Abstract
Background and Aim
This review investigates the role of gastrointestinal and hepatic manifestations in COVID‐19, particularly with regard to the prevalence of isolated gastrointestinal (GI) symptoms.
Methods
We searched PubMed, Embase, and Cochrane library for COVID‐19 publications from 1 December 2019 to 18 May 2020. We included any study that reported the presence of GI symptoms in a sample of >5 COVID‐19 patients. Data collection and risk of bias assessment were performed independently by two reviewers. Where ≥3 studies reported data sufficiently similar to allow calculation of a pooled prevalence, we performed random effects meta‐analysis.
Results
This review included 17 776 COVID‐19 patients from 108 studies. Isolated GI symptoms only occurred in 1% (95% confidence interval [CI] 0–6%) of patients. GI symptoms were reported in 20% (95% CI 15–24%) of patients. The most common were anorexia (21%, 95% CI 15–27%), diarrhea (13%, 95% CI 11–16%), nausea or vomiting (8%, 95% CI 6–11%), and abdominal pain (4%, 95% CI 2–6%). Transaminase elevations were present in 24% (95% CI 17–31%) of patients. Higher prevalence of GI symptoms were reported in studies published after 1st April, with prevalence of diarrhea 16% (95% CI 13–20), nausea or vomiting 12% (95% CI 8–16%), and any GI symptoms 24% (95% CI 18–34%). GI symptoms were associated with severe COVID‐19 disease (odds ratio [OR] 2.1, 95% CI 1.3–3.2), but not mortality (OR 0.90, 95% CI 0.52–1.54).
Conclusions
Patients with isolated GI symptoms may represent a small but significant portion of COVID‐19 cases. When testing resources are abundant, clinicians should still consider testing patients with isolated GI symptoms or unexplained transaminase elevations for COVID‐19. More recent studies estimate higher overall GI involvement in COVID‐19 than was previously recognized.
Keywords: COVID‐19, gastrointestinal, meta‐analysis, SARS‐CoV‐2, systematic review
This review is a systematic review and meta‐analysis to characterize the prevalence of gastrointestinal (GI) and hepatic involvement in COVID‐19 and specifically assess the prevalence of GI symptoms in isolation. This review included 17 776 COVID‐19 patients from 108 studies. Isolated GI symptoms occurred in 1% (95% confidence interval 0–6%) of patients.
Introduction
The novel coronavirus SARS‐CoV‐2, also known as coronavirus 2019 (COVID‐19), originated in Wuhan, China during the fall of 2019 before rapidly disseminating throughout the world and being declared a pandemic by the World Health Organization in March, 2020. COVID‐19 was initially considered a primary respiratory illness with symptoms of shortness of breath, cough, and fever. However, there is an increasing appreciation of the ability of SARS‐CoV‐2 to produce a wide range of symptoms, 1 including GI (gastrointestinal) symptoms like diarrhea, nausea, vomiting, and abdominal pain. 2
Current CDC testing guidelines for COVID‐19 recognize the diversity of symptoms possible and advise testing for patients displaying fever, cough, shortness of breath, chills, myalgia, new loss of taste or smell, vomiting, diarrhea, or sore throat. 1 In practice, respiratory symptoms are prioritized by both patients and healthcare workers for testing and pre‐emptive isolation precautions. 3 In accordance with the worldwide effort to arrest the spread of this virus and mitigate its effects on the global population, the aim of this systematic review and meta‐analysis is to determine 1 whether isolated GI symptoms warrant testing for COVID‐19, and 2 whether GI symptoms, alone or in combination with respiratory symptoms, are associated with severe disease and/or mortality.
Methods
Search strategy and study selection
PubMed, Embase, and the Cochrane library were searched from 1 December 2019 to 18 May 2020 with the help of a medical librarian (full search terms and inclusion criteria in supplement). The study protocol was registered with PROSPERO (CRD42020182644).
We included any study that reported the presence or absence of GI manifestations in a sample of ≥5 COVID‐19 positive patients, including diarrhea, nausea, vomiting, anorexia (loss of appetite), abdominal pain, or liver injury, with or without the presence of typical respiratory viral symptoms. Two authors independently reviewed each article for inclusion, extracted data, and assessed the risk of bias (ROB) using the Newcastle Ottawa Scale.
Quantitative analysis
Where ≥3 studies reported data sufficiently similar to allow calculation of a pooled prevalence, we performed random effects meta‐analysis. The primary outcome was the proportion of COVID‐19 positive patients who experienced a period of isolated GI symptoms. We also calculated pooled proportions of patients with any experience of a GI symptom including diarrhea, abdominal pain, nausea/vomiting, and anorexia (loss of appetite). We performed a separate analysis of proportions of COVID‐19 positive patients with elevated alanine transaminase (ALT) or aspartate transaminase (AST) as well as pooled mean AST and ALT, weighted according to number of patients in each study with transaminase measurements. In a secondary analysis, we calculated the odds ratio (OR) of severe COVID‐19 versus non‐severe COVID‐19 based on the presence of GI symptoms. The Freeman‐Tukey double arcsine transformation was used in the meta‐analysis of proportions. 4 Heterogeneity was assessed qualitatively and quantitatively (using the I2 statistic) and explored using stratified and subgroup analyses. Covariates included risk of bias, study location, publication date, type of healthcare setting (inpatient vs outpatient), COVID‐19 case definition, pediatric versus adult patients, and whether the study focused on a unique subpopulation or health condition. We assessed for small‐study effects in the primary analysis visually with funnel plots, as well as the Egger test for funnel plot asymmetry. Analyses were performed in STATA 14.2 (College Station, TX, USA). Additional details of study selection and analysis are described in eMethods.
