Dear Editor,
As of September 30, 2020, the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) pandemic has affected almost 14 million people in 213 countries on five continents. South Africa's first case was reported on March 5, 2020. Since then, we have had a total of 672 572 cases and 16 667 fatalities. During this time, the utility of hematological, coagulation, and biochemical investigations as predictors of the severity of infection has been established. In particular, hematological investigations namely; the full blood count (FBC) and differential (DIFF) have emerged as important prognostic markers of disease severity and progression. 1 , 2 In addition to quantitative hematological abnormalities, several authors have described abnormal morphological features of peripheral blood cells. 3 , 4 These findings have been confirmed with electron microscopy and are consistent with the viral effects, immune dysregulation, and systemic inflammation caused by coronavirus disease 2019 (COVID‐19). 3 Nonetheless, there is a paucity of data from resource‐limited settings. Indeed, local studies are warranted. South Africa has a high burden of chronic infections namely Human immunodeficiency virus (HIV) and Tuberculosis and other pre‐existing inflammatory states are established risk factors for severe COVID‐19. This study therefore aims to describe the characteristic peripheral blood morphological features associated with COVID‐19 infection in a resource‐limited setting.
One hundred and two patients consecutively admitted to the Charlotte Maxeke Johannesburg Academic Hospital, Johannesburg in South Africa between 03/08/2020 and 21/08/2020 were assessed for eligibility on the basis of a positive real‐time polymerase chain reaction from the upper respiratory tract (nasopharyngeal and oropharyngeal swab) for COVID‐19. The study was approved by the Human Research Ethics Committee of the University of the Witwatersrand (approval number M‐200462). Information such as age, gender, and ward was obtained from the laboratory information system. The following study measurements were collected on admission; FBC, DIFF, d‐dimer, prothrombin time (PT), and partial thromboplastin time (PTT). In 30 (29.4%) patients, sputum was tested on admission for Mycobacterium tuberculosis. In addition, 70 (68.6%) patients were tested for HIV. In the subgroup of HIV‐infected patients, testing for CD4 and HIV viral load (VL) was performed. FBC, DIFF, and peripheral blood smear (PBS) analysis were performed using the Sysmex XN 9000 hematology analyzer and automated slidemaker (Sysmex Corporation, Kobe, Japan). PBS was examined by two independent hematology specialists, and a third if discrepant, according to the Clinical and Laboratory Standards Institute recommendations using standard light microscopy. 5 Analysis of plasma for the coagulation assays was performed on the STA‐R Max ® automated coagulation analyzer (Diagnostica Stago, Asnières sur Seine, France). CD4 count and HIV VL were measured using the Cytomics FC 500 MPL Flow Cytometry System (Beckman Coulter, Gent, Belgium) and the Cobas 6800/8800 system (Roche Diagnostics, Indianapolis, USA), respectively. The lower limit of detection was 50 copies/mL. Sputum was analyzed on the Gene expert MTB/RIF system (Cepheid, Sunnyvale, California). These analyzers are compliant with local and international proficiency testing.
Data were analyzed using Statistica® software (version 13.2, Palo Alto, California, USA). The continuous data showed a non‐Gaussian distribution and was presented as median [interquartile range (IQR)]. Statistical comparisons were performed using chi‐squared or two‐tailed Fischer exact test for categorical variables. For statistical comparisons of continuous variables, the Mann‐Whitney U test was used. Correlations were performed by Spearman's rank method as a measure of linear association between two variables. Statistical significance was set at a P value of <.05.
The study consisted of a cohort of 102 patients of whom 25 (24.5%) were admitted to the intensive care unit (ICU). The median [IQR] age of the study population was 49 [29] years with a male: female ratio of 1.2:1, consistent with international reports. 6 There were 21 HIV‐infected patients; 10 (47.6%) with virologic suppression and a median CD4 count of 339 [336] × 109/L. According to the estimated HIV prevalence of 19.07% in South African adults, this finding does not suggest increased rates of hospitalization in this patient population. One (3.3%) HIV negative patient was co‐infected with tuberculosis. At the time of this report, 17 (16.7%) patients had recovered and were discharged.
