Abstract
The COVID‐19 pandemic is a global public health issue. Neurological complications have been reported in up to one‐third of affected cases, but their distribution varies significantly in terms of prevalence, incidence and phenotypical characteristics. Variability can be mostly explained by the differing sources of cases (hospital vs. community‐based), the accuracy of the diagnostic approach and the interpretation of the patients’ complaints. Moreover, after recovering, patients can still experience neurological symptoms. To obtain a more precise picture of the neurological manifestations and outcome of the COVID‐19 infection, an international registry (ENERGY) has been created by the European Academy of Neurology in collaboration with European national neurological societies and the Neurocritical Care Society and Research Network. ENERGY can be implemented as a stand‐alone instrument for patients with suspected or confirmed COVID‐19 and neurological findings or as an addendum to an existing registry not targeting neurological symptoms. Data are also collected to study the impact of neurological symptoms and neurological complications on outcomes. The variables included in the registry have been selected in the interests of most countries, to favour pooling with data from other sources and to facilitate data collection even in resource‐poor countries. Included are adults with suspected or confirmed COVID‐19 infection, ascertained through neurological consultation, and providing informed consent. Key demographic and clinical findings are collected at registration. Patients are followed up to 12 months in search of incident neurological manifestations. As of 19 August, 254 centres from 69 countries and four continents have made requests to join the study.
Keywords: COVID‐19, SARS‐CoV‐2, neurological diseases, complications, outcome, registry, EAN
Map of Europe including the location of the centres participating in the European Academy of Neurology registry.

HISTORICAL BACKGROUND AND RATIONALE
The present pandemic attributed to SARS‐CoV‐2 virus has become a global public health problem (https://covid19.who.int). This highly contagious coronavirus (more than 21 million people resulting positive, as of 17 August), is responsible in humans for different expressions of infection severity and involvement of organs/systems, including the nervous system [1]. Neurotropism is a common feature of coronaviruses [2] and can be due to a direct action on the nervous tissue and/or to an indirect action through the activation of cytokine cascade and immune‐mediated mechanisms [3, 4, 5]. Additionally, neurological symptoms can occur as a complication secondary to systemic illnesses or from the exacerbation of pre‐existing neurological conditions. Neurological manifestations include symptoms reflecting the overall severity of the disease (as documented by encephalitis and encephalopathy) or more specific syndromic entities (e.g., stroke or Guillain–Barré syndrome) that might be the result of the peculiar mechanisms of action of the virus [6].
Neurological manifestations have been reported in about one‐third of adult and elderly cases, as indicated in the first clinical series from Wuhan, China [7]. Children have also been shown to be affected, although to a lesser extent [8]. Data are mostly driven by case reports and small series that are illustrated in a comprehensive review [9]. Headache, myalgia, anosmia, fatigue/sleepiness are the most frequently reported symptoms [10]. Amongst severe manifestations, altered mental status [11], stroke [12, 13] and peripheral nerve involvement [14] can be the most frequent. Mild complaints and subclinical findings are not uncommon and might indicate that neurological findings are more common than expected and are underdiagnosed unless accurate screening methods are adopted. This suggests that an active search might be followed by even higher numbers. Moreover, it is becoming evident that several symptoms persist after recovering from COVID‐19 infection [15]. This new evidence suggests further and careful surveillance and monitoring.
In published reports, the distribution of neurological symptoms, signs and diseases varied significantly in terms of prevalence, incidence and phenotypical characteristics. This variability can be mostly explained by the differing sources of cases (hospital vs. community‐based), the accuracy of the diagnostic approach, and the subjective interpretation of the patients’ complaints by the attending physicians. Thus, a standardized approach is needed to provide a clearer outline of the spectrum of neurological disorders comparing the main clinical aspects of COVID‐19 disease in different countries and verifying whether differences, if any, can be attributed to differences in environmental and genetic factors. The approach also allows for the evaluation of the severity of illness across resource settings to examine the role of critical illness or prolonged hospitalization on symptoms and draw conclusions regarding causality between viral infection and neurological manifestations. A registry represents the ideal instrument for this purpose.
A REGISTRY AS AN INSTRUMENT FOR A STANDARDIZED DATA COLLECTION
Registries are the instruments used to detect and define the spectrum of a given disease in population‐based samples or in specific settings. The demographic and clinical characteristics of the individuals to be included in a registry are pre‐defined. The source(s) of cases is(are) identified. Each patient is assigned a unique identifier. The diagnosis, and any other factor deemed important for the description of a registered case, is defined using commonly accepted and unanimously applied criteria. The data are collected in compliance with these pre‐assigned criteria. To preserve the representativeness of the sample, all patients eligible for inclusion and releasing an informed consent are registered. If a follow‐up is required, patients are assessed at specific time points for the identification of any incident complication. Attrition can be minimized through an active and accurate search of the individuals qualifying for inclusion and, where a follow‐up is needed, to be invited at follow‐up visits.
