Abstract
Thrombotic complications in patients with prior COVID‐19 infection raises concern for a persistent hypercoagulable state among these patients. Thus, there is a dire need for further research aimed at anticoagulation guidelines for the same.
Keywords: aortic thrombosis, COVID‐19, patent foramen ovale, renal infarction
Thrombotic complications in patients with prior COVID‐19 infection raises concern for a persistent hypercoagulable state among these patients. Thus, there is a dire need for further research aimed at anticoagulation guidelines for the same.
1. INTRODUCTION
A 39‐year‐old female presented to the ED with severe worsening back pain. Investigation revealed an aortic thrombus and right kidney infarct which was attributed to hypercoagulable state secondary to COVID‐19 infection.
In wake of recent COVID‐19 pandemic, the medical community is facing new diagnostic challenges everyday. The full spectrum of clinical manifestations of COVID‐19 virus is not yet understood. Several case studies and reports have suggested that COVID‐19 infection leads to disruption in hemostasis and a hypercoagulable state. 1 , 2 However, the mechanism and long‐term consequences of this remain unclear. Here, we attempt to shed light on an atypical presentation of what is believed to be a late sequela of COVID‐19 infection in a young woman who presented to the hospital with right‐sided flank pain and was found to have an aortic thrombus with right renal infarcts.
2. CASE
A 39‐year‐old female presented with dull aching pain in the right flank which was nonradiating and worsened with cough and deep inspiration. On initial encounter, patient was in no apparent distress and vitals were remarkable for a blood pressure of 179/117. Examination revealed an alert and oriented young obese female with mild right‐sided costovertebral angle tenderness. Lungs were clear on auscultation, and cardiac examination was remarkable for a fixed splitting S2. The extremities were warm and well perfused without evidence of edema. Computed tomography with and without angiography revealed a thrombus within the descending aorta proximal to the diaphragmatic hiatus along with smaller mural thrombi noted proximal to the bilateral renal arteries and wedge‐shaped areas of nonenhancement in the right kidney, compatible with embolic infarcts (Figure 1, Video S1). Patient denied any recent viral illness, family history suggestive of hypercoagulable disorder, or any hospitalizations in the past.
Figure 1.
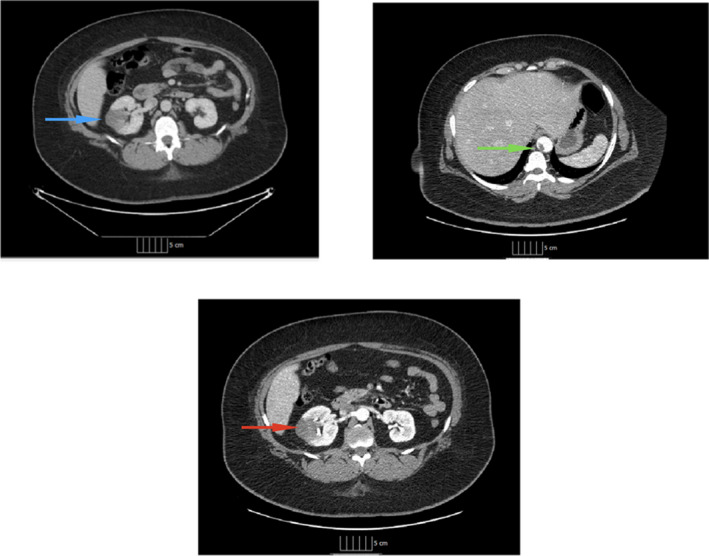
Computed tomography of the abdomen with and without angiography. Computed tomography with and without angiography showing site of aortic thrombus, renal thrombus, and renal infarct. The blue arrow points to the site of hypoattenuation noted in the right kidney on a noncontrast scan. The green arrow and the red arrow show the aortic thrombus and area of renal infarction, respectively, on angiography
Past medical history was significant for poorly controlled hypertension and use of oral contraceptives. The differential at this point included an in situ versus embolic thrombosis of aorta and renal artery. Common initial laboratory values were within normal limits except for a WBC count of 14 and PTT elevation to 46.1 seconds. EKG revealed normal sinus rhythm, and cardiac troponin was within normal limits. The computed tomography angiogram also noted innumerable hyperattenuating lesions throughout the liver, most of which exhibited peripheral rim enhancement with central hypoattenuation. The chest was remarkable for a right hilar lymph node measuring up to 1.5 cm and right upper lobe and lower lobe pulmonary nodules measuring up to 6mm. Another set of focused laboratory tests (Table 1) showed an erythrocyte sedimentation rate (ESR) of 70, C‐reactive protein (CRP) of 18.74, lactic acid dehydrogenase (LDH) of 480, a positive lupus anticoagulant screen, and a positive beta‐2 glycoprotein with a titer of 54.9. Patient screened negative for other common inherited thrombophilias. Patient's COVID‐19 PCR was negative, but was positive for COVID IgG antibody. An echocardiogram revealed a normal ejection fraction (EF) of 66% ‐ 70%, and a saline contrast study showed a moderate‐sized patent foramen ovale.
