Abstract
Increased morbidity and mortality from coronavirus disease 2019 (COVID‐19) in people with obesity have illuminated the intersection of obesity with impaired responses to infections. Although data on mechanisms by which COVID‐19 impacts health are being rapidly generated, there is a critical need to better understand the pulmonary, vascular, metabolic, and immunologic aspects that drive the increased risk for complications from COVID‐19 in people with obesity. This review provides a broad overview of the intersection between COVID‐19 and the physiology of obesity in order to highlight potential mechanisms by which COVID‐19 disease severity is increased by obesity and identify areas for future investigation toward developing tailored therapy for people with obesity who develop COVID‐19.
Study Importance.
What is already known?
-
►
Obesity is a risk factor for severe coronavirus disease 2019 (COVID‐19).
What does this review add?
-
►
We provide a broad overview of the potential mechanisms by which pulmonary physiology, metabolic dysfunction, adipose tissue biology, and inflammatory pathways may modify COVID‐19 outcomes in people with obesity.
How might these results change the direction of research or the focus of clinical practice?
-
►
Our review highlights gaps in clinical and research knowledge that need to be addressed to improve the prevention and treatment of COVID‐19 for people with obesity.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) is the third highly pathogenic coronavirus to emerge in the past 2 decades, preceded by Middle East respiratory syndrome coronavirus (MERS‐CoV) in 2012 and severe acute respiratory syndrome coronavirus 1 in 2002. Age, obesity, and type 2 diabetes mellitus (DM) are established risk factors for coronavirus disease 2019 (COVID‐19) severity (1). Obesity has been identified as an independent risk factor for severe disease, admission, need for invasive mechanical ventilation, and mortality from COVID‐19 in cohorts from China, the US, and Europe, with an adjusted hazard ratio of 1.92 for COVID‐19‐related death with BMI > 40 kg/m2 (2, 3). DM is closely linked to obesity, and at least two large retrospective studies have identified DM as an independent risk factor for increased mortality from COVID‐19 (3, 4). The dissection of the mechanisms by which obesity and DM independently contribute to COVID‐19 disease risk is fraught with methodologic challenges (5), and these physiologic modifiers likely participate in complex interactions to potentiate disease.
Interestingly, obesity may be a protective factor for non‐COVID‐19‐associated acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) (6). Meta‐analyses have suggested that although people with obesity have an increased risk for progressing to ALI/ARDS compared with people without obesity, people with obesity also have a reduced risk for mortality from ALI/ARDS. Significant debate persists regarding the potential confounders and mechanisms behind these associations (7). Furthermore, there is debate regarding whether ARDS associated with COVID‐19 is unique and different than non‐COVID‐19 ARDS (8, 9). Nonetheless, in light of the current pandemic, the links between obesity and COVID‐19 severity beg the following question: if obesity is a protective factor for non‐COVID‐19‐related lung disease, why is COVID lung disease different in this respect? In other words, more globally speaking, what are the mechanisms by which obesity increases risk for COVID‐19 disease severity and mortality?
In this review, we will summarize current theories regarding the intersection of COVID‐19 disease and obesity physiology with a focus on the pulmonary, metabolic, adipose tissue, and immune aspects of COVID‐19 that may interface with obesity to shift the risk curve for mortality and morbidity toward more severe disease. We acknowledge that the rapid accumulation of new information about COVID‐19 may clarify some of these mechanisms and refute others over time. However, we hope to broaden the framework for the discussion of obesity and COVID‐19 and point out new areas for research.
Clinical Course of COVID‐19
Core to understanding why people with obesity are more susceptible to morbidity from SARS‐CoV‐2 is the identification of the key steps of disease progression that lead to severe COVID‐19, which have been previously reviewed (10, 11). Coronaviruses are lipid membrane‐enveloped, positive‐sense, single‐strand RNA viruses, named for the crown‐like structure of spike proteins surrounding the virion as observed under electron microscopy. In the early stages of infection, SARS‐CoV‐2 binds to nasal and bronchial epithelial cells where viral replication is initiated. SARS‐CoV‐2 binds the angiotensin converting enzyme 2 (ACE2) on the cell membrane, followed by cleavage of viral capsid spike (S) protein by the host transmembrane serine protease 2 (TMPRSS2), followed in turn by viral fusion and the release of the viral nucleocapsid into the cell cytoplasm. The virus then uses host cell machinery to synthesize the viral replication/transcription complex, which associates with autophagosome‐like intracellular double membrane vesicles. This complex mediates replication, transcription, and translation of viral genomic RNA into structural and accessory proteins in the endoplasmic reticulum, followed by transport to the Golgi apparatus for virion assembly, budding, and release through the cell plasma membrane (Figure 1).
Figure 1.
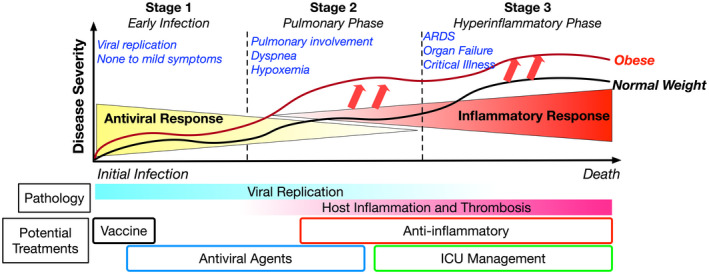
Clinical progression of COVID‐19. SARS‐CoV‐2 infection progresses through stages starting with Stage 1, which includes initial asymptomatic or mild symptoms associated with active viral replication. Some individuals progress to Stage 2, which includes increased pulmonary involvement with symptoms of dyspnea and hypoxemia and opacities observed with chest imaging that often must be managed with hospitalization. A subset of individuals in Stage 2 progress to more severe disease, Stage 3, which is associated with respiratory failure, elevated markers of systemic inflammation, and multisystem organ failure. Relative to normal‐weight individuals (indicated by the black line), people with obesity (indicated by the red line) have a higher risk for progressing to Stage 2 and 3 and have more severe disease at each stage. ARDS, acute respiratory distress syndrome; COVID‐19, coronavirus disease 2019; ICU, intensive care unit; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2.
Individuals in the early stages of infection (Stage 1) are asymptomatic and may only develop mild symptoms. Some will progress to more severe disease with symptoms of dyspnea (i.e., shortness of breath) and decreased oxygen saturation typically 1 week after symptom onset (Stage 2). This phase of illness is characterized by inflammatory lung infiltrates, pulmonary edema that appears as ground glass opacities on chest computed tomography imaging, and signs of impaired gas exchange. Epidemiologically, severe COVID‐19 is defined as dyspnea, tachypnea (respiratory rate > 30 breaths/min), blood oxygen saturation < 93%, a ratio of the partial pressure of arterial oxygen to the fraction of inspired oxygen (Pao2:Fio2) < 300 mm Hg, and/or infiltrates in more than 50% of the lung field (11). A total of 14% of individuals present with severe COVID‐19, and 5% become critically ill (Stage 3), with organ failure associated with markers of systemic inflammation (12).
