wright (Craigavon Area Hospital); Naveed Kara, Holt Walters (Darlington Memorial Hospital); Richard Williams, Alex Walkden (Derriford Hospital, Plymouth); Muhammad Quraishi, Nicola Stobbs (Doncaster Royal Infirmary); Michail Chatzimichalis, Emily Elston (Dorset County Hospital, Dorchester); Sameer Khemani, Sean Fang (East Surrey Hospital, Redhill); Paul Kirkland, Rishi Vasanthan (Eastbourne District General Hospital); Mohammed Miah, Kristina Lee (Fairfield General Hospital, Greater Manchester); Claire Mclarnon, Mark R Williams (Freeman Hospital, Newcastle); Okechukwu Okonkwo, Zahir Mughal (Gloucestershire Royal Hospital); Yakubu Karagama, Carol Xie (Guy's Hospital); Mriganka De, Aakash Amlani (Heartlands Hospital, Birmingham); Patrick Jassar, Han Cao (Hull Royal Infirmary); Sachin Patil, Billy Wong (Ipswich Hospital); Carl Philpott, Sheneen Meghji (James Paget University Hospital, Great Yarmouth); Sudip Das, Simon Cole (Leicester Royal Infirmary); Ananth Vijendren, Munira Ally (Lister Hospital, Stevenage); Prasad Kothari, Eyal Schechter (Luton and Dunstable University Hospital); Baskaran Ranganathan, Rajeev Advani (Manchester Royal Infirmary (MRI)); Shamim Toma, Adam Haymes (Medway Maritime Hospital, Gillingham); Adam Shakir, Darren Yap (Milton Keynes University Hospital); Rhodri Costello, Jennifer Wallace (Morriston Hospital, Swansea); Edward Chisholm, Shilpa Ojha (Musgrove Park Hospital, Taunton); Patrick Spielmann, Richard Steven (Ninewells Hospital, Dundee); Mrinal Supriya, Elizabeth Mathew (Northampton General Hospital); Ajmal Masood, Samuel Dewhurst (Peterborough City Hospital); Victoria Ward, Ayman Darwich (Pinderfields Hospital, Wakefield); Shalini Patiar, Zsofia Nemeth (Princess Alexandra Hospital, Harlow); Roland Terry, Rohan Vithlani (Princess Royal University Hospital, Orpington); Duncan Bowyer, Ding Yang (Princess Royal, Telford); Peter Monksfield, Peter Corbett (Queen Elizabeth Hospital Birmingham); Azher Siddiq, Joshua D Whittaker (Queen's Hospital Burton); Yujay Ramakrishnan, Wai Sum Cho (Queen's Medical Centre, Nottingham (QMC)); Angus Cain, Bobby Mondal (Raigmore Hospital, Inverness); Steve Izzat, Amru Ainine (Royal Albert Edward Infirmary, Wigan); Dilip Nair, Shawn Tan (Royal Berkshire Hospital, Reading); Anu Daudia, Jennifer Gilchrist (Royal Blackburn Hospital); Neil Tan, Min Kim (Royal Cornwall Hospital, Truro); Vijay Singh, Emma Hallett (Royal Glamorgan Hospital, South Wales); Jaydip Ray, Beverley Yu (Royal Hallamshire Hospital, Sheffield); John DeCarpentier, Bhargavi Chandrasekar (Royal Preston Hospital); Sanjiv Bhimrao, Michael Eastwood (Royal Stoke University Hospital); Vishnu S Sunkaraneni, Chang Woo Lee (Royal Surrey County Hospital, Guildford); Andrew Moore, Prajwal Shetty (Royal Sussex County Hospital, Brighton); Thomas Mawby, Elspeth Bisson (Royal United Hospital, Bath); Mudit Jindal, Alexander Yao (Russells Hall Hospital, Dudley); Marcel Geyer, Omnya Mohammed (Salisbury District Hospital); Huw Jones, Aria Amir Ghasemi (Southampton General Hospital); Aaron Trinidade, Alistair Hardy (Southend University Hospital); Sarah Little, Tiffany Munroe‐Gray (St George's Hospital, London); Alex Bennett, Lucy Li (St John's Hospital, Livingston); Mamoona Khalid‐Raja, George McNally (Stepping Hill Hospital, Greater Manchester); George Thomas, Mohamed Elmorsy (The James Cook University Hospital, Middlesbrough); Clare Williams, Matthew Zammit (The Royal Liverpool University Hospital); Kay Seymour, Elinor Warner (The Royal London Hospital); Chris Potter, Rachel Easto (Torbay Hospital); Azhar Shaida, Mohamed Elshahhat (University College London Hospital); Dheeraj Karamchandani, Charn Gill (University Hospital Coventry and Warwickshire (UHCW)); Irfan Syed, Abigail Walker (University Hospital Lewisham, London); David Walker, Kirsten Stewart (Victoria Hospital, Fife); Mark Simmons, Ahmad K. Abou‐Foul (Walsall Manor Hospital); Srinivasalu Bathala, Hannah Emerson (Warrington Hospital); John Almeyda, Agamemnon Pericleous (West Middlesex University Hospital, London); Fahmy Fahmy, Anna I Kaleva (West Suffolk Hospital, Bury St Edmunds); Ram Moorthy, James Bates (Wexham Park Hospital, Slough); Joseph Wasson, Anya Selwyn (William Harvey Hospital, Ashford); Charles Daultrey, Sanjay Patel (Worcestershire Royal Hospital); Derrick Siau, Rupali Sawant (Wythenshawe Hospital, Greater Manchester); Phillip Moore, Senthil Kumar Rajamanickan (Ysbyty Gwynedd Hospital, Bangor).
Supporting information
Supplementary Material
Supplementary Material
Supplementary Material
ACKNOWLEDGEMENTS
The following individuals are acknowledged for their contributions to local data collection: Aakash Amani, Abdelrahman Elsebiey, Abdullah Al‐Atrakchi, Adnan Kamath, Afnan Ahmed, Ahmad Saadya, Alison Liu, Amr Allam, Andrew Taggart, Angela McGreevy, Anna Hong, Antonia Tse, Ashleigh Ivy, Ayoub Goma, Bakir Al‐Dulaimy, Bhargavi Chandrasekar, Caroline Anderson, Chloe Swords, Cillian T Forde, Colin Leonard, Colm Doris, Conor McKenna, Damilola Longe, David Du, Eleanor Harrison, Elizabeth Casselden, Elliot Heward, Emily Lowe, Esmee Irvine, Eugene Omakobia, Faiza Ali, Fenella Shelton, Fergus Cooper, Frederik Priskorn, Georgina Sargent, Gillian McMeechan, Halina Viarheichyk, Irina Garlea‐Robu, Jameel Muzaffar, Jamie Patel, Jemma Butler, Jonathan Bird, Jonathan Dalton, Joseph Doyle, Katherine Velamail, Kenneth Lai, Liang Kim, Lilian Sangobowale, Louise Evans, Madeline Leadon, Marissa Cheah, Martin Guichard‐Wheatley, Matthew Williams, Michael Robinson, Muralidhar Lalam, Nicola Wooles, Nilesh Vakharia, Nirijhar Chanda, Patrick Hanna, Peter Gaskell, Ravine Gill, Robin Gurung, Ryan Johnston, Sara Mahmood, Sergiy Nevgod, Shawn Tan, Shivun Khosla, Shradda Jain, Shubhendu Kulshrestha, Simran Chopra, Sinead Philip, Tharsika Myuran, Thomas Haigh, Thomas Heycock, Wan Fang Woon, Waqas Jamil, Yadsan Devabalan, Yi Hang Tan, Zuhaib Khan.