PLANNING AND DEVELOPMENT OF A EUROPEAN REGISTRY
The European Academy of Neurology (EAN) has been active since the start of the COVID‐19 outbreak with a number of activities to promote knowledge, research and international collaborations [10]. Starting in April 2020, a Task Force was assembled, including clinicians and epidemiologists from various countries (the authors of the present report). One of the projects of this Task Force was to develop a European registry.
Objectives of a European COVID‐19 registry
The overall aim of a European COVID‐19 registry is to provide epidemiological data on the spectrum of neurological symptoms and signs in patients with COVID‐19 infection reported by neurologists or other key referents in outpatient services, emergency rooms or hospital departments. The registry can be implemented as a stand‐alone instrument for patients with suspected or confirmed COVID‐19 and neurological findings or as an addendum to an existing registry not targeting neurological signs and symptoms. More specific primary objectives are (1) to evaluate the prevalence of neurological manifestations in patients with suspected or confirmed COVID‐19 disease; and (2) to assess the general characteristics of these neurological manifestations. Secondary objectives are (1) to gain epidemiological data on neurological manifestations of the COVID‐19 infection in different countries in Europe and, where available, in non‐European countries; and (2) to study the impact of neurological symptoms and neurological complications on outcomes.
An accurate search was made of existing national registries and databases to identify the variables most commonly collected, with a threefold purpose: (1) to select the data considered of primary interest by most countries; (2) to favour data pooling and meta‐analyses on common variables from differing sources; and (3) to focus on variables that could be easily collected even in resource‐poor countries. The variables identified were critically appraised and only those on which there was full agreement were retained. For each variable, a definition was provided resulting from widely accepted criteria or, where not available, fully agreed by the group. The collection of the data was kept to a minimum to prevent attrition and loss of data due to the constraints posed by the outbreak.
Patients to be registered
A patient was eligible for inclusion provided that all the following criteria were satisfied: (1) age 18 years or older; (2) symptoms suggesting confirmed COVID‐19 infection; (3) case ascertainment through neurological consultation; and (4) patient's informed consent (according to the requirements of local regulatory agencies).
Study conduct
All neurologist members of the EAN or its affiliated national societies are entitled to participate and register all consecutive patients fulfilling the inclusion criteria of patients they are asked to visit. No additional investigations were needed beside a detailed neurological examination and a list of the most common variables recorded in the pandemic. To expand the numbers of patients to be registered, data collection will be both prospective and retrospective. A number of general symptoms and signs of infection, a list of prominent comorbidities and a number of neurological diseases, selected amongst those identified in previous series and case reports and accompanied by proper definitions, have been included in a semi‐structured case report form (Table 1). All registered patients with neurological symptoms are asked to be followed for 12 months, with telephone calls at 6 and 12 months to verify the vital status, the functional abilities and identify neurological symptoms, signs or diagnoses that might have occurred after the acute phase of the disease. The neurologist (or a designated partner of the local study team) is required to be in charge of the follow‐up.
TABLE 1.
List of variables included in the registry
| General and demographic | Site of visit, source of contagion, results of testing (where available), year of birth, sex, height, weight, smoking, duration of infection |
| History | Arterial hypertension, diabetes, cardiovascular disease, chronic kidney disease, chronic liver disease, chronic bronchial/pulmonary disease, anaemia, cancer, immune‐mediated disease, other non‐neurological (specify), neurological disease |
| General COVID‐19 complications | Dyspnoea, pneumonia, cardiovascular disease, renal insufficiency/dialysis, coagulation disorder/disseminated intravascular coagulopathy, septic shock, extracorporeal membrane oxygenation, other (specify) |
| Admission | Hospital, intensive care unit (mechanical ventilation) |
| New neurological manifestations | Date of onset, headache, hyposmia/hypogeusia, dysautonomia, vertigo, myalgia, sleep disturbances, excessive daytime sleepiness/hypersomnia, cognitive impairment, dysexecutive syndrome, hyperactive delirium, hypoactive delirium/acute encephalopathy, stupor/coma, syncope, seizures/status epilepticus, meningitis/encephalitis, stroke, movement disorders, ataxia, spinal cord disorder, peripheral neuropathy, other (specify) |
| Diagnostic tests | CSF, CT/MRI |
| Outcome | Modified Rankin Scale at discharge; if death, date, autopsy |
| 6‐month follow‐up | Modified Rankin Scale, new neurological manifestations; if death, date, autopsy |
| 12‐month follow‐up | Modified Rankin Scale, new neurological manifestations; if death, date, autopsy |
Statistical analysis plan
Statistical analyses will be performed in conformity with two separate plans. The first plan refers to countries adhering to the EAN registry. The plan includes descriptive statistics to be performed on all variables collected in the registry. Inferential statistics are also included using conventional univariate and multivariate methods. Cross‐tabulations were pre‐planned to correlate each symptom, sign and neurological diagnosis to demographics and the other clinical variables, including comorbidities and the main complications of infection. These data will be presented in the entire sample and for each country separately. The neurological diagnoses made at the time of the infection will be contrasted to the status at last observation (recovered, alive with functional impairment, dead). Multivariate analyses will also be performed using logistic regression models with status at last observation (alive/dead) as the dependent variable and neurological diagnoses as the independent variables, adjusting for demographics, comorbidities, setting and country. Follow‐up data will be analysed in survivors with Kaplan–Meier curves using the occurrence of a neurological diagnosis as the outcome variable and demographics and comorbidities as prognostic predictors. Comparisons will be tested with the log‐rank test and independent prognostic predictors will be assessed using Cox hazard models, adjusting for setting and country. Retrospective and prospective data will be analysed separately and compared. The modality for data collection (retrospective vs. prospective) will also be included in multivariable analysis models. The significance will be set at the 5% level (p = 0.05).