Results
Search results and study characteristics
Our search identified 771 unique articles, of which 108 met inclusion criteria (Fig. 1). These comprised 17 776 COVID‐19 positive patients from 16 countries on three continents, including 13 769 patients from Asia. (Table S1, Supporting information) The majority of studies focused on adult patients (78 studies) and the hospital setting (102 studies). Many studies focused on specific hospitalized subpopulations, such as healthcare workers, family clusters, and orthopedic, pregnant, or critically ill patients (Table S1). All but nine studies 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 defined COVID‐19 cases exclusively by positivity on an upper respiratory swab polymerase chain reaction (PCR). There were only three prospective studies, 14 , 15 , 16 and none were determined to have low risk of bias (Table S2).
Figure 1.
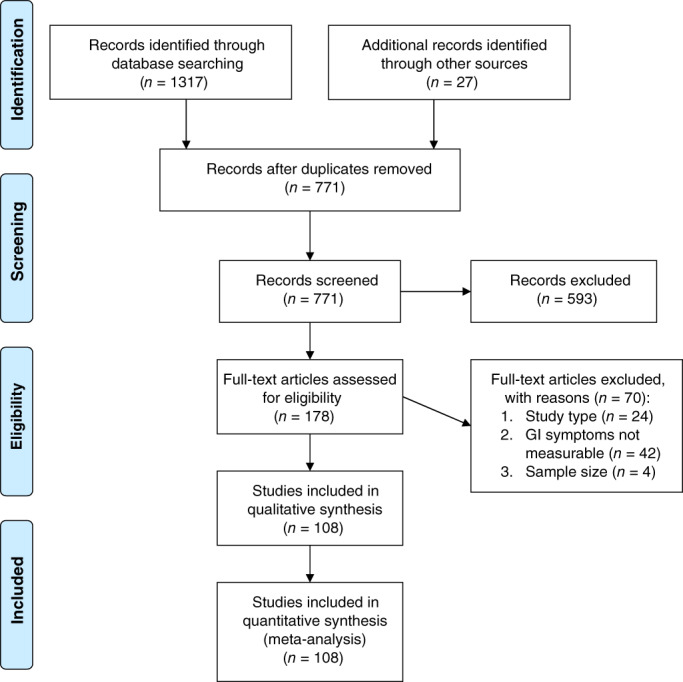
PRISMA flow diagram.
Prevalence of luminal GI symptoms
The most common GI symptoms recorded in studies were diarrhea (98 studies), nausea and/or vomiting (69 studies), abdominal pain (39 studies), and anorexia/loss of appetite (22 studies). The overall prevalence of any GI symptom in COVID‐19 patients was 20% (95% CI 15–24%) using the definitions of each study, though with a very large number of studies and consequent heterogeneity (I2 94%). When “loss of appetite” was not included in the definition of GI symptoms (leaving only diarrhea, abdominal pain, and nausea or vomiting), GI symptoms only occurred in 17% (95% CI 13–21%), vs 32% (95% CI 22–43%) when including anorexia as a GI symptom (P = 0.01, Figure S1). Findings were similar when examining experience of GI symptoms specifically as part of initial presentation (19%, 95% CI 14–25%, Figure S2).
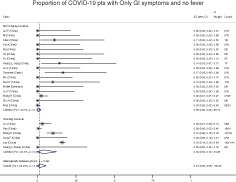
Twenty‐five studies, including 2870 COVID patients, reported on our primary outcome of patients who endorsed isolated GI symptoms at any point in the disease course without concurrent respiratory symptoms. Overall 2% (95% CI 0–6%, I2 63%) of COVID‐19 patients experienced isolated GI symptoms, with negligible difference whether the definition included anorexia (Figure S3) or whether the allowance of fever was defined (Figure S4). One percent (95% CI 0–6%, I2 88%) had only GI symptoms without fever, with greater heterogeneity and a non‐significant trend toward higher prevalence if anorexia was included (0%, 95% CI 0–2%, I2 30% if no anorexia; 4%, 95% CI 0–12%, I2 94% if anorexia included, Fig. 2). The prevalence of GI symptoms in 124 studies that reported GI symptoms as the only symptom at first illness presentation was also similar (4%, 95% CI 0–10%, I2 94%). GI symptoms lasted a mean of 4.6 (±1.9) days, based on four studies, but patients presented later in their disease course (mean of 13.2 [±5.4] days after onset of first symptoms). No study reported on a cohort of patients who were definitively known to never have developed respiratory or systemic symptoms. Notably, several 17 studies required the presence of respiratory symptoms for inclusion, and therefore would not have detected isolated GI symptoms. Likewise, there were also studies focused on GI symptoms that did not report an overall denominator of COVID‐19 positive patients. 3 , 18 , 19 , 20 , 21 , 22 In analyses of funnel plots, there was no evidence of small‐study effects (such as publication bias) for the outcome of proportion of patients with only GI symptoms (Egger test P = 0.12, Figure S5).