A challenge in the field lies in understanding at what point in this disease progression from mild to severe disease obesity leaves its mark and shifts patients toward severe disease (Figure 1). Are the antiviral responses that prevent progression from Stage 1 to Stage 2 disease impaired in people with obesity? Do differences in the sensation of dyspnea delay people with obesity from seeking care when in Stage 2? Do differences in the inflammatory responses to SARS‐CoV‐2 in critical illness in Stage 3 increase clinical severity in the context of obesity? And finally, can tailored clinical management for patients with obesity and severe COVID‐19 impact outcomes?
Pulmonary Physiology in People with Obesity Relevant to COVID‐19
People with obesity have multiple alterations in pulmonary physiology that may contribute to increased COVID‐19 severity (Figure 2) (13), and impaired ventilatory drive is linked to increased susceptibility to severe COVID‐19 disease (14). Therefore, with a decreased sensation of respiratory difficulty, people with obesity may delay care or present with more severe disease.
Figure 2.
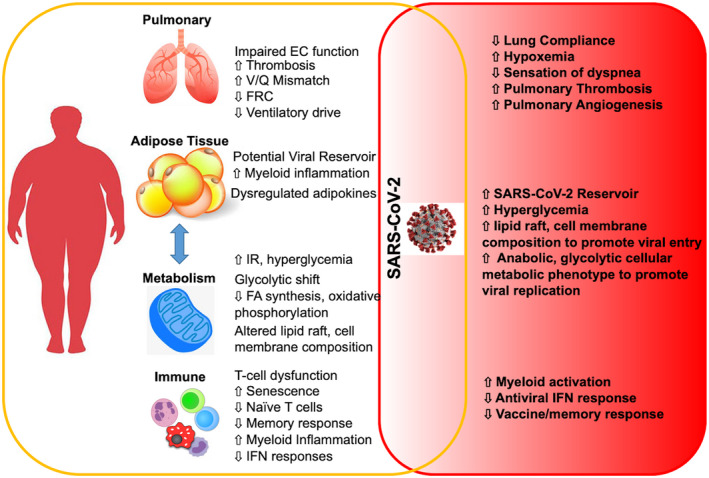
Obesity‐associated alterations in physiology and immunity that amplify responses to SARS‐CoV‐2. Obesity has broad effects on pulmonary physiology, adipose tissue biology, metabolism, and immune system function. COVID‐19 exploits these impairments in normal homeostasis leading to more severe disease and/or requiring different clinical management approaches (e.g., ventilator management) compared with people without obesity. COVID‐19, coronavirus disease 2019; EC, endothelial cells; FA, fatty acid; FRC, function residual capacity; IFN, interferon; IR, insulin resistance; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2; V/Q, lung ventilation/perfusion.
Changes in pulmonary function in people with obesity include decreased functional residual capacity, increased pleural pressure, and reduced lung and chest wall compliance (15). Baseline mismatch of ventilation and perfusion (V/Q) is observed in individuals with severe obesity (16), and V/Q mismatch has been shown to be a significant component of the severe hypoxemia seen in COVID‐19 disease (17), suggesting that it is another mechanism by which obesity may lead to more severe disease. The clinical progression of lung disease in COVID‐19 has been described to progress from an “L” phase, with low elastance (high compliance), low V/Q ratio, and low lung weight, to an “H” phase in 20‐30% of patients characterized by high elastance (low compliance), right‐to‐left shunt (decreased perfusion of unventilated areas), and high lung weight (18). In many ways, people with obesity are predisposed to pulmonary mechanics associated with “H” phase characteristics (low compliance, higher V/Q mismatch), potentially making them more susceptible to progression to more severe disease.
Another potential reason for worse outcomes may be because of a lack of evidence‐based tailored approaches to the ventilator management of people with obesity once they progress to ARDS. The NIH National Heart, Lung, and Blood Institute ARDS Network has made a significant impact on the ventilator management of patients with ARDS by standardizing protocols based on large, randomized clinical trials (19). Unfortunately, severe obesity (BMI > 35 kg/m2) has been an exclusion criterion for many NIH National Heart, Lung, and Blood Institute ARDS Network studies, leading to significant gaps in the understanding of how to best manage patients with obesity using evidence‐based approaches. Some groups have employed specialized ventilator management teams for individuals with class III obesity and ARDS to titrate care based on more in‐depth pulmonary measures of compliance and pleural pressures. Such strategies have shown decreased mortality compared with standard ventilator management (20). As we learn more about respiratory management in COVID‐19, there will likely be a need to tailor management to the unique physiology of people with obesity.
Dysregulated Pulmonary Responses to Infection in Obesity
Although the mechanisms underlying the association between ARDS and obesity are unknown, multiple studies have identified molecular characteristics of the lungs of individuals with obesity relevant to COVID‐19. Lungs from patients who died from COVID‐19 ARDS demonstrate pulmonary thrombosis and vascular angiogenesis that are unique compared with ARDS without COVID‐19, suggesting that pulmonary small vessel disease may be a key contributor to mortality (21). Supporting this, COVID‐19 individuals with elevated D‐dimers, a marker of thrombosis, and poor lung compliance have a higher mortality rate than those with low D‐dimers and normal lung compliance. This suggests an interaction between pulmonary physiology and thrombosis that contributes to COVID‐19 mortality (22). The link between obesity and endothelial dysfunction is well recognized, but gaps exist in our understanding of the effect of obesity on pulmonary vascular biology.
Rodent models of obesity and diabetes demonstrate that hyperglycemia increases pulmonary vascular permeability, inflammation, and remodeling that may exacerbate COVID‐19 lung inflammation (23). Mouse models suggest that circulating factors from obese mice and free fatty acids upregulate endothelial cell adhesion molecules and worsen lipopolysaccharide‐induced ALI potentially through endoplasmic reticulum stress (24). Mouse models of MERS‐CoV infection have revealed altered kinetics of perivascular inflammation in high‐fat diet and diabetic mice that ultimately result in a more severe inflammatory response in the lungs of obese mice (25). Early stages of infection are associated with a decrease in lung monocytes and T cells. In the later stages of illness, pulmonary inflammation persists longer after infection in diabetic mice compared with nondiabetic mice, independent of viral load. These findings suggest that obesity blunts early host‐inflammatory responses to MERS‐CoV infection in the lungs, ultimately impairing resolution of inflammation, thus leading to severe disease.
Interactions between infected pulmonary epithelial cells and inflammatory leukocytes control the degree and nature of the pulmonary inflammatory response. Single‐cell RNA sequencing studies comparing mild and severe disease have demonstrated that COVID‐19 is associated with the induction of ACE2 expression in bronchial epithelial cells that correlates with interferon (IFN) responses (26). Bioinformatic evaluation of chemokine ligand‐receptor networks have suggested that COVID‐19 severity is associated with increased epithelial–macrophage communication. Obesity is associated with increased ACE2 expression on bronchial epithelial cells of patients with chronic obstructive pulmonary disease, suggesting that obesity may potentiate SARS‐CoV‐2 entry and inflammation (27).