A separate plan (still to be discussed with partners in charge of independent data collections and willing to share their data) will consider two options: (1) inclusion of anonymized individual patient data; (2) a meta‐analysis of aggregated data.
Sample size calculation
The primary end‐points of the EAN registry are to determine the prevalence of neurological manifestations in COVID‐19 patients. The hypotheses tested by this registry are exploratory; hence a sample size calculation was not performed.
Implementation of the registry
When the protocol (Annex 1) and the case report form (Annex 2) were in final form, an extensive correspondence was started with the national societies affiliated to the EAN and with individual members. The goal was to advertise the registry and encourage countries and individuals to use this instrument. As of 17 August 2020, a total of 254 centres from four continents declared their willingness to participate. A heatmap of the participating sites is illustrated in Figure 1. The profile of each centre will be provided through the completion of an ad hoc form (Annex 3) that will also include the setting where the patient was registered.
FIGURE 1.
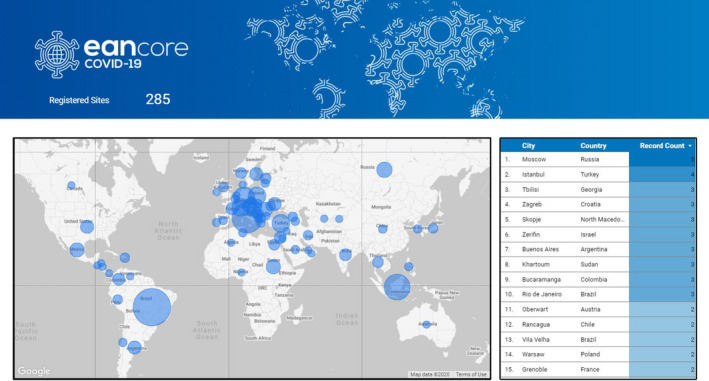
Map of Europe including the location of the centres participating in the European Academy of Neurology registry [Colour figure can be viewed at wileyonlinelibrary.com]
Whilst the EAN registry was distributed to the participating sites, a discussion was started with countries using their own registries in Europe with the intent to organize data sharing (individual patient data) or pooling (meta‐analysis).
In parallel, an intensive collaboration was also started with the Global Consortium Study of Neurological Dysfunction in COVID‐19 (GCS‐NeuroCOVID), centrally coordinated through the University of Pittsburgh in the United States. Endorsed by the Neurocritical Care Society and the Neurocritical Care Research Network, at the end of March 2020 the GCS‐NeuroCOVID investigators designed and implemented Tier‐1, basic pragmatic cohort studies to determine neurological manifestations of COVID‐19 and their prevalence amongst the hospitalized COVID‐19 patient population. This will be followed by a Tier‐2 study which will further characterize main phenotypes of neurological manifestations of COVID‐19 along with evaluation of long‐term outcome, and an experimental/translational Tier‐3 study to investigate potential disease mechanisms and biomarkers [16, 17]. The GCS‐NeuroCOVID consortium also developed a paediatrics study arm to examine neurological features of COVID‐19 across the full lifespan. Currently, GCS‐NeuroCOVID consortium has 123 adult and 96 paediatric sites representing all continents.
The similarities in scientific aims and the complementary global outreach of the two large consortia (EAN and GCS‐NeuroCOVID) generated an ideal opportunity for global collaboration. The two consortia plan to combine data in a general analysis which will provide important information on the global and regional burden of neurological manifestations of the COVID‐19 pandemic. A Memorandum of Understanding outlining the terms of this collaboration was established and jointly signed by leaders of the EAN, the Neurocritical Care Society and the Neurocritical Care Research Network. This collaboration led to the planning of joint activities aimed at promoting harmonization of data collection, to be extended to children and adolescents, and to include a more detailed search of data during follow‐up.
At the same time, the World Health Organization promoted a global forum aimed at identifying the most important issues to be investigated at a global level, in terms of improved knowledge of the spectrum of COVID‐19 infection and its neurological acute and late complications, and the detection of failures in the provision of care. Representatives from both the EAN and the GCS‐NeuroCOVID consortium investigators serve on this important mission.