Figure 2.
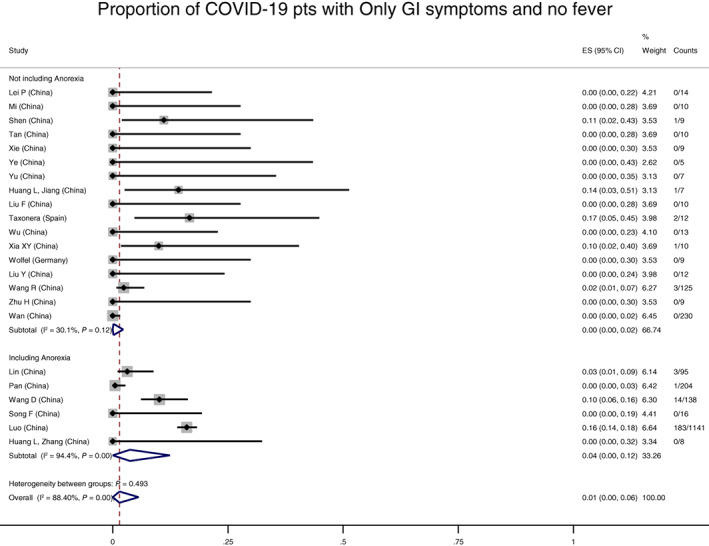
Proportion of coronavirus disease (COVID)‐19 patients with only gastrointestinal (GI) symptoms, without fever. Overall prevalence of COVID‐19 infection with a period of only GI symptoms without fever was 1% (95% confidence interval 0–6%). Whether anorexia was included in the definition of GI symptoms did not affect the proportion with GI symptoms in a statistically significant manner (P = 0.49).
Regarding specific luminal symptoms, diarrhea occurred in 13% (95% CI 11–16%, I2 94%) of COVID‐19 positive individuals, with significantly lower rates from studies examining cohorts identified by respiratory symptoms (9% vs 15% in cohorts not restricted to respiratory syndromes, P < 0.01, Figure S6). Nausea or vomiting was present in 10% (95% CI 7–12%, I2 94%, Figure S7) of patients. We assumed that all who vomited also had nausea, but the proportion of patients from 34 studies specifically reporting vomiting was 4% (95% CI 3–6%, I2 83%, Figure S8). Abdominal pain occurred in 4% (95% CI 2–6%, I2 93%, Figure S9) of patients, and anorexia in 21% (95% CI 15–27%, I2 97%, Figure S10). Diarrhea, when defined, was usually ≥3 loose bowel movements per day, but few studies quantified the severity of any GI symptoms.
In subgroup analyses to explore heterogeneity, the most significant covariates were geography and date of publication. Studies from Europe or North America reported higher proportions of all GI symptoms, which in bivariate analyses were statistically significant for diarrhea (Asia 11%, 95% CI 9–14%; West 24%, 95% CI 18–31%, Figure S11), nausea or vomiting (Asia 8%, 95% CI 6–10%; West 18%, 95% CI 15–22%, Figure 12), abdominal pain (Asia 2%, 95% CI 1–3%; West 12%, 6–20%, Figure S13), and any GI symptoms (Asia 17%, 95% CI 13–22%; West 31%, 95% CI 20–43%, Figure S14), though not for anorexia (P = 0.14, Figure S15). These results were driven by the majority of Asian studies from China, with similarly significant differences when comparing Chinese versus non‐Chinese studies (including 5 studies total from Singapore, Korea, Taiwan, and Macau). 4 , 23 , 24 , 25 , 26 , 27 Regarding date of publication, the most recently published studies reported higher proportions of GI symptoms, which were statistically significant for diarrhea (after 1 April, 16%, 95% CI 13–20%; on or before 1 April, 9%, 95% CI 7–12%, Figure S16), nausea or vomiting (after 1 April, 12%, 95% CI 8–16%; on or before 1 April, 6%, 95% CI 4–9%, Figure S17), and any GI symptoms (after 1 April, 24%, 95% CI 18–34%; on or before 1 April, 13%, 95% CI 9–19%, Figure S18), but not for abdominal pain or anorexia, though the effects were all in the same direction (Figures S19–20). Some of the effect of geography and publication date covaried, in that the majority of earlier published studies were from China. In a logit multivariable meta‐regression model incorporating both variables, publication date was no longer statistically significant for any of the GI symptoms (P = 0.14 for diarrhea, P = 0.72 for nausea/vomiting), though retained the same direction of effect (Diarrhea 14%, 95% CI 10–17%, after 1 April; 10%, 95% CI 9–13% before 1 April; Nausea/vomiting 8%, 95% CI 6–12% after 1 April; 8%, 95% CI 5–11% before 1 April). Asian studies still reported statistically significant lower rates of diarrhea and nausea or vomiting, however, even when adjusted for publication date (P = 0.02 and 0.01 respectively). There were no significant differences in the proportions of patients with any GI symptom according to the other study characteristics evaluated (eResults).