Glucose Metabolism and COVID‐19
Viral infection has diverse effects on host cellular metabolism. It was observed decades ago that influenza virus infection induces a shift in cellular metabolism toward a glycolytic phenotype (28); more recent data have demonstrated that an inhibition of glycolysis reduces influenza virus infection in in vitro cell culture models and in human bronchial epithelial cells in vivo and in vitro (29). The effects of viral infection on glucose metabolism are complex and include maladaptive responses that enhance viral replication along with adaptive responses that potentiate host immunity. For example, in a murine model of acute cytomegalovirus (CMV) infection, lean animals maintained glycemic control because of compensatory hyperinsulinemia that directly potentiated cytotoxic (CD8+) T‐cell‐mediated antiviral immunity. In contrast, prediabetic obese animals responded to viral infection with a loss of glycemic control, demonstrating that similar responses in the context of prediabetes can tip systemic metabolism toward frank diabetes with attendant adverse effects (30). Human data support these observations, with acute viral infection inducing systemic skeletal muscle insulin resistance in healthy humans without resulting in hyperglycemia (30), whereas, in contrast, people with obesity and/or diabetes are at risk for loss of glycemic control and DM complications in response to influenza infection (31).
Poor glycemic control has been identified as a risk factor for mortality from COVID‐19 (4). Obesity and DM may contribute to COVID‐19 risk by placing patients at increased risk for loss of hyperglycemic control and DM complications in response to viral disease. SARS‐CoV‐2 also appears to have direct effects on cell metabolism that may enhance infection or promote inflammation. SARS‐CoV‐2 infection of monocytes induces a shift toward a glycolytic cellular metabolic phenotype that promotes viral replication, enhances inflammatory cytokine release from monocytes, and impairs T‐cell function (32). SARS‐CoV‐2 therefore appears to have multiple effects on glucose metabolism at both cellular and systemic levels that act to potentiate disease. Given data implicating dysregulation of glucose metabolism in COVID‐19 and increased disease severity in DM patients, tight glucose control has been recommended for DM patients with COVID‐19, although certain hypoglycemic drugs, such as metformin and sodium‐glucose co‐transporter 2 inhibitors, may be contraindicated because of risk for acidosis (33). Nonetheless, metformin has been linked to reduced mortality in COVID‐19 infections in three large observational studies to date (34, 35). Multiple mechanisms have been proposed for these effects, including improvement in glucose control and insulin resistance, activation of 5' AMP‐activated protein kinase signaling with reduced reactive oxygen species formation, and anti‐inflammatory effects, among others (36). Peroxisome proliferator‐activated receptor gamma agonists (thiazolidinediones) have also been suggested as therapy for COVID and have been shown to reduce mortality in patients with non‐COVID viral lung disease (37). In addition to hypoglycemic effects, thiazolidinediones have important anti‐inflammatory properties, which may reduce cytokine storm.
Lipid Metabolism and COVID‐19
Numerous studies have provided a snapshot of the metabolic effects of COVID‐19, and we are struck by the significant parallels between the dysregulation of metabolites in COVID‐19 and in people with obesity independent of viral infection. Such overlap suggests that patients with obesity represent a vulnerable metabolic phenotype with respect to COVID‐19, owing to specific preexisting metabolic derangements that predispose patients to efficient viral infection and replication and that are amplified by SARS‐CoV‐2 infection.
Therefore, we posit that individuals with preexisting metabolic derangements related to obesity and diabetes experience a further exacerbation of similar derangements induced by COVID‐19. The additive effects of these insults make patients with obesity more likely to reach a threshold beyond which severe disease ensues. Proteomic and metabolomic analysis of sera from patients with or without COVID‐19 has demonstrated a dysregulation of macrophage signaling, platelet degranulation, and complement system pathways along with decreased levels of glycerophospholipids, sphingolipids, and fatty acids, molecules that are critical for lipid raft formation required for SARS‐CoV‐2 cell entry (38). In a separate mass spectrometry study, the plasma lipidome of COVID‐19 patients, relative to healthy controls, was characterized by increased levels of monosialodihexosyl ganglioside (GM3) and increased GM3 exosomes, levels of which correlated directly with disease severity and indirectly with CD4+ T‐cell counts (39). Additionally, reduced levels of acylcarnitines and tricarboxylic acid cycle metabolites, progressive reductions in circulating polyunsaturated fatty acid phosphatidylcholines (PUFA‐PCs), which have been shown to be correlated with increasing COVID‐19 disease severity, and increased levels of sphingomyelins have been noted in severe COVID‐19 disease.
Metabolic Derangements Common to Obesity and COVID‐19
Although the mechanistic significance of these alterations remains unclear, many serum lipidome and metabolome changes observed in COVID‐19 parallel similar observations in other viral diseases and are also independently associated with obesity and DM, suggesting potential mechanisms linking obesity to COVID‐19 disease severity. For example, increased GM3 exosome membrane composition is also seen notably in Ebola virus disease (40), and the observed negative correlation between GM3 exosomes and CD4+ T‐cell counts in COVID‐19 suggests that GM3 exosomes may mediate communication between viral‐infected cells and immune cells with immunosuppressive effects on T cells. Similarly, the observed lower serum levels of acylcarnitines, tricarboxylic acid cycle metabolites, and fatty acids in COVID‐19 patients are consistent with decreased entry of fatty acids into mitochondrial β‐oxidation pathways and defects in oxidative cellular energy metabolism, pathways that have been well documented to be impaired in obesity independent of COVID‐19 (41). The observed decreased levels of PUFA‐PC in COVID‐19, which are associated with high‐density lipoprotein synthesis, similarly implicate aberrations in high‐density lipoprotein synthesis as a risk factor for severe COVID‐19 disease, a pathway also known to be dysregulated in obesity independent of infection (42). Furthermore, decreases in PUFA‐PCs are also associated with ARDS mortality independent of COVID‐19 and obesity (43). The dysregulation of sphingolipids and sphingomyelins in SARS‐CoV‐2 infection are of interest, as these lipid moieties, critical to viral entry through lipid rafts, are increased in plasma lipidomic studies in obesity and DM independent of infection (44). Finally, lysophosphatidylcholines, which play diverse roles in regulating intracellular trafficking in all cells as well as specific roles in regulating endothelial cell function, inflammation, and cholesterol synthesis (45), are increased in COVID‐19 sera (39), and these lipid moieties are also dysregulated in sera from patients with obesity and DM (46).
ACE2 and TMPRSS2 proteins localize to cholesterol‐ and sphingolipid‐rich lipid rafts in the cell plasma membrane, which are required for efficient infection, suggesting the targeting of these lipid moieties. Cholesterol‐depleting agents, such as methyl‐β‐cyclodextrin, reduce infection by type I feline coronavirus, parainfluenza virus, and severe acute respiratory syndrome coronavirus 1 replication in cell culture models (47). Statins have also been proposed as a potential treatment, and although data regarding their use for a wide range of infectious diseases in heterogeneous patient populations are mixed, a number of studies have suggested efficacy (48, 49). Other drugs that regulate cholesterol and fatty acid synthesis and trafficking, including amiodarone, haloperidol, orlistat, and triacsin C, have shown promise as off‐label antiviral agents in in vitro models of coronavirus infection (50). More targeted therapy may result from ongoing lipidomic and metabolomic studies. For example, Yan et al. used ultra performance liquid chromatography‐mass spectrometry lipidomics to demonstrate perturbed levels of glycerophospholipids and fatty acids, including arachidonic acid in an in vitro model of human coronavirus 229E infection of Huh‐7 and VeroE6 cells, and showed that exogenous supplement of arachidonic acid inhibited viral replication in this model and in a similar model of MERS‐CoV infection (51). Along similar lines, the Food and Drug Administration‐approved “sphingomimetic” drug FTY720 is currently being studied as treatment for COVID‐19 in clinical trials (ClinicalTrials.gov NCT04280588) (52).