Expected short‐term and long‐term findings
The activation of the EAN registry and its interaction with several other surveillance systems in and outside Europe will provide a number of short‐term and long‐term findings. In the short term, a more complete picture will be offered of the spectrum of the disease and its neurological complications, comparing the various settings where the patients were registered. The demographic and clinical profile of registered patients will be compared across European countries to detect similarities and differences. Demographic and clinical characteristics of patients from countries with differing proportions of affected individuals and COVID‐related deaths will also be compared. Using the settings for denominators, prevalence and incidence of neurological signs and diseases will also be calculated, separating the neurological manifestations of the infection from well‐defined syndromic entities. Registered data will also be used to plan focused studies and the established registry can serve as a critical infrastructure to facilitate global research in future unanticipated events.
CONFLICTS OF INTEREST
None declared.
Appendix 1.
EAN NEURO‐COVID TASK FORCE
Tom Jenkins, Sheffield Institute for Translational Neuroscience, University of Sheffield, and Sheffield Teaching Hospitals NHS Foundation Trust, Sheffield, UK; Tim von Oertzen, Kepler Universitäts Klinikum, Linz, Austria; Benedetta Bodini, Department of Neurology, Sorbonne University, Saint‐Antoine Hospital, APHP, Paris, France; Antonella Marcerollo, Walton Centre NHS Foundation Trust, Liverpool, UK; School of Psychology, Faculty of Health and Life Sciences, University of Liverpool, Liverpool, UK; Martin Rakusa, Department of Neurology, University Medical Centre Maribor, Maribor, Slovenia; Riccardo Soffietti, Department of Neuroscience, Division of Neuro‐Oncology, University of Turin, Turin, Italy; Alex Twardzik, EAN Head Office, Vienna, Austria; Celia Oreja‐Guevara, Department of Neurology, Hospital Clınico San Carlos, Madrid; Departamento de Medicina, Facultad de Medicina, Universidad Complutense de Madrid (UCM), Madrid; IdISSC, Madrid, Spain; Daniel Bereczki, Department of Neurology, Semmelweis University, Budapest, Hungary; Luis Maia, Hospital Santo António, Department of Neurology, Centro Hospitalar Universitario do Porto, Porto, Portugal; Serefnur Ozturk, Department of Neurology, Faculty of Medicine, Selcuk University, Konya, Turkey; Antonio Pisani, Department of Brain and Behavioral Sciences, University of Pavia, Italy; Johann Sellner, Department of Neurology, Landesklinikum Mistelbach‐Ganserndorf, Mistelbach; Department of Neurology, Christian Doppler Medical Center, Paracelsus Medical University, Salzburg, Austria; Pille Taba, Department of Neurology and Neurosurgery, Institute of Clinical Medicine, University of Tartu, Estonia; Giovanni Diliberto, Department of Pathology and Immunology, Geneva Faculty of Medicine, Geneva, Switzerland; Didier Leys, Hôpital Roger Salengro CHU, Lille, France; Anna Sauerbier, Department of Neurology, University Hospital Cologne, Cologne, Germany; National Parkinson Foundation International Centre of Excellence, King's College Hospital, London, UK; Francesco Cavallieri, Neurology Unit, Neuromotor and Rehabilitation Department, Azienda USL‐IRCCS di Reggio Emilia, Italy; Alberto Priori, Department of Neurology, Division of Neurology, ‘Aldo Ravelli’ Research Center, University of Milan and ASST Santi Paolo e Carlo, Milan; Marialuisa Zedde, Neurology Unit, Neuromotor and Rehabilitation Department, Azienda USL‐IRCCS di Reggio Emilia, Italy.
Annex 1.
STUDY PROTOCOL

Core Scientific Committee
Claudio Bassetti, Neurology Department, University Hospital, 3010 Bern, Switzerland
Ettore Beghi, Department of Neuroscience, Istituto di Ricerche Farmacologiche Mario Negri, Milan, Italy
Raimund Helbok, Department of Neurology, Neurocritical Care Unit, Innsbruck, Austria Elena Moro, Division of Neurology, Centre Hospitalier Universitaire, Grenoble, France Pille Taba, Neurology Clinic of Tartu ‐ University Hospital
Michael Crean. Press & Communication, European Academy of Neurology, Vienna, Austria
Advisory Committee
All members of the EAN Covid‐19 Task Force
Enlarged Scientific Committee
Two representatives from Italy, Spain and Portugal; one representative from any other participating country.
