Abstract
Aim
This study aimed to describe the change in surgical practice and the impact of SARS‐CoV‐2 on mortality after surgical resection of colorectal cancer during the initial phases of the SARS‐CoV‐2 pandemic.
Method
This was an international cohort study of patients undergoing elective resection of colon or rectal cancer without preoperative suspicion of SARS‐CoV‐2. Centres entered data from their first recorded case of COVID‐19 until 19 April 2020. The primary outcome was 30‐day mortality. Secondary outcomes included anastomotic leak, postoperative SARS‐CoV‐2 and a comparison with prepandemic European Society of Coloproctology cohort data.
Results
From 2073 patients in 40 countries, 1.3% (27/2073) had a defunctioning stoma and 3.0% (63/2073) had an end stoma instead of an anastomosis only. Thirty‐day mortality was 1.8% (38/2073), the incidence of postoperative SARS‐CoV‐2 was 3.8% (78/2073) and the anastomotic leak rate was 4.9% (86/1738). Mortality was lowest in patients without a leak or SARS‐CoV‐2 (14/1601, 0.9%) and highest in patients with both a leak and SARS‐CoV‐2 (5/13, 38.5%). Mortality was independently associated with anastomotic leak (adjusted odds ratio 6.01, 95% confidence interval 2.58–14.06), postoperative SARS‐CoV‐2 (16.90, 7.86–36.38), male sex (2.46, 1.01–5.93), age >70 years (2.87, 1.32–6.20) and advanced cancer stage (3.43, 1.16–10.21). Compared with prepandemic data, there were fewer anastomotic leaks (4.9% versus 7.7%) and an overall shorter length of stay (6 versus 7 days) but higher mortality (1.7% versus 1.1%).
Conclusion
Surgeons need to further mitigate against both SARS‐CoV‐2 and anastomotic leak when offering surgery during current and future COVID‐19 waves based on patient, operative and organizational risks.
Keywords: cancer, colon cancer, COVID‐19, pandemic, rectal cancer, SARS‐CoV‐2, surgery, surgical oncology
What does this paper add to the literature?
Mortality associated with anastomotic leak and postoperative SARS‐CoV‐2 during the COVID‐19 pandemic was extremely high. A relatively small change in stoma practice was seen. Surgeons need to robustly mitigate against both SARS‐CoV‐2 and anastomotic leak when offering surgery during future waves of COVID‐19, based on patient, operative and organizational factors.
1. INTRODUCTION
During the early phases of the COVID‐19 pandemic there was uncertainty about the impact of perioperative SARS‐CoV‐2 on surgical patients and a growing scarcity of intensive care capacity [1, 2]. Guidelines emerged which recommended changing anastomotic practice in favour of forming a defunctioning stoma or end stoma in patients who would have previously only had an anastomosis [3, 4, 5, 6]. The first anticipated benefit was to diminish the severity and volume of postoperative anastomotic leaks during a time when the impact of the novel coronavirus was unknown [7]. The second was to reduce the requirement for intensive care when hospital resources were being redirected to the pandemic response [8]. The third was to reduce complications that lead to increased length of hospital stay, in order to release bed space and minimize the risk of nosocomial infection [9, 10].
Subsequent data have confirmed the detrimental effect of perioperative SARS‐CoV‐2, showing a 51.2% rate of postoperative pulmonary complications and a 30‐day mortality rate of 23.8% [11]. Despite outbreaks, cancer surgery must continue in order to prevent an overwhelming number of delayed operations, a possible increase in emergency procedures and a significant decline in population health [12].
The extent of new stoma formation during the first phases of the pandemic and the subsequent patient‐related outcomes are unknown. The impact of anastomotic leak and postoperative SARS‐CoV‐2 infection on mortality are also unknown. This study aimed to fill these knowledge gaps and to produce patient‐level outcome data that would inform patient selection and informed consent.
2. METHOD
2.1. Study design
This was a planned specialty analysis of adult patients undergoing elective colonic and rectal cancer resection in a prospective international multicentre cohort study of patients undergoing elective surgery without preoperative suspicion of SARS‐CoV‐2 [13]. Study approvals for participating hospitals were secured by local principal investigators before entry into the study and data collection. The study protocol was either registered as a clinical audit with institutional review or a research study obtaining ethical committee approval, dependent on local and national requirements. Data were collected online and stored on a secure server running the Research Electronic Data Capture (REDCap) web application [14] based in the University of Birmingham, UK. Any hospital performing elective colon or rectal cancer surgery in countries affected by the COVID‐19 pandemic was eligible to participate. Hospitals were required to collect data on consecutive eligible patients from the date of their first recorded case of COVID‐19 until 19 April 2020.
2.2. Patients and procedures
All adult patients (aged 18 years and over) who underwent elective colonic or rectal cancer resectional surgery with curative intent were eligible. Palliative operations, including those where the tumour was left in situ (e.g. formation of an end stoma without resection or bypass procedures), were excluded. Consecutive eligible patients were identified from multidisciplinary team meetings, operating lists and outpatient or telemedicine clinics. The day of surgery was defined as day zero, with patients followed up for 30 days postoperatively using routine follow‐up pathways. Patients who had an operation for suspected cancer which was subsequently shown to be a preinvasive lesion after histological examination (e.g. high‐grade dysplasia, carcinoma in situ) were still included in this study. However, patients who had an operation for a suspected cancer but who had a histologically benign lesion were excluded. Elective surgery was defined as any surgery booked in advance of a planned admission to hospital [15].
Patients who were suspected of having, or confirmed to have, SARS‐CoV‐2 infection at the time of surgery (through nasopharyngeal swab and quantitative reverse transcription polymerase chain reaction, CT thorax or clinical symptoms consistent with COVID‐19) were excluded from these analyses.
2.3. Data variables
Baseline patient characteristics included age, sex and American Society of Anesthesiologists (ASA) physical status classification [16]. Age was collected as deciles of years as a categorical variable. ASA status was analysed as grades 1–2 versus grades 3–5. Disease characteristics included baseline tumour, node, metastases (TNM) stage prior to surgery, or neoadjuvant treatment. The TNM stage was used to calculate the patient’s baseline cancer disease stage. Disease stages were grouped for analysis as Stage I or Stage II versus Stage III or Stage IV. For patients with cancers involving the rectum, data on neoadjuvant radiotherapy and the duration of therapy (long‐course or short‐course radiotherapy) were also analysed. Operative variables collected included the operative procedure performed, if a defunctioning or end stoma was formed, the operative approach (minimally invasive, minimally invasive converted to open, or open), the specialty and grade of the lead surgeon (consultant or trainee, colorectal or general surgeon) and whether a stapled or hand‐sewn technique was used for the anastomosis, where applicable. We did not specify the precise nature of minimally invasive surgery as there are many variants, but we know from previous international studies that >95% of minimally invasive operations are laparoscopic [17, 18]. For analysis, operative procedures were grouped anatomically into right resection, left resection, rectal resection and total/subtotal/panproctocolectomy. A full list of operative procedures is included in Table S1 in the Supporting Information.
2.4. Outcomes
The primary outcome measure was mortality within the 30 days following surgery. Secondary outcome measures were anastomotic leak, admission to critical care (including high‐dependency areas), postoperative SARS‐CoV‐2 infection and total length of hospital stay up to 30 days after surgery. Postoperative SARS‐CoV‐2 infection was defined as a positive swab or CT thorax in line with locally implemented protocols, or a clinical diagnosis of symptoms in keeping with COVID‐19 in patients where no swab test or CT scan was available.
2.5. Change in anastomotic practice due to COVID‐19
Data were collected on the intraoperative decision on stoma formation. Where patients had a stoma, surgeons were asked if this was their ‘normal practice’ or a ‘change in practice due to COVID‐19’. The group with a stoma created as a change in practice were labelled ‘COVID‐end‐stoma’ or ‘COVID‐defunctioning‐stoma’ for tables and analyses. If the patient had a stoma formed and the surgeon indicated a ‘change in practice due to COVID‐19’, they were asked to list all the reasons that applied to that case for this change (Figure S1).
2.6. Prepandemic data
Prepandemic data on colorectal cancer surgery were obtained from published European Society of Coloproctology (ESCP) 2015 Right Hemicolectomy Audit [19, 20, 21] and the 2017 Left Colon, Sigmoid and Rectal Resections Audit [18, 22] Data. Data from 5792 patients from 54 countries undergoing segmental resection for a colonic or rectal cancer were used for comparison with the equivalent cohort undergoing surgery during the pandemic. These data provided a contemporaneous and detailed comparison of case selection and outcomes during the pandemic and prepandemic periods. Data were not presented in these studies for total or subtotal colectomy, so no comparison was made with these operation types. TNM staging data were not available from the 2015 Right Hemicolectomy Audit and therefore comparison was not made in that field.
2.7. Statistical analysis
The study was conducted according to guidelines set by the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) statement for observational studies [23]. The chi‐square test was used to compare differences in categorical data apart from when cell sizes were small, when Fisher's exact tests were used. Continuous nonparametric data are presented as medians and interquartile ranges and median differences between groups were compared using the Mann–Whitney U‐test. Missing data are included in summary tables.
For the primary outcome of 30‐day mortality, a multilevel logistic regression was used to evaluate the impact of postoperative SARS‐CoV‐2 and anastomotic leak on death after surgery, summarized using odds ratios (ORs) with 95% confidence intervals (95% CIs). Country was included in the model as a random effect. The model also included clinically plausible preoperative and intraoperative factors in order to adjust for covariates and reduce the risk of confounding factors (age, sex, ASA grade, disease stage and operation type). Chi‐square tests and Fisher's exact tests were using to compare outcomes for those with a COVID‐stoma and those without. Similar methods were used to compare pandemic data with published prepandemic data. Analysis was performed used Stata SE version 16.1, (StataCorp, Texas, USA).
3. RESULTS
3.1. Patients and disease characteristics
This analysis included 2073 patients undergoing resection of a colonic or rectal cancer in 270 hospitals from 40 countries (Table S2) Of these patients, 1236 (59.6%) were men (Table 1). Overall, 1420 patients (68.7%) were ASA grades 1–2 and 1288 (62.1%) patients had disease Stage I–II. Of 947 patients who had an operation involving the rectum (including panproctocolectomy), 89 (9.4%) received short‐course and 206 (21.8%) received long‐course neoadjuvant radiotherapy.
TABLE 1.
Patients and disease characteristics stratified by operation
| Right‐side resection (n = 724) | Left‐side resection (n = 367) | Rectal resection (n = 935) | Total/subtotal panproctocolectomy (n = 47) | |||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| Sex | ||||||||
| Female | 343 | 47.4% | 135 | 36.8% | 343 | 36.7% | 16 | 34.0% |
| Male | 381 | 52.6% | 232 | 63.2% | 592 | 63.3% | 31 | 66.0% |
| ASA grade | ||||||||
| 1–2 | 454 | 62.7% | 244 | 66.5% | 686 | 73.4% | 36 | 76.6% |
| 3–5 | 269 | 37.2% | 123 | 33.5% | 244 | 26.1% | 11 | 23.4% |
| Missing | 1 | 0 | 5 | 0 | ||||
| Age (years) | ||||||||
| <50 | 42 | 5.8% | 25 | 6.8% | 96 | 10.3% | 11 | 23.4% |
| 50–69 | 268 | 36.9% | 187 | 51.0% | 495 | 52.9% | 16 | 34.0% |
| ≥70 | 414 | 57.3% | 155 | 42.2% | 344 | 36.8% | 20 | 42.6% |
| Disease stage | ||||||||
| I–II | 512 | 70.7% | 216 | 71.1% | 482 | 51.5% | 33 | 70.2% |
| III | 181 | 25.0% | 78 | 21.3% | 385 | 41.2% | 9 | 19.1% |
| IV | 31 | 4.3% | 28 | 7.6% | 68 | 7.3% | 5 | 10.6% |
| Neoadjuvant radiotherapya | ||||||||
| Short course | 89 | 9.5% | 0 | 0 | ||||
| Long course | 205 | 21.9% | 1 | 2.9% | ||||
| None | 641 | 68.6% | 33 | 97.1% | ||||
| Approach | ||||||||
| Laparoscopic | 395 | 54.6% | 231 | 62.9% | 540 | 57.8% | 19 | 40.4% |
| Open | 298 | 41.1% | 109 | 29.7% | 355 | 38.0% | 24 | 8.5% |
| Conversion | 31 | 4.3%% | 27 | 7.4% | 40 | 4.3% | 4 | 8.5% |
| Anastomotic technique | ||||||||
| Stapled | 527 | 77.3% | 298 | 89.2% | 619 | 92.1% | 30 | 88.2% |
| Hand sewn | 155 | 22.7% | 36 | 10.8% | 53 | 7.9% | 4 | 11.8% |
| No anastomosis | 37 | 30 | 255 | 13 | ||||
| Missing | 5 | 3 | 8 | 0 | ||||
| Seniority | ||||||||
| Colorectal consultant | 488 | 67.5% | 263 | 71.7% | 732 | 78.3% | 38 | 80.8% |
| Colorectal trainee | 61 | 8.4% | 14 | 3.8% | 55 | 5.9% | 3 | 6.4% |
| General surgery consultant | 126 | 17.4% | 65 | 17.7% | 124 | 13.3% | 6 | 12.8% |
| General surgery trainee | 43 | 6.1% | 23 | 6.3% | 18 | 1.9% | 0 | 0 |
| Missing | 5 | 2 | 6 | 0 | ||||
Abbreviation: ASA, American Society of Anesthesiologists.
