Case report
This 17‐year‐old male initially presented to the Accident and Emergency department with a 3‐week history of tender, cervical lymphadenopathy, parotid gland enlargement and fevers. In the weeks preceding his hospital attendance he reported poor appetite, weight loss and fatigue. He had no known allergies, took no regular medications and was fully up to date with all his childhood vaccinations. On direct questioning, the patient reported some preceding loss of smell, approximately 2 months previously. There was no history of any respiratory symptoms. He was attending school and was a keen athlete. He had been given three courses of antibiotics to treat any co‐existing bacterial infection with no improvement and was using ibuprofen/paracetamol, as needed, for the fevers.
Blood tests confirmed a bicytopenia (Plts 61 × 109/l, neutrophils 0·5 × 109/l), erythrocyte sedimentation rate 6 mm/h, lactate dehydrogenase 782 U/l, and c‐reactive protein < 4 mg/ml. He had a transaminitis (alanine aminotransferase (ALT) 303 U/L). A glandular fever screen was negative (Epstein‐Barr virus IgG and IgM and cytomegalovirus IgG and IgM all negative). HIV serology was negative. Mumps polymerase chain reaction detected no viral RNA, but IgG antibody was detected. Toxoplasma IgG antibody was not detected.
Chest X‐ray showed no mediastinal widening and the patient’s lungs and pleural spaces were clear, with normal cardiomediastinal contours. Cervical ultrasound showed multiple, pathologically enlarged left‐sided neck lymph nodes from levels I to V. The largest individual lymph node was located within level V, measuring 1·3 cm in the short axis dimension. There was also an enlarged left intraparotid lymph node measuring 1.1 cm. The left parotid gland demonstrated a heterogeneous, pseudonodular echo pattern, suggestive of coexistent parotid inflammation. The right‐sided neck lymph nodes were normal; the appearance of the submandibular, right parotid and thyroid glands were unremarkable.
Abdominal ultrasound found no hepatomegaly or splenomegaly, with unremarkable kidneys, pancreas, gallbladder, and urinary bladder.
Multiple 18‐gauge core needle biopsies were undertaken of a representative left‐sided level V lymph node. On analysis, this showed a necrotising lymphadenitis with no follicular hyperplasia, atypia or malignancy; no lymphoid malignancy was detected. Special stains performed on the biopsy showed no evidence of acid‐fast bacilli or fungi. The biopsy findings, in conjunction with the clinical presentation and leukopenia, were in keeping with kikuchi‐fujimoto disease (KFD) (Figs 1 and 2).
Given the history of anosmia, a SARS‐COV‐2 (COVID‐19) RNA swab was arranged, which was negative, but a serum SARS‐COV‐2‐IgG antibody test was positive, consistent with previous exposure to COVID‐19.
On a subsequent clinic review the patient was improving with no further fevers or night sweats. The lymph nodes were reducing in size and there was no ongoing parotid swelling. His blood test results showed improvement in the cytopenias (neutrophils 1·9 × 109/l, platelets 116 × 109/l) and transaminitis (ALT 65 U/l). He had no personal or family history of autoimmune conditions. Anti‐nuclear antibodies and rheumatoid factor were negative, and a liver autoimmune profile was negative. His sense of smell has still not returned to date.
Fig 1.
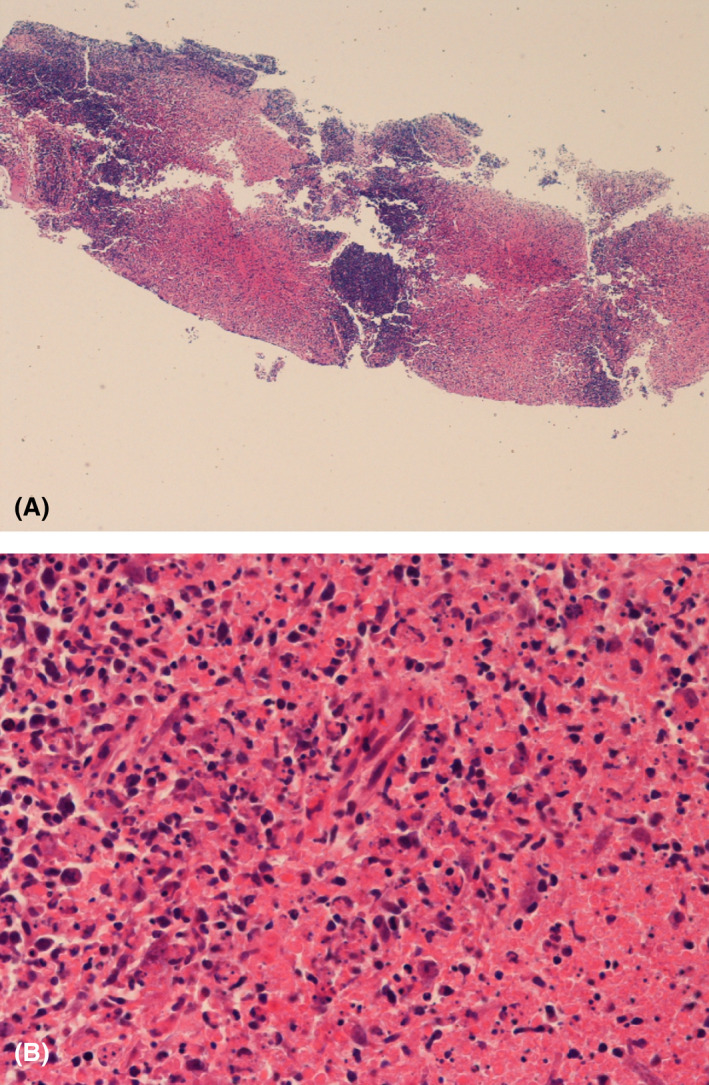
(A) Haemotoxylin and Eosin (H&E) stain (40× magnification) showing zones of necrosis. (B) H&E (400× magnification) of necrotic area demonstrating absence of neutrophils and crescentic histiocytes.