Data Management
Lalit Kaltenbach, Department of Medical Statistics, Informatics and Health Economics, Medical University of Innsbruck, Austria
Giorgia Giussani, Department of Neuroscience, Istituto di Ricerche Farmacologiche Mario Negri IRCCS, Milan, Italy
Statistical analysis
Department of Medical Statistics, Informatics and Health Economics, Medical University of Innsbruck, Austria
Elisa Bianchi, Department of Neuroscience, Istituto di Ricerche Farmacologiche Mario Negri IRCCS, Milan, Italy
Table of Changes
| Date | Page/Section | Description of Change |
|---|---|---|
| July 29 | Pg. 6/procedure section | Sentence added which allows for inclusion of retrospective patient data |
| September 7 | Pg. … | Secondary objectives: Added “… and non‐European countries” |
| Pg. … | Methodology: “Included will be all COVID‐19 patients whom the neurologists have been asked to visit or are available in the local registries and fulfilled the inclusion criteria. Both retrospective and prospective cases are eligible for inclusion.” | |
| Pg. … | CRF: Added “Final COVID‐19 status” | |
| September 19 | Pg. 8 | Information on data sharing with third countries. |
Background & Rationale
An unexpected outbreak caused by COVID‐19 virus is devastating the world population and the global economy. Europe is at present the continent with the highest number of affected individuals and deaths. Despite the exponential increase in the number of infections, information available on the full spectrum of the disease is still insufficient. Recent reports strongly suggest that COVID‐19 infection spreads to organs other than the respiratory system, including the central and peripheral nervous system. The involvement of the nervous system can be due to a direct action of the virus on the nervous tissue and/or to an indirect action through the activation of immune‐mediated mechanisms. Moreover, the need for prolonged intensive care management in severe COVID‐19 patients leads to well‐known adverse effects on the central and peripheral nervous system, including post Intensive Care Unit (ICU) syndrome and ICU acquired weakness.
At present, information available on the involvement of the nervous system during this outbreak is based on case reports and retrospective clinical series. These sources are open to selection bias, although there are indicators that neurological complications in COVID‐19 patients are associated with a worse outcome. In addition, both differences in the dissemination of the infection across Europe and variability of measures adopted to contrast the outbreak prevent a correct surveillance of the clinical characteristics of the infection, including the occurrence of neurological disorders.
Currently available information can provide a picture of the rich spectrum of symptoms, signs, and diagnoses associated with COVID‐19 infection. However, in the light of the wide differences in timing and severity of the outbreak across Europe, it is impossible to define the association between the impairment of neurological functions and the outcome of the infection. Consequently, to adopt adequate preventing measures without a systematic collection of the information in a well‐defined cohort of patients is very challenging. Only a registry can shed some light on the burden and general characteristics of neurological complications of the COVID‐19 outbreak and the association of these complications with the demographic and clinical features of the affected individuals.
Objectives
The main objective of this international Registry is to provide epidemiological data on neurological manifestations (symptoms/signs and diagnoses) in patients with COVID‐19 infection reported by neurologists in outpatient services, emergency rooms, and hospital departments. The EAN registry can be implemented as stand‐alone registry for COVID‐19 patients or as an addendum to an existing registry not targeting neurologic signs and symptoms.
-
Primary objectives are:
To evaluate the prevalence of neurological manifestations in patients with confirmedCOVID‐19 disease
To assess the general characteristics of the neurological manifestations.
-
Secondary objectives are:
To collect epidemiologic data on neurological manifestations of the COVID‐19 infection in European and non‐European countries
To evaluate the prevalence of neurological manifestations in patients with suspected COVID‐19 disease
To study the outcome of neurological manifestations in COVID‐19 patients (including the incidence of new neurological manifestations)
To evaluate the incidence of neurological manifestations during follow‐up.
Working hypotheses
Neurological manifestations are relatively common in COVID‐19 patients
There may be variability in neurological manifestations among different countries
Neurological manifestations and complications contribute to worse outcome in confirmed COVID‐19 patients.
Promoter
The Registry is promoted and endorsed by the European Academy of Neurology (EAN).
Participants to the Registry
National Neurological Societies or divisions of Neurology from individual academic centres can apply to participate to the ENERGY Consortium.
Study design
Methodology
Neurologists are asked to implement this study protocol in their institution/clinic, to assess and record demographic and other data, neurologic symptoms and signs according to the annexed electronic Case Record Form (eCRF) in confirmed and suspected COVID‐19 patients. Included will be all COVID‐19 patients whom the neurologists have been asked to visit or are available in the local registries and fulfilled the inclusion criteria. Both retrospective and prospective cases are eligible for inclusion.
The minimum requirement is to register COVID‐19 patients with neurological symptoms and/or signs and/or defined neurological disorders (see inclusion criteria). However, the inclusion of ALL patients with confirmed COVID‐19 infection is encouraged to provide the numbers for the calculation of the fraction of the affected population attributable to neurological disorders and the comparison of the overall spectrum of the disease in people with and without neurological manifestations. In centres accepting to include all COVID‐19 patients, another physician may be assigned as the person in charge of registration.
Patients
Inclusion criteria
For all COVID‐19 patients
Age 18 or older
Symptoms suggesting COVID‐19 infection OR confirmed COVID‐19 infection
Provided informed consent (according to the requirements of local regulatory agencies).
For COVID‐19 patients with neurological signs, symptoms and/or defined neurological disorders
Age 18 or older
Symptoms suggesting COVID‐19 infection OR confirmed COVID‐19 infection
Neurological evaluation/consultation
Provided informed consent (according to the requirements of local regulatory agencies).
Exclusion criteria
Symptoms suggesting other (pulmonary/systemic) infection than COVID‐19 AND other confirmed infection.
