Abstract
Background
As the global coronavirus pandemic (COVID‐19) spreads across the world, new clinical challenges emerge in the hospital landscape. Among these challenges, the increased risk of coinfections is a major threat to the patients. Although still in a low number, due to the short time of the pandemic, studies that identified a significant number of hospitalised patients with COVID‐19 who developed secondary fungal infections that led to serious complications and even death have been published.
Objectives
In this scenario, we aim to determine the prevalence of invasive fungal infections (IFIs) and describe possible associated risk factors in patients admitted due to severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection.
Patients/Methods
We designed an open prospective observational study at the Rey Juan Carlos University Hospital (Mostoles, Spain), during the period from February 1 to April 30, 2020.
Results
In this article, we reported seven patients with COVID‐19‐associated pulmonary aspergillosis (CAPA) who had a poor prognosis. Severely ill patients represent a high‐risk group; therefore, we must actively investigate the possibility of aspergillosis in all of these patients. Larger cohort studies are needed to unravel the role of COVID‐19 immunosuppressive therapy as a risk factor for aspergillosis.
Conclusions
As the pandemic continues to spread across the world, further reports are needed to assess the frequency of emergent and highly resistant reemergent fungal infections during severe COVID‐19. These coinfections are leading a significant number of patients with COVID‐19 to death due to complications following the primary viral disease.
Keywords: COVID‐19, invasive aspergillosis, lung disease, opportunistic mycosis
1. INTRODUCTION
Invasive fungal infections (IFIs) have an incidence of approximately 4.7 per 1000 patients and have been associated with high morbidity and mortality in critically ill and immunocompromised patients. The most important fungi isolated are Candida spp., Aspergillus spp., Cryptococcus spp. and Pneumocystis spp. 1 Although studies indicate an increase in IFI, the incidence is underestimated in clinical settings. 2
Invasive aspergillosis has been reported in critically ill patients with influenza. In the study by Schauwvlieghe et al, authors identified influenza as an independent risk factor for invasive pulmonary aspergillosis (IPA), associated with high mortality. 3 In previous coronavirus disease outbreaks (severe acute respiratory syndrome, SARS and Middle East Respiratory Syndrome, MERS), some patients experienced clinical complications caused by IFI, especially Aspergillus spp. 4
A few cases of IPA have been published in critical infection cases caused by the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). However, the global incidence is underestimated, and more evidence is needed. A series of factors, such as lung damage caused by the SARS‐CoV‐2 infection, medical interventions and immunosuppressive treatments used could explain the increased risk of IFI in Coronavirus Disease 2019 (COVID‐19)‐patients. It is clinically relevant to understand the risk factors associated with IFI in patients with COVID‐19 and to determine a group of high‐risk patients. Several concomitant factors play a role in the development of these coinfections. 5 , 6 , 7 , 8
We hypothesised that the use of immunosuppressors could increase the risk of the patients to develop probable IPA. 9 In this scenario, we aim to determine the prevalence of IFIs and describe possible associated risk factors in patients admitted due to severe SARS‐CoV‐2 infection.
2. METHODS
2.1. Study design
We designed an open prospective observational study, which was conducted at the Rey Juan Carlos University Hospital (HURJ), during the period from February 1 to April 30, 2020 (Figure 1). The HURJ is a centre integrated into the public health network of Madrid (Spain), which serves a population of approximately 174,000 inhabitants in the southern area of Madrid. It has 358 individual hospital beds, with the possibility of doubling the number according to the demand, including those of the Pneumology service. The Pneumology service has a Respiratory Intermediate Care Unit (RICU). Before the COVID‐19 pandemic, our RICU was integrated into the ICU and had four beds. The patients were attended by a pneumonologist in the morning and by another in the afternoon. Patient assistance at night and on weekends was performed by the ICU personnel. The Pneumology hospital area, prior to COVID‐19, had 12 hospital beds, with the possibility of doubling the amount to 24 beds, according to admission needs. During the COVID‐19 pandemic, the RICU was unified with the Pneumology hospitalisation section, reaching 45 hospital beds to attend semi‐critical patients.
Figure 1.