Of patients who had an operation involving the rectum.
Of the 2073 patients, 785 (37.9%) had an open approach and 1186 (57.2%) had a minimally invasive approach. In 102 (4.9%) minimally invasive surgery was attempted with conversion to an open operation. Of patients who had an anastomosis, 85.6% (1474/1722) had a stapled anastomosis. The lead surgeon in the majority of operations was a colorectal consultant (1522/2060, 73.9%), with a trainee as lead operator in 10.5% of procedures (217/2060).
3.2. Change in anastomosis (COVID‐stoma) and outcomes
The overall rate of stoma formation was 34.2% (708/2073), which was more frequent than the rate of 27.2% in the prepandemic era (1573/5792). The change in practice of patients having a COVID‐stoma was small: 4.3% (90/2073) of all patients (Table 2). Of patients with a new COVID‐stoma, 70% (63/90) had an end stoma, far greater than the prepandemic rate for end stoma formation of 43.6% (686/1573) (Table 5). Colorectal trainees were more likely to be the named lead surgeon when defunctioning COVID‐stomas were formed (8.3%, 11/133) when compared with colorectal consultants (0.9%, 13/1521) and general surgical consultants (0.6%, 2/322) Table 2. This contrasts with the prepandemic era when a colorectal trainee was the named lead surgeon in 4.4% (97/2218) of procedures where a stoma was formed. More COVID‐end‐stomas were formed in patients undergoing rectal resections, in those who had an open approach to surgery and in those who received either no neoadjuvant therapy or long‐course neoadjuvant radiotherapy (Table 2). This is also reflected in an increase in the number of end stoma formations in rectal resections during the pandemic era (27.3%, 255/935) when compared with the prepandemic era (23.7%, 613/2579) and a decrease of formation of anastomosis without a defunctioning stoma during the pandemic (37.4%, 350/935) compared with prepandemic levels (42.8%, 1103/2579). The proportion of COVID‐stomas compared with all stomas is shown in Table S3.
TABLE 2.
Additional number of stomas formed due to COVID‐19 in relation to all patients undergoing surgery
| COVID‐defunctioning‐stoma/all operations | COVID‐end‐stoma/all operations | |||
|---|---|---|---|---|
| n | % | n | % | |
| Overall | ||||
| New COVID‐stomas | 27/2073 | 1.3% | 63/2073 | 3.0% |
| Sex | ||||
| Female | 11/837 | 1.3% | 24/837 | 3.1% |
| Male | 16/1236 | 1.3% | 39/1236 | 3.2% |
| ASA grade | ||||
| 1–2 | 23/1420 | 1.6% | 36/1420 | 2.5% |
| 3–5 | 4/647 | 0.6% | 26/647 | 4.0% |
| Age (years) | ||||
| <50 | 3/174 | 1.7% | 2/174 | 1.1% |
| 50–69 | 15/966 | 1.6% | 31/966 | 3.2% |
| ≥70 | 9/933 | 1.0% | 30/933 | 3.2% |
| Operation | ||||
| Right resection | 1/724 | 0.1% | 10/724 | 1.4% |
| Left resection | 2/367 | 0.5% | 7/367 | 1.9% |
| Rectal resection | 24/935 | 2.5% | 45/935 | 4.8% |
| Total/subtotal/panproctocolectomy | 0/47 | 0 | 1/47 | 2.1% |
| Disease stage | ||||
| I–II | 11/838 | 1.3% | 31/838 | 3.4% |
| III | 13/653 | 2.0% | 30/653 | 4.6% |
| IV | 3/133 | 2.3% | 2/133 | 1.5% |
| Neoadjuvant radiotherapya | ||||
| Short course | 3/89 | 3.4% | 1/89 | 1.1% |
| Long course | 5/206 | 2.4% | 9/206 | 4.4% |
| None | 16/674 | 2.4% | 35/674 | 5.2% |
| Approach | ||||
| Minimally invasive | 11/1185 | 0.9% | 18/1185 | 1.5% |
| Open | 15/786 | 1.9% | 42/786 | 5.3% |
| Minimally invasive converted to open | 1/102 | 0.9% | 3/102 | 2.9% |
| Anastomotic techniqueb | ||||
| Stapled | 25/1474 | 1.7% | N/A | N/A |
| Hand sewn | 2/248 | 0.8% | N/A | N/A |
| Seniority | ||||
| Colorectal consultant | 13/1521 | 0.9% | 45/1521 | 3.0% |
| Colorectal trainee | 11/133 | 8.3% | 3/133 | 2.3% |
| General surgery consultant | 2/322 | 0.6% | 11/322 | 3.4% |
| General surgery trainee | 1/84 | 1.2% | 5/84 | 6.0% |
Percentage (%) is the increased number of new stomas (COVID‐stoma) formed during the COVID‐19 pandemic out of the total number of patients who had an operation in each group.
Of patients who had an operation involving the rectum.
Of patients who had an anastomosis.
Of all rectal resections, 7.4% (69/935) received a COVID‐stoma (Figure 1), representing 76.7% of all COVID‐stomas (n = 90). In right colonic resections, 11 COVID‐stomas were formed (1.5% of 724 right resections), nine were formed in left colonic resections (2.5% of 367 left resections) and one COVID‐stoma was formed from the total/subtotal/panproctocolectomy group (2.1% of 47; Table 2).
FIGURE 1.
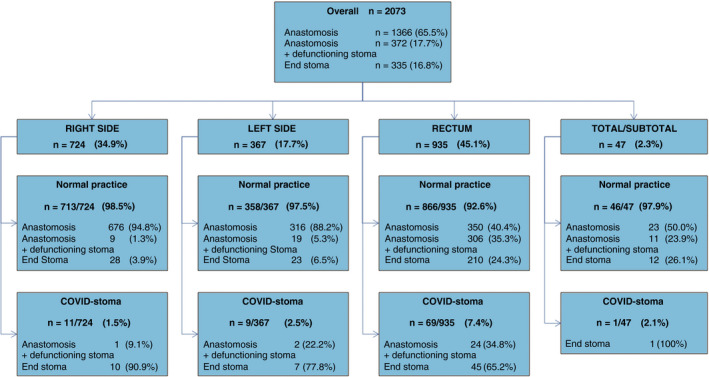
Flowchart of the type of stoma‐anastomosis configuration broken down by operative region and if patients had a change in stoma practice due to COVID‐19 (COVID‐stoma)
There were slight but nonsignificant differences in patients who had a COVID‐stoma compared with those who did not (Table 3), including a slight increase in anastomotic leak (7.4% versus 4.9%) and intensive care usage (29.9% versus 22.5%) and slight decrease in mortality (1.1% versus 1.9%). There was shorter length of stay in the group with a COVID‐stoma (4.5 days versus 6.0 days). Similarly, no difference in outcomes was observed in patients undergoing COVID‐stoma when stratified by cancer location (Table S4).
TABLE 3.
Outcomes stratified by additional stoma formation due to COVID‐19 (COVID‐stoma)
| Normal practice | COVID‐stoma | P | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Anastomotic leaka | |||||
| No | 1627 | 94.9% | 25 | 92.6% | 0.390 |
| Yes | 84 | 4.9% | 2 | 7.4% | |
| Intensive care | |||||
| No | 1537 | 77.5% | 64 | 71.1% | 0.157 |
| Yes | 446 | 22.5% | 26 | 29.9% | |
| Death | |||||
| No | 1946 | 98.1% | 89 | 98.9% | 1.000 |
| Yes | 37 | 1.9% | 1 | 1.1% | |
| Postoperative SARS‐CoV‐2 | |||||
| No | 1909 | 96.3% | 86 | 95.6% | 0.579 |
| Yes | 74 | 3.7% | 4 | 4.4% | |
| Length of stay (days)b | 6 (4–8) | 4.5 (4–6.5) | 0.270 | ||
Of patients who had an anastomosis.
Median (interquartile range).
3.3. Reasons for COVID‐stoma formation
The reason for change in practice was explored in patients who had a COVID‐stoma (stoma formation as a direct result of COVID‐19; n = 90). Surgeons were permitted to give more than one reason for change. There was a total of 147 responses. The most common reasons reported for formation of COVID‐stoma were ‘recommendation from specialty associations’ (44%, 64/147; Figure S1) and ‘to avoid possible complications requiring critical care’ (39%, 57/147). ‘Wish to reduce length of inpatient stay’ was given in 10% (14/147) and ‘fear of patient suffering COVID‐19 postoperatively’ was given in 6% (9/147) of responses. Only 2% (3/147) cited ‘Lack of access to postoperative intensive care’ and one cited ‘very difficult working conditions of full PPE’ as the reasons for COVID‐stoma.
3.4. Outcomes after surgery
Overall, 38 (1.8%) patients died within 30 days of surgery, 78 (3.8%) patients developed postoperative SARS‐CoV‐2 and 86 (4.9%) patients had an anastomotic leak. Mortality rates are presented in Figure 2, and show an increasing relationship with both anastomotic leak and SARS‐CoV‐2 infection. In risk‐adjusted analyses, significant predictors of 30‐day mortality were postoperative SARS‐CoV‐2, anastomotic leak, male sex, age over 70 years, cancer disease Stage IV and having a total/subtotal/panproctocolectomy (see Table 4 for adjusted ORs).
FIGURE 2.
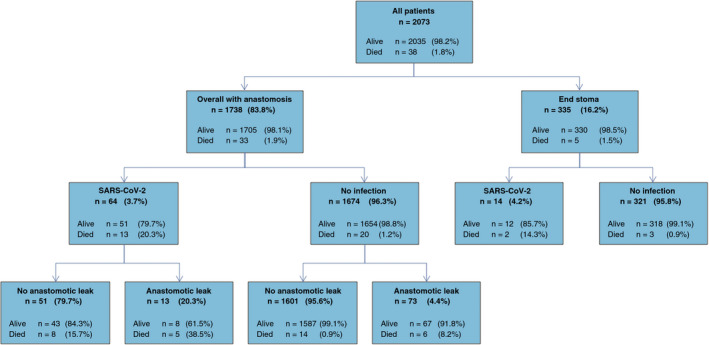
Flowchart of mortality related to postoperative SARS‐CoV‐2 and if an anastomotic leak occurred
TABLE 4.