Procedure
Patients’ inclusion can be performed prospectively, at the time of the visit or at patient’s discharge, whichever is most convenient; or retrospectively, provided that all inclusion criteria are satisfied. Visits can be performed anywhere in the context of health care facilities (outpatient services, emergency rooms, hospital departments). If at the time of the visit, the clinical picture of the patient is incomplete, the neurologist is invited to contact the caring physician upon discharge to complete the e‐CRF. The collection of the data will be kept to a minimum to prevent attrition and loss of data due to the constraints posed by the outbreak. No additional investigations are needed besides a detailed neurological examination and common variables recorded in this pandemic. The registration of the patients will continue until the end of the outbreak.
All registered patients with neurological symptoms will be followed up to 12 months, with telephone calls at 6 and 12 months, to verify clinical conditions, functional abilities, and identify neurological manifestations that might have occurred after the acute phase of the disease. The neurologist (or a designated partner of the local study team) will oversee the follow‐up.
A guide is annexed to this protocol to define each variable and facilitate data collection in the e‐CRF.
Statistical analysis plan
Descriptive statistics will be performed on all variables collected in the registry. Inferential statistics will include univariate and multivariate analyses. Cross‐tabulations will be performed for each symptom, sign and neurological diagnosis against demographics and the other clinical variables, including comorbidities and the main complications of infection. These data will be presented in the entire sample and for each country separately. The neurological diagnoses made at the time of the infection will be contrasted to the status at last observation (recovered, alive with functional impairment, dead). The prevalence of neurological symptoms, signs and diagnoses will be calculated using the number of neurological consultations as denominator and symptoms/signs and, separately, neurological diagnoses as a group. Multivariate analyses will be also performed using logistic regression models with status at last observation (alive with or without functional impairment/dead) as the dependent variable and neurological diagnoses as the independent variables, adjusting for demographics, comorbidities, centre and country. Follow‐up data will be analysed in survivors with Kaplan‐Meier curves using the occurrence of a neurological diagnosis as the outcome variable and demographics and comorbidities as prognostic predictors. Comparisons will be tested with Log‐rank and independent prognostic predictors will be assessed using Cox’s hazard models, adjusting for centre and country. The significance will be set at the 5% level (p = 0.05).
Sample size calculation. The primary endpoints of this registry are to determine the prevalence and the general characteristics of neurological manifestations in COVID 19 patients. The hypotheses of this registry are exploratory; hence a sample size calculation has not been performed.
Benefit and risk ratio
ENERGY will not interfere with the diagnostic and therapeutic decisions made by the attending physicians for the management of the disease. There may be a benefit for patients undergoing neurological examination by early identification of neurological symptoms and signs which may result in a specific treatment. Therefore, the detection of complications may lead to a better management of patients included in this registry.
Consecutive data collection will result in a better understanding of neurological disease manifestations and complications in suspected and confirmed COVID‐19 positive patients. This will be important for an early identification of core neurological symptoms during the pandemic.
Collection of data
Routinely captured data will be collected in a web‐based eCRF (REDCap) and stored in a password‐ protected database not accessible directly from the internet. The password is provided to every participating site. Each centre will be assigned a numeric code generated by the central database. The data will be securely stored at the Department of Medical Statistics, Informatics and Health Economics, Medical University of Innsbruck, Austria contracted by the EAN central office. All procedures will comply with the EU Regulation 2016/679 (DSGVO, engl. GDPR) on the protection of natural persons regarding personal data processing and movement.
Ethical standards
The Principal Investigators (PIs) will ensure that the study is conducted in full conformity with the Declaration of Helsinki and Good Clinical Practices.
Ethics committee
The protocol will be submitted by the PI to the local ethics committees (ECs). Any amendment to the protocol will require review and approval by the EC before the changes are implemented to the survey. Only individual data collected after the patient’s informed consent will be used. Every eligible patient will be assigned an anonymized code.
Data confidentiality
Participants’ and centres’ confidentiality is strictly held in trust by the participating investigators. All medical or administrative staff with an access to the data is subject to a duty of confidentiality and data protection. Therefore, the study protocol, documentation, data, and all other information generated will be held in strict confidentiality agreement protocols.
The study sponsor (European Academy of Neurology) and representatives of local authorities may inspect all documents and records required to be maintained by the local investigator for the participants in this registry. Research data of the registry, which is for purposes of statistical analysis and scientific reporting, will be transmitted to the Data Managers and the Statisticians of the registry. For this purpose, data will be de‐identified and anonymized at input into the eCRF by the local centres/PIs. Individual participants and their research data will be identified by a unique identification number. The eCRF system used by clinical sites and by research staff will be secured and password protected. In the situation when a centre would be temporary not able to access the eCRF or complete it, a paper‐based CRF will be available on demand. To keep administration and data correctness on a high level, this possibility should only rarely be used. These records will be entered in the eCRF at the EAN central office in collaboration with the research staff of the Medical University of Innsbruck and the Mario Negri Institute of Milan.
