Abstract
Background
The incidence of candidemia in our hospital has been stable over an 18‐year period (1.3 episodes per 1000 admissions). Since March 2020, we have observed an increase in cases of candidemia.
Methods
In March 2020, the hospital was prepared to receive patients with COVID‐19, with cancellation of elective procedures, discharge of less sick patients and the activation of beds for COVID‐19. We compared the incidence of candidemia in 2 periods: from January 2019 to February 2020 (period 1) and from March to September 2020 (period 2).
Results
We diagnosed 41 episodes of candidemia, 16 in period 1 and 25 in period 2 (9 COVID‐19 patients). Compared with non‐COVID‐19 patients, COVID‐19 patients with candidemia were more likely to be under mechanical ventilation (100% vs. 34.4%, P < .001). The median number of monthly admissions in period 1 and 2 was 723 (interquartile range 655‐836) and 523 (interquartile range 389‐574), respectively. The incidence of candidemia (per 1000 admissions) was 1.54 in period 1 and 7.44 in period 2 (P < .001). In period 2, the incidence of candidemia (per 1000 admissions) was 4.76 if we consider only cases of candidemia in non‐COVID‐19 patients, 2.68 if we consider only cases of candidemia in COVID‐19 patients and 14.80 considering only admissions of patients with COVID‐19.
Conclusions
The increase in the incidence of candidemia in our hospital may be attributed to 2 factors: a reduction in the number of admissions (denominator) and the occurrence of candidemia in COVID‐19 patients.
Keywords: candidemia, Candida, deep fungal infection, epidemiology, systemic infection, candidemia
1. INTRODUCTION
Candidemia is a frequent bloodstream infection worldwide, with high crude mortality rates. 1 , 2 The disease affects particularly patients with serious conditions requiring intensive care, with various risk factors for candidemia, including exposure to broad‐spectrum antibiotics, corticosteroids and other immunosuppressive agents and invasive procedures such as intravascular catheters, mechanical ventilation and dialysis. 3 Indeed, about 40% of patients are in an intensive care unit (ICU) at the time the diagnosis of candidemia is established. 4 , 5
The emergence of the COVID‐19 pandemic brought new challenges for healthcare workers worldwide. 6 , 7 Among patients with COVID‐19 who require hospitalisation, many develop severe acute respiratory distress syndrome, are admitted to an ICU and are exposed to various factors associated with candidemia. 3 Therefore, candidemia could be a potential complication of patients with COVID‐19 cared in ICUs. A series of 989 patients with COVID‐19 admitted to a hospital in Spain reported four cases of candidemia among 88 coinfections and superinfections. 8 Another series from Italy reported 21 cases of candidemia in patients with COVID‐19 and found a higher incidence of candidemia in COVID‐19 patients compared with a historical cohort. 9
In Brazil, the first case of COVID‐19 was diagnosed in February 2020, 10 and the incidence increased exponentially since March. 11 Accordingly, hospitals in the large cities in Brazil started to implement changes in their routine to be prepared for the increased need of hospital beds for patients with COVID‐19.
The incidence of candidemia in our hospital has been stable over a 21‐year period, with an overall incidence of 1.3 episodes per 1000 admissions. 12 In March 2020, the hospital was prepared to receive patients with COVID‐19, with the discharge of stable patients, cancellation of medical and surgical elective procedures, the deactivation of regular beds and activation of additional beds for COVID‐19, including ICU beds. In this study, we report an increase in the incidence of candidemia since March 2020.
2. PATIENTS AND METHODS
The study was conducted in Hospital Universitário Clementino Fraga Filho, a tertiary care university‐affiliated public hospital located in the city of Rio de Janeiro. In February 2020, the hospital had 280 active beds (16 of ICU), distributed to different medical and surgical specialties, taking care of patients with complex diseases such as cancer, hematopoietic and solid organ transplantation, autoimmune diseases and chronic degenerative diseases. The present study is part of an active study that characterises the epidemiology of candidemia in the hospital, approved by the Hospital Ethical Committee (number 30/03).
In early March, the hospital administration implemented changes in the routine work to adapt for the upcoming increase in cases of COVID‐19 requiring hospitalisation and intensive care. The changes comprised cancellation of all surgical and medical procedures that were not considered urgent, discharge of stable patients, deactivation of 50 regular beds and cancellation of routine outpatient visits. In addition, two new areas for patients with COVID‐19 were created, with 100 additional beds, including 25 for intensive care.
