Abstract
Aim
This study determined the influence of the COVID‐19 pandemic on the occurrence of multisystem inflammatory syndrome in children (MIS‐C) and compared the main characteristics of MIS‐C and Kawasaki disease (KD).
Methods
We included patients aged up to 18 years of age who were diagnosed with MIS‐C or KD in a paediatric university hospital in Paris from 1 January 2018 to 15 July 2020. Clinical, laboratory and imaging characteristics were compared, and new French COVID‐19 cases were correlated with MIS‐C cases in our hospital.
Results
There were seven children with MIS‐C, from 6 months to 12 years of age, who were all positive for the virus that causes COVID‐19, and 40 virus‐negative children with KD. Their respective characteristics were as follows: under 5 years of age (14.3% vs. 85.0%), paediatric intensive care unit admission (100% vs. 10.0%), abdominal pain (71.4% vs. 12.5%), myocardial dysfunction (85.7% vs. 5.0%), shock syndrome (85.7% vs. 2.5%) and mean and standard deviation C‐reactive protein (339 ± 131 vs. 153 ± 87). There was a strong lagged correlation between the rise and fall in MIS‐C patients and COVID‐19 cases.
Conclusion
The rise and fall of COVID‐19 first wave mirrored the MIS‐C cases. There were important differences between MIS‐C and KD.
Keywords: COVID‐19, Kawasaki disease, multisystem inflammatory syndrome, pandemic, PIMS
Abbreviations
- COVID‐19
coronavirus disease 2019
- IgG
immunoglobulin G
- KD
Kawasaki disease
- MIS‐C
multisystem inflammatory syndrome in children
- PIMS‐TS
paediatric multisystem inflammatory syndrome temporally associated with COVID‐19
- RT‐PCR
reverse‐transcriptase polymerase chain reaction
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- WHO
World Health Organization
Key Notes .
France saw a strong lagged correlation between the rise and fall of multisystem inflammatory syndrome (MIS‐C) in children and the first wave of COVID‐19.
The study also compared seven children with MIS‐C, who were all positive for the virus that causes COVID‐19, and 40 with Kawasaki disease who did not have the virus.
The authors concluded that MIS‐C patients clearly demonstrated a different clinical picture to patients with Kawasaki disease.
1. BACKGROUND
More than one million people have died since the start of the COVID‐19 pandemic, 1 but children have been relatively spared, in terms of both the incidence of the disease and the severity. Patients under 20 years of age have been reported to represent less than 2.0% of patients. 2 However, on 27 April 2020, the UK National Health Service issued an alert after a small number of children presented with an inflammatory syndrome related to COVID‐19. These children were extremely ill and had similar features to atypical Kawasaki disease (KD), toxic shock syndrome 3 and KD shock syndrome. 4 The UK Royal College of Paediatrics and Child Health defined these cases as paediatric multisystem inflammatory syndrome temporally associated with COVID‐19 (PIMS‐TS). 3 The US Centers for Disease Control and Prevention immediately defined it as multisystem inflammatory syndrome in children (MIS‐C) 5 and the World Health Organization (WHO) named it multisystem inflammatory syndrome in children and adolescents, temporally related to COVID‐19. 6 In May and June 2020, reports from Italy, 7 the UK, 4 , 8 France, 9 , 10 Spain, 11 Switzerland 12 and the United States 13 , 14 , 15 , 16 described groups of children fulfilling these descriptions. These reports used different terminologies: MIS‐C, PIMS‐TS, Kawasaki‐like disease or a combination of these. The announcement of this syndrome has raised important global concerns in the public and, in particular, among parents.
It has not been clearly demonstrated that this inflammatory condition is caused by the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) that results in COVID‐19. However, the temporal associations described in different countries, and the confirmation of the SARS‐CoV‐2 infection in most patients presenting with this inflammatory condition, provide a strong argument that they are linked. 17 , 18 The reports from different countries have described some differences, but they have also pointed out some similarities between these patients and patients with KD. Relatively, few patients have been reported to date and that is why more data are needed on this syndrome, for a deeper insight into this condition and to better understand the local kinetics of the evolution of the COVID‐19 pandemic and the occurrence of MIS‐C.
We observed an outbreak of MIS‐C in our paediatric university hospital in Paris, as in many other centres, during the first wave of the COVID‐19 pandemic. This study had two aims. The first was to describe the kinetics of the MIS‐C cases and the COVID‐19 pandemic in France, in order to determine whether they were related and what influence the pandemic had on MIS‐C cases. The second was to describe the clinical, laboratory, echocardiographic and imaging characteristics of the children involved in this MIS‐C outbreak, who were all tested positive for SARS‐CoV‐2, to patients with KD.
2. METHODS
2.1. Patients and definitions
We retrospectively reviewed the records of all patients up to 18 years of age who had been admitted to the University Pediatric Hospital Armand Trousseau in Paris with a diagnosis of KD or Kawasaki‐like disease from 1 January 2018 to 15 July 2020. The paediatric emergency department in this tertiary paediatric hospital is visited by 55,000 patients a year. Children and adolescents fulfilling the MIS‐C, 5 PIMS‐TS 3 or WHO 6 definitions of an inflammatory multisystem syndrome were also included. KD is an acute vasculitis of unknown origin that presents as a febrile illness and predominantly affects children under 5 years of age. Diagnosis was based on the clinical criteria described in the 2017 guidelines issued by the American Heart Association. 19 Patients who meet the case definition are said to have complete KD, namely typical or classic KD, whereas those who do not have sufficient principal clinical findings may be diagnosed with incomplete or atypical KD. 19 The diagnosis of classic KD is based on fever for five or more days, together with at least four of the five principal clinical features. These are as follows: (a) oral changes, including erythema, cracked lips, a ‘strawberry tongue’ and diffuse erythema of the oropharyngeal mucosa, (b) a bilateral bulbar conjunctival infection, (c) erythematous rash, (d) erythema and oedema of the hands and feet and (e) cervical lymphadenopathy of at least 1.5 cm in diameter. If coronary artery abnormalities are detected, the diagnosis of KD is considered to be confirmed in most cases. 19 Other clinical, laboratory and echocardiographic findings can support the diagnosis of incomplete KD if a patient's clinical presentation suggests KD, but their clinical features do not meet the epidemiological case definition. KD shock syndrome has been defined by Kanegaye et al 20 as: initiation of volume expansion and infusion of vasoactive agents or transfer to intensive care due to systolic hypotension for age or a decrease of at least 20% in systolic blood pressure from baseline or clinical signs of poor perfusion.