Prevalence of hepatic inflammation
Sixty‐two studies reported liver enzymes on some participants, with an overall prevalence of an abnormal transaminase in 24% of patients with COVID‐19 (95% CI 17–31%, I2 96%, Fig. 3). Similar to luminal symptoms, Asian studies reported lower proportions of elevated transaminases, although there was no significant difference in liver injury by study publication date (Figures S21–22). The average ALT was 34.8 (±16.1) units per liter (U/L), and AST 39.0 U/L (±17.3) among all COVID‐19 patients, from 44 and 43 studies, respectively. The weighted average AST:ALT ratio was 1.15 (±0.20). When restricted to only those patients with abnormal levels, average ALT was 48.7 U/L (±22.4) and AST 47.3 U/L (±23.9), although the weighted average AST:ALT ratio from each of the 11 studies reporting mean AST and ALT values in this subgroup was 1.06 (±0.32).
Figure 3.
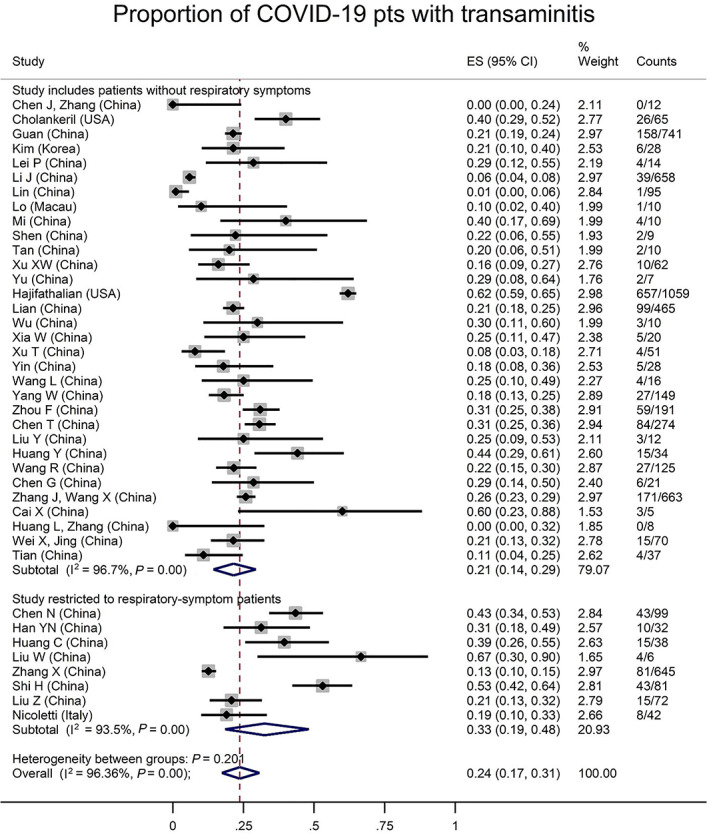
Proportion of all coronavirus disease (COVID)‐19 patients with elevated transaminases, by studies restricted to patients with respiratory symptoms or not. Overall prevalence of elevated transaminases in COVID‐19 infection was 24% (95% confidence interval 17–31%). This proportion was 33% in studies restricted to patients with respiratory symptoms, and only 21% in studies including patients without respiratory symptoms (P = 0.20).
Clinical outcomes
There was a mix of clinical severity among the studies, with 971/11 943 of overall patients experiencing mortality (75 studies), 489/8390 mechanical ventilation (59 studies), and 697/8257 intensive care unit (ICU) admission (62 studies). Random‐effects for the proportions of these outcomes from studies that included hospitalized patients of any severity were 3% (95% CI 1–5%), 4% (95% CI 2–5%), and 6% (95% CI 4–9%), respectively. From seven studies that reported these clinical outcomes according to the presence of GI symptoms, 2 , 10 , 17 , 28 , 29 , 30 , 31 odds of death was not significantly different based on the presence of GI symptoms (OR 0.90, 95% CI 0.52–1.54, Fig. 4). Exclusion of a single outlier study where GI symptoms were also associated with older age 31 revealed a significant negative association of GI symptoms with mortality (OR 0.68, 95% CI 0.48–0.97), although this was driven by two large North American studies (Fig. 4). 29 , 30
Figure 4.