Table 1.
Laboratory values
| Laboratory | Value | Reference range |
|---|---|---|
| Hb | 12.6 | 12.0‐16.0 g/dl |
| WBC | 14.5 | 4.8‐10.8 K/ul |
| Plt | 471 | 150‐400 k/ul |
| INR | 1 | 0.9‐1.2 |
| PT | 11.7 | 9.9‐13.2sec |
| aPTT | 46.1 | 24.5‐32.3 sec |
| COVID‐19 PCR | Negative | Negative |
| COVID‐19 IgG | Positive | Negative |
| ESR | 70 | 0‐20 mm/hr |
| CRP | 18.74 | 0.00‐0.40 mg/dl |
| Lupus screen | Positive | Negative |
| Protein S activity | 66 | 60%‐140% |
| Protein C Functional assay | 109 | 75.2%‐159.8% |
| Factor V assay | 95 | 50%‐150% |
| Antithrombin | 99 | 76.8%‐117.5% |
| Sodium | 136 | 135‐149 MMOL/L |
| Potassium | 4.1 | 3.4‐4.8 MMOL/L |
| Chloride | 95 | 93‐105 MMOL/L |
| Bicarbonate | 25 | 23‐32 MMOL/L |
| Creatinine | 1.1 | 0.3‐1.1 mg/dL |
| BUN | 11 | 7‐21 mg/dL |
| Calcium | 9.1 | 8.2‐10.1 mg/dL |
| Anion Gap | 16 | 4‐14 |
| LDH | 408 | 108‐199 IU/L |
Results of the laboratory tests carried out at admission.
Abbreviations: aPTT, Activated partial thromboplastin time; BUN, Blood urea nitrogen; CRP, C‐reactive protein; ESR, Erythrocyte sedimentation rate; Hb, Hemoglobin; LDH, Lactate dehydrogenase; PCR, Polymerase chain reaction; Plt, Platelet count; PT, Prothrombin time; WBC, White blood cell count.
Patient received adequate analgesia and over the course of her hospital stay remained hemodynamically stable, reported improvement in pain, and was also assessed by vascular surgery who recommended no surgical intervention. After due consultation with hematology, the patient was started on therapeutic anticoagulation with apixaban and discharged to home.
3. DISCUSSION
Since the outbreak of COVID‐19 from the Wuhan province of China, there has been a catastrophic global impact of unprecedented scale. Since July 2020, estimates have been around 15,785,641 cases and 640,016 deaths worldwide. 3 Given the current trend of this global health catastrophe, there is a dire need for better understanding of this disease.
Recent data suggest that the clinical manifestations of the COVID‐19 are mainly respiratory; however, it has a wide range of clinical presentations and affects multiple organ systems. 4 Since we are still in the process of learning the clinical profile of COVID‐19, classifying symptoms or presentations as typical versus atypical at this point may be difficult. However, for the purpose of this discussion we assume all presentations other than those of a typical viral respiratory infection as atypical. Table 2 illustrates some of the atypical symptoms/presentations associated with COVID‐19 infection. 5 , 6
Table 2.