The hematological characteristics of the patients are shown in Table 1. A lymphopenia (defined as an absolute lymphocyte count < 1.0 × 109/L) was seen in 49 (48.0%) patients, with a severe lymphopenia (absolute lymphocyte count < 0.5 × 109/L) in 19 (18.6%). In the subgroup of HIV‐infected patients, no significant difference in the median absolute lymphocyte count relative to uninfected patients was observed (1.09 [0.97] × 109/L and 1.32 [1.34] × 109/L, respectively, P = .286). On further analysis, in the HIV‐infected group with VL > 50 copies/mL, a lower median absolute lymphocyte count was found relative to the HIV‐infected group with virological suppression (0.58 [1.13] × 109/L and 1.16 [0.56] × 109/L, respectively, P = .286). Specifically, a lower median CD4 count was observed relative to the HIV‐infected group with virological suppression (88 [452] × 109/L and 339 [336] × 109/L, respectively, P = .107). Nonetheless, this was not significantly different. Patients admitted to ICU presented with a higher median platelet count and d‐dimer relative to those admitted to the general ward (P < .041 and P < .009, respectively).
TABLE 1.
Baseline laboratory characteristics of the study patients
| Variable | General ward admissions (n = 77) |
Intensive care unit admissions (n = 25) |
P value |
|---|---|---|---|
| White cell count (×109/L) | 10.04 [7.00] | 11.04 [4.76] | .242 |
| Hemoglobin (g/L) | 113.00 [53.00] | 112.00 [32.00] | .676 |
| Mean cell volume (fl) | 90.30 [10.30] | 87.40 [8.80] | .444 |
| Mean cell hemoglobin (pg) | 28.60 [3.20] | 28.60 [2.30] | .333 |
| Red cell distribution width (%) | 15.60 [3.50] | 15.90 [2.20] | .920 |
| Platelet count (×109/L) | 235 [209] | 280 [258] | .041 |
| Mean platelet volume (fl) | 10.70 [1.80] | 10.85 [2.00] | .846 |
| Absolute neutrophil count (×109/L) | 7.79 [5.76] | 9.02 [4.67] | .665 |
| Absolute lymphocyte count (×109/L) | 0.97 [0.30] | 1.30 [0.45] | .463 |
| Absolute monocyte count (×109/L) | 0.42 [0.79] | 0.47 [0.41] | .543 |
| Absolute immature granulocyte count (×109/L) | 0.07 [0.18] | 0.08 [0.20] | .940 |
| Neutrophil to lymphocyte ratio | 8.37 [8.76] | 6.35 [5.45] | .588 |
| Platelet‐to‐lymphocyte ratio | 185.82 [274.15] | 292.31 [249.88] | .239 |
| Prothrombin time (s) | 15.30 [3.40] | 14.80 [2.3] | .527 |
| Partial thromboplastin time (s) | 39.0 [10.80] | 40.1 [9.00] | .872 |
| D‐dimer (mg/L) | 1.35 [1.61] | 2.22 [3.76] | .009 |
P<0.05 bold indicates significant variables
On PBS examination, atypical lymphocytes, which are well described in the setting of viral infections, were observed in 59 (57.8%) patients. Specifically, plasmacytoid lymphocytes were described in 21 (20.6%) patients whereas large granular lymphocytes were less frequent (n = 11, 10.8%; Figure 1). Other common morphological findings included; dysplastic neutrophils (n = 60, 58.8%), a myeloid left shift (n = 40, 39.2%), leuco‐erythroblastic reaction (n = 18, 17.6%), large or giant platelets (n = 31, 30.4%), and red cell fragments ≥ 1% (n = 12, 11.8%; Figure 2).
FIGURE 1.
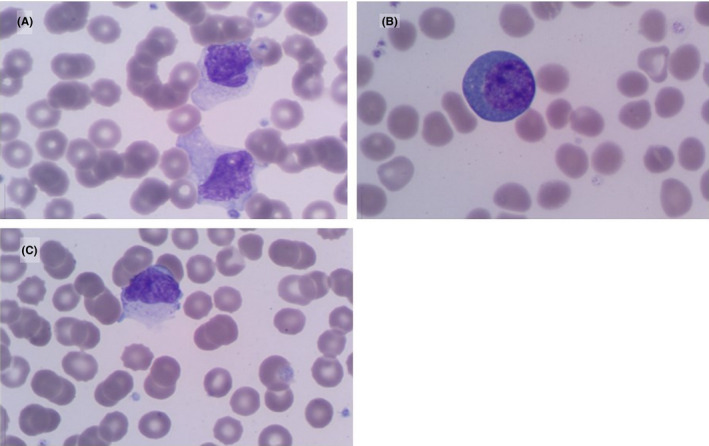
Peripheral blood morphological findings of lymphocytes in patients with COVID‐19 Atypical lymphocytes (A) including plasmacytoid lymphocytes (B) and large granular lymphocytes (C) (May‐Grünwald Giemsa, 1000× magnification)
FIGURE 2.