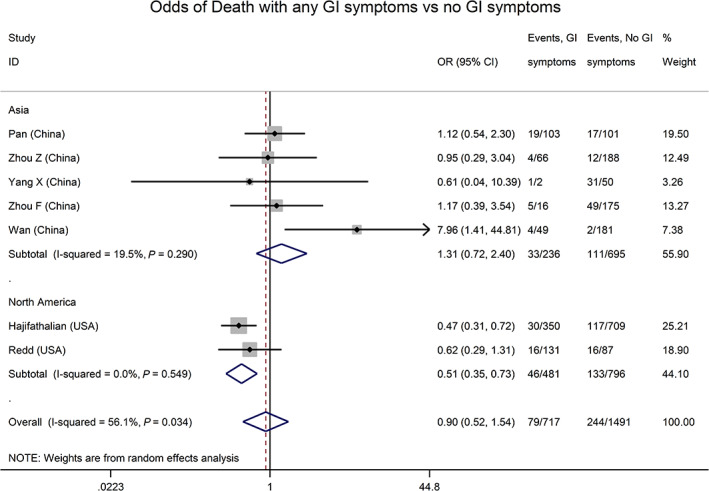
Odds of mortality in coronavirus disease (COVID‐19) infection with gastrointestinal (GI) symptoms, by continent. Odds of mortality were not significantly different (odds ratio [OR] 0.90, 95% confidence interval [CI] 0.52–1.54) in COVID‐infected patients with GI symptoms compared to those without. In the two US studies, however, the pooled odds ratio for mortality with GI symptoms was 0.51 (95% CI 0.35–0.73),versusOR 1.31 (95% CI 0.72–2.40) in studies from China (P = 0.04 for difference).
A largely non‐overlapping group of 12 studies, 14 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 all from China, reported on the presence of severe versus non‐severe COVID‐19 41 in association with GI manifestations. Overall, patients with any GI symptoms had greater odds of severe COVID‐19 related disease than those without GI symptoms (OR 2.07, 95% CI 1.34–3.18, I2 41%, Figure S23). Several outliers were very small studies (5 with <5 subjects with GI symptoms), but excluding these did not affect the point estimate or the significance of the result (OR 2.18, 95% CI 1.38–3.45, I2 56%). The associations of severe COVID‐19 with individual symptoms were in the same direction, although not statistically significant (Figures S24–26).
Discussion
This meta‐analysis represents the largest study of gastrointestinal manifestations of COVID‐19 to date, with over 17 000 patients from 108 studies. Overall, GI symptoms are common in COVID‐19, and highest in publications from outside of China and after 1 April, with one in four patients reporting some GI symptom, including 16% with diarrhea and 12% with nausea or vomiting in the most recent studies. One quarter of patients also demonstrate transaminase elevations. Presentation with GI symptoms without any respiratory symptoms or fever is uncommon but remains clinically relevant for a pandemic of this magnitude, occurring in 1% of patients.
Our primary analysis demonstrating that 1% (95% CI 0–6%) of COVID‐19 patients present with isolated GI symptoms (up to 2% if presence of fever allowed) is lower than reported in previous studies. For example, an earlier review by Mao et al. reported that 10% of COVID‐19 patients presented with only GI symptoms, and a matched cohort study from a single hospital in Wuhan by Han et al. reported isolated GI symptoms in 23% of COVID‐19 patients. 3 , 42 However, the Han et al. study was not able to estimate a true prevalence of GI symptoms among all COVID‐19 patients. Furthermore, our review identifies and includes several studies with zero prevalence of isolated GI symptoms that were not included in the Mao review. Even if the overall prevalence of isolated GI symptoms is relatively low, 1% represents hundreds of cases daily in the US alone, with both clinical and public health implications. 43 , 44
Our study differentiates between GI symptoms with and without anorexia, with the reported prevalence of GI symptoms varying from 28% when anorexia was included to 17% when excluding this symptom. Anorexia may be the result of systemic inflammation rather than a true digestive symptom. 45 The inclusion of this non‐specific infectious symptom may artificially elevate the prevalence of GI symptoms reported in some studies, 2 , 46 a distinction the clinician should keep in mind when interpreting this literature.
In exploring the significant heterogeneity inherent in a review of large numbers of studies, geography and publication date emerged as significantly associated with reported prevalence of GI symptoms. The prevalence of diarrhea and nausea/vomiting in publications printed after 1 April was nearly twice that of publications before 1 April and in publications from Europe or North America compared to Asia. Given that earlier cases and publications occurred in Asia, the effect of country versus publication date is challenging to disentangle. As COVID‐19 was first described as a respiratory illness, comprehensive collection of symptom data (by investigators as well as the clinicians populating the medical records) occurred to a lesser extent earlier in the pandemic. The results of the largest studies from our review are illustrative. Guan et al. (February 2020) reported 3.8% of patients had diarrhea and 5% nausea and/or vomiting. However, study investigators reported incomplete documentation of disease course due to overwhelmed medical infrastructure and clinically—rather than research‐driven data collection. 47 Cholankeril et al. (April 2020) reported higher prevalences of diarrhea (12%) and nausea/vomiting (12%), but the authors acknowledged testing criteria had not included extrapulmonary symptoms at the time. 48 In contrast, Hajifathalian (New York study from May) clearly defined GI symptoms and systematically evaluated patients for extrapulmonary manifestations. They reported even higher prevalence of diarrhea (22%). 29 We suggest that the pooled prevalence from the most recent studies (diarrhea 16%, 95% CI 13–20%; nausea/vomiting 12%, 95% CI 8–16%) are therefore the most accurate estimates of the true prevalence of GI symptoms in SARS‐CoV2.