Data sharing & ownership
Where ENERGY is an addendum to other registries or databases, formal collaborations can be activated with European and international organisations to share common variables in the intent to provide a broad European and even worldwide picture and favour comparisons. For countries with independent registries/databases and that wish to share their data but are unwilling to use this registry, data will be compared in aggregate using pre‐specified statistical plans. The data collected by individual centres will be accessible to these centres without restriction. All participants should be registered as active members of the EAN Neuro‐COVID Registry Consortium.
The data collected can be also used to test scientific hypotheses forwarded by any active member. However, these hypotheses should be illustrated in ad‐hoc protocols to be submitted for approval to the Registry Core Scientific Committee. The scientific reports should be published on behalf of the EAN and the affiliated neurological societies.
Participating sites will be informed of any data sharing agreement with organisations in countries not associated to the European Union.
Publication, and Authorship
Data will be made available to the scientific community by means of abstract or scientific papers submitted to peer‐reviewed journals. Authorship of the main manuscript will follow the ICMJE recommendations that base authorship on the following four criteria:
Substantial contributions to the conception or design of the work or the acquisition, analysis, or interpretation of data for the work, AND
Drafting the work or revising it critically for important intellectual content, AND
Final approval of the version to be published, AND
Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
A writing committee composed by the Core Scientific Committee will draft the work and will be authors of the manuscript. All publications will be made in the name of ENERGY Consortium. All those who satisfy the criteria for authorship will be listed as authors. Each centre will be mentioned at least by the name of one author and listed "on behalf of the ENERGY consortium" in the main publications in PubMed. Additional authors will be listed based on the contribution of each site to the registry. Each author’s contribution within the Consortium will be specified.
Case Record Form
Centre ID Patient’s code Site of visit
Hospital
Emergency room
Outpatient service
Other (spec)
Year of birth
Sex
Height
Weight
Smoking (no/yes)
Source of contagion
Occupation
Family member
Social
Travel
Other (specify)
Date of first symptoms of infection
Final COVID‐19 status Comorbidities in history (no/yes)
If yes, check all that apply
Arterial hypertension
Diabetes
Cardiovascular disease
Chronic kidney disease
Chronic liver disease
Chronic bronchial/pulmonary disease
Anemia
Cancer
Immune‐mediated disease
Other non‐neurological (specify)
Neurological disease Premorbid Modified Rankin Scale
Relevant COVID‐19 complications (not present in history) (No/Yes) If yes, check all that apply
Dyspnea
Pneumonia
Cardiovascular disease
Renal insufficiency/dialysis
Coagulation disorder/disseminated intravascular coagulopathy
Septic shock
Extracorporeal membrane oxygenation
-
Other (specify)
Hospital admission (no/yes)
ICU admission (no/yes)
Mechanical ventilation (No/Yes)
New neurological symptoms/signs/diagnoses (no/yes)
If yes:
Date of onset of neurological symptoms/signs
Check all that apply and state if related/unrelated to COVID‐19
Headache
Hyposmia/hypogeusia
Dysautonomia
Vertigo
Myalgia
Sleep disturbances
Excessive daytime sleepiness/hypersomnia
Cognitive impairment
Dysexecutive syndrome
Hyperactive delirium
Hypoactive delirium/acute encephalopathy
Stupor/coma
Syncope
Seizures/status epilepticus
Meningitis/Encephalitis
Stroke
Movement disorders
Ataxia
Spinal cord disorder
Peripheral neuropathy
Other (specify)
Diagnostic tests
CSF (No/Yes)
CT/MRI (No/Yes)
Outcome
Modified Rankin Scale at discharge
If patient died, date of death
If death, autopsy (No/Yes)
Follow‐up
6 months
Modified Rankin Scale
Occurrence of new neurological issues (No/Yes)
If yes, date of onset and specification
If patient died, date of death
If death, autopsy (No/Yes)
12 months
Modified Rankin Scale
Occurrence of new neurological issues (No/Yes)
If yes, date of onset and specification
If patient died, date of death
If death, autopsy (No/Yes)
Annex 2.
CASE REPORT FORM









Annex 3.
PROFILE OF PARTICIPATING CENTRE
General characteristics of the participants in the ENERGY registry
| Investigator name and surname | |
| Centre name | |
| Centre’s ID | /__/__/__/ |
| University hospital | /__/ |
| General hospital | /__/ |
| Outpatient service | /__/ |
| Other (specify) | /__/__/ |
| Town | /__/__/ |
| Country | /__/__/ |
| Does the institution have a record of all patients assessed for COVID infection? | /Yes//No/ |
| Number of patients assessed in the institution for COVID infection | |
| Estimated | /__/__/__/ |
| Calculated (absolute numbers) | /__/__/__/ |
| Number of patients with confirmed COVID infection | |
| Estimated | /__/__/__/ |
| Calculated (absolute numbers) | /__/__/__/ |
| CENTRE profiling | |
| Data first patient entered | /__/__//__/__//__/__/ |
| Data last patient entered | /__/__//__/__//__/__/ |
| Consecutive patients with neurological problems | /R//P/ |
| Consecutive patients with neurological problems seen by a neurologist | /R//P/ |
| Consecutive patients admitted to the hospital/unit | /R//P/ |
| Non‐consecutive patients seen by a neurologist | /R//P/ |
| Non‐consecutive patients admitted to the hospital evaluated by a neurologist | /R//P/ |
| Non‐consecutive patients admitted to the hospital/unit | /R//P/ |
P, prospective; R, retrospective.