Adipose Tissue as a Target for SARS‐CoV‐2 Infection
Adipose tissue plays a central role in metabolism, immune, and endocrine function and, as such, is a prime candidate for regulating metabolic and inflammatory responses to viral infection. Adiposity, specifically visceral adiposity, which is strongly linked to metabolic disease, has been identified as a risk factor for COVID‐19 disease severity (53), yet underlying mechanisms remain poorly defined. Adipose tissue may serve as a direct target for SARS‐CoV‐2, and SARS‐CoV‐2 RNA can be detected in visceral (omental) adipose tissue of humans with COVID‐19 (54); however, whether infectious virions are present remains unknown. Precedent for direct adipotropy of other viral infection exists: H5N1 targets white adipose tissue and is adipotropic in murine models (55), whereas CMV, adenovirus, and respiratory syncytial virus infect adipocytes in vitro (56). Although it predominantly targets airway epithelial cells, influenza A also infects immune and nonimmune cells, including preadipocytes, in mice (57). Other data have demonstrated infection of nonadipocyte adipose‐tissue‐resident cells by multiple viruses, including adipose tissue macrophages and lymphocytes by influenza, adenovirus, and human immunodeficiency virus (56, 58, 59). ACE2 expression is high in adipose relative to other tissues (60) and adipocyte expression of ACE2 is upregulated in obesity and DM. Increased expression of ACE2 in adipose tissue has been linked to worse clinical outcomes of COVID‐19 (61, 62). In summary, these observations suggest a potential mechanism for SARS‐CoV‐2 viral adipotropy. If adipose tissue is a reservoir for persistent SARS‐CoV‐2 infections in people with obesity, antiviral and vaccine approaches to combat disease may have to be titrated based on adiposity.
A heightened state of inflammation is a dominant feature of adipose tissue as adipocytes, preadipocytes, and adipose tissue leukocytes, most notably adipose tissue macrophages, are a source of inflammatory cytokines in vivo. Other viral infections increase adipose tissue inflammatory cytokine release. Adenovirus‐36, respiratory syncytial virus, or CMV infection induces increased adipocyte interleukin 6 (IL‐6) cytokine secretion in vitro (56), which is further increased in the presence of coculture with macrophages, suggesting that adipose tissue resident leukocytes synergize with adipocytes to potentiate cytokine expression. Visceral adipose tissue, relative to subcutaneous adipose tissue, secretes higher levels of inflammatory cytokines independent of obesity, including tumor necrosis factor alpha (TNF‐α), IL‐6, and IL‐1β (63), all of which are cytokines implicated in COVID‐19 cytokine storm. These observations suggest that increased inflammatory cytokine expression from visceral adipose tissue in obesity may contribute to cytokine storm and increased COVID‐19 severity.
Viral infection may regulate adipocyte browning as well. Influenza A infection of murine 3T3L1 adipocytes induces browning and the upregulation of genes such as uncoupling protein 1 (Ucp1) (57). Irisin, an adipokine that induces browning, reduces adipocyte expression in vitro of a number of genes that are induced in the lung by COVID‐19 infection (64). IFN and IFN‐response genes play a critical role in viral defense and are specifically implicated in host responses to coronaviruses (65), and they have been implicated in browning as well, with some IFN‐response genes (IFI27) promoting (66) whereas others (IRF3) inhibit (67) adipocyte browning. The directionality of IFN effects on browning therefore depends upon the specific mediators involved. IL‐6, a key mediator of COVID‐19‐related cytokine storm (68, 69), is increased in serum and adipose tissue in obesity (70) and promotes browning in adipocytes (71). Whether adipocyte browning is an adaptive or maladaptive response to viral‐induced inflammation remains unclear, but it is possible that browning may be an adaptive response that potentiates IFN‐ and IL‐6‐mediated immune responses within adipose tissue. Alternatively, browning may potentiate excessive cytokine release (e.g., IL‐6, secretion of which, from white adipose tissue, is increased in browning) (71) and contribute to disease severity, depending upon the phase of infection. Further research will clarify these issues, but these observations suggest that adipocyte browning‐inflammation cross talk plays a role in host response to viral infection.
Although much attention focuses on canonical white adipose tissue anatomic depots, including visceral and subcutaneous sites, ectopic adipocytes, which are present in most tissues and increased in obesity, may also be a target for SARS‐CoV‐2. Epicardial adipose tissue has been suggested as a target for infection and a contributor to COVID‐19‐associated myocarditis (72), although, to date, data directly demonstrating viral infection of this adipose tissue depot are lacking. Excess perinephric adipose tissue and liver steatosis are risk factors for disease severity in young COVID‐19 patients (73). Dedifferentiation of dermal adipocyte‐like cells into lipofibroblasts contribute to fibrosis in fibrotic skin disease, and investigators have suggested that a similar dedifferentiation of pulmonary adipocyte‐like cells may contribute to COVID‐19 disease severity by promoting pulmonary fibrosis (74). Interestingly, in a separate study, leptin has been shown to promote fibrosis in a murine model of ARDS (75), suggesting a role for fibrosis in mediating lung disease in obesity. Although direct evidence implicating pulmonary lipofibroblasts or leptin in COVID‐19 lung disease is so far lacking, ectopic adipocytes are increased in multiple tissues in obesity and may contribute to disease severity through multiple mechanisms, including, but not limited to, fibrosis.
Adipose‐tissue‐based therapy for COVID‐19 is an active area of research. Specifically, adipose tissue stem cells (i.e., preadipocytes) are a type of mesenchymal stem cell (MSC) with diverse immunomodulatory, anti‐inflammatory, and regenerative properties. MSC therapy for COVID‐19 is under active study. Over 30 clinical trials are currently in progress studying MSC therapy for COVID‐19 (76, 77), of which at least 5 study adipose tissue MSC (NCT04313647, NCT04276987). To date, clinical efficacy has not been demonstrated, but trials have yet to mature. Any putative therapeutic effects of adipose tissue MSC may not be adipose tissue specific, as multiple data suggest that MSC from other tissues have diverse therapeutic potential. Further research will be necessary to clarify this issue in COVID‐19 disease. Nonetheless, adipose tissue represents a readily available source of expandable MSC that has potential as a therapeutic tool.
Obesity‐Induced Inflammation as a Potentiator of COVID‐19 Disease
The biphasic clinical course of severe COVID‐19 from initial mild symptoms to progression toward ARDS suggests a dysregulation of the immune response to viral infection. IFN responses are crucial to antiviral responses, and impaired production of type I IFNs is a feature of severe COVID‐19 disease (78). Further emphasizing the importance of proper IFN production, patients with severe COVID‐19 have been found to harbor neutralizing autoantibodies to IFN and to be enriched for loss of function mutations in genes critical to proper IFN signaling (79, 80). Estimates suggest that innate defects in IFN production may account for up to 14% of individuals with severe COVID‐19. The importance of IFN in COVID‐19 intersects with the observations of impaired IFN responses to immunologic stimuli in people with obesity, which is believed to contribute to impaired antiviral immunity (81). Leptin has been shown to impair IFN responses via suppressor of cytokine signaling 3 (SOCS3), suggesting a potential mechanism by which obesity and dysfunctional IFN responses are linked (82).