Extra‐pulmonary manifestations and atypical presentations of COVID‐19
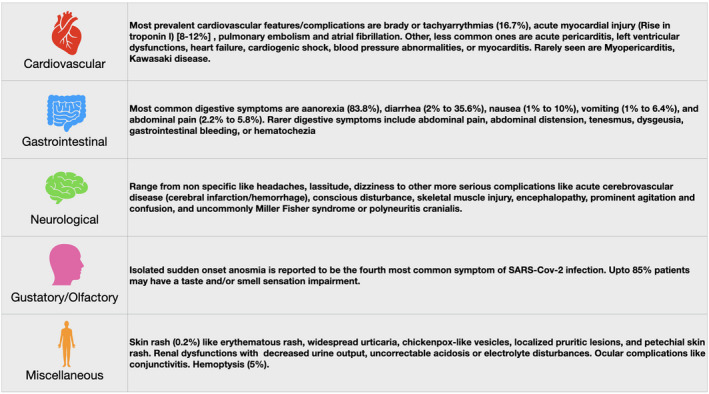
Diverse system involvement and symptomatology in COVID‐19 patients.
Abbreviation: SARS‐Cov‐2, Severe acute respiratory syndrome coronavirus 2.
Diverse system involvement and symptomatology in COVID‐19 patients.
Abbreviation: SARS‐Cov‐2, Severe acute respiratory syndrome coronavirus 2.
One of the well‐known effects of the COVID‐19 infection is a hypercoagulable state resulting in various thrombotic events despite standard thromboprophylaxis. These thrombotic events are seen to be both venous and/or arterial resulting in events like deep vein thrombosis (DVTs), pulmonary embolism (PEs), and even ischemic strokes. 7 , 8 , 9
Derangements of the clotting cascade have been observed as well, which may result in altered laboratory values, one of which is an elevated aPTT. 7 , 10 Being part of common initial laboratory panel in most healthcare settings, an abnormal value may result in delay in initiation of adequate anticoagulation therapy, in part due to fear of a bleeding diathesis. However, a good percentage of COVID‐19 patients with elevated aPTT also test positive for lupus anticoagulant. In fact, a recent study demonstrated up to 91% of these patients test positive for lupus anticoagulant. 7 , 11 Other abnormalities of the coagulation system are also fairly common, including but not limited to increased von Willebrand (vWF) activity, vWF antigen, and factor VIII levels, while factor XII levels can be often low. 5 , 7 , 8
Many lupus anticoagulant assays are sensitive to CRP levels and lead to false‐positive results in conditions where CRP is markedly elevated such as COVID‐19. 12 The need to anticoagulate patients with COVID‐19 infection has been widely recognized even in the absence of randomized trials. Incidence of thrombotic complications is high among all COVID‐19 patients but significantly more so among patients in the intensive care units (incidence 1.1% in non‐ICU patients vs. 79% in ICU patients). 7 Autopsy studies have shown most patients showed macro‐ and microvascular thrombosis in lungs among other vital organs. 7 Currently, the American Society of Hematology recommends that all hospitalized patients with COVID‐19 should be treated with prophylactic doses of low molecular weight heparin (LMWH) or fondaparinux (recommended over unfractionated heparin), while therapeutic doses should be reserved for intubated patients who develop sudden clinical and laboratory findings consistent with PE, physical findings consistent with thrombosis, and patients with respiratory failure, particularly when D‐dimer and/ or fibrinogen levels are very high. 13 , 14
4. CONCLUSION
This case raises the question of lingering sequelae of COVID‐19 after seroconversion and lack of active infection. Given that the patient had a patent foramen ovale, it is unclear at this point whether the thrombosis was in situ and purely arterial or whether it migrated from the venous system. Regardless of its origin, there seems to be a higher risk of thrombotic events among such patients. This notion reinforces the need of adequate anticoagulation among COVID‐19 patients. However, currently there are no guidelines regarding anticoagulation for this population of patients who are not hospitalized and have remote history of infection. It seems that there is a critical need for further research among patients who have “cleared” COVID‐19 infection and now have antibodies for the same. This however may be a difficult task since a large number of such patients may be asymptomatic and thus present to the hospital only after they show complications for thromboembolism.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
Sanchit V. Kundal: served as a member of primary team, was involved in the management of patient, interviewed and examined the patient, collected and compiled data, reviewed relevant research articles, and composed the manuscript. Emmanuel U. Emeasoba: reviewed the relevant research articles and contributed to the discussion, and also assisted in compiling the manuscript. Chad Harris: reviewed the relevant research articles and contributed to the discussion. Gurchetan Randhawa: reviewed the relevant research articles and contributed to the discussion. Mariya Astashkevich: reviewed the relevant research articles and contributed to the discussion.