Adjusted and unadjusted regression model of predictors for 30‐day mortality
| Mortality | Univariable | Multivariable | P | |||||
|---|---|---|---|---|---|---|---|---|
| n | % | OR | 95% CI | OR | 95% CI | |||
| Anastomotic leak | No | 27/1954 | 1.4% | – | – | |||
| Yes | 11/93 | 11.8% | 9.21 | 4.32–19.64 | 6.01 | 2.58–14.06 | <0.001 | |
| SARS‐CoV−2 | No | 23/1995 | 1.2% | – | – | |||
| Yes | 15/78 | 19.2% | 20.41 | 10.17–41.00 | 16.90 | 7.86–36.38 | <0.001 | |
| Age (years) | <70 | 13/1140 | 1.1% | – | – | |||
| >70 | 25/933 | 2.7% | 2.39 | 1.21–4.69 | 2.87 | 1.32–6.20 | 0.008 | |
| Sex | Female | 7/837 | 0.8% | – | ||||
| Male | 31/1236 | 2.5% | 3.05 | 1.34–6.96 | 2.46 | 1.01–5.93 | 0.045 | |
| ASA gradea | 1–2 | 19/1420 | 1.3% | – | – | |||
| 3–5 | 19/647 | 2.9% | 2.23 | 1.17–4.24 | 1.57 | 0.76–3.26 | 0.223 | |
| Disease stage | I–II | 17/1288 | 1.3% | – | ||||
| III | 15/653 | 2.3% | 1.76 | 0.87–3.54 | 2.00 | 0.91–4.20 | 0.088 | |
| IV | 6/132 | 4.6% | 3.56 | 1.38–9.19 | 3.43 | 1.16–10.21 | 0.026 | |
| Operation | Right resection | 9/724 | 1.2% | – | – | |||
| Left resection | 6/367 | 1.6% | 1.32 | 0.47–3.74 | 1.45 | 0.47–4.48 | 0.524 | |
| Rectal resection | 19/935 | 2.0% | 1.65 | 0.74–3.66 | 1.60 | 0.65–3.93 | 0.302 | |
| Total/subtotal/panproctocolectomy | 4/47 | 8.5% | 7.39 | 2.19–24.96 | 9.06 | 2.21–37.15 | 0.002 | |
Statistically significant P values are indicated in bold.
American Society of Anesthesiologists (ASA) physical status classification [16].
3.5. Case selection during the pandemic
Pandemic data are compared with prepandemic data from ESCP‐published cohort data in Table 5. There were few differences between patient characteristics across different operations. Overall, during the pandemic, patients selected for surgery were fitter (with lower ASA grade), more stomas were formed and a stapled technique was used more frequently than hand‐sewn anastomosis (Table 5). Outcomes following surgery during the pandemic included fewer anastomotic leaks and admissions to critical care; however, mortality was higher during the pandemic than in prepandemic era (Table 5).
TABLE 5.
Comparison of patient and disease characteristics and outcomes of patients undergoing elective cancer operations currently (during the pandemic) alongside composite data from the ESCP 2015 and 2017 audits (prepandemic)
| Right | Prepandemic | During pandemic | P‐value | Left | Prepandemic | During pandemic | P‐value | Rectum | Prepandemic | During pandemic | P‐value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | Sex | Sex | |||||||||
| Male | 1151 (51.7%) | 381 (52.6%) | 0.676 | Male | 589 (59.6%) | 232 (63.2%) | 0.228 | Male | 1617 (62.7%) | 592 (63.3%) | 0.738 |
| Female | 1074 (48.3%) | 343 (47.4%) | Female | 400 (40.4%) | 135 (36.8%) | Female | 962 (37.3%) | 343 (36.7%) | |||
| Age (years) | Age (years) | Age (years) | |||||||||
| <50 | 104 (4.7%) | 42 (5.8%) | 0.312 | <50 | 64 (6.5%) | 25 (6.8%) | 0.434 | <50 | 210 (8.1%) | 96 (10.3%) | 0.061 |
| 50–69 | 876 (39.3%) | 268 (37.0%) | 50–69 | 469 (47.4%) | 187 (50.1%) | 50–69 | 1336 (51.8%) | 495 (52.9%) | |||
| ≥70 | 1245 (56.0%) | 414 (57.2%) | ≥70 | 456 (46.1%) | 155 (42.1%) | ≥70 | 1033 (40.1%) | 344 (36.8%) | |||
| ASA grade | ASA grade | ASA grade | |||||||||
| 1–2 | 1379 (62.0%) | 454 (62.8%) | 0.694 | 1–2 | 617 (62.7%) | 244 (66.5%) | 0.198 | 1–2 | 1685 (66.0%) | 686 (73.8%) | <0.001 |
| 3–5 | 846 (38.0%) | 269 (37.2%) | 3–5 | 367 (37.3%) | 123 (33.5%) | 3–5 | 868 (34.0%) | 244 (26.2%) | |||
| Approach | Disease stage | Disease stage | |||||||||
| Minimally invasive | 1211 (54.4%) | 395 (54.7%) | <0.001 | I–II | 468 (50.8%) | 261 (71.1%) | <0.001 | I–II | 1421 (56.8%) | 479 (51.5%) | <0.001 |
| Open | 813 (36.5%) | 298 (41.0%) | III | 375 (40.6%) | 78 (21.4%) | III | 821 (32.8%) | 383 (41.2%) | |||
| Conversion | 201 (9.1%) | 31 (4.3%) | IV | 79 (8.6%) | 28 (9.6%) | IV | 261 (10.4%) | 68 (7.3%) | |||
| Operation | Approach | Neoadjuvant radiotherapy | |||||||||
| Anastomosis | 2194 (98.6%) | 677 (93.5%) | <0.001 | Minimally invasive | 519 (53.6%) | 231 (62.9%) | 0.001 | Short course | 177 (7.2%) | 89 (9.5%) | 0.001 |
| Anastomosis + defunction | 6 (0.3%) | 10 (1.4%) | Open | 356 (36.8%) | 109 (29.7%) | Long course | 679 (27.5%) | 205 (21.9%) | |||
| End stoma | 25 (1.1%) | 37 (5.1%) | Conversion | 93 (9.6%) | 27 (7.4%) | None | 1611 (58.1%) | 641 (68.6%) | |||
| Anastomotic techniquea | Operation | Approach | |||||||||
| Stapled | 1381 (62.8%) | 527 (77.3%) | <0.001 | Anastomosis | 922 (93.3%) | 316 (86.1%) | <0.001 | Minimally invasive | 1315 (54.2%) | 540 (57.8%) | <0.001 |
| Hand sewn | 819 (37.2%) | 155 (22.7%) | Anastomosis + defunction | 18 (1.8%) | 21 (5.7%) | Open | 867 (35.8%) | 355 (38.0%) | |||
| End stoma | 48 (4.9%) | 30 (8.2%) | Conversion | 243 (10.0%) | 40 (4.2%) | ||||||
| Seniority | Anastomotic techniquea | Operation | |||||||||
| Colorectal surgeon | 1465 (58.3%) | 488 (67.9%) | <0.001 | Stapled | 685 (72.9%) | 298 (89.2%) | <0.001 | Anastomosis | 1103 (42.8%) | 350 (37.4%) | 0.012 |
| Colorectal trainee | 333 (13.2%) | 61 (8.5%) | Hand sewn | 255 (27.1%) | 36 (10.8%) | Anastomosis + defunction | 863 (33.5%) | 330 (35.3%) | |||
| General surgeon | 467 (18.6%) | 126 (17.5%) | End stoma | 613 (23.7%) | 255 (27.3%) | ||||||
| General surgical trainee | 250 (9.9%) | 44 (6.1%) | |||||||||
| Anastomotic leaka | Seniority | Anastomotic techniquea | |||||||||
| No | 2056 (93.5%) | 662 (96.4%) | 0.005 | Colorectal surgeon | 705 (71.3%) | 263 (72.1%) | <0.001 | Stapled | 1811 (92.1%) | 619 (92.1%) | 0.998 |
| Yes | 144 (6.5%) | 25 (3.6%) | Colorectal trainee | 88 (8.9%) | 14 (3.8%) | Hand sewn | 155 (7.9%) | 53 (7.9%) | |||
| General surgeon | 170 (17.2%) | 65 (17.8%) | |||||||||
| General surgical trainee | 26 (2.6%) | 23 (6.3%) | |||||||||
| Intensive care | Anastomotic leaka | Seniority | |||||||||
| No | 1605 (72.1%) | 578 (79.8%) | <0.001 | No | 869 (92.5%) | 323 (95.9%) | 0.031 | Colorectal surgeon | 2078 (80.7%) | 732 (78.8%) | 0.087 |
| Yes | 620 (27.9%) | 158 (20.2%) | Yes | 71 (7.5%) | 14 (4.1%) | Colorectal trainee | 112 (4.4%) | 55 (5.9%) | |||
| General surgeon | 355 (13.8%) | 124 (13.4%) | |||||||||
| General surgical trainee | 31 (1.2%) | 18 (1.9%) | |||||||||
| Death | Intensive care | Anastomotic leaka | |||||||||
| No | 2188 (98.3%) | 715 (98.8%) | 0.155 | No | 693 (70.1%) | 299 (81.5%) | <0.001 | No | 1786 (90.8%) | 636 (93.5%) | 0.030 |
| Yes | 37 (1.7%) | 9 (1.2%) | Yes | 295 (29.9%) | 68 (18.5%) | Yes | 180 (9.2%) | 44 (6.5%) | |||
| Length of stay (days), median (IQR) | 7 (5–10) | 6 (4–8) | <0.001 | Death | Intensive care | ||||||
| No | 982 (99.3%) | 361 (98.4%) | 0.254 | No | 1707 (66.2%) | 692 (74.0%) | <0.001 | ||||
| Yes | 7 (0.7%) | 6 (1.6%) | Yes | 870 (33.8%) | 243 (26.0%) | ||||||
| Length of stay | 7 | 6 | <0.001 | Death | |||||||
| (days), median (IQR) | (5‐9) | (4‐8) | No | 2559 (99.2%) | 916 (98.0%) | 0.261 | |||||
| Yes | 20 (0.8%) | 19 (2.0%) | |||||||||
| Length of stay | 8 | 7 | <0.001 | ||||||||
| (days), median (IQR) | (6‐11) | (5‐11) | |||||||||
Statistically significant P values are indicated in bold.
Of patients who had an anastomosis.
In patients who had an anastomotic leak, mortality was 8.6%, (6/70) in the pandemic data. In the prepandemic data, the mortality in those who had a leak was 6.6% (26/395).
4. DISCUSSION
Mortality associated with an anastomotic leak and postoperative SARS‐CoV‐2 during the first waves of the COVID‐19 pandemic was extremely high. A small change in stoma practice was observed, with fewer than 5% of patients receiving a COVID‐stoma when they would usually have had an anastomosis only. Although those patients did not suffer any adverse outcomes, those measures alone did not reduce the overall complication rates seen in this study. In comparison with published mortality data following perioperative SARS‐CoV‐2 infection alone, the relative risk of death was almost 60% higher in combination with anastomotic leak (24.1% versus 34.8%) [11].
Comparison with previous ESCP cohort data identifies some of the selection bias that took place during these phases of the pandemic. There was an increased use of stapled anastomosis, fewer admissions to intensive care and a shorter length of stay. These all suggest efforts by surgeons and patients to reduce the duration of surgery, resource usage and hospital stay. Rectal cancer patients undergoing surgery seemed to be fitter than in data from the ESCP audits, with a higher proportion of patients of ASA grades 1–2. Slightly fewer patients underwent neoadjuvant therapy compared with before the pandemic, which suggests a greater element of delayed surgery or ‘watch and wait’ strategies during the pandemic. Outcomes from patients who had neoadjuvant therapies and were either delayed or did not have surgery are awaited. There may be an increased flow of patients ‘postpandemic’, both needing surgery and needing monitoring, who will require additional support from already strained surgical systems.
This study had limitations. First, this was an observational study in the first phase of the pandemic, where guideline implementation was incomplete. Data on implementation of guidelines by each hospital or country were not captured in this study. Second, the absolute change in practice presented was small, so firm conclusions cannot be drawn around the safety of the wider adoption of risk‐averse practices. Third, comparison with the prepandemic ESCP audit dataset may be biased through undetected patient‐, hospital‐ and country‐level differences that could preclude direct comparison, therefore the results must be interpreted with caution and firm conclusions should not be drawn. Fourth, data were not presented for patients who had surgery delayed due to COVID‐19 or had an alternative treatment strategy. We therefore present an incomplete picture of the care of colorectal cancer patients during the pandemic. Fifth, change in practice to COVID‐stoma was reported by the surgeon and is therefore subjectively reported. We attempted to overcome this by comparing the total stoma rate with prepandemic rates, showing an increased rate of stoma formation during the pandemic. Sixth, despite guidance and concerns around aerosolization, this study showed that laparoscopic approaches continued. The reasons for this, including surgeon and patient attitudes, deserve further exploration by way of additional qualitative research. Finally, although case selection and more elective stomas can potentially reduce postoperative risks, further robust strategies are needed to mitigate against morbidity and mortality and further exploration is required.