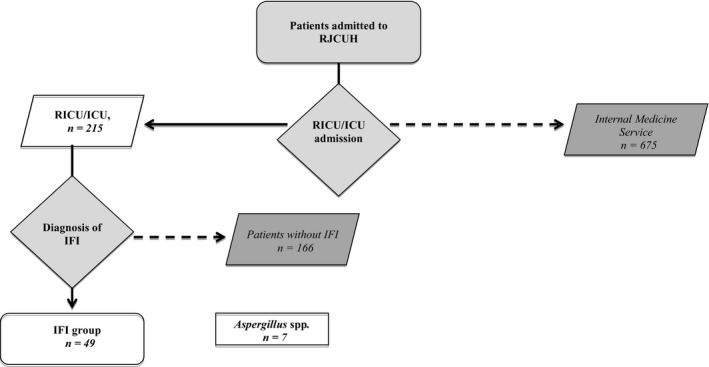
Flow chart of the study. ICU, Intensive Care Unit; IFI, Invasive fungal infection; RICU, Respiratory Intermediate Care Unit; RJCUH, Rey Juan Carlos University Hospital
The study presented here was approved by the Ethics Committee of the Fundación Jiménez Díaz Health Research Institute (EO102‐20‐HRJC). This ethics committee covers four hospitals that belong to the same hospital group (Jiménez Díaz Foundation, Rey Juan Carlos University Hospital, Infanta Elena University Hospital, and Villalba Hospital). This work is a retrospective observational study and, in view of the pandemic situation, informed consent was not requested from the patients. The confidentiality regarding subject personal data was maintained.
2.2. Sample design
We included 215 adult patients respectively admitted to the RICU or to the intensive care unit (ICU), with a confirmed diagnosis of severe pneumonia caused by SARS‐CoV‐2 (Figure 1). IFI was diagnosed according to the criteria of the European Organization for Research and Treatment of Cancer/Mycoses Study Group. 10
Patients without a microbiological diagnosis of SARS‐CoV‐2 or IFI, and those from whom the planned data was not collected were excluded from the database.
In order to confirm the presence of a SARS‐CoV‐2 infection, smear samples were collected from the upper respiratory tract, at the moment of admission, and processed for RT‐PCR (‘Primerdesign Ltd COVID‐19 genesig® Real‐Time PCR assay’, HAIN Lifescience, Chandler's Ford, UK). A serological diagnosis was made using the COVID‐19 rapid Biozek Medical Test with a specificity of 98% for IgG and of 96% for IgM and a sensitivity of 100% for IgG and 85% for IgM (BIOZEK, Apeldoorn, Netherlands).
2.3. Microbiological diagnosis
According to our protocol, from all patients admitted to the RICU or ICU, samples of respiratory secretions and nasal, oropharyngeal, rectal, urine and skin exudates were collected every two weeks. Samples of sputum, from patients with sputum production, were collected and processed for microbiology analysis.
To obtain additional samples of respiratory secretions by a direct aspiration (bronchoaspirates, BAS) or by completing a bronchoalveolar lavage (BAL), a bronchoscopy was performed. For the BAL, we instilled three 50 ml aliquots of sterile physiological solution (150 ml in total) in the area with radiological involvement.
Patients with a sustained fever above 38ºC, despite the use of prescribed antibiotics, had blood samples collected for blood cultures. Similarly, we sent the tip of the removed central catheters of these patients to the microbiology laboratory. Fungal species were identified by the analysis of the ITS1‐5.8S‐ITS2 region amplified by PCR and sequenced according Sanger et al (1977).
For the diagnosis of fungal infections, the detection of the Aspergillus galactomannan antigen was carried out in the BAL sample by using the Platelia™ Aspergillus EIA assay (Bio‐Rad, Marnes La Coquette, France). We considered that the result was positive when the Aspergillus‐galactomannan antigen value was ≥0.5.
2.4. Variables
We obtained the data from the electronic medical record system of our centre, which allows the access to medical and nursing comments, in addition to laboratory and radiology exams.
We collected the data related to the socio‐demographic situation of the patients as well as their baseline health situation. During the inpatient stay, the data regarding the clinical course of the disease, the treatment for COVID‐19 and the unfavourable evolution of the disease, including the occurrence of a major complication, admission to the ICU, and death were analysed.