Multisystem inflammatory syndrome in children cases have been defined in three ways by the US Centers for Disease Control and Prevention 5 : first, a patient under the age of 21 years presenting with fever, laboratory evidence of inflammation, evidence of clinically severe illness requiring hospitalisation and the involvement of two or more organs, namely cardiac, renal, respiratory, haematological, gastrointestinal, dermatological or neurological; second no alternative plausible diagnoses; and third being positive for current or recent SARS‐CoV‐2 infections or COVID‐19 exposure in the 4 weeks before the onset of symptoms. Some of these patients may fulfil all, or some, of the criteria for Kawasaki disease. 5 The comparisons of the MIS‐C, PIMS‐TS and WHO definitions are shown in Table S1.
2.2. Data collected
Data on patient demographics, clinical, laboratory, echocardiographic, imaging, therapeutic, and outcomes were collected for patients seen from 1 January 2018 to 15 July 2020. We collected data using a standardised form from both the hospital's electronic health record system and paper health records. For patients seen from 1 April 2020 to 15 July 2020, we collected data on SARS‐CoV‐2 infections and close COVID‐19 contacts and determined whether patients fulfilled the MIS‐C, 5 PIMS‐TS, 3 WHO 6 definitions or the classic or incomplete KD criteria. 19 Since data collection on race and ethnicity are not allowed in France, these were not available. Although the above definitions are very similar, we chose to use the definition for MIC‐S in our hospital because we felt that the link suggested with a SARS‐CoV‐2 infection was more straightforward, Table S1.
2.3. Treatment
As MIS‐C is a new syndrome, patients who were affected were treated according to our hospital protocol for KD. The first‐line treatment was intravenous immunoglobulins at 2 g/kg as a single infusion given over 10–12 hours. If apyrexia was not obtained within 36 hours, or fever reappeared <7 days after initial treatment, the treating physician could decide on a second dose of intravenous immunoglobulins and/or the use of corticosteroids. 19
2.4. Detecting SARS‐CoV‐2
We identified SARS‐CoV‐2 ribonucleic acid using reverse‐transcriptase polymerase chain reaction (RT‐PCR) on nasopharyngeal swabs or stool samples. Samples were obtained within the first 48 hours of admission. We used the Allplex nCoV assay (Seegene Inc, Seoul, South Korea) and the Bosphore v2 nCoV assay (Anatolia Geneworks, Istanbul, Turkey). We identified SARS‐Cov‐2 IgG antibodies using the Alinity‐i SARS‐CoV‐2 IgG (Abbott Molecular, Illinois, USA). Serology was performed during hospitalisation for patients admitted from 1 April to 15 July 2020. Positive RT‐PCR or serology results defined patients with a proven SARS‐CoV‐2 infection.
2.5. Comparing MIS‐C and KD patients
All the MIS‐C patients had the SARS‐CoV‐2 infection. They were compared to KD patients, including those who did not have the viral infection during the pandemic phase of the study.
2.6. Evolution of the pandemic and occurrence of MIS‐C
To determine the relationship between the occurrence of MIS‐C and the evolution of the COVID‐19 pandemic in France, which had 66.5 million inhabitants in January 2020, 21 we obtained official French COVID‐19 data from Public Health France. The cumulative confirmed COVID‐19 cases for the whole country were collected and for every week from the beginning of the epidemic, as well as the mean daily number of new cases. These data were used to construct chronological curves that were analysed according to the occurrence of MIS‐C.
In order to test the influence of the number of new COVID‐19 cases in our region on the occurrence of MIS‐C cases, we obtained data on COVID‐19 visits to emergency departments (ED) from the Oscour surveillance network. This surveillance network automatically collects anonymous, real‐time, demographic, and diagnostic data from 86% of all French emergency departments. These COVID‐19 data, available at Public Health France, enabled us to calculate the daily and weekly number of COVID‐19 ED visits of patients of all ages in the whole Paris region from the beginning of the pandemic and correlate them to the occurrence of MIS‐C cases in children in our hospital.
2.7. Statistical analysis
The characteristics of the two groups were compared using the Student t test for continuous variables and the chi‐square test or Fisher's exact test, as necessary, for categorical variables. The strength of the relationship between the number of new COVID‐19 cases and the number of MIS‐C cases was tested with Spearman's rho correlation coefficient. A linear regression model was used to measure the extent of the explained variance. Analyses were performed with SPSS, version 18 (SPSS Inc, Chicago, USA). A p value of < 0.05 was considered statistically significant.
2.8. Ethical approval
This study was approved by the Ethics Committee for Research of the Sorbonne University. Parents were informed that their children's data would be anonymously used and could ask for them to be excluded from the analysis; none of them did so.
3. RESULTS
The study comprised 47 patients admitted to the hospital from 1 January 2018 to 15 July 2020. These included seven patients diagnosed with MIS‐C and 40 diagnosed with KD.