Clear data and safe strategies are needed to continue to provide safe surgery during future pandemic waves. This study highlights several patient, operative and organizational factors that may bring benefit and need further testing. At a patient level, selection of fitter patients, who will benefit most from curative surgery during peaks of pandemics, is logical. This has been previously recommended to both conserve critical care capacity and avoid exposing high‐risk patients to nosocomial SARS‐CoV‐2 transmission [3, 9]. At an operative level, the avoidance of leaks seems paramount. Forming stomas alone is not necessarily the solution, as they carry their own risks and morbidity. Selecting lower‐risk patients for anastomosis, use of defunctioning stomas and more liberal use of end stomas in high‐risk patients might be best supported through formal risk stratification for anastomotic leak [23, 24]. At an organizational level, the prevention of postoperative SARS‐CoV‐2‐related infections is paramount. This seems best approached by identifying preoperative, presymptomatic carriers (i.e. preoperative swab testing) and by providing COVID‐19‐free surgical pathways. Both of these areas require further evidence to best define exactly which measures they include (e.g. number of swabs, role of computed tomography of the thorax, components of COVID‐19‐free pathways). With an estimated 3 000 000 cancer operations postponed around the world [12], and more accruing during second waves, efficient measures to safely discharge patients early and protect them from the risk of in‐hospital transmission should continue.
5. DATA AVAILABILITY SHARING
Data‐sharing requests will be considered by the management group upon written request to the corresponding author. If agreed, de‐identified participant data will be available, subject to a data‐sharing agreement.
CONFLICT OF INTEREST
There are no conflicts of interest to declare.
Funding information
This report was funded by a National Institute for Health Research (NIHR) Global Health Research Unit Grant (NIHR 16.136.79) using UK aid from the UK Government to support global health research, The Association of Coloproctology of Great Britain and Ireland, Bowel & Cancer Research, Bowel Disease Research Foundation, Association of Upper Gastrointestinal Surgeons, British Association of Surgical Oncology, British Gynaecological Cancer Society; European Society of Coloproctology, NIHR Academy, Sarcoma UK, Vascular Society for Great Britain and Ireland and Yorkshire Cancer Research. The funders had no role in study design, data collection, analysis and interpretation or writing of this report. The views expressed are those of the authors and not necessarily those of the National Health Service, the NIHR or the UK Department of Health and Social Care.
Supporting information
Appendix 1.
1.1. Writing group (*denotes joint first authors)
Elizabeth Li*, James C. Glasbey*, Dmitri Nepogodiev*, Joana F. F. Simoes*, Omar M. Omar, Mary L. Venn, Jonathan P. Evans, Kaori Futaba, Charles H. Knowles, Ana Minaya‐Bravo, Helen Mohan, Manish Chand, Peter Pockney, Salomone Di Saverio, Neil Smart, Abigail Vallance, Dale Vimalachandran, Richard J. W. Wilkin, Aneel Bhangu (Overall guarantor).
1.2. Statistical analysis
Omar M. Omar (Lead statistician), Elizabeth Li, James C. Glasbey, Aneel Bhangu.
1.3. CovidSurg Operations Committee
Kwabena Siaw‐Acheampong, Ruth A. Benson, Edward Bywater, Daoud Chaudhry, Brett E. Dawson, Jonathan P. Evans, James C. Glasbey, Rohan R. Gujjuri, Emily Heritage, Conor S. Jones, Sivesh K. Kamarajah, Chetan Khatri, Rachel A. Khaw, James M. Keatley, Andrew Knight, Samuel Lawday, Elizabeth Li, Harvinder S. Mann, Ella J. Marson, Kenneth A. McLean, Siobhan C. Mckay, Emily C. Mills, Dmitri Nepogodiev, Gianluca Pellino, Maria Picciochi, Elliott H. Taylor, Abhinav Tiwari, Joana F. F. Simoes, Isobel M. Trout, Mary L. Venn, Richard J. W. Wilkin, Aneel Bhangu.
1.4. International Cancer Leads (*denotes specialty principal Investigator)
James C. Glasbey (Chair); Colorectal: Neil J. Smart*, Ana Minaya‐Bravo*, Jonathan P. Evans, Gaetano Gallo, Susan Moug, Francesco Pata, Peter Pockney, Salomone Di Saverio, Abigail Vallance, Dale Vimalchandran.
1.5. Dissemination Committee
Joana F. F. Simoes (Chair); Tom E. F. Abbott, Sadi Abukhalaf, Michel Adamina, Adesoji O. Ademuyiwa, Arnav Agarwal, Murat Akkulak, Ehab Alameer, Derek Alderson, Felix Alakaloko, Markus Albertsmeiers, Osaid Alser, Muhammad Alshaar, Sattar Alshryda, Alexis P. Arnaud, Knut Magne Augestad, Faris Ayasra, José Azevedo, Brittany K. Bankhead‐Kendall, Emma Barlow, David Beard, Ruth A. Benson, Ruth Blanco‐Colino, Amanpreet Brar, Ana Minaya‐Bravo, Kerry A. Breen, Chris Bretherton, Igor Lima Buarque, Joshua Burke, Edward J. Caruana, Mohammad Chaar, Sohini Chakrabortee, Peter Christensen, Daniel Cox, Moises Cukier, Miguel F. Cunha, Giana H. Davidson, Anant Desai, Salomone Di Saverio, Thomas M. Drake, John G. Edwards, Muhammed Elhadi, Sameh Emile, Shebani Farik, Marco Fiore, J. Edward Fitzgerald, Samuel Ford, Tatiana Garmanova, Gaetano Gallo, Dhruv Ghosh, Gustavo Mendonça Ataíde Gomes, Gustavo Grecinos, Ewen A. Griffiths, Madalegna Gründl, Constantine Halkias, Ewen M. Harrison, Intisar Hisham, Peter J. Hutchinson, Shelley Hwang, Arda Isik, Michael D. Jenkinson, Pascal Jonker, Haytham M. A. Kaafarani, Debby Keller, Angelos Kolias, Schelto Kruijff, Ismail Lawani, Hans Lederhuber, Sezai Leventoglu, Andrey Litvin, Andrew Loehrer, Markus W. Löffler, Maria Aguilera Lorena, Maria Marta Madolo, Piotr Major, Janet Martin, Hassan N. Mashbari, Dennis Mazingi, Symeon Metallidis, Ana Minaya‐Bravo, Helen M. Mohan, Rachel Moore, David Moszkowicz, Susan Moug, Joshua S. Ng‐Kamstra, Mayaba Maimbo, Ionut Negoi, Milagros Niquen, Faustin Ntirenganya, Maricarmen Olivos, Kacimi Oussama, Oumaima Outani, Marie Dione Parreno‐Sacdalanm, Francesco Pata, Carlos Jose Perez Rivera, Thomas D. Pinkney, Willemijn van der Plas, Peter Pockney, Ahmad Qureshi, Dejan Radenkovic, Antonio Ramos‐De la Medina, Keith Roberts, April C. Roslani, Martin Rutegård, Irène Santos, Sohei Satoi, Raza Sayyed, Andrew Schache, Andreas A Schnitzbauer, Justina O. Seyi‐Olajide, Neil Sharma, Richard Shaw, Sebastian Shu, Kjetil Soreide, Antonino Spinelli, Grant D Stewart, Malin Sund, Sudha Sundar, Stephen Tabiri, Philip Townend, Georgios Tsoulfas, Gabrielle H. van Ramshorst, Raghavan Vidya, Dale Vimalachandran, Oliver J. Warren, Duane Wedderburn, Naomi Wright, EuroSurg, European Society of Coloproctology (ESCP), Global Initiative for Children’s Surgery (GICS), GlobalSurg, GlobalPaedSurg, ItSURG, PTSurg, SpainSurg, Italian Society of Colorectal Surgery (SICCR), Association of Surgeons in Training (ASiT), Irish Surgical Research Collaborative (ISRC), Transatlantic Australasian Retroperitoneal Sarcoma Working Group (TARPSWG), Italian Society of Surgical Oncology (SICO).
1.6. Collaboratoring authors (*denotes site principal investigators)
Argentina: Alurralde C., Caram E. L., Eskinazi D* (Sanatorio 9 De Julio Sa); Badra R., García J.S., Lucchini S.M.* (Sanatorio Allende).
Australia: Cecire J., Salindera S.*, Sutherland A. (Coffs Harbour Health Campus); Ahn J.H., Chen S., Gauri N., Jang S., Jia F., Mulligan C., Yang W., Ye G., Zhang H. (Concord Repatriation General Hospital); Moss J.*, Richards T., Thian A., Vo U. G. (Fiona Stanley Hospital); Bagraith K., Chan E., Ho D., Jeyarajan E., Jordan S., Nolan G. J., Von Papen M., Wullschleger M. (Gold Coast University Hospital); Egoroff N., Gani J., Lott N., Pockney P.* (John Hunter Hospital); Phan D., Townend D.* (Lismore Base Hospital); Bong C., Gundara J.* (Logan Hospital); Bowman S.*, Guerra G. R. (Queen Elizabeth II Jubilee Hospital); Dudi‐Venkata N. N., Kroon H. M.*, Sammour T. (Royal Adelaide Hospital); Mitchell D.*, Swinson B. (Royal Brisbane and Women’s Hospital).
Austria: Messner F., Öfner D.* (Medical University of Innsbruck); Emmanuel K., Grechenig M., Gruber R., Harald M., Öhlberger L., Presl J.*, Wimmer A. (Paracelsus Medical University Salzburg).
Barbados: Barker D., Boyce R., Doyle A., Eastmond A., Gill R., O’Shea M., Padmore G.*, Paquette N., Phillips E., St John S., Walkes K. (Queen Elizabeth Hospital).
Belgium: Flamey N., Pattyn P.* (Az Delta); Oosterlinck W.*, Van den Eynde J., Van den Eynde R. (Uz Leuven).
Bulgaria: Sokolov M.* (University Hospital Alexandrovska).
Canada: Boutros M.*, Caminsky N. G., Ghitulescu G. (Jewish General Hospital); Boutros M.*, Demyttenaere S.*, Garfinkle R. (St Mary’s Hospital); Nessim C.*, Stevenson J. (The Ottawa Hospital).
Croatia: Bačić G., Karlović D., Kršul D., Zelić M.* (University Hospital Center Rijeka); Bakmaz B., Ćoza I., Dijan E., Katusic Z., Mihanovic J.*, Rakvin I. (Zadar General Hospital).
Cyprus: Frantzeskou K., Gouvas N.*, Kokkinos G., Papatheodorou P., Pozotou I., Stavrinidou O., Yiallourou A.* (Nicosia General Hospital).
Czechia: Martinek L., Skrovina M.*, Szubota I. (Hospital and Oncological Centre Novy Jicin).
Denmark: Ebbehøj A. L., Krarup P., Schlesinger N., Smith H.* (Bispebjerg Hospital).
Egypt: Al Sayed M., Ashoush F.*, Elazzazy E., Essam E., Eweda M., Hassan E., Metwalli M., Mourad M., Qatora M. S., Sabry A.*, Samih A., Samir Abdelaal A., Shehata S.*, Shenit K. (Alexandria Main University Hospital); Attia D., Kamal N., Osman N.* (Alexandria Medical Research Institute); Alaa S., Hamza H. M., M. elghazaly S., Mohammed M. M.*, Nageh M. A., Saad M. M.*, Yousof E. A. (Assiut University Hospital); Eldaly A. S.* (El‐Menshawy Hospital); Amira G., Sallam I.*, Sherief M., Sherif A. (Misr Cancer Center); Ghaly G.*, Hamdy R., Morsi A., Salem H.*, Sherif G. (National Cancer Institute); Abdeldayem H., Abdelkader Salama I.*, Balabel M., Fayed Y., Sherif A. E.* (National Liver Institute, Menoufia University).
Finland: Kauppila J. H.*, Sarjanoja E. (Länsi‐Pohja Central Hospital); Helminen O., Huhta H., Kauppila J. H.* (Oulu University Hospital).