An episode of candidemia (see definition below) was identified by looking at the records of the microbiology laboratory. Once an episode was identified, patients were followed for 30 days from the date of the incident candidemia. We used a standardised case report form and a dictionary of terms to collect information about the episode of candidemia, including demographics, medical ward, underlying medical condition, co‐morbidities, coexisting exposures (antibiotics, immunosuppressive agents, parenteral nutrition, mechanical ventilation, surgery), treatment and the outcome (30‐day mortality).
An episode of candidemia was defined as the first isolation (incident candidemia) of Candida species from a blood culture in a patient with signs of infection. New positive blood cultures within 30 days from the incident candidemia were considered part of the same episode. Blood cultures were obtained at the discretion of the attending physician and were processed using the automated system Bactec (Becton Dickinson, NJ, USA). Species identification was performed using CHROMagar (Becton Dickinson, NJ, USA), germ tube and microscopic morphology on cornmeal Tween 80 agar, complemented by biochemical tests using the Vitek 2 cards (BioMérieux AS, Marcy l´ Etoile, France).
In order to evaluate changes in the incidence of candidemia, we compared the incidence from January 2019 to February 2020 (period 1) with the incidence from March to September 2020 (period 2) using the chi‐square test. We also compared the clinical characteristics of episodes of candidemia in patients with and without COVID‐19. Categorical and continuous variables were compared using Fisher's exact test and Mann‐Whitney U test, respectively. All statistical analyses were performed in the SPSS software (IBM SPSS Statistics version 21) and Epi‐Info (Centers for Disease Control and prevention, version 7.2.2.6).
3. RESULTS
During the study period, we observed 41 episodes of candidemia in 41 patients: 16 in period 1 and 25 in period 2. The median age of the 41 patients was 62 years (interquartile range 54‐71), and 51.2% were male. Twenty patients (48.8%) were in the ICU at diagnosis of candidemia. Candida albicans (41.5%) was the most frequent agent of candidemia (Table 1). Ten patients did not receive treatment because they died before the diagnosis of candidemia. Treatment was anidulafungin in 28 and fluconazole in three patients. The 30‐day mortality rate in the entire cohort was 61.0% (51.6% in patients who received treatment).
TABLE 1.
Characteristics of candidemic patients with and without COVID‐19
| Variable |
Total n = 41 |
COVID‐19 n = 9 |
Non‐COVID‐19, period 1 n = 16 |
Non‐COVID‐19, period 2 n = 16 |
|---|---|---|---|---|
| Gender Male : Female | 21:20 | 5:4 | 9:7 | 7:9 |
| Age, median (interquartile range) | 62 (17‐87) | 62 (54‐87) | 62.5 (46‐67) | 58 (53‐73) |
| Time (days) from admission to candidemia, median (range) | 15 (0‐71) | 17 (0‐22) | 22 (11.5‐32) | 13 (2.5‐19) |
| Admission in ICU | 20 (48.8) | 7 (77.8) | 8 (50.0) | 5 (31.2) |
| Cancer | 11 (26.8) | 1 (11.1) | 6 (37.5) | 4 (25.0) |
| Haematologic malignancy | 4 | 0 | 2 | 2 |
| Solid tumour | 7 | 1 | 4 | 2 |
| Diabetes | 16 (39.0) | 3 (33.3) | 6 (37.5) | 7 (43.8) |
| Chronic renal failure | 13 (31.7) | 1 (11.1) | 7 (43.8) | 5 (31.3) |
| Chronic dialysis | 7 (17.0) | 0 | 4 (25.0) | 3 (18.8) |
| Liver disease | 6 (14.6) | 1 (11.1) | 2 (12.5) | 3 (18.8) |
| Neurologic disease | 9 (22.0) | 2 (22.2) | 5 (31.3) | 2 (12.5) |
| Cardiac disease | 28 (68.3) | 6 (66.7) | 10 (62.5) | 12 (75.0) |
| Lung disease | 6 (14.6) | 0 | 1 (6.3) | 5 (31.3) |
| Surgery | 10 (24.4) | 0 | 6 (37.5) | 4 (25.0) |