3.1. Description of the MIS‐C outbreak
Between 13 April and 15 July 2020, seven children diagnosed with MIS‐C and 10 children diagnosed with typical or atypical KD were admitted to our hospital. The combined incidence of MIS‐C and typical and atypical KD during that period was 5.7 patients per month. In comparison, 30 children diagnosed with typical or atypical KD were admitted from January 2018 to March 2020, with a mean incidence of 1.1 patients per month. Figure 1, panel A shows all the children with typical or atypical KD and the children with MIS‐C, admitted by month, and their need for a PICU stay from 1 January 2018 to 15 July 1 2020. The main characteristics of the seven MIS‐C patients are shown in Table 1. There were two males aged 0.5 and 12.6 years and five females aged 5.8, 6.0, 7.5, 8.3 and 11.3 years.
FIGURE 1.
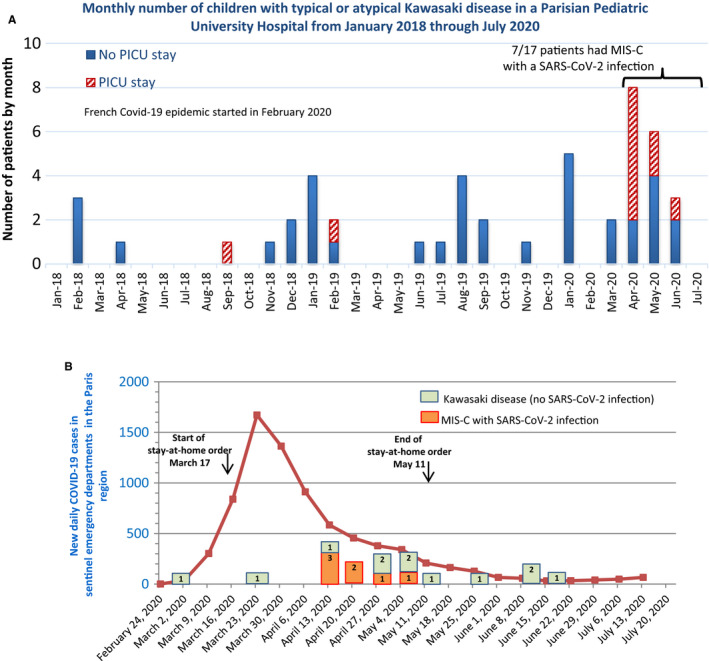
Panel A. Monthly occurrence of KD cases and MIS‐C in our paediatric university hospital in Paris from January 2018 to July 2020. Red dashed bars indicate cases admitted to the paediatric intensive care unit (PICU). Panel B. Daily number of new cases of COVID‐19 reported for the Paris region from March to July 2020 and the weekly number of KD, and MIS‐C cases who tested positive for SARS‐CoV‐2, displayed by the week of occurrence, admitted to the same hospital (bars). Numbers within the bars indicate the number of patients
TABLE 1.
Description of the seven children with MIS‐C children who presented to the paediatric university hospital during the first wave of the COVID‐19 pandemic
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | |
|---|---|---|---|---|---|---|---|
| Date of hospitalisation | 13 April | 19 April | 19 April | 20 April | 20 April | 28 April | 5 May |
| Age (years) | 8.3 | 12.6 | 5.8 | 11.3 | 0.5 | 6.0 | 7.5 |
| Sex | Female | Male | Female | Female | Male | Female | Female |
| SARS‐CoV‐2 infection | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| RT‐PCR (nasal swab/stool) | Neg/ND | Neg/Neg | Pos/ND | Neg/Neg | Neg/ND | Neg/ND | Neg/ND |
| Serology (IgG) | Pos | Pos | Pos | Pos | Pos | Pos | Pos |
| COVID‐19 contact a | No | Yes | No | Yes | Yes | Yes | Yes |
| Other respiratory viruses b | No | No | ND | No | No | No | No |
| PICU stay in days | 3 | 2 | 8 | 12 | 2 | 7 | 8 |
| Met PIMS‐TS definition | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Met WHO definition | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Kawasaki major criteria c | |||||||
| Type of presentation | Classic | Incomplete | Incomplete | Incomplete | Incomplete | Classic | Incomplete |
| Number of major criteria | 4 | 3 | 3 | 1 | 3 | 5 | 2 |
| Extremity changes | Yes | No | Yes | Yes | No | Yes | No |
| Rash | Yes | Yes | Yes | No | Yes | Yes | Yes |
| Conjunctivitis | Yes | Yes | No | No | Yes | Yes | No |
| Oral changes | Yes | Yes | Yes | No | Yes | Yes | Yes |
| Cervical lymphadenopathy | No | No | No | No | No | Yes | No |
| Days of fever at diagnosis | 5 | 6 | 7 | 4 | 7 | 5 | 6 |
| Other signs and symptoms | |||||||
| Abdominal pain | Yes | Yes | Yes | Yes | No | No | Yes |
| Other | Vomiting, stiff neck | Vomiting, diarrhoea | Vomiting, diarrhoea, sterile pyuria | Vomiting | Hydrocele, irritability | Cervical pain | Vomiting, diarrhoea |
| Echocardiography | Abnormal | Abnormal | Abnormal | Abnormal | Normal | Abnormal | Abnormal |
| Aneurism | No | No | No | No | No | No | No |
| Ejection fraction | 25% | 55% | 31% | 55% | 70% | 25% | 30% |
| Mitral regurgitation | Yes | No | No | No | No | No | Yes |
| Pericardial effusion | Yes | No | No | Yes | No | No | Yes |
| Electrocardiography | Normal | Normal | ST segment elevation | ST segment depression | Normal | Normal | Normal |
| Myocardial dysfunction | Yes | Yes | Yes | Yes | No | Yes | Yes |
| KDSS | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Chest X‐ray | Normal | Normal |
Right pleural effusion, Cardiomegaly |
Cardiomegaly | Bilateral alveolar opacities | Cardiomegaly | Cardiomegaly, bilateral perihilar alveolar opacities |