France: Beyrne C., Jouffret L.*, Marie‐Macron L. (Centre Hospitalier Avignon); Lakkis Z.*, Manfredelli S. (CHU Besançon); Chebaro A.*, El Amrani M., Lecolle K., Piessen G.*, Pruvot F. R., Zerbib P. (CHU Lille); Ballouhey Q.*, Barrat B., Taibi A. (Chu Limoges); Bergeat D., Merdrignac A. (CHU Rennes – General Surgery); Le Roy B., Perotto L. O., Scalabre A.* (Chu Saint Etienne); Aimé A., Ezanno A.*, Malgras B. (Hia Begin); Bouche P. A.*, Tzedakis S.* (Hôpital Cochin – APHP); Cotte E., Glehen O., Kepenekian V., Passot G. (Hopital Lyon Sud); D’Urso A., Mutter D., Seeliger B.* (Strasbourg University Hospitals/IHU‐Strasbourg); Bonnet S., Denet C., Fuks D., Laforest A., Pourcher G., Seguin‐Givelet A.*, Tribillon E. (Institut Mutualiste Montsouris); Duchalais E.* (Nantes University Hospital).
Germany: Bork U.*, Fritzmann J., Praetorius C., Weitz J., Welsch T. (Carl‐Gustav‐Carus University Hospital, TU Dresden); Beyer K., Kamphues C.*, Lauscher J. C., Loch F. N., Schineis C. (Charité University Medicine, Campus Benjamin Franklin); Becker R.*, Jonescheit J. (Heilig‐Geist Hospital Bensheim); Pergolini I., Reim D.* (Klinikum Rechts der Isar TUM School of Medicine); Boeker C., Hakami I.*, Mall J.* (KRH Nordstadt‐Siloah Hospitals); Albertsmeier M.*, Kappenberger A., Schiergens T., Werner J. (LMU Klinikum Campus Innenstadt); Nowak K.*, Reinhard T.* (Romed Klinikum Rosenheim); Kleeff J., Michalski C., Ronellenfitsch U.* (University Hospital Halle (Saale)); Bertolani E., Königsrainer A.*, Löffler M. W., Quante M.*, Steidle C., Überrück L., Yurttas C. (University Hospital Tuebingen); Izbicki J., Nitschke C., Perez D., Uzunoglu F. G.* (University Medical Center Hamburg–Eppendorf).
Greece: Antonakis P., Contis I., Dellaportas D., Gklavas A., Konstadoulakis M., Memos N.*, Papaconstantinou I.*, Polydorou A., Theodosopoulos T., Vezakis A. (Aretaieion Hospital); Antonopoulou M. I., Manatakis D. K.*, Tasis N. (Athens Naval and Veterans Hospital); Arkadopoulos N., Danias N., Economopoulou P., Frountzas M., Kokoropoulos P., Larentzakis A., Michalopoulos N.*, Parasyris S., Selmani J., Sidiropoulos T., Vassiliu P. (Attikon University General Hospital); Bouchagier K.*, Klimopoulos S., Paspaliari D., Stylianidis G. (Evaggelismos General Hospital); Baxevanidou K., Bouliaris K., Chatzikomnitsa P., Efthimiou M., Giaglaras A., Kalfountzos C.*, Koukoulis G., Ntziovara A. M., Petropoulos K., Soulikia K., Tsiamalou I., Zervas K., Zourntou S. (General Hospital of Larissa ‘Koutlimpaneio and Triantafylleio’); Baloyiannis I., Diamantis A., Perivoliotis K., Tzovaras G.* (General University Hospital of Larissa); Christidis P., Ioannidis O.*, Loutzidou L. (George Papanikolaou General Hospital of Thessaloniki); Karaitianos I.*, Tsirlis T. (Henry Dunant Hospital Center); Charalabopoulos A., Liakakos T., Baili E., Schizas D.*, Spartalis E., Syllaios A., Zografos C. (Laiko University Hospital); Athanasakis E., Chrysos E., Tsiaoussis I., Xenaki S.*, Xynos E.* (University Hospital of Heraklion Crete and Interclinic Hospital of Crete).
Hong Kong: Futaba K.*, Ho M. F., Hon S. F., Mak T. W. C., Ng S. S. M. (Prince of Wales Hospital); Foo C. C.* (Queen Mary Hospital).
Hungary: Banky B.*, Suszták N. (Szent Borbála Kórház).
India: Bhat G. A., Chowdri N. A., Mehraj A.*, Parray F., Shah Z. A., Wani R. (Sher‐I‐Kashmir Institute of Medical Sciences); Ahmed Z., Bali R., Bhat M. A., Laharwal A., Mahmood M., Mir I., Mohammad Z., Muzamil J., Rashid A.* (SMHS Hospital, Government Medical College).
Ireland: Aremu M.*, Canas‐Martinez A., Cullivan O., Murphy C., Owens P., Pickett L. (Connolly Hospital Blanchardstown); Corrigan M.*, Daly A., Fleming C.*, Jordan P., Killeen S., Lynch N., O’Brien S., Syed W. A. S., Vernon L. (Cork University Hospital); Fahey B. A., Larkin J. O.*, McCormick P., Mehigan B. J., Mohan H., Shokuhi P., Smith. J (St James’s Hospital); Bashir Y., Bass G. A., Connelly T. M., Creavin B., Earley H., Elliott J. A.*, Gillis A. E., Kavanagh D. O., Neary P. C., O’Riordan J. M., Reynolds I. S., Rice D., Ridgway P. F., Umair M., Whelan M. (Tallaght University Hospital); Corless K., Finnegan L., Fowler A., Hogan A., Lowery A.*, McKevitt K.*, Ryan É. (University Hospital Galway); Coffey J. C., Cunningham R. M., Devine M., Nally D.*, Peirce C. (University Hospital Limerick); Hardy N. P., Neary P. M., O’Malley S.*, Ryan M. (University Hospital Waterford/University College Cork).
Italy: Macina S.* (ASST Mantua); Mariani N. M.*, Opocher E., Pisani Ceretti A. (ASST Santi Paolo E. Carlo); Bianco F.* (ASST Papa Giovanni XXIII – Bergamo); Marino M. V.*, Mirabella A., Vaccarella G. (Azienda Ospedaliera Ospedali Riuniti Villa Sofia‐Cervello); Agostini C., Alemanno G., Bartolini I., Bergamini C., Bruscino A., De Vincenti R., Di Bella A., Fortuna L., Maltinti G., Muiesan P.*, Prosperi P.*, Ringressi M. N., Risaliti M., Taddei A.*, Tucci R. (Azienda Ospedaliera Universitaria Careggi); Campagnaro T.*, Guglielmi A., Pedrazzani C., Rattizzato S., Ruzzenente A., Turri G. (Azienda Ospedaliera Universitaria Integrata di Verona); Bellora P., D’Aloisio G., Ferrari M., Francone E., Gentilli S.*, Nikaj H. (Azienda Ospedaliero Universitaria Maggiore della Carità); Bianchini M., Chiarugi M., Coccolini F., Di Franco G., Furbetta N., Gianardi D., Guadagni S., Morelli L.*, Palmeri M., Tartaglia D.* (Azienda Ospedaliero Universitaria Pisana); Anania G.*, Carcoforo P.*, Chiozza M., De Troia A., Koleva Radica M., Portinari M., Sibilla M. G., Urbani A. (Azienda Ospedaliero Universitaria San’anna); Fabbri N., Feo C. V.*, Gennari S., Parini S., Righini E. (Azienda Unità Sanitaria Locale di Ferrara, Università di Ferrara); Annessi V., Castro Ruiz C., Montella M. T., Zizzo M.* (Azienda Unità Sanitaria Locale – IRCCS di Reggio Emilia); Grossi U., Novello S., Romano M., Rossi S., Zanus G.* (Ca’ Foncello Treviso – DISCOG – Università di Padova); Esposito G., Frongia F., Pisanu A., Podda M.* (Cagliari University Hospital); Belluco C., Lauretta A.*, Montori G., Moras L., Olivieri M.; Feo C. F., Perra T.*, Porcu A.*, Scanu A. M. (Cliniche San Pietro, Aou Sassari); Aversano A., Carbone F., Delrio P.*, Di Lauro K., Fares Bucci A., Rega D.*, Spiezio G. (Colorectal Surgical Oncology Unit – Istituto Nazionale Tumori Fondazione, Pascale IRCCS); Calabrò M.*, Farnesi F., Lunghi E. G., Muratore A.*, Pipitone Federico N. S. (Edoardo Agnelli); De Palma G. D., Luglio G.*, Pagano G., Tropeano F. P. (Federico II University Hospital); Baldari L.*, Boni L.*, Cassinotti E.* (Fondazione IRCCS Ca’ Granda – Ospedale Maggiore Policlinico di Milano); Cosimelli M., Fiore M.*, Guaglio M.*, Sorrentino L. (Fondazione IRCCS Istituto Nazionale dei Tumori, Milano); Agnes A., Alfieri S., Belia F., Biondi A., Cozza V., D’Ugo D., De Simone V., Litta F., Lorenzon L., Marra A. A., Marzi F., Parello A., Persiani R., Ratto C., Rosa F., Scrima O., Sganga G. (Fondazione Policlinico Universitario Agostino Gemelli IRCCS); Belli A.*, Izzo F., Patrone R. (HPB Surgical Oncology Unit – Istituto Nazionale Tumori Fondazione, Pascale IRCCS); Carrano F. M., Carvello M. M., Di Candido F., Maroli A., Spinelli A.* (Humanitas Clinical and Research Center IRCCS, Rozzano (Mi) and Humanitas University, Department of Biomedical Sciences, Pieve Emanuele (Mi)); Aprile A., Batistotti P., Massobrio A., Pertile D., Scabini S.*, Soriero D. (IRCCS Ospedale Policlinico San Martino); De Manzoni Garberini A.* (Ospedale Civile Spirito Santo); Federico P., Maida P., Marra E., Marte G., Petrillo A., Tammaro P., Tufo A.* (Ospedale del Mare); Berselli M.*, Borroni G.*, Cocozza E., Conti L., Desio M., Rizzi A. (ASST Sette Laghi‐Varese); Baldi C.*, Corbellini C., Sampietro G. M. (Ospedale di Rho – ASST Rhodense); Baldini E.*, Capelli P., Conti L., Isolani S. M., Ribolla M. (Ospedale Guglielmo da Saliceto Piacenza); Bondurri A., Colombo F.*, Ferrario L., Guerci C., Maffioli A. (Ospedale Luigi Sacco Milano); Armao T., Ballabio M.*, Bisagni P., Gagliano A., Longhi M., Madonini M., Pizzini P. (Ospedale Maggiore di Lodi); Mochet S.*, Usai A. (Ospedale Regionale Umberto Parini); Bianco F.*, Incollingo P. (Ospedale S. Leonardo – Asl Napoli 3 Sud, Castellammare di Stabia); Mancini S., Marino Cosentino L.*, Sagnotta A.* (Ospedale San Filippo Neri); Nespoli L. C., Tamini N.* (Ospedale San Gerardo); Anastasi A., Bartalucci B., Bellacci A., Canonico G.*, Capezzuoli L., Di Martino C., Ipponi P., Linari C., Montelatici M., Nelli T., Spagni G., Tirloni L., Vitali A. (Ospedale San Giovanni di Dio); Abate E., Casati M.*, Casiraghi T., Laface L., Schiavo M. (Ospedale Vittorio Emanuele III – Carate Brianza); Arminio A., Cotoia A., Lizzi V.*, Vovola F. (Ospedali Riuniti Azienda Ospedaliera Universitaria Foggia); Vergari R.* (Ospedali Riuniti di Ancona); D’Ugo S.*, Depalma N., Spampinato M. G. (Vito Fazzi, Leece); Brachini G., Chiappini A., Cicerchia P. M., Cirillo B., De Toma G., Fiori E., Fonsi G. B., Iannone I., La Torre F., Lapolla P.*, Meneghini S., Mingoli A., Sapienza P., Zambon M. (Policlinico Umberto I Sapienza University of Rome); Capolupo G. T.*, Mazzotta E. (Policlinico Universitario Campus Bio Medico of Rome); Gattolin A., Migliore M., Rimonda R., Sasia D.*, Travaglio E. (Regina Montis Regalis Hospital, Mondovì); Cervellera M., Gori A., Sartarelli L., Tonini V.* (S. Orsola‐Malpighi Hospital); Chessa A.*, Fiorini A., Norcini C. (San Giovanni di Dio); Colletti G., Confalonieri M., Costanzi A.*, Frattaruolo C., Mari G., Monteleone M. (San Leopoldo Mandic); De Nardi P.*, Parise P., Vignali A. (San Raffaele Scientific Institute, Milan); Belvedere A., Bernante P., Jovine E., Neri J., Parlanti D., Pezzuto A. P., Poggioli G., Rottoli M.*, Tanzanu M., Violante T. (IRCCS Azienda Ospedaliero – Universitaria di Bologna; Alma Mater Studiorum University of Bologna); Borghi F., Cianflocca D., Di Maria Grimaldi S., Donati D., Gelarda E., Giraudo G., Giuffrida M. C., Marano A.*, Palagi S., Pellegrino L., Peluso C., Testa V.* (Santa Croce E. Carle Hospital, Cuneo); Agresta F.*, Prando D.*, Zese M.* (Santa Maria degli Angeli Hospital ULSS 5 – Adria); Armatura G.*, Frena A., Scotton G.* (St Moritz Hospital); Gallo G.*, Sammarco G., Vescio G. (University ‘Magna Graecia’ of Catanzaro); Di Marzo F.* (Valtiberina); Fontana T.* (‘Vittorio Emanuele’ – Gela).