Broad evidence indicates that severe COVID‐19 disease is associated with amplified inflammatory responses in both innate and adaptive immunity that contributes to disease severity. Single‐cell profiling of bronchoalveolar lavage fluid from patients with COVID‐19 indicates an abundance of monocyte‐derived macrophages and neutrophils in severe disease and an association of clonal CD8+ T cells in bronchoalveolar lavage fluid with moderate disease (83). In serial analysis of blood samples from a range of COVID‐19 severity, severe COVID‐19 was characterized by a disappearance of nonclassical CD14LowCD16High monocytes, accumulation of major histocompatibility complex, Class II, DRlow (HLA‐DRLow) classical monocytes, the generation of immature neutrophils, and the upregulation of calprotectins (S100A8/S100A9) (84). Early COVID‐19 disease is associated with exaggerated myeloid activation in the blood, identified by an elevated neutrophil/lymphocyte ratio at presentation for those who progress to severe disease with evidence of emergency hematopoiesis and an elevated number of marker of proliferation Ki‐67+ monocytes (85). Therefore, it is clear that severe COVID‐19 is associated with both qualitative and quantitative alterations in peripheral blood monocytes and neutrophils that may represent exaggerated inflammatory responses and contribute to disease severity. These observations align with the longstanding association between myeloid cell activation (i.e., myelopoiesis) and obesity and diabetes (86).
Independent, single‐cell analysis of blood samples has also demonstrated specific derangements in adaptive immune cells that are associated with COVID‐19 disease severity (87). Whereas moderate disease is associated with a vigorous immune response with an expansion of CD4 and CD8 effector T cells, severe COVID‐19 is associated with evidence of exhausted T‐cell responses, dysregulated IFN signatures, and evidence of broader T‐cell expansion. The importance of adequate T‐cell responses in preventing severe COVID‐19 disease has been emphasized in independent studies that evaluated T cells in conjunction with SARS‐CoV‐2‐neutralizing antibodies in acute and convalescent patients (88). Coordinated CD4+ and CD8+ T‐cell responses were associated with mild disease, whereas uncoordinated T‐cell responses and a loss of naïve T cells were observed in elderly patients and patients with poor COVID‐19 outcomes. Peripheral blood naïve T cells decrease with age and are believed to be a marker of a reduced capacity of the thymus to maintain a T‐cell pool capable of generating robust immunity to infection (89). Importantly, obesity has been associated with a loss of naive T cells in adults and children, suggesting that impairment in adaptive immunity in individuals with obesity may contribute to COVID‐19 susceptibility (90).
Adipokines such as leptin and adiponectin have been shown to modulate pulmonary inflammation. Elevated leptin levels are associated with increased mortality in patients with ARDS (91). Leptin has also been shown to induce an inflammatory phenotype in murine alveolar macrophages, suggesting that increased leptin in obesity may potentiate pulmonary inflammation in COVID‐19 (92). Adiponectin inhibits IL‐6 expression by murine pulmonary endothelial cells in vitro and reduces lung inflammation in murine ARDS models in vivo (93). Although the potential for adipokines to potentiate pulmonary inflammation in COVID‐19 is attractive, data directly implicating leptin, adiponectin, and other adipokines in COVID‐19 are lacking so far, which may suggest a dominant effect of other unique features of pulmonary physiology in people with obesity.
Treatment Considerations for COVID‐19 for People with Obesity
One important unanswered question is whether weight loss might attenuate risk of severe COVID‐19 disease. Weight loss has been shown to reduce the expression of ACE2 receptor in subcutaneous adipose tissue (94), suggesting a mechanism by which weight loss may decrease viral burden in COVID‐19, assuming that adipose tissue is a reservoir for viral replication. In contrast, despite weight loss, inflammation persists within adipose tissue in formerly obese mice and humans who have had obesity (95, 96), a finding that might be expected to attenuate beneficial effects of weight loss on COVID‐19. A single study has examined predictors of COVID‐19 infection and disease severity in a cohort of patients who had undergone bariatric surgery and found that COVID‐19 correlated with persistent DM and greater weight loss after surgery, and that intensive care unit admission correlated with persistent DM after surgery (97). These data suggest that DM remission may be more important than weight loss per se in modifying COVID‐19 severity.
In terms of existing treatment options for COVD‐19, currently dexamethasone treatment has been the most consistently supported for reducing mortality (98) and is broadly used as an anti‐inflammatory approach. The effect of dexamethasone on attenuating IL‐6 production from monocytes is impaired in people with obesity, suggesting that this strategy may have to be modified based on BMI (99).
The pursuit of a vaccine as a public health measure is crucial in the prevention of SARS‐CoV‐2 infection and spread. Since the preparation of this review, several vaccines have shown high efficacy in prevention of COVID‐19 disease, and applications for Food and Drug Administration emergency‐use authorization are pending. The distribution of vaccines may start as early as late 2020. However, a reduction in response to vaccination for other viral illnesses, such as influenza, is well documented in people with obesity because of impairments in T‐cell memory responses (100). Impaired T‐cell immunity is related to abnormal T‐cell metabolism and generation of an exhausted/senescent T‐cell phenotype in people with obesity and diabetes similar to other states of chronic immune activation (101). The decrease in naïve T cells for people with obesity, which is linked to poor vaccine responses (102), suggests that the wide range of SARS‐CoV‐2 vaccine strategies under investigation may be less robust in people with obesity and that vaccine regimens may have to be modified based on BMI.
Future Directions and Opportunities
The constantly shifting impact of SARS‐CoV‐2 on the health of individuals, communities, and society cannot be overstated. Unfortunately, social distancing measures and the increased time at home needed to address the public health threat of SARS‐CoV‐2 may only worsen the prevalence of obesity, as 22% of survey respondents reported weight gain and increased eating (103). Therefore, we may note an increase in the prevalence of people with obesity as a risk group for severe COVID‐19. In addition, we are just beginning to understand the long‐term implications of COVID‐19 on health and the ways in which mild, moderate, and severe COVID‐19 may impact people with obesity. Therefore, the need to improve COVID‐19 outcomes in people with obesity spans prevention, early symptom management, and acute intensive care settings and provides opportunities for research at many stages that the obesity research community is uniquely positioned to address.
Funding agencies
This work supported by NIH Grants R01DK115190 (RWO, CNL), R01DK090262 (CNL), and Veterans Affairs Grant I01CX001811 (RWO).
Disclosure
The authors declared no conflict of interest.
Contributor Information
Robert W. O’Rourke, Email: rorourke@med.umich.edu.
Carey N. Lumeng, Email: clumeng@umich.edu.