We found liver inflammation to be even more prevalent than GI symptoms with 24% of patients having elevated transaminases. The mechanism of liver injury in COVID‐19 patients is not yet well understood. While angiotensin‐converting enzyme 2 (ACE 2) receptors exist on bile duct epithelium and hepatocytes, liver injury is more likely due to immune activation 49 , 50 than direct viral cytopathic effect. 51 , 52 Many cases of hepatic dysfunction in severe COVID‐19 are likely multifactorial, including insults from hypoxia, hypoperfusion, thrombosis, and hepatotoxic medications. The average transaminase level even in patients with abnormal levels was not severely elevated (<50 U/L), which suggests that liver inflammation is mild and not a major part of COVID‐19 related inflammation. Finally, synthetic liver dysfunction clearly linked to COVID‐19 infection has yet to be described. 53
This review also examined the relationship between GI symptoms and clinical outcomes in COVID‐19, with mixed results. Pooled data from 12 Chinese studies yielded an association between GI symptoms and severe COVID‐19, similar to prior reviews. 42 , 54 However, patients with GI symptoms showed a trend toward lower mortality. The main reason for this apparent inconsistency is likely the different sets of studies reporting each outcome, as the inverse relationship with mortality was driven by two large US studies, 29 , 54 also published after prior reviews. 42 Regardless, the association with severe disease is limited by heterogeneous definitions of what constitutes “severe,” and possibly other factors such as late‐onset GI symptoms resulting from treatments in patients that are more ill. The small number of studies evaluating the association of GI symptoms with outcomes indicate that more research is needed in this area.
Our meta‐analysis has notable limitations. As a review of a large number of observational studies, there is a great degree of heterogeneity, which is only partially explained by subgroup analyses. The prevalence estimates of less variably defined outcomes—such as isolated GI symptoms, diarrhea, and vomiting—are therefore more reliable than the outcomes of “any” GI symptoms or anorexia. The majority of included studies are Chinese and from hospitalized settings, limiting the generalizability of these findings to other regions and less severely ill patients. Lastly, no studies had low risk of bias. This rating was usually due to lack of COVID‐19 negative comparison groups, suboptimal measurement of GI symptoms, and inadequate follow‐up of COVID‐19 patients. In fact, recent articles have drawn attention to the poor methodology of many early COVID‐19 studies, driven by overwhelmed healthcare systems in need of rapid information dissemination with abbreviated peer review. 55 , 56 These limitations notwithstanding, our review is the largest to‐date of the peer‐reviewed literature, including separate subgroup analyses of literature published since 1 April, to provide the most updated estimates of the prevalence of GI and hepatic effects of COVID‐19 infection.
We advocate broad testing for COVID‐19—including for patients presenting with isolated GI symptoms or unexplained elevations of LFTs—when testing resources are plentiful. While COVID‐19 with isolated GI symptoms may not be common enough (1–2%) to warrant testing on this basis alone when testing resources are scarce, these patients should consider self‐quarantine to monitor for development of other symptoms, including fever, cough, and dyspnea. The significant role of digestive symptoms in COVID‐19 is clear, but many knowledge gaps regarding their pathophysiology and prognostic value remain. Further research should proceed through well‐designed prospective studies.
Supporting information
Appendix S1. Supporting information.
Acknowledgments
We would like to thank Mr Mark McKone and Ms Janine Tillett for providing their medical librarian expertise and for developing the search strategy.
Michael K Dougherty shares co‐first authorship.
Declaration of conflict of interest: None.
Author contribution: Robert D Dorrell, Eric L Barash, Asher E Lichtig performed data extraction and risk assessments. Michael K Dougherty performed data analysis and co‐wrote the paper with Robert D Dorrell. Elizabeth T Jensen and Steven B Clayton provided oversight of the study. All authors contributed to the conceptualization of the study, interpretation of study results, and editing of manuscript drafts.
Guarantor of the article: Dr Elizabeth T Jensen MPH, PhD.
