Abstract
In the literature, many cases of coronavirus disease 2019 (COVID‐19) positive pregnancies have been observed, mostly with mild findings, but there is limited evidence about perinatal transition and early COVID‐19 positive newborns. In this case, severe acute respiratory syndrome coronavirus 2 reverse transcription–polymerase chain reaction results were studied from samples obtained from the placenta, amniotic fluid, cord blood and postoperative breast milk – that were obtained while avoiding contamination and preserved appropriately – of a cesarean section performed under anesthesia on a woman with previous cesarean section and gestational diabetes mellitus history. This patient who presented to our emergency gynecology clinic with membrane rupture was infected with severe acute respiratory syndrome coronavirus 2 two weeks before delivery but was not treated as the disease was asymptomatic. In addition, literature data in line with this topic were evaluated to demonstrate that there was generally no perinatal transmission over 34 weeks of gestation.
Keywords: coronavirus disease 2019, pregnancy, reverse transcription–polymerase chain reaction, severe acute respiratory syndrome coronavirus 2, vertical transmission
Introduction
In this case report, we evaluate the potential of perinatal transmission from mother to fetus and safety of post‐partum breastfeeding in a mother who had coronavirus disease 2019 (COVID‐19) pneumonia and was diagnosed by computed tomography (CT) findings despite a negative nasopharyngeal swab sample taken at the late stage of the disease. This study aimed to show that there is no perinatal severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) transmission in the third trimester especially over 34 weeks of gestation.
Case Report
On April 21, 2020, all members of a family with definite COVID‐19 contact history and sharing the same house with a patient (husband had COVID‐19 symptoms) presented to adult and child emergency clinics and SARS‐CoV‐2 reverse transcription–polymerase chain reaction (RT–PCR) samples were taken. The family was notified over phone that nasopharyngeal swab results were positive in all family members (wife, 1.5‐year‐old child and mother), but the results of the patient were not available at the time. During this time, the husband and mother with advancing symptoms received inpatient treatment for 5 and 12 days, respectively. Patient anamnesis revealed that she had no symptoms since then, did not use mask in the same house with her 1.5‐year‐old child, did not follow isolation rules and stayed in quarantine under these conditions. Fourteen days later, on May 4, 2020, she was admitted to Umraniye Training and Research Hospital Emergency Obstetrics Clinic with complaints of leaking vaginal fluid. The patient was 42 years old, G2P1 37 weeks in vitro fertilization pregnant, had diet‐regulated gestational diabetes mellitus (GDM) and had a previous cesarean section history. Preoperative nasopharyngeal swab was taken again from the patient without further complaints. Delivery was carried out by cesarean section under spinal anesthesia in accordance with all procedures and with protective equipment. A baby girl weighing 3580 g, with 1 min and 5 min APGAR score of 8/10 was delivered. Perioperative intra‐abdominal acid was not monitored, RT–PCR samples were taken from the clear amniotic fluid, cord blood and placenta under appropriate conditions while avoiding contamination (Fig. 1). These samples were preserved at −20°C until the breast milk sample was obtained. After the breast milk sample was obtained from the patient, all the samples were delivered to the COVID‐19 GLAB Diagnostic Center of Health Institutes of Turkey. As the test results submitted 14 days ago were not available, postoperative thoracic noncontrast CT was performed until the second set of results became available and diffuse peripheral and peribronchovascular ground‐glass appearance in nodular form was observed in both the lungs (Fig. 2). The result was interpreted as a thoracic noncontrast CT with typical findings in terms of COVID‐19 and was evaluated in favor of moderate–severe parenchymal involvement. Results of the first RT–PCR sample could not be accessed due to a software error in the public health management system and the second RT–PCR result performed 2 weeks later was negative. It was learned that during this 14‐day period, the pregnant woman had contact with her COVID‐19 positive, untreated 1.5‐year‐old son without following isolation rules owing to lack of symptoms. Upon moderate–severe involvement in thoracic CT, the mother was isolated, and cefazolin, enoxaparin sodium, azithromycin and hydroxychloroquine treatment was initiated. Swab sample was taken from the newborn and the mother, and the baby was given to the mother for breastfeeding while the mother was wearing a mask. The baby was admitted to the intensive care unit after developing feeding intolerance and vomiting 6 h after being breastfed. Meanwhile, favipiravir was added to the treatment of the mother (no chronic disease, no risk factor other than GDM, asymptomatic and had stable vital signs) due to a sudden decrease in oxygen saturation level to 90 mmHg on postoperative day 1 and monitoring of moderate–severe involvement in thoracic CT compliant with COVID‐19 radiological findings. On postoperative day 3, oxygen saturation reached normal levels. SARS‐CoV‐2 nucleic acid tests that were studied using the naso‐oropharyngeal swab samples taken twice from the mother and the baby during their hospitalization were negative. The baby was followed up for 1 week at the neonatal intensive care unit, and its general condition improved without starting any treatment. The baby and its mother were discharged 1 week after its birth. Patient consent was obtained for all the results of the mother and baby to be used in this case report. CFX96 Touch Real‐Time PCR Detection System was used to determine RT–PCR results. Our scientific research application No. 2020‐05‐10T08_41_14 was approved by the Ministry of Health of the Republic of Turkey.