| Others imaging findings | Pleural effusion | Peritoneal effusion, terminal ileitis | Peritoneal effusion | Peritoneal and pleural effusion. Typical COVID‐19 opacities on chest CT (subpleural areas of focal consolidation and ground glass opacities) | ND | Pleural effusion, retropharyngeal abscess on cervical CT | ND |
| Laboratory values d | |||||||
| Leucocytes count/µl | 16,090 | 9,040 | 19,800 | 6,670 | 1,660 | 4,730 | 22,120 |
| Neutrophil count/µl | 14,270 | 8,260 | 16,800 | 5,940 | 150 | 3,590 | 19,470 |
| Lymphocyte count/µl | 770 | 510 | 1,500 | 315 | 1,380 | 710 | 880 |
| Haemoglobin, g/dl | 8,9 | 13.7 | 8.2 | 7.2 | 7.6 | 10.1 | 7.9 |
| Platelet count/µl | 281,000 | 227,000 | 210,000 | 122,000 | 102,000 | 102,000 | 187,000 |
| C‐reactive protein, mg/L | 134 | 340 | 183 | 315 | 261 | 310 | 390 |
| Procalcitonin, ng/ml | 9.83 | 8.62 | 242.44 | 63.51 | 50.79 | 13.08 | NA |
| Fibrinogen, mg/dl | 7.01 | 7.21 | 6.39 | 7.08 | 2.18 | 6.16 | 7.56 |
| Ferritin, ng/ml | 334 | NA | NA | NA | 470 | NA | NA |
| Triglycerides, mg/dl | 1.14 | NA | NA | NA | 1.38 | NA | NA |
| D‐dimer, ng/ml | 4,407 | NA | 13,942 | >20,000 | 2,660 | 2,604 | 1,416 |
| ALT, U/L | 15 | 38 | 21 | 46 | 6 | <5 | 32 |
| AST, U/L | 29 | 71 | 115 | 77 | 11 | 21 | 62 |
| Lipase U/L | 128 | 153 | 45 | 168 | NA | 15 | 42 |
| Albumin (lowest), g/L | 19.7 | 24.5 | 18 | 19.1 | 21 | 18.4 | 20.6 |
| Sodium (lowest), mEq/L | 128 | 129 | 133 | 136 | 133 | 130 | 123 |
| LDH, U/L | 487 | 581 | 337 | NA | 300 | NA | NA |
| Creatinine, µmol/L | 24 | 81 | 180 | 68 | 22 | 32 | 174 |
| Troponin e , ng/L | 125 | 4,607 | 654 | 545 | 10 | 340 | 1,793 |
| BNP, ng/L | 2,305 | 870 | 19,013 | 3,773 | 189 | 3140 | >5,000 |
| Treatment | |||||||
| Tracheal intubation | No | No | Yes | Yes | No | No | Yes |
| Inotropes | Yes | No | Yes | Yes | No | Yes | Yes |
| IVIG doses | 1 | 1 | 2 | 2 | 2 | 2 | 1 |
| Corticosteroids | Yes | Yes | No | No | No | Yes | Yes |
| Outcome | Favourable | Favourable | Favourable | Favourable | Favourable | Favourable | Favourable |
ALT, alanine aminotransferase (ref <32 U/L); AST, aspartate aminotransferase (ref <32 U/L; BNP, brain‐type natriuretic peptide (normal value <100 ng/L); COVID‐19, coronavirus disease 2019; CT, computed tomography; IgG, immunoglobulin G antibodies; IVIG, intravenous immunoglobulins (2 g/kg intravenously); LDH, lactate dehydrogenase (ref 290–580 U/L).
Abbreviations: IVIG, intravenous immunoglobulin; KDSS, Kawasaki disease shock syndrome; MIS‐C, multisystem inflammatory syndrome in children; NA, not available; ND, not done; Neg, negative; PICU, paediatric intensive care unit; PIMS‐TS, paediatric multisystem inflammatory syndrome temporally associated with COVID‐19; Pos, positive; RT‐PCR, reverse transcription‐polymerase chain reaction; WHO, World Health Organization.
Close contact with patient with confirmed or highly suspected SARS‐CoV‐2.
PCR on nasal swab for other respiratory viruses: adenovirus, bocavirus, coronavirus 229E, NL63 and OC43, enterovirus, influenza virus A H1 and H3, influenza virus B, metapneumovirus, parainfluenza virus 1, 2, 3 and 4, rhinovirus, respiratory syncytial virus A and B. *Do these all need to be listed?*
Five major clinical criteria from American Heart Association.
Highest value before diagnosis during hospital stay unless indicated otherwise.
Troponin normal values: < 34 ng/ml.
All seven MIS‐C patients tested positive for the SARS‐CoV‐2 infection: one had a positive RT‐PCR from a nasopharyngeal swab and all seven had positive immunoglobulin G (IgG) serology. A known COVID‐19 contact was found in five of these seven children and these are detailed in Table 2. Two of the seven children with MIS‐C also fulfilled the criteria for classic KD. The use of the PIMS‐TS and WHO definitions yielded the same results as the MIS‐C definition. All seven MIS‐C patients were admitted to the PICU: the two boys stayed for 2 days and the five girls for three to 12 days. None of them had coronary aneurisms. An abnormal echocardiography was found in six of the seven MIS‐C patients, mainly due to a low ejection fraction or mitral regurgitation. All seven children received intravenous immunoglobulins and four also received corticosteroids. The outcomes were favourable in all seven children (Table 1).
TABLE 2.