INTEGRATE (The UK ENT Trainee Research Network) . Admission avoidance in tonsillitis and peritonsillar abscess: A prospective national audit during the initial peak of the COVID‐19 pandemic. Clin Otolaryngol. 2021;46:363–372. 10.1111/coa.13680
Contributor Information
INTEGRATE (The UK ENT Trainee Research Network):
John C Hardman, Chloe Swords, John P J Rocke, Abigail Walker, James E Bryan, Kristijonas Milinis, Rajeev G Mathew, Gareth H Jones, Oliver McLaren, Matthew E Smith, James E Bryan, John C Hardman, Kristian Hutson, Gareth H Jones, Rajeev G Mathew, Oliver McLaren, Kristijonas Milinis, John P J Rocke, Anna Slovick, Matthew E Smith, Chloe Swords, Abigail Walker, John C Hardman, James E Bryan, Kristijonas Milinis, Matthew E Smith, George McNally, Andrea Burgess, Muhammad Shakeel, Anas Gomati, Manohar Bance, James E Bryan, Jeffrey Lancaster, Natalie Maple, Catherine Smyth, Colm Dorris, Andrew Kelly, David McCrory, Yogesh MBhatt, Guled M Jama, Montio Morgan, Victoria Perkins, Paul Spraggs, Thomas Geyton, Yohanna Takwoingi, Srinish Gopala‐Krishnan, David Strachan, Robert Taylor, Mark Puvanendran, Matthew Egan, Catherine Rennie, Nicholas Cereceda‐Monteoliva, Arun Cardozo, Antonia Tse, Duncan McRae, Omar T Burgan, Ekambar Reddy, Brendan Wright, Naveed Kara, Holt Walters, Richard Williams, Alex Walkden, Muhammad Quraishi, Nicola Stobbs, Michail Chatzimichalis, Emily Elston, Sameer Khemani, Sean Fang, Paul Kirkland, Rishi Vasanthan, Mohammed Miah, Kristina Lee, Claire Mclarnon, Mark R Williams, Okechukwu Okonkwo, Zahir Mughal, Yakubu Karagama, Carol Xie, Mriganka De, Aakash Amlani, Patrick Jassar, Han Cao, Sachin Patil, Billy Wong, Carl Philpott, Sheneen Meghji, Sudip Das, Simon Cole, Ananth Vijendren, Munira Ally, Prasad Kothari, Eyal Schechter, Baskaran Ranganathan, Rajeev Advani, Shamim Toma, Adam Haymes, Adam Shakir, Darren Yap, Rhodri Costello, Jennifer Wallace, Edward Chisholm, Shilpa Ojha, Patrick Spielmann, Richard Steven, Mrinal Supriya, Elizabeth Mathew, Ajmal Masood, Samuel Dewhurst, Victoria Ward, Ayman Darwich, Shalini Patiar, Zsofia Nemeth, Roland Terry, Rohan Vithlani, Duncan Bowyer, Ding Yang, Peter Monksfield, Peter Corbett, Azher Siddiq, Joshua D Whittaker, Yujay Ramakrishnan, Wai Sum Cho, Angus Cain, Bobby Mondal, Steve Izzat, Amru Ainine, Dilip Nair, Shawn Tan, Anu Daudia, Jennifer Gilchrist, Neil Tan, Min Kim, Truro, Vijay Singh, Emma Hallett, Jaydip Ray, Beverley Yu, John DeCarpentier, Bhargavi Chandrasekar, Sanjiv Bhimrao, Michael Eastwood, Vishnu S Sunkaraneni, Chang Woo Lee, Andrew Moore, Prajwal Shetty, Thomas Mawby, Elspeth Bisson, Mudit Jindal, Alexander Yao, Marcel Geyer, Omnya Mohammed, Huw Jones, Aria Amir Ghasemi, Aaron Trinidade, Alistair Hardy, Sarah Little, Tiffany Munroe‐Gray, Alex Bennett, Lucy Li, Mamoona Khalid‐Raja, George McNally, George Thomas, Mohamed Elmorsy, Clare Williams, Matthew Zammit, Kay Seymour, Elinor Warner, Chris Potter, Rachel Easto, Azhar Shaida, Mohamed Elshahhat, Dheeraj Karamchandani, Charn Gill, Irfan Syed, Abigail Walker, David Walker, Kirsten Stewart, Mark Simmons, Ahmad K Abou‐Foul, Srinivasalu Bathala, Hannah Emerson, John Almeyda, Agamemnon Pericleous, Fahmy Fahmy, AnnaI Kaleva, Ram Moorthy, James Bates, Joseph Wasson, Anya Selwyn, Charles Daultrey, Sanjay Patel, Derrick Siau, Rupali Sawant, Phillip Moore, and Senthil Kumar Rajamanickan
DATA AVAILABILITY STATEMENT
Research data are not shared.
REFERENCES
- 1. NHS Digital . NHS hospital episode statistics in England and Wales. 2017.