A severe pneumonia was identified when there was failure of one or more organs, when the oxygen saturation measured by pulse oximetry was <90% in ambient air, or when the respiratory rate was >30 breaths per minute. In acute respiratory distress syndrome (ARDS) cases, the classification was made according to when the partial pressure of arterial Oxygen (PaO2)/ fraction of inspired oxygen (FiO2) scored as mild (200 mm Hg < PaO2 / FiO2 < 300 mm Hg), moderate (100 mm Hg < PaO2 / FiO2 < 200 mm Hg) or severe (PaO2 / FiO2 < 100 mm Hg). 11
The CURB‐651 and MuLBSTA2 were used as severity indices for pneumonia, the latter being used as an indicator for viral pneumonia. According to the MuLBSTA score, we classified the patients as having a low risk (0–11 points, mortality 5.07%) or a high risk of mortality (≥12 points, mortality 33.92%) (Table S1). We considered a cut‐off point of 12 from the MuLBSTA score to differentiate between the low‐risk mortality group (0–11 points) and the high‐risk mortality group (>12 points). 12 , 13
The radiological involvement was classified according to the radiographic assessment of lung oedema (RALE). 14 To calculate the RALE score, the chest was divided into 4 quadrants. Each quadrant was scored, from zero to four, according to the extension of the alveolar opacities: zero, no involvement; one, <25% of involvement; two: 25%–50%; three: 50%–75% and four: >75%). The total result was obtained from the sum of the scores of each one of the quadrants. We analysed each lung separately, and we scored from zero to four points depending on the damage visualised in the radiological exam (maximum of eight points, adding up the punctuation of both lungs). A score below two was interpreted as mild involvement, between three and six points as moderate, and above 6 as severe involvement (Table S2).
2.5. Statistical analysis
Data was analysed using the IBM® Statistical Product and Service Solutions (spss ®) statistical software, version 20.0 (SPSS Inc, Chicago, IL, USA). Statistical summary was applied to describe socio‐demographics and clinical variables of the study. Data was presented as median (interquartile range [IQR], mean ± standard deviation [SD]) or numbers with percentages if not indicated otherwise. Differences between groups were analysed using Fisher tests. The calculation of relative risk (RR) was made using contingency tables and the results were expressed as a confidence interval (CI). For univariate analysis Student's test and Chi‐square test, were used as appropriate. Spearman's test was used for the bivariate correlation test for independent predictor selections. The level of significance will be assumed when p < .05.
3. RESULTS
From the total number of patients included in the study (n = 215), we diagnosed opportunistic IFI in 49 individuals (22.8%). Seven of these patients had an infection caused by Aspergillus spp. (A. fumigatus, n = 3; A. flavus, n = 2 and A. niger, n = 2) (Table 1). The global prevalence of aspergillosis was 5.4%. In all cases, Aspergillus spp. was isolated from respiratory samples (bronchoalveolar lavage n = 6, bronchial aspirated secretions n = 2, and serial sputum n = 1). The bronchoscopy was made during ICU admission, and the indication of a possible infection was the progression of lung infiltrates and the low ratio of PaO2/FiO2, despite medical treatment and invasive ventilation. The median age of the patients was 59.6 ± 15.21 years, and the majority was male (five male patients versus two female patients). Approximately 85.7% (n = 6) had comorbidities (high blood pressure in all cases and one patient had interstitial lung disease) (Table 1). None of the patients were immunocompromised. The main comorbidities identified in the patients were morbid obesity, high blood pressure and sleep apnoea. None of the patients were immunocompromised (Table 1). Five patients with IPA were admitted to the ICU, approximately in the 14th day after the symptoms started. All of them needed orotracheal intubation and parenteral nutrition. The outcomes were more adverse in IPA patients, with a high mortality (86% vs 37%, p‐value = .002) and more extended hospital stay (31.8 vs 15.7 days, p‐value = .038).
Table 1.