Description of close contacts with COVID‐19 in seven children with MIS‐C during the 2 months before the onset of their disease
|
Patient No. |
Lockdown respected a |
Contact with confirmed or suspected COVID‐19 |
Symptoms between contact and MIS_C diagnosis | Day 1 Fever | Day 1 Hospital | Contact to COVID‐19 (days) | ||
|---|---|---|---|---|---|---|---|---|
| Yes/No | Day 1 of contact | Setting | ||||||
| 1 | Yes | No | NA | NA | None | 8 April | 13 April | |
| 2 | Yes | Yes | 7 March | Adult, party | None | 13 April | 19 April | 37 |
| 3 | Yes | No | NA | NA | None | 14 April | 19 April | |
| 4 | Yes | Yes | 17 March | Grandfather, household | None | 18 April | 20 April | 32 |
| 5 | Yes | Yes | 17 March | Mother b , household | Cough, laryngitis on 1 April | 15 April | 20 April | 34 |
| 6 | Yes | Yes | 30 March | Adult cousin, household | None | 23 April | 28 April | 24 |
| 7 | Yes | Yes | 5 March | Father, household | None | 30 April | 5 May | 58 |
Abbreviation: MIS‐C, multisystem inflammatory syndrome in children.
France's lockdown started on 17 march 2020.
Child's mother continued to go to work and got COVID‐19
3.2. Comparison of MIS‐C patients and KD patients
From 1 January 2018 to 15 July 2020, we identified 40 children who presented with typical or atypical KD, including 10 who presented during the COVID‐19 lockdown. None of them tested positive for SARS‐Cov‐2. We compared their patient demographics, clinical, laboratory, echocardiographic, therapeutic and outcomes with the seven children with MIS‐C, who all tested positive for SARS‐CoV‐2 (Table 3). Compared to the children with KD, the children with MIS‐C were older, and a lower proportion were under 5 years of age (14.3% vs. 85.0%, p < 0.001). The MIS‐C patients were admitted to the PICU more frequently (100% vs. 10.0%, p < 0.001) and had higher frequencies of abdominal pain (71.4% vs. 12.5%, p = 0.003), myocardial dysfunction (85.7% vs. 5.0%, p < 0.001) and KD shock syndrome (85.7% vs. 2.5%, p < 0.001). The patients with MIC‐C also had a higher mean +/− standard deviation of C‐reactive protein (339 ± 131 vs. 153 ± 87, p < 0.001) and a lower mean +/− standard deviation of serum sodium (130 ± 4.2 vs. 134 ± 3.0, p = 0.011).
TABLE 3.
Comparison between 40 typical or atypical Kawasaki disease patients and seven MIS‐C patients with a SARS‐CoV‐2 infection
|
Kawasaki disease (no SARS‐CoV‐2 infection) N = 40 |
MIS‐C with SARS‐CoV‐2 infection N = 7 |
p value | |
|---|---|---|---|
| Age, years | 2.3 (2.0) | 7.5 (4.0) | 0.013 |
| Age, younger than 5 years, n (%) | 34 (85.0%) | 1 (14.3%) | 0.001 |
| Sex, male, n (%) | 26 (65.0%) | 3 (42.9%) | 0.403 |
| PICU stay, n (%) | 4 (10.0%) | 7 (100.0%) | <0.001 |
| Kawasaki major criteria a | |||
| ≥4, n (%) | 17 (42.5%) | 2 (28.6%) | 0.685 |
| Number of major criteria | 3.2 (1.2) | 2.9 (1.5) | 0.503 |
| Other symptoms | |||
| Abdominal pain, n (%) | 5 (12.5%) | 5 (71.4%) | 0.003 |
| Aneurisms or dilated coronaries, n (%) | 7 (17.5%) | 0 (0.0%) | 0.573 |
| Ejection fraction b below 40%, n (%) | 1 (2.5%) | 4 (57.1%) | 0.001 |
| Mitral regurgitation, n (%) | 10 (25.0%) | 2 (28.6%) | 1 |
| Pericardial effusion, n (%) | 11 (27.0%) | 1 (14.3%) | 0.659 |
| Abnormal electrocardiography, n (%) | 0 (0.0%) e | 4 (57.1%) | 0.001 |
| Myocardial dysfunction, n (%) | 2 (5.0%) | 6 (85.7%) | <0.001 |
| KDSS, n (%) | 1 (2.5%) | 6 (85.7%) | <0.001 |
| Laboratory values c | |||
| White blood cell count/µl | 14,873 (4 583) | 11,444 (7 903) | 0.303 |
| Neutrophil count/µl | 9,572 (4 948) | 9,783 (7,205) | 0.925 |
| Lymphocyte count/µl | 3,790 (2,149) | 866 (434) | <0.001 |
| Haemoglobin, g/dl | 10.0 (1.1) | 9.1 (2.2) | 0.322 |
| Platelet count/µl | 398,175 (170 885) | 175,857 (69,247) | <0.001 |
| CRP, mg/L | 153 (87) | 339 (131) | <0.001 |
| CRP, >250 mg/L, n (%) | 8 (20.0%) | 6 (85.7%) | 0.002 |
| ALT, U/L | 56 (67) | 23 (16) | 0.206 |
| AST, U/L | 53 (76) | 55 (37) | 0.951 |
| Albumin (lowest), g/L | 28.1 (4.5) | 20.2 (2.2) | <0.001 |
| Sodium (lowest), mEq/L | 134 (3) | 130 (4.2) | 0.011 |
| Sodium below 132 mEq/L, n (%) | 8 (20.0%) | 4 (57.1%) | 0.06 |
| Creatinine, µmol/L | 23.2 (8.5) | 83 (68.0) | 0.059 |
| Treatment | |||
| Inotropic support, n (%) | 1 (2.5%) | 5 (71.4%) | <0.001 |
| IVIG (number of doses) d | 1.2 (0.4) | 1.6 (0.5) | 0.041 |
| Corticosteroids, n (%) | 7 (17.5%) | 4 (57.1%) | 0.042 |
| Favourable outcome, n (%) | 10 (100.0%) | 7 (100.0%) | 1 |
Values are means (SD) unless indicated otherwise.