Japan: Kanemitsu Y.*, Moritani K. (National Cancer Center Hospital).
Jordan: Al Abdallah M.*, Ayasra F., Ayasra Y., Qasem A. (Al‐Basheer Hospital); Fahmawee T., Hmedat A., Obeidat K.* (King Abdullah University Hospital); Abou Chaar M. K., Al‐Masri M.*, Al‐Najjar H., Alawneh F. (King Hussein Cancer Center).
Libya: Alkadeeki G.*, Al Maadany F. S. (Al‐Jalaa Hospital); Aldokali N., Senossi O., Subhi M. T. (Alkhadra Hospital); Burgan D.*, Kamoka E., Kilani A. I. (National Cancer Institute, Sabratha); Ellojli I.*, Kredan A. (Tripoli University Hospital).
Lithuania: Bradulskis S., Dainius E., Kubiliute E., Kutkevičius J., Parseliunas A., Subocius A., Venskutonis D.* (Lithuanian University of Health Sciences Kaunas Clinical Hospital).
Madagascar: Rasoaherinomenjanahary F.*, Razafindrahita J. B., Samison L. H. (Joseph Ravoahangy Andrianavalona Hospital).
Malaysia: Hamdan K. H., Ibrahim M. R., Tan J. A., Thanapal M. R.* (Hospital Kuala Lumpur); Amin Sahid N., Hayati F.*, Jayasilan J., Sriram R. K.*, Subramaniam S. (Queen Elizabeth Hospital and Universiti Malaysia Sabah, Kota Kinabalu, Sabah); Che Jusoh M. A., Hussain A. H., Mohamed Sidek A. S., Mohd Yunus M. F., Soh J. Y., Wong M., Zakaria A. D.*, Zakaria Z. (School of Medical Sciences and Hospital, Universiti Sains Malaysia); Fathi N. Q., Xavier R. G., Roslani A. C.* (University Malaya Medical Centre).
Mexico: Buerba G. A., Mercado M. Á.*, Posadas‐Trujillo O. E., Salgado‐Nesme N., Sarre C. (Instituto Nacional de Ciencias Médicas y Nutrición ‘Salvador Zubirán’).
Morocco: Amrani L., El Ahmadi B., El Bouazizi Y., Majbar A. M., Benkabbou A., Mohsine R., Souadka A.* (Institut National d’Oncologie, Université Mohammed V Rabat).
Netherlands: Hompes R.*, Meima‐van Praag E. M., Pronk A. J. M., Sharabiany S. (Amsterdam UMC, University of Amsterdam); Grotenhuis B.*, Hartveld L. (Antoni Van Leeuwenhoek Ziekenhuis); Posma‐Bouman L.* (Slingeland Ziekenhuis); Derksen T., Franken J., Oosterling S.* (Spaarne Gasthuis); Konsten J.*, Van Heinsbergen M. (Viecuri Medisch Centrum).
Nigeria: Olaogun J.* (Ekiti State University Teaching Hospital); Abdur‐Rahman L.*, Adeyeye A.*, Bello J., Olasehinde O., Popoola A. (University of Ilorin Teaching Hospital).
Pakistan: Jamal A., Kerawala A. A.* (Cancer Foundation Hospital); Memon A. S.*, Nafees Ahmed R., Rai .L* (Dr Ruth K. M. Pfau Civil Hospital); Ayub B., Ramesh P., Sayyed R.* (Patel Hospital); Butt U. I.*, Kashif M., Qureshi A.*, Farooka M. W.*, Ayyaz M.* (Services Hospital Lahore); Ayubi A., Waqar S. H.* (Pakistan Institute of Medical Sciences).
Poland: Major P. (Jagiellonian University Medical College).
Portugal: Azevedo C., Machado D., Mendes F.* (Centro Hospitalar Cova da Beira); De Sousa X.* (Centro Hospitalar de Setúbal); Fernandes U., Ferreira C.*, Guidi G., Marçal A., Marques R., Martins D., Vaz Pereira R., Vieira B. (Centro Hospitalar de Trás‐Os‐Montes e Alto Douro, EPE); Afonso J., Almeida J. I., Almeida‐Reis R.*, Correia de Sá T., Costa M. J. M. A., Fernandes V., Ferraz I., Lima da Silva C., Lopes L., Machado N., Marialva J., Nunes Coelho M., Pereira C., Ribeiro A., Ribeiro C. G., Santos R., Saraiva P., Silva R., Tavares F., Teixeira M. (Centro Hospitalar do Tamega e Sousa); Almeida A. C., Amaral M. J., Andrade R., Camacho C., Costa M., Lázaro A.*, Nogueira O., Oliveira A., Ruivo A., Silva M., Simões J. (Centro Hospitalar e Universitário de Coimbra); Devezas V., Jácome F., Nogueiro J., Pereira A., Santos‐Sousa H.*, Vaz S. (Centro Hospitalar e Universitário de São João); Pinto J., Tojal A.* (Centro Hospitalar Tondela‐Viseu); Cardoso P.*, Cardoso N., Domingos J. C., Henriques P., Manso M. I., Martins dos Santos G., Martins R., Morais H.*, Pereira R., Revez T., Ribeiro R., Ribeiro V. I., Soares A. P., Sousa S., Teixeira J. (Centro Hospitalar Universitário do Algarve – Unidade de Faro); Amorim E., Baptista V. H., Cunha M. F.*, Sampaio da Nóvoa Gomes Miguel I. I. (Centro Hospitalar Universitário do Algarve – Unidade de Portimão); Bandovas J. P., Borges N.*, Chumbinho B., Figueiredo de Barros I., Frade S., Gomes J., Kam da Silva Andrade A., Pereira Rodrigues A., Pina S., Silva N.*, Silveira Nunes I., Sousa R. (Centro Hospitalar Universitário Lisboa Central); Azevedo P., Costeira B., Cunha C., Garrido R.*, Miranda P., Peralta Ferreira M., Sousa Fernandes M. (Hospital Beatriz Angelo); Galvão D., Vieira A.* (Hospital de Santo Espirito da Ilha Terceira); Patrício B., Santos P. M. D. D.*, Vieira Paiva Lopes A. C. (Hospital de Torres Vedras – Centro Hospitalar do Oeste); Cunha R., Faustino A., Freitas A., Mendes J. R.*, Parreira R. (Hospital do Divino Espírito Santo); Abreu da Silva A.*, Claro M., Costa Santos D., Deus A. C., Grilo J. V. (Hospital do Litoral Alentejano); Borges F.*, Corte Real J., Henriques S., Lima M. J., Matos Costa P. (Hospital Garcia de Orta); Brito da Silva F., Caiado A.*, Fonseca F. (Instituto Português de Oncologia de Lisboa Francisco Gentil); Ângelo M., Baiao J. M., Martins Jordão D.*, Vieira Caroço T. (IPO Coimbra); Baía C., Canotilho R., Correia A. M., Ferreira Pinto A. P., Peyroteo M., Videira J. F.* (IPO Porto).
Réunion: Kassir R.*, Sauvat F. (CHU Réunion).
Romania: Bezede C., Chitul A., Ciofic E., Cristian D., Grama F.* (Coltea Clinical Hospital); Bonci E.*, Gata V.*, Titu S.* (Prof. Dr. Ion Chiricuta Institute of Oncology).
Russia: Garmanova T., Kazachenko E., Markaryan D., Rodimov S., Tsarkov P.*, Tulina I. (Clinic of Coloproctology and Minimally Invasive Surgery, Sechenov Medical State University); Litvina Y., Provozina A. (Immanuel Kant Baltic Federal University, Regional Clinical Hospital); Agapov M.*, Galliamov E., Kakotkin V., Kubyshkin V., Kamalov A., Semina E. (Moscow Research and Educational Center, Lomonosov Moscow State University).
Saudi Arabia: Alshahrani M.*, Alsharif F., Eskander M. (Aseer Central Hospital); Alharthi M., Aljiffry M., Basendowah M., Malibary N.*, Nassif M., Saleem A., Samkari A., Trabulsi N.* (King Abdulaziz University Hospital); Al Awwad S.*, Alghamdi M.*, Alnumani T.* (King Fahad General Hospital); Al Habes H., Alqannas M.*, Alyami M.*, Alzamanan M., Cortés Guiral D.*, Elawad A. (King Khalid Hospital); AlAamer O., Alselaim N.* (King Saud Bin Abdulaziz University for Health Sciences, King Abdullah International Medical Research Center, Ministry of National Guard, Health Affairs, General Surgery Department); Al‐Khayal K., Alhassan N., Alobeed O., Alshammari S., Bin Nasser A.*, Bin Traiki T., Nouh T.*, Zubaidi A. M. (King Saud University).
Serbia: Aleksić L., Antic A., Barisic G.*, Ceranic M., Grubač Ž., Jelenkovic J., Kecmanović D., Kmezić S., Knezevic D.*, Krivokapic Z.*, Latinčić S., Markovic V.*, Matić S.*, Miladinov M., Pavlov M.*, Pejovic I., Tadic B., Vasljević J., Velickovic D. (Clinic for Digestive Surgery, Clinical Centre of Serbia); Buta M., Cvetkovic A., Gacic S., Goran M., Jeftic N., Markovic I.*, Milanović M., Nikolic S., Pejnovic L., Savković N., Stevic D., Vucic N., Zegarac M. (Institute for Oncology and Radiology of Serbia); Karamarkovic A., Kenic M., Kovacevic B., Krdzic I.* (Zvezdara University Medical Center).
Singapore: Lieske B.* (National University Hospital).
South Africa: Almgla N.*, Boutall A., Herman A., Kloppers C.*, Nel D., Rayamajhi S. (Groote Schuur Hospital).