Summarised socio‐demographic variables (gender, age, admission to ICU), number of comorbidities, and COVID‐19 symptoms
| Sex | Age (years old) | Comorbidities | Time from start symptoms (Days a | COVID‐19 symptoms | ICU (OTI) Time OTI | Complications | Death (days) Cause of death | Treatment b | Antifungal therapy | Isolate | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Case 1 | Male | 75 | Sleep apnoea syndrome, Basal‐cell carcinoma, Diabetes mellitus type 2, arterial hypertension, dyslipidemia, COPD | 20 | Dyspnoea, cough, and fever | YES (YES) 5 days | Pneumothorax, sepsis, atrial fibrillation, severe myopathy and acute renal failure | YES (25) Sepsis | Tocilizumab (600 mg) Methylprednisolone (40 mg/day) Interferon β‐1b | No treatment | A. fumigatus |
| Case 2 | Male | 42 | Morbid obesity | 12 | Cough, sputum, dyspnoea, and fever | YES (YES) 11 days | Thoracic bleeding, sepsis, severe myopathy, acute renal failure, multiorgan failure and atrial fibrillation | YES (23) Sepsis | Methylprednisolone (40 mg/day) | Itraconazole (IV): 200 mg Q12H × 48 h. Subsequent, daily starting day 3 200 mg Q24H | A. fumigatus |
| Case 3 | Female | 60 | Morbid obesity, peptic ulcer, extrinsic allergic alveolitis | 10 | Fever, malaise, diarrhoea and headache | YES (YES) 10 days | Sepsis, atrial fibrillation, severe myopathy and acute renal failure | YES (20) | Lopinavir/Ritonavir (400 mg/100 mg) | No treatment | A. niger |
| Case 4 | Female | 58 | Sleep apnoea syndrome, and arterial hypertension | 15 | Fever, malaise, cough and dyspnoea | YES (YES) 7 days | Acute renal failure, and severe myopathy | NO | Tocilizumab (1.600 mg) Methylprednisolone (120 mg daily ‐ for 3 days) | Itraconazole (IV): 200 mg Q12H × 48 h. Subsequent, daily starting day 3 200 mg Q24H until the ninth day PO: 200 mg Q24H for 60 days | A. flavus |
| Case 5 | Male | 70 | Diabetes mellitus type 2, and arterial hypertension | 11 | Fever, malaise, cough and dyspnoea | NO (NO) | Atrial fibrillation, and pulmonary embolism | YES (42) Organ failure | Tocilizumab (600 mg) Methylprednisolone (120 mg daily ‐ for 3 days) | Itraconazole IV: 200 mg Q12H × 48 h. Subsequent, daily starting day 3 200 mg Q24H | A. fumigatus |
| Case 6 | Male | 55 | Diabetes mellitus type 2, and arterial hypertension | 10 | Dyspnoea, cough, and fever | YES (YES) 5 days | Acute renal failure | NO (28) | Tocilizumab (600 mg) Methylprednisolone (120 mg daily ‐ for 3 days) | Amphotericin B deoxycholate (ABD) (IV) 5 mg / kg / day | A. niger |
| Case 7 | Male | 57 | No known comorbidity | 12 | Dyspnoea, cough, and fever | YES (YES) 18 days | Pneumothorax, thoracic bleeding, pulmonary infarction, sepsis, acute renal failure, severe myopathy and Broncho‐pulmonary fistula | YES (30) Sepsis | Tocilizumab (600 mg) Methylprednisolone (250 mg daily ‐ for 3 days) | Itraconazole (IV): 200 mg Q24H until the ninth day PO: 200 mg Q24H for 60 days | A. flavus |
Abbreviations: COPD, Chronic Obstructive Pulmonary Disease; COVID‐19, Coronavirus Disease 2019; ICU, Intensive Care Unit; IV (Intravenous), administration within or into a vein or veins; OTI, Orotracheal intubation; PO (Oral), Administration to or by way of the mouth.
‘Time from start symptoms’ refers to the time from the beginning of the symptoms to hospital admission.
All patients received Azithromycin 500 mg for 3 days, Hydroxychloroquine sulphate 5 mg/kg (310 mg base) for 7 days and third‐generation cephalosporins for 7 days.
Radiological involvement was more severe in IPA patients than in patients without IPA, and clinical scores of severity followed the same trend (lower PaO2/FiO2 value: 136.4 vs 150.6; high MuLBSTA score punctuation: 11.2 vs 10, without significant differences. There was a significant number of complications 100% vs 70%, p‐value ≤ .05 (Table 2, Tables S1 and S2).
Table 2.