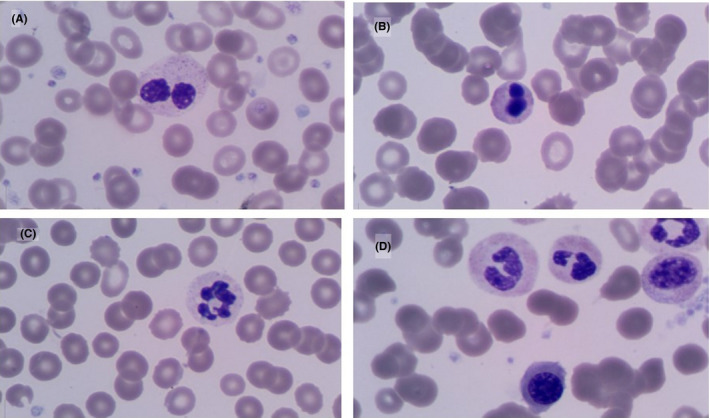
Peripheral blood morphological findings of neutrophils in patients with COVID‐19. Neutrophils with hyposegmented nuclei (pseudopelgers) (A), apoptotic chromatin (B) and hypogranular cytoplasm (C); leuco‐erythroblastic reaction (D) (May‐Grünwald Giemsa, 1000× magnification)
The neutrophil to lymphocyte (NLR) ratio showed a weak direct correlation with age (r = 0.32, P < .002) and PT (r = 0.27, P < .017).
Hospital and ICU admission are largely determined by patients’ clinical signs and symptoms of COVID‐19 as well as comorbidities. However, several laboratory parameters have been identified which have the potential to expedite the assessment of disease severity and triaging in a resource‐limited setting. In particular, lymphopenia was a common finding, similar to other coronavirus infections. This has been shown to correlate with the severity of COVID‐19 infection. 7 This was characterized by PBS features of lymphocyte activation. Highly pleomorphic lymphocytes were observed, ranging in size from 15 to 30 µm. Many were large with abundant cytoplasm which was often vacuolated and occasionally granular. The nuclei were predominantly lobulated with clumped chromatin. In addition, occasional plasmacytoid lymphocytes were observed. Lymphopenia is also a characteristic finding of suppressed immune function associated with HIV infection. Nonetheless, in the subgroup of 21 (20.6%) HIV‐infected patients, no significant difference in the median absolute lymphocyte count relative to uninfected patients was observed. This is consistent with recent data from developed countries of HIV‐infected patients on suppressive antiretroviral therapy. 8 In contrast, in our study, only 10 (47.6%) of the HIV‐infected admissions were virologically suppressed. Although no significant difference in the median absolute lymphocyte count or CD4 count was found in HIV‐infected patients stratified according to HIV VL, larger clinical studies are required to understand the effect of COVID‐19 infection on low CD4 T‐cell function.
In addition to the viral effects, recent data suggest that neutrophilia is an expression of systemic inflammation and cytokine storm which play an important role in the pathogenesis of COVID‐19 infection. A neutrophilia in conjunction with an increase in the NLR was observed in the majority (n = 59, 57.8%) of hospitalized patients. Nonetheless, no significant difference was found between general ward and ICU admissions (P = .665). In contrast, recent data from China have identified the NLR, a well‐known marker of systemic inflammation, in conjunction with age > 50 years as a reliable indicator of disease severity. 9 On PBS examination, a myeloid left shift was described in 40 (39.2%) study patients. A leuco‐erythroblastic reaction (n = 18, 17.6%) was less commonly observed. More interestingly, morphological features of infection‐related dysplasia including, pseudopelgers, apoptotic chromatin, and hypogranular cytoplasm were a prominent feature in circulating granulocytes (n = 60, 58.8%). These findings, which can be attributed to inflammatory cytokines, direct infection of hematopoietic stem and progenitor cells or changes in the bone marrow microenvironment in the setting of infection, complement previous similar studies. 3 , 4
Furthermore, the cytokine storm associated with COVID‐19 is responsible for activation of coagulation. In conjunction with hypoxia, inflammation and upregulated tissue factor expression contribute to pulmonary microvascular thrombosis associated with severe infection. 10 In the current study, platelet counts and d‐dimer levels of ICU admissions were significantly elevated (P < .041 and P < .009, respectively). Similarly, other studies have reported that the d‐dimer is the strongest independent predictor of mortality in COVID‐19 patients. 11 In addition to a thrombocytosis, a subsequent increased mean platelet volume (MPV) and higher platelet‐to‐lymphocyte ratio (PLR) have also been reported in association with severe infection. 12 The MPV and PLR represent accessible, efficient and cost‐effective surrogate markers of platelet activation, and systemic inflammation in severe infection. Nonetheless, in this study, although a higher MPV and PLR were observed among ICU admissions, this did not achieve statistical significance. The MPV and PLR require further evaluation in a prospective multi‐center study in order to determine their use for risk stratification and clinical decision‐making in a resource‐limited setting.