ETHICAL APPROVAL
Ethic committee was not consulted for approval as the case report was written with due permission from the patient and with all possible efforts to maintain complete anonymity.
Supporting information
Video S1
ACKNOWLEDGMENTS
The authors thank the patient for providing consent to publish this case report. Published with written consent of the patient.
Kundal SV, Emeasoba EU, Harris C, Randhawa G, Astashkevich M. Aortic thrombosis and renal infarction in a young female with patent foramen ovale and COVID‐19 antibody. Clin Case Rep.2021;9:345–349. 10.1002/ccr3.3527
Twitter handle @thenewyorkmd.
DATA AVAILABILITY STATEMENT
Not applicable.
REFERENCES
- 1. Panigada M, Bottino N, Tagliabue P, et al. Hypercoagulability of COVID‐19 patients in Intensive Care Unit. A report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemos. 2020;18(7):1738–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Spiezia L, Boscolo A, Poletto F, et al. COVID‐19‐Related Severe Hypercoagulability in Patients Admitted to Intensive Care Unit for Acute Respiratory Failure. Thromb Haemost. 2020;120(6):998‐1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. WHO COVID‐19 situation report 188. Webpage ‐ https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports
- 4. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet (London, England). 2020;395(10229):1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Abobaker A, Raba AA, Alzwi A. Extrapulmonary and atypical clinical presentations of COVID‐19. J Med Virol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baj J, Karakuła‐juchnowicz H, Teresiński G, et al. COVID‐19: specific and non‐specific clinical manifestations and symptoms: the current state of knowledge. J Clin Med. 2020;9(6):1753–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Singhania N, Bansal S, Nimmatoori DP, Ejaz AA, Mccullough PA, Singhania G. Current Overview on Hypercoagulability in COVID‐19. Am J Cardiovasc Drugs. 2020;20(5):393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Helms J, Tacquard C, Severac F, et al. High risk of thrombosis in patients with severe SARS CoV‐2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46(6):1089–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Llitjos JF, Leclerc M, Chochois C, et al. High incidence of venous thromboembolic events in anticoagulated severe COVID‐19 patients. J Thromb Haemost. 2020;18(7):1743–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Klok FA, Kruip MJHA, Van der meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID‐19. Thromb Res. 2020;191:145–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bowles L, Platton S, Yartey N, et al. Lupus Anticoagulant and Abnormal Coagulation Tests in Patients with Covid‐19. N Engl J Med. 2020;383(3):288‐290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schouwers SM, Delanghe JR, Devreese KM. Lupus Anticoagulant (LAC) testing in patients with inflammatory status: does C‐reactive protein interfere with LAC test results? Thromb Res. 2010;125(1):102‐104. [DOI] [PubMed] [Google Scholar]
- 13. Kreuziger LB, et al.COVID‐19 and VTE/Anticoagulation: Frequently Asked Questions. [Webpage], (2020) April 17, 2020 [cited 2020; Version 2.1].
- 14.Lee, et al.COVID‐19 and Pulmonary Embolism: Frequently Asked Questions. [Webpage], April 9, 2020; Version 1.0: Available from: https://www.hematology.org/covid-19/covid-19-and-pulmonary-e
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1
Data Availability Statement
Not applicable.