References
- 1. CDC . Coronavirus Disease (COVID‐19) Centers for Disease Control and Prevention: US Department of Health and Human Services, 2020. Available from URL: https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fcases-updates%2Fsummary.html
- 2. Pan L, Mu M, Yang P et al Clinical characteristics of COVID‐19 patients with digestive symptoms in Hubei, China: a descriptive, cross‐sectional, multicenter study. Am. J. Gastroenterol. 2020; 115: 766–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Han C, Duan C, Zhang S et al Digestive symptoms in COVID‐19 patients with mild disease severity: clinical presentation, stool viral RNA testing, and outcomes. Am. J. Gastroenterol. 2020; 115: 916–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta‐analysis of binomial data. Arch Public Health. 2014; 72: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li J, Wang X, Chen J, Zuo X, Zhang H, Deng A. COVID‐19 infection may cause ketosis and ketoacidosis. Diabetes Obes. Metab. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shao F, Xu S, Ma X et al In‐hospital cardiac arrest outcomes among patients with COVID‐19 pneumonia in Wuhan, China. Resuscitation. 2020; 151: 18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shen Q, Guo W, Guo T et al Novel coronavirus infection in children outside of Wuhan, China. Pediatr. Pulmonol. 2020; 55: 1424–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tang X, Du R, Wang R et al Comparison of hospitalized patients with acute respiratory distress syndrome caused by COVID‐19 and H1N1. Chest. 2020; 158: 195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang X, Cai H, Hu J et al Epidemiological, clinical characteristics of cases of SARS‐CoV‐2 infection with abnormal imaging findings. Int. J. Infect. Dis. 2020; 94: 81–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhou Z, Zhao N, Shu Y, Han S, Chen B, Shu X. Effect of gastrointestinal symptoms in patients with COVID‐19. Gastroenterology. 2020; 158: 2294–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tan ND, Qiu Y, Xing XB, Ghosh S, Chen MH, Mao R. Associations between angiotensin converting enzyme inhibitors and angiotensin II receptor blocker use, gastrointestinal symptoms, and mortality among patients with COVID‐19. Gastroenterology. 2020; 159: 1170–1172.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wei XY, Jing D, Jia B et al Characteristics of in peripheral blood of 70 hospitalized patients and 8 diarrhea patients with COVID‐19. Int. J. Med. Sci. 2020; 17: 1142–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang J, Liu P, Wang M et al The clinical data from 19 critically ill patients with coronavirus disease 2019: a single‐centered, retrospective, observational study. Z. Gesundh Wiss. 2020: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huang C, Wang Y, Li X et al Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020; 395: 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wölfel R, Corman VM, Guggemos W et al Virological assessment of hospitalized patients with COVID‐2019. Nature. 2020; 581: 465–9. [DOI] [PubMed] [Google Scholar]
- 16. Huang L, Zhang X, Zhang X et al Rapid asymptomatic transmission of COVID‐19 during the incubation period demonstrating strong infectivity in a cluster of youngsters aged 16‐23 years outside Wuhan and characteristics of young patients with COVID‐19: a prospective contact‐tracing study. J. Infect. 2020; 80: e1–e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhou F, Yu T, Du R et al Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020; 395: 1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hossain R, Lazarus MS, Roudenko A et al CT scans obtained for nonpulmonary indications: associated respiratory findings of COVID‐19. Radiology. 2020; 296: 173–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Klopfenstein T, Kadiane‐Oussou NJ, Royer PY, Toko L, Gendrin V, Zayet S. Diarrhea: an underestimated symptom in Coronavirus disease 2019. Clin. Res. Hepatol. Gastroenterol. 2020; 44: 282–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Saeed U, Sellevoll HB, Young VS, Sandbaek G, Glomsaker T, Mala T. Covid‐19 may present with acute abdominal pain. Br. J. Surg. 2020; 107: e186–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cai X, Ma Y, Li S, Chen Y, Rong Z, Li W. Clinical characteristics of 5 COVID‐19 cases with non‐respiratory symptoms as the first manifestation in children. Front. Pediatr. 2020; 8: 258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nicoletti A, Talarico V, Sabetta L et al Screening of COVID‐19 in children admitted to the hospital for acute problems: preliminary data. Acta Biomed. 2020; 91: 75–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pung R, Chiew CJ, Young BE et al Investigation of three clusters of COVID‐19 in Singapore: implications for surveillance and response measures. Lancet. 2020; 395: 1039–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Young BE, Ong SWX, Kalimuddin S et al Epidemiologic features and clinical course of patients infected with SARS‐CoV‐2 in Singapore. JAMA. 2020; 323: 1488–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kim ES, Chin BS, Kang CK et al Clinical course and outcomes of patients with severe acute respiratory syndrome coronavirus 2 infection: a preliminary report of the first 28 patients from the Korean cohort study on COVID‐19. J. Korean Med. Sci. 2020; 35: e142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu JY, Chen TJ, Hwang SJ. Analysis of imported cases of covid‐19 in Taiwan: a nationwide study. Int. J. Environ. Res. Public Health. 2020; 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lo IL, Lio CF, Cheong HH et al Evaluation of SARS‐CoV‐2 RNA shedding in clinical specimens and clinical characteristics of 10 patients with COVID‐19 in Macau. Int. J. Biol. Sci. 2020; 16: 1698–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yang X, Zhao J, Yan Q, Zhang S, Wang Y, Li Y. A case of COVID‐19 patient with the diarrhea as initial symptom and literature review. Clin. Res. Hepatol. Gastroenterol. 