Spain: Paniagua García Señorans M.*, Vigorita V. (Álvaro Cunqueiro Hospital); Acrich E., Baena Sanfeliu E., Barrios O., Golda T.*, Santanach C., Serrano‐Navidad M., Sorribas Grifell M., Vives R. V. (Bellvitge University Hospital); Escolà D., Jiménez A.* (Comarcal Alt Penedés); Cayetano Paniagua L., Gómez Fernández L.* (Consorci Sanitari de Terrassa); Collera P., Diaz Del Gobbo R., Farre Font R., Flores Clotet R., Gómez Díaz C. J.*, Guàrdia N., Guariglia C. A., Osorio A., Sanchez Jimenez R., Sanchon L., Soto Montesinos C. (Fundació Althaia – Xarxa Assistencial Universitària de Manresa); Alonso‐Lamberti L., García‐Quijada J., Jimenez Miramón J., Jimenez V.*, Jover J. M., Leon R., Rodriguez J. L., Salazar A., Valle Rubio A. (Getafe University Hospital); Aguado H.* (Hellín Hospital); Bravo Infante R., De Lacy F. B., Lacy A. M.*, Otero A., Turrado‐Rodriguez V.*, Valverde S. (Hospital Clinic Barcelona); Anula R., Cano‐Valderrama O., Del Campo Martín M., Díez‐Valladares L., Domínguez I., Dziakova J., García Alonso M., García Romero E., Gómez Latorre L., Muguerza J.M.*, Pizarro M. J., Saez Carlin P., Sánchez del Pueblo C., Sánchez‐Pernaute A., Sanz Ortega G., Sanz‐Lopez R., Torres A. (Hospital Clínico de Madrid); Garcés‐Albir M.*, Lopez F.*, Martín‐Arévalo J., Moro‐Valdezate D.*, Pla Marti V. (Hospital Clínico Universitario de Valencia); Beltrán de Heredia J., De Andrés Asenjo B.*, Gómez Sanz T., Jezieniecki C., Nuñez del Barrio H., Ortiz de Solórzano Aurusa F. J., Romero de Diego A., Ruiz Soriano M., Trujillo Díaz J., Vázquez Fernández A. (Hospital Clínico Universitario de Valladolid); Lora‐Cumplido P., Sosa M. V.* (Hospital de Cabueñes); Gonzalez‐Gonzalez E., Minaya Bravo A. M.* (Hospital del Henares); Alonso de la Fuente N., Jimenez Toscano M.* (Hospital del Mar); Grau‐Talens E. J., Martin‐Perez B.* (Hospital Don Benito‐Villanueva); Benavides Buleje J. A., Carrasco Prats M.*, Giménez Francés C.*, Muñoz Camarena J. M., Parra Baños P. A., Peña Ros E., Ramirez Faraco M., Ruiz‐Marín M.*, Valero Soriano M. (Hospital General Reina Sofía); Estaire Gómez M.*, Fernández Camuñas Á., Garcia Santos E. P., Jimenez Higuera E., Martínez‐Pinedo C., Muñoz‐Atienza V., Padilla‐Valverde D.*, Picón Rodríguez R., Sánchez‐García S., Sanchez‐Pelaez D. (Hospital General Universitario de Ciudad Real); Colombari R. C., del Valle E., Fernández M., Lozano Lominchar P.*, Martín L., Rey Valcarcel C., Zorrilla Ortúzar J. (Hospital General Universitario Gregorio Marañón); Alcaide Matas F., García Pérez J. M., Troncoso Pereira P.* (Hospital Mateu Orfila); Mora‐Guzmán I.* (Hospital Santa Bárbara); Achalandabaso Boira M.*, Sales Mallafré R. (Hospital Universitari de Tarragona Joan XXIII); Marín H., Prieto Calvo M., Villalabeitia Ateca I.* (Hospital Universitario Cruces); De Andres Olabarria U., Durán Ballesteros M., Fernández Pablos F. J., Ibáñez‐Aguirre F. J., Sanz Larrainzar A., Ugarte‐Sierra B.* (Hospital Universitario de Galdakao); Correa Bonito A., Delgado Búrdalo L., Di Martino M.*, García Septiem J.*, Maqueda González R., Martin‐Perez E. (Hospital Universitario de la Princesa); Calvo Espino P.*, Guillamot Ruano P. (Hospital Universitario de Móstoles); Colao García L., Díaz Pérez D.*, Esteban Agustí E., Galindo Jara P., Gutierrez Samaniego M.*, Hernandez Bartolome M. A.*, Serrano González J. (Hospital Universitario de Torrejón de Ardoz); Alonso Poza A., Diéguez B., García‐Conde M., Hernández‐García M., Losada M.* (Hospital Universitario del Sureste); Alvarez E., Chavarrias N., Gegúndez Simón A., Gortázar S., Guevara J., Prieto Nieto M. I., Ramos‐Martín P., Rubio‐Perez I.*, Saavedra J., Urbieta A. (Hospital Universitario la Paz); Cantalejo Diaz M., De Miguel Ardevines M. D. C., Duque‐Mallén V.*, Gascon Ferrer I., González‐Nicolás Trébol M. T., Gracia‐Roche C., Herrero Lopez M., Martinez German A., Matute M., Sánchez Fuentes N., Sánchez‐Rubio M., Santero‐Ramirez M. S., Saudí S. (Hospital Universitario Miguel Servet); Blazquez Martin A., Diez Alonso M.*, Hernandez P., Mendoza‐Moreno F., Ovejero Merino E., Vera Mansilla C. (Hospital Universitario Principe de Asturias); Acebes García F., Bailón M., Bueno Cañones A. D., Choolani Bhojwani E., Marcos‐Santos P., Miguel T., Pacheco Sánchez D., Pérez‐Saborido B., Sanchez Gonzalez J., Tejero‐Pintor F. J.* (Hospital Universitario Río Hortega); Cano A., Capitan‐Morales L., Cintas Catena J., Gomez‐Rosado J.*, Oliva Mompean F., Pérez Sánchez M. A., Río Lafuente F. D., Torres Arcos C., Valdes‐Hernandez J. (Hospital Universitario Virgen Macarena); Cholewa H., Frasson M., Martínez Chicote C., Sancho‐Muriel J.* (Hospital Universitario Y Politécnico la Fe); Abad Gurumeta A., Abad‐Motos A., Martínez‐Hurtado E., Ripollés‐Melchor J.*, Ruiz Escobar A. (Infanta Leonor University Hospital); Cuadrado‐García A.*, Garcia‐Sancho Tellez L.*, Heras Aznar J.*, Maté P., Ortega Vázquez I.*, Picardo A. L., Rojo López J. A., Sanchez Cabezudo Noguera F.*, Serralta de Colsa D.* (Infanta Sofía University Hospital); Cagigas Fernandez C., Caiña Ruiz R., Gomez Ruiz M., Martínez‐Pérez P., Poch C., Santarrufina Martinez S.*, Valbuena Jabares V. (Marqués de Valdecilla University Hospital); Blas Laina J. L., Cros B., Escartin J.*, Garcia Egea J., Nogués A., Talal El‐Abur I., Yánez C. (Royo Villanova); Cagigal Ortega E. P., Cervera I., Díaz Peña P., Gonzalez J., Marqueta De Salas M., Perez Gonzalez M.*, Ramos Bonilla A., Rodríguez Gómez L. (Severo Ochoa University Hospital); Blanco‐Colino R., Espin‐Basany E.*, Pellino G. (Vall d’Hebron University Hospital).
Sri Lanka: Arulanantham A., Bandara G. B. K. D., Jayarajah U.*, Ravindrakumar S., Rodrigo V. S. D. (District General Hospital Chilaw); Srishankar S.* (Teaching Hospital Anuradhapura).
Sudan Ali Adil A. K. (Al‐Rajhi).
Sweden: Älgå A.*, Heinius G., Nordberg M., Pieniowski E. (Stockholm South General Hospital); Löfgren N., Rutegård M.* (Umea University Hospital).
Switzerland: Arigoni M., Bernasconi M., Christoforidis D.*, Di Giuseppe M., La Regina D., Mongelli F. (Ente Ospedaliero Cantonale); Chevallay M., Dwidar O., Gialamas E., Sauvain M. (Pourtales Neuchatel Hospital); Adamina M.*, Crugnale A. S., Guglielmetti L., Peros G. (Kantonsspital Winterthur).
Turkey: Aghayeva A.*, Hamzaoglu I., Sahin I. (Acibadem Altunizade Hospital); Akaydin E., Aliyeva Z., Aytac E., Baca B., Ozben V.*, Ozmen B. B. (Acibadem Atakent Hospital); Arikan A. E.*, Bilgin I. A.*, Kara H., Karahasanoğlu T., Uras C. (Acibadem Maslak Hospital); Dincer H. A., Erol T. (Hacettepe University Hospital); Alhamed A., Ergün S.*, Özçelık M. F., Sanli A. N., Uludağ S. S.*, Velidedeoglu M.*, Zengin A. K. (Istanbul Universty – Cerrahpaşa Medical Faculty); Bozkurt M. A., Kara Y.*, Kocataş A. (Kanuni Sultan Suleyman Training and Research Hospital); Azamat İ. F., Balik E.*, Buğra D., Kulle C. B. (Koç University Medical School); Gözal K., Güler S. A., Köken H., Tatar O. C.*, Utkan N. Z., Yıldırım A., Yüksel E. (Kocaeli University Teaching Hospital); Akin E., Altintoprak F.*, Cakmak G., Çelebi F., Demir H., Dikicier E., Firat N., Gönüllü E., Kamburoğlu M. B., Küçük I. F., Mantoglu B. (Sakarya University Faculty of Medicine); Çolak E.*, Kucuk G. O., Uyanik M. S. (Samsun Training and Research Hospital); Göksoy B.* (Sehit Prof. Dr. İlhan Varank Training and Research Hospital); Bozkurt E., Mihmanli M., Tanal M.*, Yetkin S. G. (Sisli Hamidiye Etfal Training and Research Hospital); Akalin M., Arican C., Avci E. K., Aydin C., Demirli Atıcı S.*, Emiroglu M., Kaya T.*, Kebabçı E., Kilinc G., Kirmizi Y., Öğücü H., Salimoğlu S., Sert İ., Tugmen C., Tuncer K., Uslu G., Yeşilyurt D. (University of Health Sciences Tepecik Training and Research Hospital); Yildiz A.* (Yildirim Beyazit University Yenimahalle Training and Research Hospital).
Uganda: Lule H.*, Oguttu B.* (Kampala International University Teaching Hospital).