The findings of this study must be interpreted in light of certain limitations. First, this was a single‐center study which is a specialist facility. As such, the study patients represent a highly selective group from the total pool of COVID‐19 infections in South Africa. A second limitation of this study is as a result of limited resources, only patients with severe COVID‐19 pneumonia were hospitalized during the study period. Thirdly, we acknowledge that therapies administered, for example, steroids could potentially influence our results. Lastly, a large proportion of patients were still admitted at the time of this report. As such mortality and discharge outcomes could not be reported.
In conclusion, we have described how the characteristic peripheral blood findings of COVID‐19 infection can be used to assess the extent of the viral effects, immune dysregulation and systemic inflammation of COVID‐19.
CONFLICTS OF INTEREST
The author(s) declare no conflict of interests with respect to the authorship and/or publication of this article.
AUTHOR'S CONTRIBUTION
As co‐first authors, ES and TD contributed equally to study design, data collection, entry and analysis and writing of the manuscript. BL contributed to study design and critical review of the manuscript. All authors revised the manuscript and approved the final version.
ACKNOWLEDGEMENTS
We thank the laboratory staff at CMJAH for their bravery in the fight against SARS‐CoV‐2 and their efforts toward saving lives. We thank RC Janet for proofreading the manuscript.
REFERENCES
- 1. Fan BE, Chong VCL, Chan SSW, et al. Hematologic parameters in patients with COVID‐19 infection. Am J Hematol. 2020;95(6):E131‐E134. 10.1002/ajh.25774 [DOI] [PubMed] [Google Scholar]
- 2. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China. Lancet. 2020;395(10223):497‐506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lüke F, Orsó E, Kirsten J, et al. Coronavirus disease 2019 induces multi‐lineage, morphologic changes in peripheral blood cells. eJHaem. 2020;1(1):376‐383. 10.1002/jha2.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zini G, Bellesi S, Ramundo F, et al. Morphological anomalies of circulating blood cells in COVID‐19. Am J Hematol. 2020;95(7):870‐872. 10.1002/ajh.25824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Koepke A, vanAssendelft OW, Brindza LJ, et al. Reference Leukocyte (WBC) Differential Count (Proportional) and Evaluation of Instrumental Methods. H20–A2; Wayne, PA: CLSI; 2007. [Google Scholar]
- 6. Guan W‐J, Ni Z‐Y, Hu YU, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708‐1720. 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen R, Sang L, Jiang M, et al. Longitudinal hematologic and immunologic variations associated with the progression of COVID‐19 patients in China. J Allergy Clin Immunol. 2020;146(1):89‐100. 10.1016/j.jaci.2020.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vizcarra P, Pérez‐Elías MJ, Quereda C, et al. Description of COVID‐19 in HIV‐infected individuals: a single‐centre, prospective cohort. Lancet HIV. 2020;7(8):e554‐e564. 10.1016/S2352-3018(20)30164-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ma A, Cheng J, Yang J, et al. Neutrophil‐to‐lymphocyte ratio as a predictive biomarker for moderate‐severe ARDS in severe COVID‐19 patients. Crit Care. 2020;24(1):288. 10.1186/s13054-020-03007-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Iba T, Levy JH, Levi M, et al. Coagulopathy in COVID‐19. J Thromb Haemost. 2020;18(9):2103‐2109. 10.1111/jth.14975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wu C, Chen X, Cai Y, et al. Pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934. 10.1001/jamainternmed.2020.0994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chan AS, Rout A. Use of neutrophil‐to‐lymphocyte and platelet‐to‐lymphocyte ratios in COVID‐19. J Clin Med Res. 2020;12(7):448‐453. 10.14740/jocmr4240 [DOI] [PMC free article] [PubMed] [Google Scholar]