| Mechanical ventilation | 20 (48.8) | 9 (100) | 4 (25.0) | 7 (43.8) |
| Parenteral nutrition | 2 (4.9) | 0 | 2 (12.5) | 0 |
| Central venous catheter | 39 (95.1) | 9 (100) | 16 (100) | 14 (87.5) |
| Hypotensive | 24 (58.5) | 8 (88.9) | 7 (43.8) | 9 (56.3) |
| Fever | 20 (48.8) | 2 (22.2) | 9 (56.3) | 9 (56.3) |
| Candida species | ||||
| C albicans | 17 (41.5) | 5 (55.6) | 7 (43.8) | 5 (31.3) |
| C tropicalis | 10 (24.4) | 2 (22.2) | 3 (18.8) | 5 (31.3) |
| C parapsilosis | 7 (17.1) | 0 | 4 (25.0) | 3 (18.8) |
| C glabrata | 4 (9.8) | 1 (11.1) | 2 (12.5) | 1 (6.3) |
| Other a | 3 | 1 | 2 | |
| Received treatment | 31 (75.6) | 6 (66.7) | 12 (75.0) | 13 (81.3) |
| Anidulafungin | 28 / 31 (90.3) | 6 / 6 (100) | 11 / 12 (91.7) | 11 / 13 (84.6) |
| Fluconazole | 3 / 31 (9.7) | 0 | 1 / 12 (8.3) | 2 / 13 (15.4) |
| 30‐day mortality | 25 (61.0) | 6 (66.7) | 9 (56.3) | 10 (62.5) |
Numbers in parenthesis represent percentage unless specified
Period 1: January 2019 to February 2020; Period 2: March to September 2020
ICU = intensive care unit
Other species – COVID‐19 patients: C famata; non‐COVID‐19 patients: C haemulonii and C albicans + C tropicalis (1 each)
The number of admissions of patients with COVID‐19 since March 2020 was 608. Among the 25 episodes of candidemia in period 2, nine occurred in patients with COVID‐19 (1.5% of patients admitted with COVID‐19). All patients with COVID‐19‐associated candidemia were under mechanical ventilation, compared with 34.4% of non‐COVID‐19 patients (P < .001). In addition, although non‐significant, COVID‐19 patients were more likely to be in an ICU (77.8% vs. 40.6%, P = .07) and to be hypotensive at diagnosis of candidemia (88.9% vs. 50.0%, P = .06) and less likely to have surgery within 30 days before candidemia (no patient vs. 31.2%, P = .08). The 30‐day mortality rate was high in both groups (66.7% in COVID‐19 and 59.4% in non‐COVID‐19 patients, Table 1).
The median number of monthly admissions in periods 1 and 2 was 723 (interquartile range 655‐835) and 523 (interquartile range 389‐574), respectively, and the median number of patients‐day was 6936 (interquartile range 6452‐7232) in period 1 and 5428 (interquartile range 4952‐6761) in period 2. As shown in Figure 1, while the number of admissions decreased in period 2 (March‐September 2020), coinciding with the preparation of the hospital to the COVID‐19 pandemic, the number of cases of candidemia increased. The overall incidence of candidemia in the 21‐month study period was 2.98 episodes per 1000 admissions, being 1.54 per 1000 admissions in period 1 and 7.44 per 1000 admissions in period 2 (P < .001, risk ratio 4.83; 95% confidence interval 2.58‐9.04). Considering the periods of March to September 2019 and 2020, the incidence was 1.28 and 7.44 per 1000 admissions, respectively (P < .001). The incidence of candidemia per 1000 patients‐day was also significantly higher in period 2 (0.17 in period 1 and 0.62 in period 2, P < .001, risk ratio 3.69, 95% confidence interval 1.97‐6.90). In period 2, the incidence of candidemia per 1000 admissions was 4.76 if we consider only cases of candidemia in patients without COVID‐19, 2.68 if we consider only cases of candidemia occurring in patients with COVID‐19, and 14.80 considering only admissions of patients with COVID‐19.
FIGURE 1.
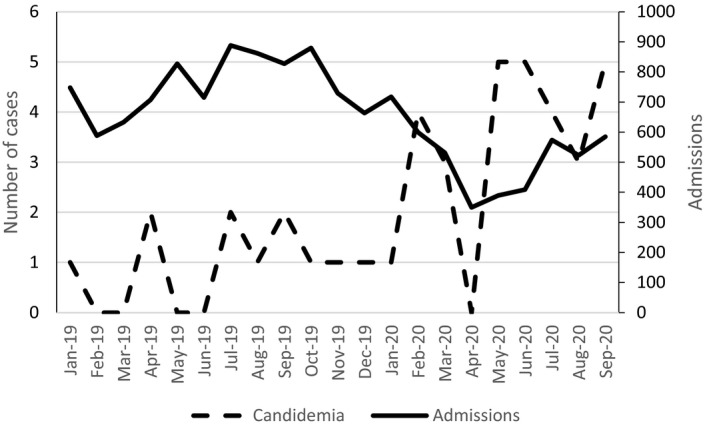
Admissions and cases of candidemia in the 21‐month study period
4. DISCUSSION
In our study, we observed a significant increase in the incidence of candidemia, coinciding with the start of the admission of patients with COVID‐19 in the hospital, with nine of 25 cases of candidemia occurring in patients with COVID‐19.