Fig 2.
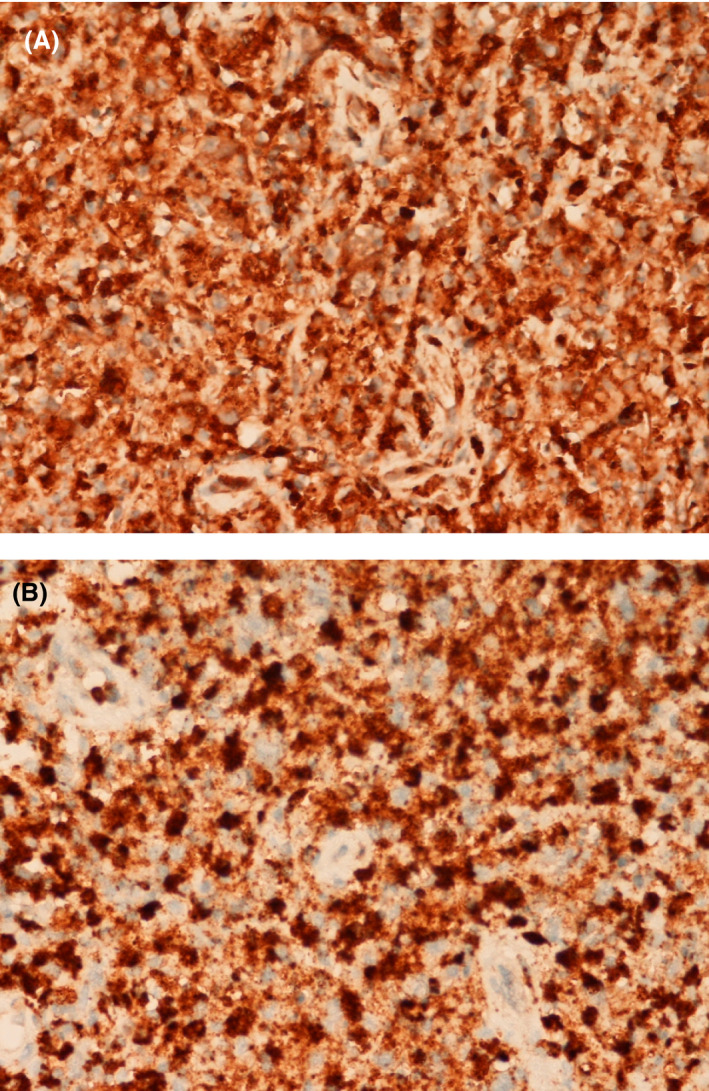
(A) CD68 (400× magnification) highlighting histiocytes. (B) Histiocytes showing expression of myeloperoxidase (400× magnification).