Figure 1.
Severe acute respiratory syndrome coronavirus 2 reverse transcription–polymerase chain reaction was negative in the breast milk, cord blood and amniotic fluid samples, respectively.
Figure 2.
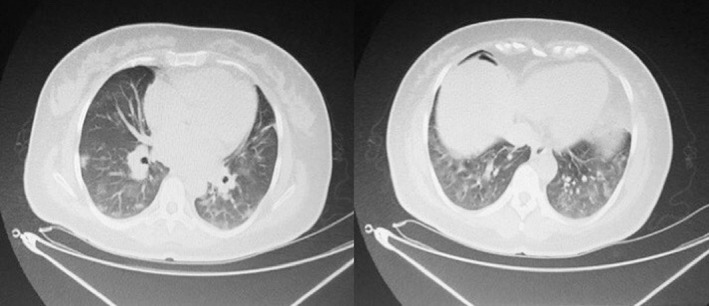
Chest computed tomography scans of asymptomatic patient.
Discussion
Pregnant women are susceptible to intracellular organisms such as viruses, especially respiratory pathogens and those that cause severe infections, due to changes in their cellular immunity. Maternal pneumonia is associated with various negative obstetric outcomes such as early membrane rupture, preterm action, intrauterine fetal distress and neonatal death. 1 In our case, wherein the procedures of sampling and delivery to the laboratory were fulfilled in accordance with WHO guidance, 2 it was shown that perinatal transmission did not occur at 37th gestational week in a mother who had asymptomatic COVID‐19 and therefore was not treated. Another study reported that SARS‐CoV‐2 RT–PCR was negative in the amniotic fluid, cord blood, breast milk and throat swab taken from 46 newborns born from COVID‐19 positive mothers, confirming our case. 3 In our case, negative RT–PCR results obtained from the naso‐oropharyngeal swab taken twice from the baby, placenta, amniotic fluid and breast milk suggested that there is no evidence of direct vertical transmission of SARS‐CoV‐2 infection in the third trimester. In our case, post‐partum low saturation occurred with COVID‐19‐compliant thoracic CT findings without pre‐partum symptoms related to SARS‐CoV‐2 infection. Lymphopenia is one of the common features of COVID‐19 pneumonia, and some researchers believe that lymphopenia is closely related to the severity of the disease or mortality. 4 Lymphopenia was not observed in our case (Table 1). It is also known that, in addition to SARS‐CoV‐2 infection, an increase in alanine aminotransferase or aspartate aminotransferase can be one of the clinical symptoms of many obstetric diseases in pregnant women. In our case, transaminases were within normal limits, suggesting that the increase in transaminase was not specific. Conversely, in a study published by Chen et al., thoracic CT showed findings of typical viral pneumonia, and the authors concluded that thoracic CT has a significant value in clinical diagnosis because of its high sensitivity and specificity. 3 In suspected and confirmed cases, thoracic CT used to support diagnosis and determine the prevalence of infection showing bilateral, peripherally localized, ground‐glass opacities in the lungs of patients developing COVID‐19 pneumonia is typical. 5 Diagnostic criteria of COVID‐19 infection include: (i) Typical thoracic CT imaging with more pronounced patchy opacities in peripheral zones of the lung, ground‐glass appearance, consolidation and infiltration; and (ii) SARS‐CoV‐2 positivity in RT–PCR tests. Negative RT–PCR test results could be due to various factors in infected persons. Furthermore, studies showing that there may be false negative RT–PCR results for COVID‐19 infection cases due to low‐negative viral titer and improper sampling and swab sites in the late stage of the disease support the diagnosis in our case. 3 The sensitivity and specificity rates of RT–PCR test that was used in Turkey still unknown. In a study in which 1014 patients were analyzed, while the PCR test makes the diagnosis in 59% of cases, specific CT findings were positive in 88% of the cases. 6 In a study conducted in Turkey, the sensitivity of CT for COVID‐19 pneumonia is 97% shown. 7 To date, there have been a few studies in the literature showing vertical transmission. In the first study, Zamaniyan et al. demonstrated that swab samples taken from a newborn of a COVID‐19 positive 32‐week mother were negative, but the amniotic fluid was positive for SARS‐CoV‐2 RT–PCR. 8 This suggested that infants born under 34 weeks may have increased fetal exposure due to lack of surfactants. In the second study, no vertical transmission was detected in the third trimester. 