The epidemiology and incidence of candidemia in our hospital have been well characterised. Comparing three 7‐year periods, we observed an overall incidence of 1.30 episodes per 1000 admissions, which was quite stable in the three periods: 1.05, 1.50 and 1.38 per 1000 admissions, respectively. Cancer was the underlying disease in about one‐third of cases, and candidemia occurred in the context of surgery (mostly abdominal) in about 40% of cases. 12
Patients presenting with severe forms of COVID‐19 are at increased risk to develop candidemia. These patients are exposed to multiple risk factors for candidemia, such as antibiotics, corticosteroids, venous catheters and dialysis. 13 In addition, enterocytes are infected by SARS‐CoV‐2 due to the high expression of the angiotensin converting enzyme‐2 receptor, 14 causing diarrhoea, nausea, vomiting and abdominal pain. 15 Disruption of mucosal barrier caused by COVID‐19 could be an additional risk factor for candidemia, by facilitation the translocation of Candida species from the gut lumen to the bloodstream. 16 Finally, the use of tocilizumab to treat COVID‐19 may further increase the risk for candidemia. 17
A review paper reported 43 published cases of invasive yeast infections in patients with COVID‐19, 40 of which were caused by Candida species. 18 The overall incidence varied from 0.7% to 23.5%, and the majority of patients with candidemia were in an ICU and had been exposed to various factors associated with candidemia, including central venous catheter, broad‐spectrum antibiotics, corticosteroids and parenteral nutrition. Remarkably, surgery was reported in only one case (vascular surgery, which is not a classical predisposing factor for candidemia). 19
In our study, baseline characteristics of patients with COVID‐19‐associated and non‐COVID‐19‐associated candidemia differed. While non‐COVID‐19 cases resembled our usual patient profile, with cancer as underlying disease and previous surgery in 31.2% of cases, only one candidemic patient with COVID‐19 had cancer (solid tumour), and none had prior surgery. In addition, all COVID‐19 patients were in ICU at diagnosis of candidemia, compared with 40.6% of non‐COVID‐19 patients.
Another important finding of the present study was the increased incidence of candidemia in period 2 (7.44 per 1000 admissions) compared with period 1 (1.54 per 1000 admissions, which was similar to the overall historical rate in the hospital). The increase in the incidence of candidemia could be explained by two factors, as shown in Figure 1: an increase in the absolute number of episodes of candidemia and a decrease in the number of admissions in period 2.
The hospital administrative team established a series of measures to comply with the expected increase in the need of hospital beds to face the emerging rise in the incidence of COVID‐19 in the city. This resulted in the deactivation of 50 regular beds and the creation of two areas for COVID‐19 patients, with 25 ICU beds and 75 regular beds. In addition, elective admissions were cancelled, including admissions for elective surgeries, which are associated with short period of hospitalisation and a high turnover of hospital beds. By contrast, even with a net increase in the number of hospital beds, the admission of patients with COVID‐19, with various complications, resulted in a lower turnover of beds. As consequence, the number of admissions reduced substantially in period 2. Therefore, one explanation for the increased incidence of candidemia is simply a mathematical issue: a reduction in the denominator. However, there was also an increase in the absolute number of cases, both in COVID‐19 and non‐COVID‐19 patients, resulting in an incidence higher than our historical incidence of 1.30 episodes per 1000 admissions (4.76 episodes per 1000 admissions in patients without COVID‐19, 2.68 in patients with COVID‐19 and 14.80 considering only admissions of patients with COVID‐19). On the other hand, the increase in the incidence of candidemia in non‐COVID‐19 patients may have occurred because during the pandemic, only the sicker (and at higher risk for candidemia) patients remained in the hospital.
An important limitation of our study is the small sample size, hampering the comparison of characteristics of COVID‐19‐associated and non‐COVID‐19‐associated candidemia. Nevertheless, it seems that while COVID‐19 patients with severe disease are at an increased risk to develop candidemia, the characteristics of candidemia in these patients are different from the usual non‐COVID‐19‐associated candidemia that occur in our hospital. Acknowledging these differences may help to early identify patients at higher risk to develop candidemia. Future studies are needed to define risk factors as well as to evaluate the role of prediction scores and fungal biomarkers in the early diagnosis of COVID‐19‐associated candidemia.