CRP, C‐reactive protein; IVIG, Intravenous immunoglobulins; KDSS, Kawasaki disease shock syndrome; MIS‐C, multisystem inflammatory syndrome in children.
American Heart Association criteria. 19
Left ventricle ejection fraction.
The highest value before diagnosis during hospital stay is reported unless indicated otherwise.
All 47 patients received IVIG.
Missing data on electrocardiography for 16 patients.
3.3. Evolution of the MIS‐C outbreak related to COVID‐19 in France
Figure 1, panel B shows the evolution of the daily number of new cases of COVID‐19 reported by the emergency departments sentinel surveillance network for the Paris region from March to July 2020, displayed by the week of occurrence. The period when French citizens were ordered to stay at home is included. The Figure also shows the MIS‐C cases and the typical and atypical KD patients from March to July, displayed by the week of hospital admission. The first MIS‐C cases occurred about 3 weeks after the peak of daily COVID‐19 cases in the Paris region. None of the patients admitted after the second week of May had a SARS‐CoV‐2 infection or fulfilled the MIS‐C criteria.
Figure 2 shows the correlation between the new weekly ED COVID‐19 cases reported for the Paris region from March to July 2020 and the weekly number of children presenting with MIS‐C and confirmed SARS‐CoV‐2 who were admitted to our hospital. For the correlation analysis, the weekly numbers of MIS‐C cases were plotted against the new weekly ED COVID‐19 cases for the region at four weekly time points before admission. Spearman's rho correlation coefficient for the same week that the children with MIS‐C were admitted was 0.367 (p = 0.102). We also looked at the 1–4 weeks before admission and they were as follows: 0.465 (p = 0.039), 0.577 (p = 0.01), 0.705 (p = 0.001) and 0.724 (p = 0.001), respectively. The R‐squared measures were highest (0.806) for the correlation between the weekly number of children with MIS‐C and the weekly new ED COVID‐19 cases reported in the Paris region 3 weeks before.
FIGURE 2.
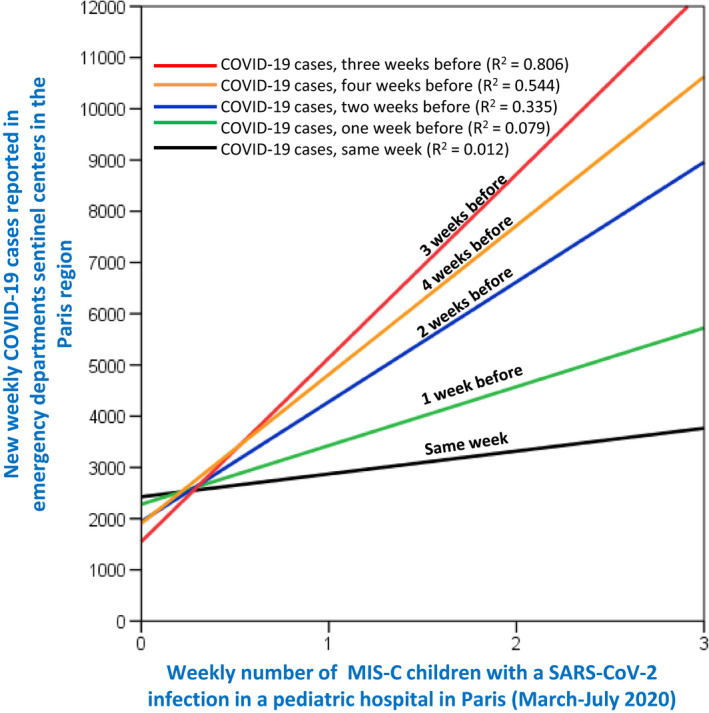
Correlation between new weekly COVID‐19 cases reported for the Paris region from March to July 2020 and weekly number of children presenting with MIS‐C and SARS‐CoV‐2 to our paediatric university hospital. The figure shows correlations between the weekly number of MIS‐C cases and weekly COVID‐19 cases that were reported in the region during the same week (black regression line, Rho 0.367, p = 0.102), 1 week before (green, Rho 0.465, p = 0.039), 2 weeks before (blue, Rho 0.577, p = 0.01), 3 weeks before (red, Rho 0.705, p = 0.001) and 4 weeks before (orange, Rho 0.724, p = 0.001). The R‐squared measure was highest for the correlation between the weekly MIS‐C and weekly new COVID‐19 cases reported 3 weeks before. Rho, Spearman's rho correlation coefficient
Figure S1 shows the COVID‐19 pandemic evolution in France from March to July 2020. This includes the official cumulative confirmed cases and new daily cases in France, as well as the 10 KD virus‐negative cases and the MIS‐C virus‐positive cases during this period, displayed by when they were first seen in our hospital. We also plotted the weekly numbers of MIS‐C cases against the new daily official COVID‐19 cases for the whole of France at four weekly time points before those admissions (Figure S2). The results were almost identical to those obtained for the COVID‐19 cases in the Paris region.
4. DISCUSSION
This study showed a strong lagged correlation between the rise and fall of the number of children and adolescents with MIS‐C and the rise and fall of the first wave of the COVID‐19 pandemic in France. To the best of our knowledge, this has not been reported before. The correlation was highest for the number of new COVID‐19 cases reported 3–4 weeks before the occurrence of MIS‐C cases. The correlations were similar when we used the number of new ED cases in the Paris region or the total number of new official cases for the whole of France. All seven of the MIS‐C patients tested positive for SARS‐CoV‐2. Compared to children with KD, MIS‐C patients were significantly older and were more likely to be admitted to the PICU. They also had a higher frequency of abdominal pain, myocardial dysfunction and KD shock syndrome, a higher C‐reactive protein and lower serum sodium. During the period of the MIS‐C outbreak, 10 children diagnosed with KD were admitted. They did not meet the MIS‐C criteria and did not test positive for SARS‐CoV‐2.