9 The other, in a study conducted in Pope Giovanni 13 Hospital, Bergamo, Italy, one of the babies born from SARS‐CoV‐2 RT–PCR positive mothers was delivered by one normal and the other by cesarean delivery. It was reported that swabs taken from newborns were negative, but nasopharyngeal swab results obtained after 7 days from both babies were positive. 10 Although it is mentioned that there is no history of contact with their mothers for both babies, this suggests that adequate protective equipment and contact was not paid attention. The baby was asymptomatic and nasopharyngeal swab results were negative, suggesting that the fetus under 34 weeks acquired the disease through the intrauterine route. In a meta‐analysis including 1271 pregnant women infected with SARS‐CoV‐2 and almost half of whom were in the third trimester, no vertical transmission was reported to any infant. 11 In our case, even if the swabs were taken postoperatively, the elevation of interleukin‐6 over C‐reactive protein, white blood cells, lymphocytes, neutrophils and procalcitonin is noteworthy. Breastfeeding is not contraindicated according to several current guidelines published internationally. In a study, it was suggested that SARS‐COV‐2 viral load was not detected in breast milk; further, benefits of breastfeeding were emphasized and it was suggested that it should not be regarded as a means of transmission. 1 In contrast, Chinese pediatricians recommend that babies born from COVID‐19 positive mother should be fed by donor mothers or fed formula. 12 In this case, we also show that there is no transmission from mother's breast milk. In our case, we only had late lung involvement due to the lack of early SARS‐CoV‐2 RT–PCR results in the period when COVID‐19 lung involvement started. Due to the absence of IgM, IgG kit throughout Turkey, we were unable to prove that the patient with negative RT–PCR results had indeed had the disease. Due to the low sensitivity of SARS‐CoV‐2 to IgM and IgG, even if we had obtained the kit, it could have only been used to support the diagnosis. Diagnosis could not be finalized in our case because of various reasons such as inadequate and inappropriate samples, obtaining the samples in the wrong period of infection, uncertainty over whether they were treated appropriately, the conditions during transport to the laboratory and miscellaneous technical reasons related to the test. Thus, this case shows that CT is highly sensitive and specific in establishing the diagnosis of COVID‐19. In the currently reported cases of COVID‐19 and pregnancy, it was observed that cases with SARS‐CoV‐2 RT–PCR positivity were treated in the hospital during the third trimester after the symptoms started and delivery was performed after the treatment was finalized. However, in our case, the patient showed no symptoms despite close contact with COVID‐19‐diagnosed family members and therefore did not receive treatment because of the absence of results in the third trimester, and she developed post‐partum symptoms 2 weeks after contact. The diagnosis of the patient was confirmed with thoracic CT due to the lack of serological (IgM, IgG) test kits in our country. In conclusion, the case presented in this report is one of the rare cases where cord blood, amniotic fluid, placenta and breast milk samples were obtained and studied together. In our case, the infant had no clinical, radiological, hematological or biochemical evidence of SARS‐CoV‐2, indicating that SARS‐CoV‐2 does not cause perinatal complications in the third trimester and especially after 34 weeks of gestation, and there is no vertical transmission. Further studies should be conducted in the first and second trimesters to find out possible perinatal transmission.
Table 1.