UK: Agilinko J., Ahmeidat A., Bekheit M.*, Cheung L. K., Kamera B. S., Mignot G., Shaikh S.*, Sharma P. (Aberdeen Royal Infirmary); Al‐Mohammad A., Ali S., Ashcroft J., Baker O., Coughlin P., Davies R. J.*, Kyriacou H., Mitrofan C. G., Morris A., Raby‐Smith W., Rooney S., Singh A., Tan X. S., Townson A., Tweedle E. (Addenbrooke’s Hospital); Angelou D., Choynowski M., McAree B.*, McCanny A., Neely D. (Antrim Area Hospital – Northern Health and Social Care Trust); Mosley F.* (Bradford Royal Infirmary); Arrowsmith L.*, Campbell W.* (Causeway Hospital); Grove T., Kontovounisios C., Warren O.* (Chelsea and Westminster Hospital); Clifford R., Eardley N., Krishnan E., Manu N., Martin E., Roy Mahapatra S., Serevina O. L., Smith C., Vimalachandran D.* (Countess of Chester Hospital); Emslie K.*, Labib P.*, Minto G., Natale J., Panahi P., Rogers L.* (Derriford Hospital); Abubakar A.*, Akhter Rahman M. M., Chan E., O’Brien H., Sasapu K.* (Diana Princess of Wales Hospital Grimsby); Ng H. J.* (Dumfries and Galloway Royal Infirmary); Day A.* (East Surrey Hospital); Hunt A., Laskar N.* (East Sussex Healthcare (Conquest Hospital and Eastbourne District General Hospital)); Gupta A.*, Steinke J., Thrumurthy S. (Epsom and St Helier University Hospitals NHS Trust); Massie E., McGivern K., Rutherford D., Wilson M.* (Forth Valley Royal Hospital); Handa S., Kaushal M., Kler A., Patel P.*, Redfern J., Tezas S. (Furness General Hospital); Aawsaj Y., Barry C., Blackwell L., Emerson H., Fisher A.*, Katory M., Mustafa A. (Gateshead Health NHS Foundation Trust); Kretzmer L.*, Lalou L., Manku B., Parwaiz I., Stafford J. (George Eliot Hospital); Abdelkarim M., Asqalan A., Gala T., Ibrahim S., Maw A.*, Mithany R., Morgan R.*, Sundaram Venkatesan G. (Glan Clwyd Hospital); Boulton A. J. (Good Hope Hospital); Hardie C., McNaught C.* (Harrogate District Hospital); Karandikar S.*, Naumann D. (Heartlands Hospital); Ayorinde J., Chase T., Cuming T., Ghanbari A., Humphreys L., Tayeh S.* (Homerton University Hospital); Aboelkassem Ibrahim A., Evans C., Ikram H., Loubani M.*, Nazir S., Robinson A., Sehgal T., Wilkins A. (Hull University Teaching Hospitals NHS Trust); Dixon J.*, Jha M., Thulasiraman S. V., Viswanath Y. K. S.* (James Cook University Hospital); Curl‐Roper T., Delimpalta C., Liao C. C. L.*, Velchuru V., Westwood E. (James Paget University NHS Foundation Trust Hospital); Bond‐Smith G.*, Mastoridis S., Tebala G. D., Verberne C. (John Radcliffe Hospital); Bhatti M. I., Boyd‐Carson H., Elsey E., Gemmill E., Herrod P.*, Jibreel M., Lenzi E., Saafan T., Sapre D., Sian T., Watson N. (King’s Mill Hospital); Athanasiou A.*, Burke J., Costigan F., Elkadi H., Johnstone J., Nahm C. (Leeds Teaching Hospitals Trust); Annamalai S., Ashmore C., Kourdouli A. (Leicester Royal Infirmary); Askari A., Cirocchi N., Kudchadkar S., Patel K., Sagar J.*, Talwar R.* (Luton and Dunstable University Hospital); Abdalla M., Ismail O., Newton K., Stylianides N.* (Manchester Royal Infirmary); Aderombi A., Bajomo O., Beatson K., Garrett W.*, Ng V. (Medway Hospital); Al‐Habsi R., Divya G S., Keeler B.* (Milton Keynes University Hospital); Egan R., Fabre I., Harries R.*, Li Z., Parkins K., Spencer N., Thompson D. (Morriston Hospital Swansea); Gemmell C., Grieco C., Hunt L.* (Musgrove Park Hospital); Mahmoud Ali F. (Newcastle Upon Tyne Hospitals NHS Foundation Trust.); Seebah K., Shaikh I.*, Sreedharan L., Youssef M.* (Norfolk and Norwich University Hospital); Shah J.* (North Manchester General Hospital); McLarty N., Mills S.*, Shenfine A. (Northumbria NHS Hospital Trust); Sahnan K. (Northwick Park Hospital); Michel M., Patil S., Ravindran S., Sarveswaran J.*, Scott L. (Pinderfields Hospital); Bhangu A.*, Cato L. D., Kamal M., Kulkarni R., Parente A., Saeed S., Vijayan D. (Queen Elizabeth Hospital Birmingham); Kaul S., Khan A. H., Khan F., Mukherjee S.*, Patel M., Sarigul M., Singh S. (Queen’s Hospital Romford); Adiamah A., Brewer H., Chowdhury A.*, Evans J., Humes D.*, Jackman J., Koh A., Lewis‐Lloyd C., Oyende O., Reilly J., Worku D. (Queens Medical Centre); Bisset C., Moug S. J.* (Royal Alexandra Hospital); Math S., Sarantitis I., Timbrell S., Vitone L.* (Royal Blackburn Hospital); Faulkner G.* (Royal Bolton Hospital); Brixton G., Findlay L., Majkowska A., Manson J.*, Potter R. (Royal Bournemouth Hospital); Bhalla A.*, Chia Z., Daliya P., Grimley E., Malcolm F. L., Theophilidou E. (Royal Derby Hospital); Daniels I. R., Fowler G., Massey L., McDermott F.*, Rajaretnam N. (Royal Devon and Exeter Hospital); Beamish A., Magowan D., Nassa H., Price C., Smith L., Solari F., Tang A. M., Williams G.* (Royal Gwent Hospital); Davies E.*, Hawkin P., Raymond T., Ryska O. (Royal Lancaster Infirmary); Baron R. D.*, Gahunia S., McNicol F.*, Russ J., Szatmary P., Thomas A. (Royal Liverpool University Hospital); Jayasinghe J. D., Knowles C., Ledesma F. S., Minicozzi A.*, Navaratne L., Ramamoorthy R., Sohrabi C., Thaha M.*, Venn M. (Royal London Hospital); Atherton R.*, Brocklehurst M., McAleer J., Parkin E.* (Royal Preston Hospital); Aladeojebi A., Ali M., Gaunt A.* (Royal Stoke University Hospital); Hammer C., Stebbing J. (Royal Surrey County Hospital); Bhasin S., Bodla A. S., Burahee A., Crichton A., Fossett R., Yassin N.* (Royal Wolverhampton NHS Trust); Brown S.*, Lee M., Newman T., Steele C. (Sheffield Teaching Hospital NHS Foundation Trust); Baker A., Konstantinou C., Ramcharan S.*, Wilkin R. J. W. (South Warwickshire NHS Foundation Trust); Lawday S., Lyons A.* (Southmead Hospital); Chung E., Hagger R., Hainsworth A., Karim A., Owen H., Ramwell A., Williams K.* (St George’s Hospital); Hall J. (Stepping Hill Hospital); Harris G., Royle T.*, Watson L. J. (Sunderland Royal Hospital); Asaad P., Brown B., Duff S.*, Khan A., Moura F., Wadham B. (The University Hospital of South Manchester); McCluney S., Parmar C.*, Shah S. (The Whittington Hospital); Babar M. S., Goodrum S., Whitmore H. (Torbay and South Devon NHS Trust); Balasubramaniam D.*, Jayasankar B.*, Kapoor S., Ramachandran A. (Tunbridge Wells Hospital); Beech N., Chand M.*, Green L., Kiconco H., McEwen R. (University College London Hospital); Pereca J.* (University Hospital Ayr); Gash K.*, Gourbault L., MacCabe T., Newton C.* (University Hospitals Bristol NHS Foundation Trust); Baig M., Bates H., Dunne N., Khajuria A., Ng V., Sarma D. R., Shortland T., Tewari N.* (University Hospitals Coventry and Warwickshire NHS Trusts); Akhtar M. A.*, Brunt A., McIntyre J., Milne K., Rashid M. M., Sgrò A., Stewart K. E., Turnbull A. (Victoria Hospital Kirkcaldy); Aguilar Gonzalez M.*, Talukder S.* (West Suffolk Hospital); Eskander P., Hanna M., Olivier J.* (Weston General Hospital); Magee C.*, Powell S.* (Wirral University Teaching Hospital); Flindall I., Hanson A., Mahendran V. (Worcestershire Royal Hospital); Green S., Lim M., MacDonald L., Miu V., Onos L., Sheridan K., Young R.* (York Teaching Hospitals NHS Trust); Alam F., Griffiths O., Houlden C., Kolli V. S., Lala A. K., Seymour Z.* (Ysbyty Gwynedd).
USA: Haynes A.*, Hill C., Leede E., McElhinney K., Olson K. A., Riley C., Thornhill M. (Dell Seton Medical Center at the University of Texas); Etchill E., Gabre‐Kidan A.*, Jenny H., Kent A., Ladd M. R., Long C., Malapati H., Margalit A., Rapaport S., Rose J., Stevens K., Tsai L., Vervoort D., Yesantharao P., Bigelow B. (Johns Hopkins Hospital); Klaristenfeld D.* (Kaiser Permanente San Diego Medical Center); Huynh K. (Kaiser Permanente West Los Angeles); Azam M., Choudhry A.*, Marx W. (SUNY Upstate University Hospital); Abel M. K., Boeck M., Chern H., Kornblith L.*, Nunez‐Garcia B., Ozgediz D., Glencer A., Sarin A., Varma M. (University of California, San Francisco (UCSF)); Abbott D., Acher A., Aiken T., Barrett J., Foley E., Schwartz P., Zafar S. N.* (University of Wisconsin); Hawkins A.*, Maiga A. (Vanderbilt University Medical Center).
REFERENCES
- 1. Ranney ML, Griffeth V, Jha AK. Critical supply shortages—the need for ventilators and personal protective equipment during the Covid‐19 pandemic. N Engl J Med. 2020;382:e41. [DOI] [PubMed] [Google Scholar]
- 2. Xie J, Tong Z, Guan X, Du B, Qiu H, Slutsky AS. Critical care crisis and some recommendations during the COVID‐19 epidemic in China. Intensive Care Med. 2020;46(5):837–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. American College of Surgeons . COVID 19: elective case triage guidelines for surgical care. Available from: https://www.facs.org/‐/media/files/covid19/guidance_for_triage_of_nonemergent_surgical_procedures.ashx. Accessed 27th March 2020
- 4. The Royal College of Surgeons of Edinburgh . Intercollegiate General Surgery Guidance on COVID‐19. Available from: https://www.rcsed.ac.uk/news‐public‐affairs/news/2020/march/intercollegiate‐general‐surgery‐guidance‐on‐covid‐19‐update. Accessed 27th March 2020
- 5. Aj B, Brown C, Abdelrahman T, Rl H, Rj E, Ansell J, et al. International surgical guidance for COVID‐19: Validation using an international Delphi process‐Cross‐sectional study. Int J Surg. 2020;79:309–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Association of Coloproctology of Great Britain and Ireland . Urgent Intercollegiate General Surgery Guidance on COVID‐19. Available from: https://www.acpgbi.org.uk/news/urgent‐intercollegiate‐general‐surgery‐guidance‐on‐covid‐19/. Accessed 27th March 2020
- 7. Lisi G, Campanelli M, Spoletini D, Carlini M. The possible impact of COVID‐19 on colorectal surgery in Italy. Colorectal Dis. 2020;22:641–2. [DOI] [PubMed] [Google Scholar]
- 8. Willan J, King AJ, Jeffery K, Bienz N. Challenges for NHS hospitals during covid‐19 epidemic. BMJ. 2020;20:m1117. [DOI] [PubMed] [Google Scholar]
- 9. COVIDSurg Collaborative . Global guidance for surgical care during the COVID‐19 pandemic. Br J Surg. 2020;107:1097–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang Y, Wang Y, Chen Y, Qin Q. Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID‐19) implicate special control measures. J Med Virol. 2020;92:568–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Collaborative C. Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS‐CoV‐2 infection: an international cohort study. Lancet. 2020;396:27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. CovidSurg Collaborative . Elective surgery cancellations due to the COVID‐19 pandemic: global predictive modelling to inform surgical recovery plans. Br J Surg. 2020. 10.1002/bjs.11746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. CovidSurg Collaborative . Elective cancer surgery in COVID‐19‐free surgical pathways during the SARS‐CoV‐2 pandemic: An International, Multicenter, Comparative Cohort Study. J Clin Oncol. 2020. JCO2001933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. National Confidential Enquiry into Patient Outcome and Death . The NCEPOD Classification of Intervention 2004. Available from: https://www.ncepod.org.uk/classification.html. Accessed 27th March 2020
- 16. American Society of Anesthesiologists . ASA Physical Status Classification System 2019. Available from: https://www.asahq.org/standards‐and‐guidelines/asa‐physical‐status‐classification‐system. Accessed 27th March 2020
- 17. The European Society of Coloproctology Collaborating Groups . The impact of conversion on the risk of major complication following laparoscopic colonic surgery: an international, multicentre prospective audit. Colorectal Dis. 2018;20(S6):69–89. [DOI] [PubMed] [Google Scholar]
- 18. The European Society of Coloproctology Collaborating Group . Safety of primary anastomosis following emergency left sided colorectal resection: an international, multi‐centre prospective audit. Colorectal Dis. 2018;20(S6):47–57. [DOI] [PubMed] [Google Scholar]
- 19. European Society of Coloproctology Collaborating Group . The impact of stapling technique and surgeon specialism on anastomotic failure after right‐sided colorectal resection: an international multicentre, prospective audit. Colorectal Dis. 2018;20:1028–40. [DOI] [PubMed] [Google Scholar]
- 20. European Society of Coloproctology Collaborating Group , Battersby N, Bhangu A, Chaudhri S, El‐Hussuna A, Frasson M, et al. Relationship between method of anastomosis and anastomotic failure after right hemicolectomy and ileo‐caecal resection: an international snapshot audit. Colorectal Dis. 2017;19:e296–311. [DOI] [PubMed] [Google Scholar]
- 21. European Society of Coloproctology Collaborating Group . Predictors for anastomotic leak, postoperative complications, and mortality after right colectomy for cancer: results from an international snapshot audit. Dis Colon Rectum. 2020;63:606–18. [DOI] [PubMed] [Google Scholar]
- 22. The European Society of Coloproctology Collaborating Group . An international multicentre prospective audit of elective rectal cancer surgery; operative approach versus outcome, including transanal total mesorectal excision (TaTME). Colorectal Dis. 2018;20(S6):33–46. [DOI] [PubMed] [Google Scholar]
- 23. Sammour T, Lewis M, Thomas ML, Lawrence MJ, Hunter A, Moore JW. A simple web‐based risk calculator (www.anastomoticleak.com) is superior to the surgeon's estimate of anastomotic leak after colon cancer resection. Tech Coloproctol. 2017;21:35–41. [DOI] [PubMed] [Google Scholar]
- 24. Frasson M, Flor‐Lorente B, Rodríguez JLR, Granero‐Castro P, Hervás D, Alvarez Rico MA, et al. Risk factors for anastomotic leak after colon resection for cancer. Ann Surg. 2015;262:321–30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data‐sharing requests will be considered by the management group upon written request to the corresponding author. If agreed, de‐identified participant data will be available, subject to a data‐sharing agreement.