Summarised scores for assessment of pneumonia severity (MuLBSTA and CURB‐65), and the radiological findings (RALE); lung failure (PaO2/FiO2, SpO2/FiO2), and outcomes (prevalence of complications, length of stay and prevalence of death)
| Aspergillus sp. group (n = 7) | Non‐Aspergillus sp. group (n = 208) | p‐value (Chi‐square test χ2) | |
|---|---|---|---|
| Age (years old) | 59.6 | 63 | .32 |
| PaO2/FiO2 (IQR) | 136.4 (71) | 148.5 (69) | .47 |
| SpO2/FiO2 (IQR) | 123.7 (64) | 162.5 (73) | .40 |
| MulBSTA score (IQR) | 11.2 (5.5) | 10 (6) | .95 |
| CURB‐65 score (IQR) | 1.7 (2) | 0.5 (2) | .032 |
| RALE score: Severe group (%) | 50 | 46 | .84 |
| Complications (%) | 100 | 70 | .05 |
| Duration of stay (days) ± SD | 32.25 ± 14 | 16.5 ± 10.5 | .038 |
| Death (%) | 86 | 37 | .002 |
Abbreviations: FiO2, Fraction of inspired oxygen; IQR, Interquartile range; PaO2, Partial Pressure of arterial Oxygen; RALE, Radiographic assessment of lungoedema; SD, standard deviation; SpO2, Oxygen saturation measured by a pulsometer.
The time since the onset of symptoms started was eleven days (IQR 26). Upon admission, all patients received a noninvasive respiratory therapy (four cases with continuous positive airway pressure and three cases with high nasal flow). In six patients, orotracheal intubation and invasive ventilation were required due to the worsening of respiratory function (Tables 1 and 2). Regarding the medical treatment received, all patients were treated with type 1β interferon (IFN), five patients were treated with Tocilizumab (71.4%), and four patients (57.1%) were treated with systemic corticosteroids (Tables 1 and 3). All patients received Azithromycin 500 mg for 3 days, hydroxychloroquine at a dose of 5 mg/kg for 7 days and 3rd generation cephalosporins for 7 days (Table 1). Interestingly, Tocilizumab was prescribed in 71.4% of the cases whereas only 33.3% was prescribed to the other group (p‐value = .05). Similarly, type 1β IFN was prescribed to 71.4% vs 20.9% patients without Aspergillus spp. (p‐value ≤ .05). A combination of Tocilizumab with corticosteroids was more frequent in IPA patients (57.1% vs 28.7%, p‐value = .18) (Table 3). From the seven patients diagnosed with IPA, three did not receive antifungal treatment for early death, three were treated with itraconazole and one of them with Amphotericin B (Table 1).
Table 3.
Pharmacological treatment of COVID‐19 patients. Treatment received by patients with and without an Aspergillus sp. coinfection
| Treatment regimen | Aspergillus sp. group (n = 7) | Non‐Aspergillus sp. group (n = 208) | p‐value |
|---|---|---|---|
| Azithromycin; n (%) | 7 (100) | 194 (93.3) | .91 |
| Hydroxychloroquine; n (%) | 3 (43) | 203 (98) | .84 |
| Lopinavir‐ritonavir; n (%) | 3 (43) | 59 (28.3) | .24 |
| Tocilizumab; n (%) | 5 (71.4) | 69 (33.3) | .05 |
| Corticosteroids; n (%) | 4 (57.1) | 187 (90) | .196 |
| Cyclosporine; n (%) | 1 (14.2) | 19 (9.2) | .55 |
| IFN type 1β; n (%) | 5 (71.4) | 9 (4.3) | .05 |
| Combined IS treatment: | 57.1% | 36% | .18 |
| TCZ + SC | 4 (57.1) | 60 (28.7) | |
| TCZ + CP | 0 | 0 | |
| SC + CP | 0 | 10 (4.7) | |
| TCZ + SC + CZ | 0 | 14 (6.7) |
Abbreviations: CP, Cyclosporine; IFN, Interferon; IS, Immunosuppressants; SC, Systemic corticosteroids; TCZ, Tocilizumab.
We identified important complications in those patients, such as acute renal failure (five cases, 71.4%), sepsis (five cases, 71.4%), atrial fibrillation (four cases, 57.1%), pulmonary embolism (three cases, 42.8%) (Table 1). Unfortunately, five patients (71.4%) died in the ICU after an average stay of 24 days (IQR 27) due to complications caused by ARDS and severe respiratory failure.