Discussion
Kikuchi‐Fujimoto disease, otherwise known as histiocytic necrotising lymphadenitis, is a rare condition that usually presents with a typical pattern of general flu‐like symptoms with posterior cervical, tender lymphadenopathy. 1 Classic examples will display blood results consistent with a low neutrophil count, with a raised erythrocyte sedimentation rate and raised C‐reactive protein. 2 In this case, there was moderate neutropenia, but normal inflammatory markers at the time of presentation. Imaging is often non‐specific with computed tomography findings often showing multiple affected lymph nodes with homogeneity and perinodal infiltration. 3
The aetiology of KFD is a subject of debate. Although multiple viruses have been linked to KFD, none have been found to be consistently linked to the condition. 4 One paper found 55 cases of KFD occurring in association with connective tissue disease (most frequently this was systemic lupus erythematosus). 5 There is also an association between human leukocyte antigen class II genes and KFD. 6 There can be difficulties in differentiating KFD from tuberculosis as the presenting features have much overlap. 7
Diagnosis remains reliant on histological analysis of a lymph node biopsy. Lymph node biopsy typically shows sheets of non‐suppurative, non‐caseating necrosis, karyorrhectic nuclear debris and proliferating mononuclear cells, including histiocytes (many with characteristic crescentic nuclei), lymphoid cells and plasmacytoid monocytes. 8 The histiocytes in KFD characteristically co‐express CD68 and myeloperoxidase (MPO), whilst plasmacytoid monocytes are CD68+ MPO‐. 9
COVID‐19 has been associated with other inflammatory conditions, including multisystem inflammatory syndrome in children (MIS‐C); however, there is a poor understanding of the mechanism behind this response currently. 10 COVID‐19 has also been implicated in immunological complications, such as macrophage activation syndrome and cytokine storm syndrome, which has led to the wider use of anti‐inflammatory treatments. 11
This case highlights an unusual presentation of COVID‐19 in a young patient. Although there is no conclusive proof that KFD was triggered by COVID‐19 in this case, the temporal link suggests an association between the virus and this rare disease. As far as we are aware, there are no previous recorded cases of KFD associated with COVID‐19 infection. Further work is needed to understand the possible correlation between this virus and KFD.
References
- 1. Veer V, Lim A, Issing W. Kikuchi‐Fujimoto disease: a case report and literature review. Case Rep Otolaryngol. 2012;2012:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bosch X, Guilabert A, Miquel R, Campo E. Enigmatic Kikuchi‐Fujimoto disease: a comprehensive review. Am J Clin Pathol. 2004;122(1):141–52. [DOI] [PubMed] [Google Scholar]
- 3. Kwon SY, Kim TK, Kim YS, Lee KY, Lee NJ, Seol HY. CT findings in Kikuchi disease: analysis of 96 cases. Am J Neuroradiol. 2004;25(6):1099–102. [PMC free article] [PubMed] [Google Scholar]
- 4. Hollingsworth HC, Peiper SC, Weiss LM, Raffeld M, Jaffe ES. An investigation of the viral pathogenesis of Kikuchi‐Fujimoto disease: lack of evidence for Epstein‐Barr virus or human herpesvirus type 6 as the causative agents. Arch Pathol Lab Med. 1994;118(2):134–40. [PubMed] [Google Scholar]
- 5. Sharma V, Rankin R. Fatal Kikuchi‐like lymphadenitis associated with connective tissue disease: a report of two cases and review of the literature. Springerplus. 2015;4:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tanaka T, Ohmori M, Yasunaga S, Ohshima K, Kikuchi M, Sasazuki T. DNA typing of HLA class II genes (HLA‐DR, ‐DQ and ‐DP) in Japanese patients with histiocytic necrotizing lymphadenitis (Kikuchi’s disease). Tissue Antigens. 1999;54(3):246–53. [DOI] [PubMed] [Google Scholar]
- 7. Nayak HK, Mohanty PK, Mallick S, Bagchi A. Diagnostic dilemma: Kikuchi’s disease or tuberculosis? BMJ Case Rep. 2013;29:2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pilichowska M, Pinkus JL, Pinkus GS. Histiocytic necrotizing lymphadenitis (Kikuchi‐Fujimoto Disease): lesional cells exhibit an immature dendritic cell phenotype. Am J Clin Pathol. 2009;131(2):174–82. [DOI] [PubMed] [Google Scholar]
- 9. Pileri SA, Facchetti F, Ascani S, Sabattini E, Poggi S, Piccioli M, et al. Myeloperoxidase expression by histiocytes in Kikuchi's and Kikuchi‐like lymphadenopathy. Am J Pathol. 2001;159(3):915–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jiang L, Tang K, Levin M, Irfan O, Morris S, Wilson K, et al. COVID‐19 and multisystem inflammatory syndrome in children and adolescents. Lancet Infect Dis. 2020;20(11):e276–e288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Soy M, Keser G, Atagündüz P, Tabak F, Atagündüz I, Kayhan S. Cytokine storm in COVID‐19: pathogenesis and overview of anti‐inflammatory agents used in treatment. Clin Rheumatol. 2020;9(7):2085–94. [DOI] [PMC free article] [PubMed] [Google Scholar]