- 2. Harcourt J, Tamin A, Lu X, et al. Severe acute respiratory syndrome coronavirus 2 from patient with coronavirus disease, United States. Emerging Infect. Dis. 2020;26:1266‐1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thamboo A, Lea J, Sommer DD, et al. Clinical evidence based review and recommendations of aerosol generating medical procedures in otolaryngology ‐ head and neck surgery during the COVID‐19 pandemic. J Otolaryngol Head Neck Surg. 2020;49:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Workman AD, Welling DB, Carter BS, et al. Endonasal instrumentation and aerosolization risk in the era of COVID‐19: simulation, literature review, and proposed mitigation strategies. Int Forum Allergy Rhinol. 2020;10:798‐805. [DOI] [PubMed] [Google Scholar]
- 5. To KK‐W, Tsang OT‐Y, Yip CC‐Y, et al. Consistent detection of 2019 novel coronavirus in saliva. Clin Infect Dis. 2020;71:841‐843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Curran J, Calder N, Yaneza M, et al. Reducing potential aerosol generation in flexible nasolaryngoscopy: a novel method. J Laryngol Otol. 2020. ePub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McNally G, Burgess A, Agrawal S, et al.ENT UK COVID‐19 adult tonsillitis & quinsy guidelines. 2020.
- 8. von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344‐349. [DOI] [PubMed] [Google Scholar]
- 9. INTEGRATE (The UK ENT research trainee network) . Admission avoidance in acute epistaxis: a prospective national audit during the initial peak of the COVID‐19 pandemic. Clin Otolaryngol. 2020. Submitted. [DOI] [PubMed] [Google Scholar]
- 10. El‐Anwar MW, Elzayat S, Fouad YA. ENT manifestation in COVID‐19 patients. Auris Nasus Larynx. 2020. ePub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Proenca‐Modena JL, Pereira Valera FC, Jacob MG, et al. High rates of detection of respiratory viruses in tonsillar tissues from children with chronic adenotonsillar disease. PLoS One. 2012;7:e42136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sidell D, Shapiro NL. Acute tonsillitis. Infect Disord Drug Targets. 2012;12:271‐276. [DOI] [PubMed] [Google Scholar]
- 13. ENT Trainee Research Collaborative – West Midlands . National prospective cohort study of peritonsillar abscess management and outcomes: the Multicentre Audit of Quinsies study. J Laryngol Otol. 2016;130:768‐776. [DOI] [PubMed] [Google Scholar]
- 14. Klug TE. Incidence and microbiology of peritonsillar abscess: the influence of season, age, and gender. Eur J Clin Microbiol Infect Dis. 2014;33:1163‐1167. [DOI] [PubMed] [Google Scholar]
- 15. Hartnett KP, Kite‐Powell A, DeVies J, et al. Impact of the COVID‐19 pandemic on emergency department visits ‐ United States, January 1, 2019‐May 30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:699‐704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bird JH, Biggs TC, King EV. Controversies in the management of acute tonsillitis: an evidence‐based review. Clin Otolaryngol. 2014;39:368‐374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ashman A, Harris R. Outpatient management of patients with acute tonsillitis and peritonsillar abscess in ninety adult patients. Clin Otolaryngol. 2017;42:720‐723. [DOI] [PubMed] [Google Scholar]
- 18. Mohammed H, Jin X, Masterson L, et al. Ambulant management of acute tonsillitis in adult patients, a study on 330 patients. Clin Otolaryngol. 2017;42:897‐901. [DOI] [PubMed] [Google Scholar]
- 19. Hardman JC, McCulloch NA, Nankivell P. Do corticosteroids improve outcomes in peritonsillar abscess? Laryngoscope. 2015;125:537‐538. [DOI] [PubMed] [Google Scholar]
- 20. Ozbek C, Aygenc E, Tuna EU, et al. Use of steroids in the treatment of peritonsillar abscess. J Laryngol Otol. 2004;118:439‐442. [DOI] [PubMed] [Google Scholar]
- 21. Chau JKM, Seikaly HR, Harris JR, et al. Corticosteroids in peritonsillar abscess treatment: a blinded placebo‐controlled clinical trial: dexamethasone in PTA. Laryngoscope. 2014;124:97‐103. [DOI] [PubMed] [Google Scholar]
- 22. Forner D, Curry DE, Hancock K, et al. Medical intervention alone vs surgical drainage for treatment of peritonsillar abscess: a systematic review and meta‐analysis. Otolaryngol Head Neck Surg. 2020; ePub ahead of print. [DOI] [PubMed] [Google Scholar]
- 23. National Institute for Health and Clinical Excellence . Clinical knowledge summary: sore throat ‐ acute. 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Supplementary Material
Supplementary Material
Data Availability Statement
Research data are not shared.