4. DISCUSSION
Severe SARS‐CoV‐2 infection causes severe lung damage (diffuse alveolar damage in the acute or organising phases and focal pulmonary microthrombi). 15 In some cases, this lung damage was added to previous comorbidities that already affected the patients (eg chronic obstructive pulmonary disease (COPD), type 2 diabetes mellitus and chronic renal failure). COVID‐19 treatment (ie biological treatments, corticosteroids or type 1β IFN) and invasive techniques performed during patient admission to the ICU (eg orotracheal intubation, central venous or arterial catheters, and parenteral nutrition) could lead to a secondary invasive fungal infection. Symptoms, such as fever, cough and dyspnoea could not pinpoint whether the patients had only COVID‐19 or an IPA coinfection. The persistence of fever is probably the most indicative symptom for the existence of an associated concomitant infection. To our knowledge, and to this date, more than 50 published cases of IPA have been identified in patients with COVID‐19. However, the clinical characteristics and risk factors are not well defined. 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 It seems to exist a trend in which patients with IPA have the worst clinical prognosis and the highest mortality. In our cohort, five of the patients that had an IPA coinfection died. Wu JH et al, showed lesions related to a fungal infection in the postmortem analysis of patients with COVID‐19. 26 In our cohort, we did postmortem studies in all deceased patients and we are unaware of any other potential causes. The radiological findings were not specifically caused by IPA, probably because the radiological findings in COVID‐19 patients overlapped those in the former, according to other authors. 27
The prevalence of IPA, published in other studies, was very similar to our data: between four and nine per cent of critically ill patients had IPA. 6 According to Van Arkel et al, the average time for the diagnosis was approximately 19 days after admission, whereas in our study this average was 12 days.
The poor prognosis of patients with IPA supports the systematic screening of Aspergillus spp. infection in critically ill patients with COVID‐19. We assume that the complexity of diagnosing IPA is due to the fact that laboratory results and findings in the radiological images are not sufficient to distinguish aspergillosis from SARS‐CoV‐2 infection.
According to our results, the significant risk factor that seems to be associated with IPA is the treatment for COVID‐19 (Tocilizumab and type 1β IFN). This is in line with the fact that cytokines act in the defence of the host against fungal infections. Patients with severe COVID‐19 have noticeable lung damage due to the viral replication and the consequent cytokine storm and complex inflammatory processes. 28 This hyperinflammatory response has been described in cases of sepsis (between 3.7%–4.3%) and half of the ARDS severe cases. 29 Recent evidence supports the role of IL‐6 as a marker for a more severe disease, 30 and the treatment with Tocilizumab seems to be an excellent choice to treat them. 31 Last but not least, there are published cases of an invasive fungal infection in patients with rheumatoid arthritis treated with Tocilizumab. This evidence can probably be extended to the patients suffering from COVID‐19. 32 , 33
In summary, patients with COVID‐19 and an invasive aspergillosis coinfection have a poor prognostic. Critically ill patients represented a high‐risk group, and we should actively investigate the possibility of aspergillosis in all of these patients. Larger cohort studies are needed in order to understand the role of immunosuppressive therapy with COVID‐19 as a risk factor for IPA.
TRANSPARENCY DECLARATION
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AUTHOR CONTRIBUTION
GONZALO SEGRELLES: Formal analysis (equal); Methodology (equal); Writing‐original draft (equal). Glauber R de S Araújo: Conceptualization (equal); Investigation (equal); Writing‐review & editing (equal). Estefania Llopis‐Pastor: Methodology (equal); Writing‐review & editing. Javier CArrillo: Investigation (equal); Writing‐review & editing (equal). Marta Hernandez: Investigation; Writing‐review & editing. Laura Rey: Investigation; Writing‐review & editing. nestor Rodriguez: Investigation; Writing‐review & editing. ines Escribano: Investigation; Writing‐review & editing. Esther Anton: Investigation; Writing‐review & editing. Celia Zamarro: Investigation; Writing‐review & editing. Mercedes Salmones: Investigation; Writing‐review & editing. Susana Frases: Conceptualization (equal); Formal analysis (equal); Investigation; Methodology (equal); Writing‐original draft (equal); Writing‐review & editing.