Patient's laboratory test results
| Preoperative results | Reference range | Postoperative results (Day 1) | |
|---|---|---|---|
| White‐cell count | 7.45 per mm3 | 4.00–10.00 | 8.2 per mm3 |
| Neutrophil | 4.6 per mm3 | 2.00–7.00 | 5.5 per mm3 |
| Lymphocyte | 2.1 per mm3 | 0.80–4.00 | 1.9 per mm3 |
| C‐reactive protein | 0.2 mg/dL | 0.0–0.5 | 2.8 mg/dL |
| Procalcitonin | <0.005 ng/mL | <0.05 | <0.005 ng/mL |
| Interleukin‐6 | x | 0.0–5.9 | 35.2 pg/mL |
| D‐Dimer | x | 0–500 | 2330 ng/mL |
| Ferritin | 134 ng/mL | 5,0–204 | 131 ng/mL |
| Potassium | 3.7 mEq/L | 3.5–5.1 | 4.0 mEq/L |
| Magnesium | 1.65 mg/dL | 1.6–2.6 | 1.62 mg/dL |
| Hemoglobin | 12.7 g/dL | 11.0–15.0 | 11 g/dL |
| Platelet | 215 per mm3 | 100–400 | 193 per mm3 |
| Glucose | 121 mg/dL | 70–105 | 75 mg/dL |
| Alanine aminotransaminase | 30 U/L | 0,0–55 | 35 U/L |
| Aspartate transaminase | 43 U/L | 5,0–34 | 47 U/L |
| Lactate dehydrogenase | 312 U/L | 125–220 | 300 U/L |
| Spot urine protein/Creatinine ratio | 0,35 mg/dL | 0.0–0.3 | x |
Disclosure
None declared.
References
- 1. Schwartz DA, Graham AL. Potential maternal and infant outcomes from (Wuhan) coronavirus 2019‐nCoV infecting pregnant women: Lessons from SARS, MERS, and other human coronavirus infections. Viruses 2020; 12: 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. WHO . Laboratory testing for 2019 novel coronavirus (2019‐nCoV) in suspected human cases. Interim guidance. 17 Jan 2020. [Cited 4 Feb 2020.] Available from URL: https://www.who.int/publications‐detail/laboratory‐testing‐for‐2019‐novel‐coronavirus‐in‐suspected‐human‐cases‐20200117
- 3. Chen H, Guo J, Wang C et al. Clinical characteristics and intrauterine vertical transmission potential of COVID‐19 infection in nine pregnant women: A retrospective review of medical records. Lancet 2020; 395: 809–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fuk‐Woo Chan J, Yuan S, Kok KH et al. A cluster of familial pneumonia associated with new coronavirus 2019 showing person‐to‐person transmission: Study of a family cluster. Lancet 2020; 395: 514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shi H, Han X, Jiang N et al. Radiological findings from 81 patients with COVID‐19 pneumonia in Wuhan, China: A descriptive study. Lancet Infect Dis 2020; 20: 425–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ai T, Yang Z, Hou H et al. Correlation of chest CT and RT‐PCR testing in coronavirus disease 2019 (COVID‐19) in China: A report of 1014 cases. Radiology 2020; 296: E32–E40. 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Erkan F, Ertürk MŞ. COVID‐19'da akciğerde klinik ve radyolojik bulgular. In: Önal AE (ed). Halk Sağlığı ve COVID‐19. 1. Baskı. Ankara: Türkiye Klinikleri, 2020; 26–34. [Google Scholar]
- 8. Zamaniyan M, Ebadi A, Aghajanpoor Mir S, Rahmani Z, Haghshenas M, Azizi S. Preterm delivery in pregnant woman with critical COVID‐19 pneumonia and vertical transmission. Prenat Diagn 2020. 10.1002/pd.5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lv Y, Gu B, Chen Y et al. No intrauterine vertical transmission in pregnancy with COVID‐19: A case report. J Infect Chemother 2020; 26: 1313–1315. 10.1016/j.jiac.2020.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Patanè L, Morotti D, Giunta MR et al. Vertical transmission of COVID‐19: SARS‐CoV‐2 RNA on the fetal side of the placenta in pregnancies with COVID‐19 positive mothers and neonates at birth. Am J Obstet Gynecol MFM 2020; 2: 100145. 10.1016/j.ajogmf.2020.100145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Diriba K, Awulachew E, Getu E. The effect of coronavirus infection (SARS‐CoV‐2, MERS‐CoV, and SARS‐CoV) during pregnancy and the possibility of vertical maternal–fetal transmission: A systematic review and meta‐analysis. Eur J Med Res 2020; 25: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang L, Shi Y, Xiao T et al. Chinese expert consensus on the perinatal and neonatal management for the prevention and control of the 2019 novel coronavirus infection. Ann Transl Med 2020; 8: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]