2020; 44: e109–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hajifathalian K, Krisko T, Mehta A et al Gastrointestinal and hepatic manifestations of 2019 novel coronavirus disease in a large cohort of infected patients from New York: clinical implications. Gastroenterology. 2020; 159: 1137–1140.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Redd WD, Zhou JC, Hathorn KE et al Prevalence and characteristics of gastrointestinal symptoms in patients with SARS‐CoV‐2 infection in the United States: a multicenter cohort study. Gastroenterology. 2020; 159: 765–767.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wan Y, Li J, Shen L et al Enteric involvement in hospitalised patients with COVID‐19 outside Wuhan. Lancet Gastroenterol. Hepatol. 2020; 5: 534–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chen Q, Quan B, Li X et al A report of clinical diagnosis and treatment of nine cases of coronavirus disease 2019. J. Med. Virol. 2020; 92: 683–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jin X, Lian JS, Hu JH et al Epidemiological, clinical and virological characteristics of 74 cases of coronavirus‐infected disease 2019 (COVID‐19) with gastrointestinal symptoms. Gut. 2020; 69: 1002–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang JJ, Dong X, Cao YY et al Clinical characteristics of 140 patients infected with SARS‐CoV‐2 in Wuhan, China. Allergy. 2020; 75: 1730–41. [DOI] [PubMed] [Google Scholar]
- 35. Zhang R, Ouyang H, Fu L et al CT features of SARS‐CoV‐2 pneumonia according to clinical presentation: a retrospective analysis of 120 consecutive patients from Wuhan city. Eur. Radiol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Xia XY, Wu J, Liu HL, Xia H, Jia B, Huang WX. Epidemiological and initial clinical characteristics of patients with family aggregation of COVID‐19. J. Clin. Virol. 2020; 127: 104360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhao XY, Xu XX, Yin HS et al Clinical characteristics of patients with 2019 coronavirus disease in a non‐Wuhan area of Hubei Province, China: a retrospective study. BMC Infect. Dis. 2020; 20: 311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li K, Wu J, Wu F et al The clinical and chest CT features associated with severe and critical COVID‐19 pneumonia. Invest. Radiol. 2020; 55: 327–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhang H, Liao YS, Gong J, Liu J, Xia X, Zhang H. Clinical characteristics of coronavirus disease (COVID‐19) patients with gastrointestinal symptoms: a report of 164 cases. Dig. Liver Dis. 2020; 52: 1076–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chen G, Wu D, Guo W et al Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Investig. 2020; 130: 2620–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020; 323: 1239–42. [DOI] [PubMed] [Google Scholar]
- 42. Mao R, Qiu Y, He JS et al Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID‐19: a systematic review and meta‐analysis. Lancet Gastroenterol. Hepatol. 2020; 5: 667–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Li R‐L, Chu S‐G, Luo Y, Huang Z‐H, Hao Y, Fan C‐H. Atypical presentation of SARS‐CoV‐2 infection: a case report. World J. Clin. Cases. 2020; 8: 1265–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chan JFW, Yuan S, Kok KH et al A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person‐to‐person transmission: a study of a family cluster. Lancet. 2020; 395: 514–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gautron L, Layé S. Neurobiology of inflammation‐associated anorexia. Front. Neurosci. 2010; 3: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Luo S, Zhang X, Xu H. Don't overlook digestive symptoms in patients with 2019 novel coronavirus disease (COVID‐19). Clin. Gastroenterol. Hepatol. 2020; 18: 1636–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Guan WJ, Ni ZY, Hu Y et al Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020; 382: 1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cholankeril G, Podboy A, Aivaliotis VI et al High prevalence of concurrent gastrointestinal manifestations in patients with SARS‐CoV‐2: early experience from California. Gastroenterology. 2020; 159: 775–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Xu Z, Shi L, Wang Y et al Pathological findings of COVID‐19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020; 8: 420–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Patel KP, Patel PA, Vunnam RR et al Gastrointestinal, hepatobiliary, and pancreatic manifestations of COVID‐19. J. Clin. Virol. 2020; 128: 104386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Li Y, Zhou W, Yang L, You R. Physiological and pathological regulation of ACE2, the SARS‐CoV‐2 receptor. Pharmacol. Res. 2020; 157: 104833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cai Q, Huang D, Yu H et al COVID‐19: abnormal liver function tests. J. Hepatol. 2020; 73: 566–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Weber S, Mayerle J, Irlbeck M, Gerbes AL. Severe liver failure during SARS‐CoV‐2 infection. Gut. 2020; 69: 1365–7. [DOI] [PubMed] [Google Scholar]
- 54. Henry BM, de Oliveira MHS, Benoit J, Lippi G. Gastrointestinal symptoms associated with severity of coronavirus disease 2019 (COVID‐19): a pooled analysis. Intern. Emerg. Med. 2020; 15: 857–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yu Y, Shi Q, Zheng P et al Assessment of the quality of systematic reviews on COVID‐19: a comparative study of previous coronavirus outbreaks. J. Med. Virol. 2020; 92: 883–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Alexander PE, Debono VB, Mammen MJ et al COVID‐19 coronavirus research has overall low methodological quality thus far: case in point for chloroquine/hydroxychloroquine. J. Clin. Epidemiol. 2020; 123: 120–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting information.