To date, SARS‐CoV‐2 has not been definitely proved to cause MIS‐C. However, MIS‐C also appeared during outbreaks of COVID‐19 in Europe 7 , 8 , 9 and the United States 13 , 14 , 15 , 16 and this link has been highly suggested. 22 The strong lagged correlation between the rise and fall of MIS‐C cases, and the rise and fall of the first wave of the COVID‐19 pandemic in France, reported in this paper further supports this association. If MIS‐C is related to SARS‐CoV‐2, the delay between the peak of the virus in the general population and the MIS‐C cases suggests a post‐infectious phenomenon, rather than a condition resulting from an acute infection. 18 Our results are consistent with previous reports 7 , 8 , 9 , 16 of MIS‐C cases. These have indicated an absence of COVID‐19 symptoms before an MISC‐C diagnosis, often negative RT‐PCR results, very frequent positive antibodies, frequent family exposure and the development of MIS‐C after a time lag of 3–6 weeks. These elements further suggest that SARS‐CoV‐2 may be acting as either a trigger or as an immune‐modulating factor. 17 All seven of the MIS‐C patients in our series tested positive for IgG antibodies, while only one tested positive for nasopharyngeal RT‐PCR. It could be argued that the presence of antibodies to SARS‐CoV‐2 does not, in itself, imply a post‐infectious process linked to MIS‐C. This is because positive antibody tests could also become more and more common in children as the COVID‐19 spreads in the population. However, this is not a likely explanation for the findings in our series. A cross‐sectional prospective study conducted in the Paris region during approximately the same period as our study found that only 10.5% of 605 asymptomatic or pauci‐symptomatic children aged 0–15 years tested positive for SARS‐CoV‐2 serology. 23 It is notable that 87.3% of those who tested positive in that study had confirmed or suspected COVID‐19 contacts. 23
We do not know the role played by lockdowns on the MIS‐C outbreaks. It has been reported that many children are infected by their parents, so it is possible that these restrictions may have enhanced close contact and eventually increase the viral load from adults to susceptible children.
While MIS‐C has been reported in several countries in Europe and in the United States, it has not been reported in China or Japan 22 where KD is more frequent. A series of KD patients in a paediatric Japanese hospital, published online in this journal in August 2020, found no increase in the incidence of KD or changes in its clinical features during the local COVID‐19 pandemic. 24 It could be that MIS‐C is such a rare condition that it has only been observed in countries with a very large number of COVID‐19 cases, such as Italy, Spain, France, the UK and the United States. 22 The fact that the MIS‐C cases in our report occurred during a shorter period than the first wave of the COVID‐19 pandemic in France, and that the cases correlated with the peak of this pandemic, is consistent with this hypothesis. Figure S1 suggests that MIS‐C cases appeared when the official number of new confirmed COVID‐19 cases was around 4,500 per day in France. In reality, the real number of new cases would have been much higher, because the testing rate during the earlier stages of the pandemic was relatively low.
A clear and consensual definition of this new multisystem inflammatory syndrome that is temporally associated with COVID‐19 is needed. The syndrome is currently referred to as PIMS‐TS or just PIMS, MIS‐C, multisystem inflammatory syndrome in children and adolescents temporally related to COVID‐19, Kawasaki‐like disease or atypical KD. In our series, the use of the MIS‐C, PIMS‐TS and WHO definitions yielded the same results. Although this inflammatory condition shares multiple clinical and laboratory features with KD, some important differences exist. 17 Cheung et al 13 reported the clinical characteristics of 17 patients with PIMS: eight who met the criteria for typical KD and five for incomplete KD. Dufort et al 16 reported that 36% of the 99 MIS‐C patients they studied also had a diagnosis of KD. The current case definition of MIS‐C is very broad and would be met by children with many infectious and inflammatory conditions. In our series, only children who tested positive for SARS‐CoV‐2 fulfilled the MIS‐C criteria. We suggest that an MIS‐C diagnosis should only be given to children who test positive for SARS‐CoV‐2, as these infected patients have a clearly different clinical picture, as confirmed by Dufort et al. 16
We did not collect racial or ethnic data, as this is not permitted in France. Previous case series have suggested that MIS‐C primarily affects people of African or Caribbean ancestry. 8 , 9 However, one case series from New York reported that twelve out of seventeen patients they studied were white. 13 Although predisposing genetic factors have been described for KD, caution is necessary when interpreting the current racial or ethnic data for MIS‐C. An alternative explanation may be that there is a higher prevalence of SARS‐CoV‐2 in some groups, with the spread of infection driven by decreased opportunities for social distancing because of dense housing. Some groups with a higher prevalence of SARS‐CoV‐2 are also more likely to be essential key workers or have to work for economic reasons. 25 Social disparities have been reported for COVID‐19.
This study had some limitations. Its retrospective design meant that there were less data on inflammatory investigations in the 30 children with KD admitted during the pre‐COVID‐19 pandemic, which precluded a larger comparison with MIS‐C patients. Second, this was a single‐centre study with few patients and the results may have been due to local variations. Third, the data on the MIS‐C outbreak were only collected for 3 months, with a short follow‐up period. However, the decline of the observed outbreak was as sharp as its rise and no new cases of MIS‐C were seen during the last 2 months of the study period. Fourth, although the correlation between the rise and fall of the MIS‐C outbreak and the COVID‐19 pandemic was very strong, it does not provide formal proof of causality. Fifth, correlations should be interpreted with caution because of the small sample size.