References
- 1. Simonnet A, Chetboun M, Poissy J, et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) requiring invasive mechanical ventilation. Obesity (Silver Spring) 2020;28:1195‐1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Seidu S, Gillies C, Zaccardi F, et al. The impact of obesity on severe disease and mortality in people with SARS‐CoV‐2: a systematic review and meta‐analysis. Endocrinol Diabetes Metab 2020:e00176. doi: 10.1002/edm2.176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID‐19‐related death using OpenSAFELY. Nature 2020;584:430‐436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhu L, She ZG, Cheng X, et al. Association of blood glucose control and outcomes in patients with COVID‐19 and pre‐existing type 2 diabetes. Cell Metab 2020;31:1068‐1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Muniyappa R, Wilkins KJ. Diabetes, obesity, and risk prediction of severe COVID‐19. J Clin Endocrinol Metab 2020;105:dgaa442. doi: 10.1210/clinem/dgaa442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ni YN, Luo J, Yu H, et al. Can body mass index predict clinical outcomes for patients with acute lung injury/acute respiratory distress syndrome? A meta‐analysis. Crit Care 2017;21:36. doi:10.1186/s13054-017-1615-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang H, Lee CC, Chou EH, et al. Mortality association between obesity and pneumonia using a dual restricted cohort model. Obes Res Clin Pract 2019;13:561‐570. [DOI] [PubMed] [Google Scholar]
- 8. Fan E, Beitler JR, Brochard L, et al. COVID‐19‐associated acute respiratory distress syndrome: is a different approach to management warranted? Lancet Respir Med 2020;8:816‐821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li X, Ma X. Acute respiratory failure in COVID‐19: is it “typical” ARDS? Crit Care 2020;24:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gandhi RT, Lynch JB, Del Rio C. Mild or moderate Covid‐19. N Engl J Med 2020;383:1757‐1766. [DOI] [PubMed] [Google Scholar]
- 11. Berlin DA, Gulick RM, Martinez FJ. Severe Covid‐19. N Engl J Med 2020;10:1056. [DOI] [PubMed] [Google Scholar]
- 12. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72,314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020;323:1239‐1242. [DOI] [PubMed] [Google Scholar]
- 13. Zwillich CW, Sutton FD, Pierson DJ, Greagh EM, Weil JV. Decreased hypoxic ventilatory drive in the obesity‐hypoventilation syndrome. Am J Med 1975;59:343‐348. [DOI] [PubMed] [Google Scholar]
- 14. Tobin MJ, Laghi F, Jubran A. Why COVID‐19 silent hypoxemia is baffling to physicians. Am J Respir Crit Care Med 2020;202:356‐360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dixon AE, Peters U. The effect of obesity on lung function. Expert Rev Respir Med 2018;12:755‐767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rivas E, Arismendi E, Agusti A, et al. Ventilation/perfusion distribution abnormalities in morbidly obese subjects before and after bariatric surgery. Chest 2015;147:1127‐1134. [DOI] [PubMed] [Google Scholar]
- 17. Lang M, Som A, Mendoza DP, et al. Hypoxaemia related to COVID‐19: vascular and perfusion abnormalities on dual‐energy CT. Lancet Infect Dis 2020;20:1365‐1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gattinoni L, Chiumello D, Caironi P, et al. COVID‐19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med 2020;46:1099‐1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brower RG, Lanken PN, MacIntyre N, et al. Higher versus lower positive end‐expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med 2004;351:327‐336. [DOI] [PubMed] [Google Scholar]
- 20. Florio G, Ferrari M, Bittner EA, et al. A lung rescue team improves survival in obesity with acute respiratory distress syndrome. Crit Care 2020;24:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid‐19. N Engl J Med 2020;383:120‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Grasselli G, Tonetti T, Protti A, et al. Pathophysiology of COVID‐19‐associated acute respiratory distress syndrome: a multicentre prospective observational study. Lancet Respir Med 2020;8;1201‐1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Willson C, Watanabe M, Tsuji‐Hosokawa A, Makino A. Pulmonary vascular dysfunction in metabolic syndrome. J Physiol 2019;597:1121‐1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shah D, Romero F, Guo Z, et al. Obesity‐induced endoplasmic reticulum stress causes lung endothelial dysfunction and promotes acute lung injury. Am J Respir Cell Mol Biol 2017;57:204‐215. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25. Kulcsar KA, Coleman CM, Beck SE, Frieman MB. Comorbid diabetes results in immune dysregulation and enhanced disease severity following MERS‐CoV infection. JCI Insight 2019;4:e131774. doi: 10.1172/jci.insight.131774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chua RL, Lukassen S, Trump S, et al. COVID‐19 severity correlates with airway epithelium‐immune cell interactions identified by single‐cell analysis. Nat Biotechnol 2020;38:970‐979. [DOI] [PubMed] [Google Scholar]
- 27. Higham A, Singh D. Increased ACE2 expression in bronchial epithelium of COPD patients who are overweight. Obesity (Silver Spring) 2020;28:1586‐1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Klemperer H. Glucose breakdown in chick embryo cells infected with influenza virus. Virology 1961;13:68‐77. [DOI] [PubMed] [Google Scholar]
- 29. Smallwood HS, Duan S, Morfouace M, et al. Targeting metabolic reprogramming by influenza infection for therapeutic intervention. Cell Rep 2017;19:1640‐1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sestan M, Marinovic S, Kavazovic I, et al. Virus‐induced interferon‐gamma causes insulin resistance in skeletal muscle and derails glycemic control in obesity. Immunity 2018;49:164‐177. [DOI] [PubMed] [Google Scholar]
- 31. Harper SA, Bradley JS, Englund JA, et al. Seasonal influenza in adults and children–diagnosis, treatment, chemoprophylaxis, and institutional outbreak management: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis 2009;48:1003‐1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Codo AC, Davanzo GG, Monteiro LB, et al. Elevated glucose levels favor SARS‐CoV‐2 infection and monocyte response through a HIF‐1alpha/glycolysis‐dependent axis. Cell Metab 2020;32:437‐446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bornstein SR, Rubino F, Khunti K, et al. Practical recommendations for the management of diabetes in patients with COVID‐19. Lancet Diabetes Endocrinol 2020;8:546‐550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cariou B, Hadjadj S, Wargny M, et al. Phenotypic characteristics and prognosis of inpatients with COVID‐19 and diabetes: the CORONADO study. Diabetologia 2020;63:1500‐1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Singh AK, Singh R. Is metformin ahead in the race as a repurposed host‐directed therapy for patients with diabetes and COVID‐19? Diabetes Res Clin Pract 2020;165:108268. doi: 10.1016/j.diabres.2020.108268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bramante CT, Ingraham NE, Murray TA, et al. Metformin and risk of mortality in patients hospitalised with COVID-19: a retrospective cohort analysis. Lancet Healthy Longev 2021;2:e34-e41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ciavarella C, Motta I, Valente S, Pasquinelli G. Pharmacological (or synthetic) and nutritional agonists of PPAR‐γ as candidates for cytokine storm modulation in COVID‐19 disease. Molecules 2020;25:2076. doi: 