CONFLICT OF INTEREST
Dr Nucci reports personal fees from Pfizer, personal fees from MSD, personal fees from Basilea, personal fees from Biotoscana, personal fees from Astellas, personal fees from Abbvie, personal fees from Amgen, personal fees from Janssen, outside the submitted work; Other authors: nothing to disclose.
AUTHOR CONTRIBUTION
Marcio Nucci: Conceptualization (equal); Formal analysis (equal); Investigation (equal); Methodology (equal); Writing‐original draft (equal); Writing‐review & editing (equal). Gloria Barreiros: Investigation (equal); Writing‐review & editing (equal). Luiz Guimarães: Investigation (equal); Writing‐review & editing (equal). Vitor Deriquehem: Investigation (equal); Writing‐review & editing (equal). Anna Castiñeiras: Investigation (equal); Writing‐review & editing (equal). Simone A Nouer: Conceptualization (equal); Investigation (equal); Methodology (equal); Writing‐review & editing (equal).
REFERENCES
- 1. Cornely FB, Cornely OA, Salmanton‐Garcia J, et al. Attributable mortality of candidemia after introduction of echinocandins. Mycoses. 2020;63:1373‐1381. [DOI] [PubMed] [Google Scholar]
- 2. Ricotta EE, Lai YL, Babiker A, et al. Invasive candidiasis species distribution and trends, United States, 2009–2017. J Infect Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Eggimann P, Garbino J, Pittet D. Epidemiology of Candida species infections in critically ill non‐immunosuppressed patients. Lancet Infect Dis. 2003;3:685‐702. [DOI] [PubMed] [Google Scholar]
- 4. Colombo AL, Guimaraes T, Sukienik T, et al. Prognostic factors and historical trends in the epidemiology of candidemia in critically ill patients: an analysis of five multicenter studies sequentially conducted over a 9‐year period. Intensive Care Med. 2014;40:1489‐1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nucci M, Queiroz‐Telles F, Alvarado‐Matute T, et al. Epidemiology of candidemia in Latin America: a laboratory‐based survey. PLoS One. 2013;8:e59373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected With SARS‐CoV‐2 admitted to ICUs of the lombardy region, Italy. JAMA. 2020;323:1574‐1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Garcia‐Vidal C, Sanjuan G, Moreno‐Garcia E, et al. Incidence of co‐infections and superinfections in hospitalized patients with COVID‐19: a retrospective cohort study. Clin Microbiol Infect. 2020. 10.1016/j.cmi.2020.07.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mastrangelo A, Germinario BN, Ferrante M, et al. Candidemia in COVID‐19 patients: incidence and characteristics in a prospective cohort compared to historical non‐COVID‐19 controls. Clin Infect Dis. 2020. 10.1093/cid/ciaa1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rodriguez‐Morales AJ, Gallego V, Escalera‐Antezana JP, et al. COVID‐19 in Latin America: The implications of the first confirmed case in Brazil. Travel Med Infect Dis. 2020;35:101613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lobo AP, Cardoso‐Dos‐Santos AC, Rocha MS, et al. COVID‐19 epidemic in Brazil: Where are we at? Int J Infect Dis. 2020;97:382‐385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Braga PR, Cruz IL, Ortiz I, Barreiros G, Nouer SA, Nucci M. Secular trends of candidemia at a Brazilian tertiary care teaching hospital. Braz J Infect Dis. 2018;22:273‐277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the New York City area. JAMA. 2020;323:2052‐2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lamers MM, Beumer J, van der Vaart J, et al. SARS‐CoV‐2 productively infects human gut enterocytes. Science. 2020;369:50‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cha MH, Regueiro M, Sandhu DS. Gastrointestinal and hepatic manifestations of COVID‐19: A comprehensive review. World J Gastroenterol. 2020;26:2323‐2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nucci M, Anaissie E. Revisiting the source of candidemia: Skin or gut? Clin Infect Dis. 2001;33:1959‐1967. [DOI] [PubMed] [Google Scholar]
- 17. Antinori S, Bonazzetti C, Gubertini G, et al. Tocilizumab for cytokine storm syndrome in COVID‐19 pneumonia: an increased risk for candidemia? Autoimmun Rev. 2020;19:102564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Arastehfar A, Carvalho A, Nguyen MH, et al. COVID‐19‐associated candidiasis (CAC): An underestimated complication in the absence of immunological predispositions? J Fungi (Basel). 2020;6:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Posteraro B, Torelli R, Vella A, et al. Pan‐echinocandin‐resistant candida glabrata bloodstream infection complicating COVID‐19: A fatal case report. J Fungi (Basel). 2020;6:163. [DOI] [PMC free article] [PubMed] [Google Scholar]