Supporting information
Table S1‐S2
ACKNOWLEDGEMENTS
We acknowledge Dr Barbara Hissa for critical reading and scientific editing of the manuscript. This work was supported by the Brazilian agencies Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) ‐ Finance Code 001 and Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ).
REFERENCES
- 1. Brown GD, Denning DW, Gow NAR, et al. Human fungal infections. Sci Transl Med. 2012;4(165):165rv13. 10.1126/scitranslmed.3004404 [DOI] [PubMed] [Google Scholar]
- 2. Das R. An overview of changing trends in systemic fungal infections. Published online 2012; http://www.webmedcentral.com/article_view/3375URL:http://www.webmedcentral.com/article_view/3386
- 3. Schauwvlieghe AFAD, Rijnders BJA, Philips N, et al. Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: a retrospective cohort study. Lancet Respir Med. 2018;6(10):782‐792. 10.1016/S2213-2600(18)30274-1 [DOI] [PubMed] [Google Scholar]
- 4. Hwang DM, Chamberlain DW, Poutanen SM, Low DE, Asa SL, Butany J. Pulmonary pathology of Severe Acute Respiratory Syndrome in Toronto. Mod Pathol. 2005;18(1):1‐10. 10.1038/modpathol.3800247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Santana MF, Pivoto G, Alexandre MAA, et al. Confirmed invasive pulmonary aspergillosis and COVID‐19: the value of postmortem findings to support antemortem management. Rev Soc Bras Med Trop. 2020;53(1):1. 10.1590/0037-8682-0401-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van Arkel ALE, Rijpstra TA, Belderbos HNA, van Wijngaarden P, Verweij PE, Bentvelsen RG. COVID‐19–associated Pulmonary aspergillosis. Am J Respir Crit Care Med. 2020;202(1):132‐135. 10.1164/rccm.202004-1038LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nasri E, Shoaei P, Vakili B, et al. Fatal invasive pulmonary aspergillosis in COVID‐19 patient with acute myeloid leukemia in Iran. Mycopathologia. 2020;1‐8. 10.1007/s11046-020-00493-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Prattes J, Valentin T, Hoenigl M, Talakic E, Reisinger AC, Eller P. Invasive pulmonary aspergillosis complicating COVID‐19 in the ICU ‐ a case report. Med Mycol Case Rep. 2020;1‐3. 10.1016/j.mmcr.2020.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Donnelly JP, Chen SC, Kauffman CA, et al. Revision and update of the consensus definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin Infect Dis. 2020;71(6):1367‐1376. 10.1093/cid/ciz1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. De Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the european organization for research and treatment of cancer/invasive fungal infections cooperative group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) C. Clin Infect Dis. 2008;46(12):1813‐1821. 10.1086/588660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: The Berlin definition. JAMA ‐ J Am Med Assoc. 2012;307(23):2526‐2533. 10.1001/jama.2012.5669 [DOI] [PubMed] [Google Scholar]
- 12. Nguyen Y, Corre F, Honsel V, et al. Applicability of the CURB‐65 pneumonia severity score for outpatient treatment of COVID‐19. J Infect. 2020;81(3):e96‐e98. 10.1016/j.jinf.2020.05.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guo L, Wei D, Zhang X, et al. Clinical features predicting mortality risk in patients with viral pneumonia: the MuLBSTA score. Front Microbiol. 2019;10:2752. 10.3389/fmicb.2019.02752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jabaudon M, Audard J, Pereira B, et al. Early changes over time in the radiographic assessment of lung edema score are associated with survival in ARDS. Chest. 2020. 10.1016/j.chest.2020.06.070. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bradley BT, Maioli H, Johnston R, et al. Histopathology and ultrastructural findings of fatal COVID‐19 infections in Washington State: a case series. Lancet. 2020;396(10247):320‐332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Van Arkel ALE, Rijpstra TA, Belderbos HNA, van Wijngaarden P, Verweij PE, Bentvelsen RG. COVID‐19‐associated pulmonary aspergillosis. Am J Respir Crit Care Med. 2020;202(1):132‐135. 