10.3390/molecules25092076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shen B, Yi X, Sun Y, et al. Proteomic and metabolomic characterization of COVID‐19 patient sera. Cell 2020;182:59‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Song JW, Lam SM, Fan X, et al. Omics‐driven systems interrogation of metabolic dysregulation in COVID‐19 pathogenesis. Cell Metab 2020;32:188‐202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kyle JE, Burnum‐Johnson KE, Wendler JP, et al. Plasma lipidome reveals critical illness and recovery from human Ebola virus disease. Proc Natl Acad Sci USA 2019;116:3919‐3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gomez‐Serrano M, Camafeita E, Lopez JA, et al. Differential proteomic and oxidative profiles unveil dysfunctional protein import to adipocyte mitochondria in obesity‐associated aging and diabetes. Redox Biol 2017;11:415‐428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Weiler Miralles CS, Wollinger LM, Marin D, Genro JP, Contini V, Morelo Dal Bosco S. Waist‐to‐height ratio (WHtR) and triglyceride to HDL‐C ratio (TG/HDL‐c) as predictors of cardiometabolic risk. Nutr Hosp 2015;31:2115‐2121. [DOI] [PubMed] [Google Scholar]
- 43. Maile MD, Standiford TJ, Engoren MC, et al. Associations of the plasma lipidome with mortality in the acute respiratory distress syndrome: a longitudinal cohort study. Respir Res 2018;19:60. doi:10.1186/s12931-018-0758-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chew WS, Torta F, Ji S, et al. Large‐scale lipidomics identifies associations between plasma sphingolipids and T2DM incidence. JCI Insight 2019;5:e126925. doi: 10.1172/jci.insight.126925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Law SH, Chan ML, Marathe GK, Parveen F, Chen CH, Ke LY. An updated review of lysophosphatidylcholine metabolism in human diseases. Int J Mol Sci 2019;20:1149. doi:10.3390/ijms20051149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kim M, Yoo HJ, Ko J, Lee JH. Metabolically unhealthy overweight individuals have high lysophosphatide levels, phospholipase activity, and oxidative stress. Clin Nutr 2020;39:1137‐1145. [DOI] [PubMed] [Google Scholar]
- 47. Li L, Yu L, Hou X. Cholesterol‐rich lipid rafts play a critical role in bovine parainfluenza virus type 3 (BPIV3) infection. Res Vet Sci 2017;114:341‐347. [DOI] [PubMed] [Google Scholar]
- 48. Douglas I, Evans S, Smeeth L. Effect of statin treatment on short term mortality after pneumonia episode: cohort study. BMJ 2011;342:d1642. doi: 10.1136/bmj.d1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Katsiki N, Banach M, Mikhailidis DP. Lipid‐lowering therapy and renin‐angiotensin‐aldosterone system inhibitors in the era of the COVID‐19 pandemic. Arch Med Sci 2020;16:485‐489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sturley SL, Rajakumar T, Hammond N, et al. Potential COVID‐19 therapeutics from a rare disease: weaponizing lipid dysregulation to combat viral infectivity. J Lipid Res 2020;61:972‐982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yan B, Chu H, Yang D, et al. Characterization of the lipidomic profile of human coronavirus‐infected cells: implications for lipid metabolism remodeling upon coronavirus replication. Viruses 2019;11:73. doi:10.3390/v11010073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lythgoe MP, Middleton P. Ongoing clinical trials for the management of the COVID‐19 pandemic. Trends Pharmacol Sci 2020;41:363‐382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Iacobellis G, Malavazos AE, Ferreira T. COVID‐19 rise in younger adults with obesity: visceral adiposity can predict the risk. Obesity (Silver Spring) 2020;28:1795. doi:10.1002/oby.22951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Safari S, Keyvani H, Alamdari NM, et al. Abdominal surgery in patients with COVID‐19: detection of SARS‐CoV‐2 in abdominal and adipose tissues. Ann Surg 2020;272:e253‐e256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Nishimura H, Itamura S, Iwasaki T, Kurata T, Tashiro M. Characterization of human influenza A (H5N1) virus infection in mice: neuro‐, pneumo‐ and adipotropic infection. J Gen Virol 2000;81:2503‐2510. [DOI] [PubMed] [Google Scholar]
- 56. Bouwman JJ, Visseren FL, Bouter KP, Diepersloot RJ. Infection‐induced inflammatory response of adipocytes in vitro. Int J Obes (Lond) 2008;32:892‐901. [DOI] [PubMed] [Google Scholar]
- 57. Ayari A, Rosa‐Calatrava M, Lancel S, et al. Influenza infection rewires energy metabolism and induces browning features in adipose cells and tissues. Commun Biol 2020;3:237. doi:10.1038/s42003-020-0965-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gu J, Korteweg C. Pathology and pathogenesis of severe acute respiratory syndrome. Am J Pathol 2007;170:1136‐1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zwezdaryk KJ, Ferris MB, Strong AL, et al. Human cytomegalovirus infection of human adipose‐derived stromal/stem cells restricts differentiation along the adipogenic lineage. Adipocyte 2016;5:53‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Li MY, Li L, Zhang Y, Wang XS. Expression of the SARS‐CoV‐2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty 2020;9:45. doi:10.1186/s40249-020-00662-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Al‐Benna S. Association of high‐level gene expression of ACE2 in adipose tissue with mortality of COVID‐19 infection in obese patients. Obes Med 2020;19:100283. doi: 10.1016/j.obmed.2020.100283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. El‐Sayed Moustafa JS, Jackson AU, Brotman SM, et al. ACE2 expression in adipose tissue is associated with COVID‐19 cardio‐metabolic risk factors and cell type composition. medRxiv. Preprint posted online August 14, 2020. doi: 10.1101/2020.08.11.20171108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Fain JN, Madan AK, Hiler ML, Cheema P, Bahouth SW. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology 2004;145:2273‐2282. [DOI] [PubMed] [Google Scholar]
- 64. de Oliveira M, De Sibio MT, Mathias LS, Rodrigues BM, Sakalem ME, Nogueira CR. Irisin modulates genes associated with severe coronavirus disease (COVID‐19) outcome in human subcutaneous adipocytes cell culture. Mol Cell Endocrinol 2020;515:110917. doi: 10.1016/j.mce.2020.110917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bergallo M, Ferrari L, Faolotto G, et al. Interferon signature in immunosuppressed patients with lower respiratory tract infections: dosage on bronchoalveolar lavage. Minerva Med 2020;111:245‐253. [DOI] [PubMed] [Google Scholar]
- 66. Jin W, Jin W, Pan D. Ifi27 is indispensable for mitochondrial function and browning in adipocytes. Biochem Biophys Res Commun 2018;501:273‐279. [DOI] [PubMed] [Google Scholar]
- 67. Kumari M, Wang X, Lantier L, et al. IRF3 promotes adipose inflammation and insulin resistance and represses browning. J Clin Invest 2016;126:2839‐2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Chen X, Zhao B, Qu Y, et al. Detectable serum severe acute respiratory syndrome coronavirus 2 viral load (RNAemia) is closely correlated with drastically elevated Interleukin 6 level in critically ill patients with coronavirus disease 2019. Clin Infect Dis 2020;71:1937‐1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Herold T, Jurinovic V, Arnreich C, et al. Elevated levels of IL‐6 and CRP predict the need for mechanical ventilation in COVID‐19. J Allergy Clin Immunol 2020;146:128‐136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Park HS, Park JY, Yu R. Relationship of obesity and visceral adiposity with serum concentrations of CRP, TNF‐alpha and IL‐6. Diabetes Res Clin Pract 2005;69:29‐35. [DOI] [PubMed] [Google Scholar]