Contributor Information
Ettore Beghi, Email: ettore.beghi@marionegri.it.
for the EAN Neuro‐COVID Task Force:
Tom Jenkins, Tim Oertzen, Benedetta Bodini, Antonella Marcerollo, Martin Rakusa, Riccardo Soffietti, Celia Oreja‐Guevara, Daniel Bereczki, Serefnur Ozturk, Antonio Pisani, Johann Sellner, Pille Taba, Giovanni Diliberto, Didier Leys, Anna Sauerbier, Francesco Cavallieri, Alberto Priori, and Marialuisa Zedde
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analysed in this study.
REFERENCES
- 1. Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID‐19): a review. JAMA. 2020;324(8):782. 10.1001/jama.2020.12839 [DOI] [PubMed] [Google Scholar]
- 2. Wu Y, Xu X, Chen Z, et al. Nervous system involvement after infection with COVID‐19 and other coronaviruses. Brain Behav Immun. 2020;87:18‐22. 10.1016/j.bbi.2020.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cheng Q, Yang Y, Gao J. Infectivity of human coronavirus in the brain. EBioMedicine. 2020;56:102799. 10.1016/j.ebiom.2020.102799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Michalicová A, Bhide K, Bhide M, Kováč A. How viruses infiltrate the central nervous system. Acta Virol. 2017;61(4):393‐400. [DOI] [PubMed] [Google Scholar]
- 5. Zubair AS, McAlpine LS, Gardin T, Farhadian S, Kuruvilla DE, Spudich S. Neuropathogenesis and neurologic manifestations of the coronaviruses in the age of coronavirus disease 2019: a review. JAMA Neurol. 2020;77(8):1018‐1027. 10.1001/jamaneurol.2020.2065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Romero‐Sánchez CM, Díaz‐Maroto I, Fernández‐Díaz E, et al. Neurologic manifestations in hospitalized patients with COVID‐19: the ALBACOVID registry. Neurology. 2020;95(8):e1060–e1070. 10.1212/WNL.0000000000009937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):1‐9. 10.1001/jamaneurol.2020.1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Garazzino S, Montagnani C, Donà D, et al. Multicentre Italian study of SARS‐CoV‐2 infection in children and adolescents. Euro Surveill. 2020;25(18):2000600: 10.2807/1560-7917.ES.2020.25.18.2000600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ellul MA, Benjamin L, Singh B, et al. Neurological associations of COVID‐19. Lancet Neurol. 2020;19(9):767‐783. 10.1016/S1474-4422(20)30221-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moro E, Priori A, Beghi E, et al. The international European Academy of Neurology survey on neurological symptoms in patients with COVID‐19 infection. Eur J Neurol. 2020;27(9):1727‐1737. 10.1111/ene.14407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Varatharaj A, Thomas N, Ellul MA, et al. Neurological and neuropsychiatric complications of COVID‐19 in 153 patients: a UK‐wide surveillance study. Lancet Psychiatry. 2020;7(10):875‐882. 10.1016/S2215-0366(20)30287-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Divani AA, Andalib S, Di Napoli M, et al. Coronavirus disease 2019 and stroke: clinical manifestations and pathophysiological insights. J Stroke Cerebrovasc Dis. 2020;29(8):104941. 10.1016/j.jstrokecerebrovasdis.2020.104941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tan YK, Goh C, Leow AST, et al. COVID‐19 and ischemic stroke: a systematic review and meta‐summary of the literature. J Thromb Thrombolysis. 2020;50(3):587‐595. 10.1007/s11239-020-02228-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Galassi G, Marchioni A. Facing acute neuromuscular diseases during COVID‐19 pandemic: focus on Guillain–Barré syndrome. Acta Neurol Belg. 2020;120(5):1067‐1075. 10.1007/s13760-020-01421-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Carfì A, Bernabei R, Landi F, Gemelli Against COVID‐19 Post‐Acute Care Study Group . Persistent symptoms in patients after acute COVID‐19. JAMA. 2020;324(6):603‐605. 10.1001/jama.2020.12603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Frontera J, Mainali S, Fink EL, et al. Global Consortium Study of neurological dysfunction in COVID‐19 (GCS‐NeuroCOVID): study design and rationale. Neurocrit Care. 2020;33(1):25‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Needhan EJ, Chou SH, Coles AJ, Menon DK. Neurological implications of COVID‐19 infections. Neurocrit Care. 2020;32(3):667‐671. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analysed in this study.