10.1164/rccm.202004-1038LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mohamed A, Rogers TR, Talento AF. COVID‐19 Associated invasive pulmonary aspergillosis: Diagnostic and therapeutic challenges. J Fungi. 2020;6(3):115. 10.3390/jof6030115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Salehi M, Ahmadikia K, Badali H, Khodavaisy S. Opportunistic fungal infections in the epidemic area of COVID‐19: A clinical and diagnostic perspective from Iran. Mycopathologia. 2020;185(4):607‐611. 10.1007/s11046-020-00472-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Verweij PE, Gangneux J‐P, Bassetti M, et al. Diagnosing COVID‐19‐associated pulmonary aspergillosis. Lancet Microbe. 2020;1(2):e53‐e55. 10.1016/S2666-5247(20)30027-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brown L‐AK, Ellis J, Gorton R, De S, Stone N. Surveillance for COVID‐19‐associated pulmonary aspergillosis. Lancet Microbe. 2020;1(4):e152. 10.1016/S2666-5247(20)30091-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Alanio A, Dellière S, Fodil S, Bretagne S, Mégarbane B. Prevalence of putative invasive pulmonary aspergillosis in critically ill patients with COVID‐19. Lancet Respir Med. 2020;8(6):e48‐e49. 10.1016/S2213-2600(20)30237-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Salehi M, Ahmadikia K, Mahmoudi S, et al. Oropharyngeal candidiasis in hospitalised COVID‐19 patients from Iran: Species identification and antifungal susceptibility pattern. Mycoses. 2020;63(8):771‐778. 10.1111/myc.13137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rutsaert L, Steinfort N, Van Hunsel T, et al. COVID‐19‐associated invasive pulmonary aspergillosis. Ann Intensive Care. 2020;10(1):71. 10.1186/s13613-020-00686-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Verweij PE, Rijnders BJA, Brüggemann RJM, et al. Review of influenza‐associated pulmonary aspergillosis in ICU patients and proposal for a case definition: an expert opinion. Intensive Care Med. 2020;46(8):1524‐1535. 10.1007/s00134-020-06091-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bulpa P, Dive A, Sibille Y. Invasive pulmonary aspergillosis in patients with chronic obstructive pulmonary disease. Eur Respir J. 2007;30(4):782‐800. 10.1183/09031936.00062206 [DOI] [PubMed] [Google Scholar]
- 26. Wu JH, Li X, Huang B, et al. Pathological changes of fatal Coronavirus disease 2019 (COVID‐19) in the lungs: report of 10 cases by postmortem needle autopsy. Zhonghua Bing Li Xue Za Zhi. 2020;49(6):568‐575. 10.3760/cma.j.cn112151-20200405-00291 [DOI] [PubMed] [Google Scholar]
- 27. Koehler P, Cornely OA, Böttiger BW, et al. COVID‐19 associated pulmonary aspergillosis. Mycoses. 2020;63(6):528‐534. 10.1111/myc.13096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang LP, Wang M, Wang Y, Zhu J, Zhang N. Focus on the 2019 novel Coronavirus (SARS‐CoV‐2). Future Microbiol. 2020;15:905‐918. 10.2217/fmb-2020-0063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID‐19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20(6):363‐374. 10.1038/s41577-020-0311-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vabret N, Britton GJ, Gruber C, et al. Immunology of COVID‐19: current state of the science. Immunity. 2020;52(6):910‐941. 10.1016/j.immuni.2020.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cala‐García JD, Sierra‐Bretón JD, Cavelier‐Baiz JE, Faccini‐Martínez ÁA, Pérez‐Díaz CE. Recovery of COVID‐19 Acute Respiratory Distress Syndrome with Tocilizumab: successful outcome in two critically ill patients. Immunotherapy. 2020;12(15):1127‐1132. 10.2217/imt-2020-0154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gastaldi R, Sicaud A, Gaudin P, Baillet A. Pulmonary Pasteurellosis in a patient treated with Tocilizumab for rheumatoid arthritis. JCR J Clin Rheumatol. 2017;23(8):451‐452. 10.1097/RHU.0000000000000592 [DOI] [PubMed] [Google Scholar]
- 33. Yamaguchi T, Ito S, Takano Y, et al. A case of disseminated sporotrichosis treated with prednisolone, immunosuppressants, and Tocilizumab under the diagnosis of rheumatoid arthritis. Intern Med. 2012;51(15):2035‐2039. 10.2169/internalmedicine.51.7342 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1‐S2