- 71. Kristof E, Klusoczki A, Veress R, et al. Interleukin‐6 released from differentiating human beige adipocytes improves browning. Exp Cell Res 2019;377:47‐55. [DOI] [PubMed] [Google Scholar]
- 72. Kim IC, Han S. Epicardial adipose tissue: fuel for COVID‐19‐induced cardiac injury? Eur Heart J 2020;41:2334‐2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Deng M, Qi YA‐O, Deng L, et al. Obesity as a potential predictor of disease severity in young COVID‐19 patients: a retrospective study. Obesity (Silver Spring) 2020;28:1815‐1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kruglikov IL, Scherer PE. The role of adipocytes and adipocyte‐like cells in the severity of COVID‐19 infections. Obesity (Silver Spring) 2020;28:1187‐1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Jain M, Budinger GR, Lo A, et al. Leptin promotes fibroproliferative acute respiratory distress syndrome by inhibiting peroxisome proliferator‐activated receptor‐gamma. Am J Respir Crit Care Med 2011;183:1490‐1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Pinky Gupta S, Krishnakumar V, Sharma Y, Dinda AK, Mohanty S. Mesenchymal stem cell derived exosomes: a nano platform for therapeutics and drug delivery in combating COVID‐19. Stem Cell Rev Rep 2021;17:;33‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Tsuchiya A, Takeuchi S, Iwasawa T, et al. Therapeutic potential of mesenchymal stem cells and their exosomes in severe novel coronavirus disease 2019 (COVID‐19) cases. Inflamm Regen 2020;40:14. doi:10.1186/s41232-020-00121-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Hadjadj J, Yatim N, Barnabei L, et al. Impaired type I interferon activity and inflammatory responses in severe COVID‐19 patients. Science 2020;369:718‐724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Bastard P, Rosen LB, Zhang Q, et al. Autoantibodies against type I IFNs in patients with life‐threatening COVID‐19. Science 2020;370:eabd4585. doi: 10.1126/science.abd4585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Zhang Q, Bastard P, Liu Z, et al. Inborn errors of type I IFN immunity in patients with life‐threatening COVID‐19. Science 2020;370:eabd4570. doi: 10.1126/science.abd4570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Teran‐Cabanillas E, Montalvo‐Corral M, Caire‐Juvera G, Moya‐Camarena SY, Hernandez J. Decreased interferon‐alpha and interferon‐beta production in obesity and expression of suppressor of cytokine signaling. Nutrition 2013;29:207‐212. [DOI] [PubMed] [Google Scholar]
- 82. Teran‐Cabanillas E, Hernandez J. Role of leptin and SOCS3 in inhibiting the type I interferon response during obesity. Inflammation 2017;40:58‐67. [DOI] [PubMed] [Google Scholar]
- 83. Liao M, Liu Y, Yuan J, et al. Single‐cell landscape of bronchoalveolar immune cells in patients with COVID‐19. Nat Med 2020;26:842‐844. [DOI] [PubMed] [Google Scholar]
- 84. Silvin A, Chapuis N, Dunsmore G, et al. Elevated calprotectin and abnormal myeloid cell subsets discriminate severe from mild COVID‐19. Cell 2020;182:1401‐1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Mann ER, Menon M, Knight SB, et al. Longitudinal immune profiling reveals key myeloid signatures associated with COVID‐19. Sci Immunol 2020;5:eabd6197. doi: 10.1126/sciimmunol.abd6197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Singer K, DelProposto J, Morris DL, et al. Diet‐induced obesity promotes myelopoiesis in hematopoietic stem cells. Mol Metab 2014;3:664‐675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Zhang JY, Wang XM, Xing X, et al. Single‐cell landscape of immunological responses in patients with COVID‐19. Nat Immunol 2020;21:1107‐1118. [DOI] [PubMed] [Google Scholar]
- 88. Rydyznski Moderbacher C, Ramirez SI, Dan JM, et al. Antigen‐specific adaptive immunity to SARS‐CoV‐2 in acute COVID‐19 and associations with age and disease severity. Cell 2020;183:996‐1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Goronzy JJ, Fang F, Cavanagh MM, Qi Q, Weyand CM. Naive T cell maintenance and function in human aging. J Immunol 2015;194:4073‐4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Mauro C, Smith J, Cucchi D, et al. Obesity‐induced metabolic stress leads to biased effector memory CD4(+) T cell differentiation via PI3K p110delta‐Akt‐mediated signals. Cell Metab 2017;25:593‐609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Ubags ND, Stapleton RD, Vernooy JH, et al. Hyperleptinemia is associated with impaired pulmonary host defense. JCI Insight 2016;1:e82101. doi: 10.1172/jci.insight.82101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Mancuso P, Canetti C, Gottschalk A, Tithof PK, Peters‐Golden M. Leptin augments alveolar macrophage leukotriene synthesis by increasing phospholipase activity and enhancing group IVC iPLA2 (cPLA2gamma) protein expression. Am J Physiol Lung Cell Mol Physiol 2004;287:L497‐L502. doi: 10.1152/ajplung.00010.2004 [DOI] [PubMed] [Google Scholar]
- 93. Konter JM, Parker JL, Baez E, et al. Adiponectin attenuates lipopolysaccharide‐induced acute lung injury through suppression of endothelial cell activation. J Immunol 2012;188:854‐863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Li L, Spranger L, Soll D, et al. Metabolic impact of weight loss induced reduction of adipose ACE‐2 ‐ potential implication in COVID‐19 infections? Metabolism 2020;113:154401. doi: 10.1016/j.metabol.2020.154401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Magkos F, Fraterrigo G, Yoshino J, et al. Effects of moderate and subsequent progressive weight loss on metabolic function and adipose tissue biology in humans with obesity. Cell Metab 2016;23:591‐601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Zamarron BF, Mergian TA, Cho KW, et al. Macrophage proliferation sustains adipose tissue inflammation in formerly obese mice. Diabetes 2017;66:392‐406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Bel Lassen P, Poitou C, Genser L, et al. COVID‐19 and its severity in bariatric surgery operated patients. Obesity (Silver Spring) 2021;29:24‐28. [DOI] [PubMed] [Google Scholar]
- 98. RECOVERY Collaborative Group ; Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with Covid‐19. N Engl J Med. 2021;384:693‐704. doi: 10.1056/NEJMoa2021436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Huang CJ, Acevedo EO, Mari DC, Randazzo C, Shibata Y. Glucocorticoid inhibition of leptin‐ and lipopolysaccharide‐induced interleukin‐6 production in obesity. Brain Behav Immun 2014;35:163‐168. [DOI] [PubMed] [Google Scholar]
- 100. Sheridan PA, Paich HA, Handy J, et al. Obesity is associated with impaired immune response to influenza vaccination in humans. Int J Obes (Lond) 2012;36:1072‐1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Yi H‐S, Kim SY, Kim JT, et al. T‐cell senescence contributes to abnormal glucose homeostasis in humans and mice. Cell Death Dis 2019;10:249. doi:10.1038/s41419-019-1494-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Čičin‐Šain L, Smyk‐Paerson S, Currier N, et al. Loss of naive T cells and repertoire constriction predict poor response to vaccination in old primates. J Immunol 2010;184:6739‐6745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Zachary Z, Brianna F, Brianna L, et al. Self‐quarantine and weight gain related risk factors during the COVID‐19 pandemic. Obes Res Clin Pract 2020;14:210‐216. [DOI] [PMC free article] [PubMed] [Google Scholar]