5. CONCLUSION
The rise and fall of the COVID‐19 epidemic were associated with the rise and fall of an outbreak of MIS‐C in children and adolescents in France. The fall in the MIS‐C cases as the first wave of the COVID‐19 pandemic reduced in intensity in France further supports their association. Although MIS‐C has been relatively rare, clinicians should be aware of this new clinical presentation, as more cases are likely if the second wave of COVID‐19 epidemic has the same impact as the first one. Because the number of patients by MIS‐C is relatively low, it is very important to carry out multicentre studies, so that we can develop a better understanding of this new condition.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
Supporting information
App S1
Carbajal R, Lorrot M, Levy Y, et al. Multisystem inflammatory syndrome in children rose and fell with the first wave of the COVID‐19 pandemic in France. Acta Paediatr.2021;110:922–932. 10.1111/apa.15667
Funding informationNo external funding.
REFERENCES
- 1. Johns Hopkins Center for Systems Science and Engineering . COVID‐19 dashboard by the center for systems science and engineering (CSSE) at Johns Hopkins University (JHU). https://coronavirus.jhu.edu/map.html. Accessed November 11, 2020.
- 2. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323(13):1239‐1242. [DOI] [PubMed] [Google Scholar]
- 3. Royal College of Paediatrics and Child Health . Guidance: paediatric multisystem inflammatory syndrome temporally associated with COVID‐19. https://www.rcpch.ac.uk/sites/default/files/2020-05/COVID-19-Paediatric-multisystem-%20inflammatory%20syndrome-20200501.pdf. Accessed November 11 2020.
- 4. Riphagen S, Gomez X, Gonzalez‐Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID‐19 pandemic. Lancet. 2020;395(10237):1607‐1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Centers for Disease Control and Prevention . Information for healthcare providers about multisystem inflammatory syndrome in children (MIS‐C). Case definition for MIS‐C. https://www.cdc.gov/mis-c/hcp/. Accessed November 11, 2020.
- 6. World Health Organization . Multisystem inflammatory syndrome in children and adolescents temporally related to COVID‐19. Published May 15, 2020. https://www.who.int/news-room/commentaries/detail/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19. Accessed November 11, 2020.
- 7. Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki‐like disease at the Italian epicentre of the SARS‐CoV‐2 epidemic: an observational cohort study. Lancet. 2020;395(10239):1771‐1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Whittaker E, Bamford A, Kenny J, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS‐CoV‐2. JAMA. 2020;324(3):259‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Toubiana J, Poirault C, Corsia A, et al. Kawasaki‐like multisystem inflammatory syndrome in children during the covid‐19 pandemic in Paris, France: prospective observational study. BMJ. 2020;369:m2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pouletty M, Borocco C, Ouldali N, et al. Paediatric multisystem inflammatory syndrome temporally associated with SARS‐CoV‐2 mimicking Kawasaki disease (Kawa‐COVID‐19): a multicentre cohort. Ann Rheum Dis. 2020;79(8):999‐1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cabrero‐Hernandez M, Garcia‐Salido A, Leoz‐Gordillo I, et al. Severe SARS‐CoV‐2 infection in children with suspected acute abdomen: a case series from a Tertiary Hospital in Spain. Pediatr Infect Dis J. 2020;39(8):e195‐e198. [DOI] [PubMed] [Google Scholar]
- 12. Belhadjer Z, Meot M, Bajolle F, et al. Acute heart failure in multisystem inflammatory syndrome in children (MIS‐C) in the context of global SARS‐CoV‐2 pandemic. Circulation. 2020;142(5):429‐436. [DOI] [PubMed] [Google Scholar]
- 13. Cheung EW, Zachariah P, Gorelik M, et al. Multisystem inflammatory syndrome related to COVID‐19 in previously healthy children and adolescents in New York City. JAMA. 2020;324(3):294‐296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kaushik S, Aydin SI, Derespina KR, et al. Multisystem inflammatory syndrome in children (MIS‐C) associated with SARS‐CoV‐2 infection: a multi‐institutional study from New York City. J Pediatr. 2020;224:24‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Feldstein LR, Rose EB, Horwitz SM, et al. Multisystem inflammatory syndrome in U.S. Children and adolescents. N Engl J Med. 2020;383(4):334‐346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dufort EM, Koumans EH, Chow EJ, et al. Multisystem inflammatory syndrome in children in New York State. N Engl J Med. 2020;383(4):347‐358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McCrindle BW, Manlhiot C. SARS‐CoV‐2‐related inflammatory multisystem syndrome in children: different or shared etiology and pathophysiology as Kawasaki disease? JAMA. 2020;324(3):246‐248. [DOI] [PubMed] [Google Scholar]
- 18. Son MBF. Pediatric inflammatory syndrome temporally related to covid‐19. BMJ. 2020;369:m2123. [DOI] [PubMed] [Google Scholar]
- 19. McCrindle BW, Rowley AH, Newburger JW, et al. Diagnosis, treatment, and long‐term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation. 2017;135(17):e927‐e999. [DOI] [PubMed] [Google Scholar]
- 20. Kanegaye JT, Wilder MS, Molkara D, et al. Recognition of a Kawasaki disease shock syndrome. Pediatrics. 2009;123(5):e783‐e789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. INSEE . Bilan démographique 2019. https://www.insee.fr/fr/statistiques/1892086?sommaire=1912926. Accessed July 22, 2020.
- 22. Rowley AH. Understanding SARS‐CoV‐2‐related multisystem inflammatory syndrome in children. Nat Rev Immunol. 2020;20(8):453‐454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cohen R, Jung C, Ouldali N, et al. Assessment of spread of SARS‐CoV‐2 by RT‐PCR and concomitant serology in children in a region heavily affected by COVID‐19 pandemic. MedRxiv. 2020. [Google Scholar]
- 24. Iio K, Uda K, Hataya H, et al. Kawasaki disease or Kawasaki‐like disease: influence of SARS‐CoV‐2 infections in Japan. Acta Paediatr. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Martinez DA, Hinson JS, Klein EY, et al. SARS‐CoV‐2 positivity rate for Latinos in the Baltimore‐Washington, DC Region. JAMA. 2020;324(4):392. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
App S1


