Abstract
Aim
To assess predictors of in‐hospital mortality in people with prediabetes and diabetes hospitalized for COVID‐19 infection and to develop a risk score for identifying those at the greatest risk of a fatal outcome.
Materials and Methods
A combined prospective and retrospective, multicentre, cohort study was conducted at 10 sites in Austria in 247 people with diabetes or newly diagnosed prediabetes who were hospitalized with COVID‐19. The primary outcome was in‐hospital mortality and the predictor variables upon admission included clinical data, co‐morbidities of diabetes or laboratory data. Logistic regression analyses were performed to identify significant predictors and to develop a risk score for in‐hospital mortality.
Results
The mean age of people hospitalized (n = 238) for COVID‐19 was 71.1 ± 12.9 years, 63.6% were males, 75.6% had type 2 diabetes, 4.6% had type 1 diabetes and 19.8% had prediabetes. The mean duration of hospital stay was 18 ± 16 days, 23.9% required ventilation therapy and 24.4% died in the hospital. The mortality rate in people with diabetes was numerically higher (26.7%) compared with those with prediabetes (14.9%) but without statistical significance (P = .128). A score including age, arterial occlusive disease, C‐reactive protein, estimated glomerular filtration rate and aspartate aminotransferase levels at admission predicted in‐hospital mortality with a C‐statistic of 0.889 (95% CI: 0.837‐0.941) and calibration of 1.000 (P = .909).
Conclusions
The in‐hospital mortality for COVID‐19 was high in people with diabetes but not significantly different to the risk in people with prediabetes. A risk score using five routinely available patient variables showed excellent predictive performance for assessing in‐hospital mortality.
Keywords: coronavirus infection, diabetes, prediabetic state
1. INTRODUCTION
Following the emergence of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) at the end of 2019 in Wuhan, China, COVID‐19 1 disease has rapidly spread across the world, achieving pandemic status.
Initial reports from China, 2 , 3 followed by the United States 4 and Europe, 5 showed that the prevalence of diabetes was as high as 20% in people hospitalized with COVID‐19. Moreover, the epidemiological data also suggested that diabetes is more frequent in people experiencing adverse clinical outcomes. 6 The prevalence of diabetes was high in people experiencing severe disease, and other studies showed higher mortality rates in people with diabetes compared with non‐diabetic cohorts. 7 In addition, another study also highlighted the high prevalence of prediabetes in people experiencing severe COVID‐19 disease. 8
Previous research has also shown that people with diabetes face an increased risk of infections, which can potentially be explained by impaired phagocytosis via neutrophils, macrophages and monocytes, impaired neutrophil chemotaxis and bactericidal activity, as well as impaired innate cell‐mediated immunity. 9 , 10 Although some observational studies suggest that good glycaemic control may accompany a reduced risk of infectious disease, there is still debate concerning this matter in the literature. 9
During the COVID‐19 lockdown phases across various countries, the question arose regarding those population groups that are at a particularly high risk of severe COVID‐19 episodes or death because they require special protection; once affected by the disease, rapid risk stratification in people with disturbed glucose metabolism is critical for planning further therapy, as well as studies investigating novel treatment approaches. Given the high prevalence of diabetes in COVID‐19, everyone with diabetes was initially considered to be part of a high‐risk population. However, while further research showed an independent impact of diabetes on outcomes in people with SARS‐CoV2 infection, 7 it became evident that age and co‐morbidities play major roles in unfavourable outcomes. 11
To untangle the contribution of diabetes by itself from associated co‐morbidities, the Austrian Diabetes Association initiated a COVID‐19 registry in people with diabetes or prediabetes with the aim of identifying those individuals at the greatest risk of lethal disease outcomes when hospitalized with SARS‐CoV‐2 infection. The objective of the current study is to analyse fatality rates in people with diabetes or prediabetes hospitalized for COVID‐19 in Austria and to develop an easily applicable score with which to identify those people at the highest risk of a fatal outcome within this patient population.
2. MATERIALS AND METHODS
2.1. Study design and population
We initiated a combined prospective and retrospective, multicentre, non‐interventional cohort study at 10 hospital sites in Austria to collect information on the characteristics of people with diabetes and confirmed SARS‐CoV‐2 infection. Nationwide ethics approval was obtained from the Ethics Committee of the Medical University of Graz, Austria (EK 32–355 ex 19/20). The study sponsor was the Austrian Diabetes Association. The study started on 15 April 2020; for this analysis we utilized data entered up to 30 June 2020.
Where possible, written informed consent was collected from living patients to participate in this study. Patients who were unable to give their consent before discharge were contacted later for permission to use their clinical data. For patients who had deceased before providing consent, or where consenting to the trial was not feasible, then the ethics committee waived the requirement for informed consent; in these cases anonymized, retrospective data were used and included in the analysis, because otherwise the data would have been biased by potentially false low mortality rates.
2.2. Study participants or inclusion criteria
People aged 18 years or older with a confirmed positive throat swab for SARS‐CoV‐2 and a confirmed diagnosis of type 1 diabetes, type 2 diabetes or prediabetes were included in the registry (either known or newly diagnosed). For this analysis we only included those hospitalized with COVID‐19. Diabetes was diagnosed according to the Austrian Diabetes Association. 12 Prediabetes was defined as an HbA1c of 5.7%‐6.4% (39‐46 mmol/mol). 12 HbA1c was measured in case increased glucose values were evident in people without known diabetes. No specific exclusion criteria were defined.
2.3. Data collection
Data collection was performed by clinical physicians and study coordinators at 10 participating centres across Austria. Data were collected from their medical files and the clinical laboratory.
This study captured and processed data using an electronic case report form developed with HybridForms, as part of a validated electronic data capture system with an audit trail and controlled levels of access (Kapsch BusinessCom and Icomedias, Graz, Austria).
2.4. Study variables
2.4.1. Outcome
The primary outcome of this study was in‐hospital mortality in patients with diabetes and confirmed diagnosis of COVID‐19.
2.4.2. Predictors
Demographic information, clinical characteristics and laboratory findings were collected from the medical record systems of the participating centres. Demographic data consisted of information regarding age and gender. Clinical characteristics included the classification of diabetes, duration of diabetes, microvascular (diabetic retinopathy and diabetic kidney disease) and macrovascular disease (stroke, myocardial infarction, chronic heart disease, arterial occlusive disease [i.e. cerebrovascular or peripheral artery disease]), as well as other co‐morbidities of interest (autoimmune disease, cancer, respiratory disease, liver disease, transplantation) and vital signs. Furthermore, current therapy to regulate blood pressure, blood sugar, blood lipids, immunity and pain were recorded. Laboratory data, available from the local laboratory at the clinical site, included HbA1c, fasting glucose, leucocytes, haemoglobin, estimated glomerular filtration rate (eGFR; the Modification of Diet in Renal Disease equation was used at three sites, and at the other sites the Chronic Kidney Disease Epidemiology Collaboration formula was used), high sensitive C‐reactive protein (CRP), inflammatory markers, liver function tests, lipid status, procalcitonin, ferritin, interleukine‐6, n‐terminal pro brain natriuretic peptide (NT‐proBNP) and troponin T. The variables recorded in the registry are those taken upon hospital admission.
2.5. Statistical analyses
All statistical analyses were performed in Stata 16.1 (StataCorp, TX, USA). Qualitative variables are presented as frequency and percentage (%) and quantitative variables as mean ± standard deviation (SD) or median and interquartile range (IQR) as appropriate. Chi‐square or Fischer exact tests were performed to compare qualitative variables and unpaired t‐tests or Mann–Whitney U tests to compare normal and non‐normal quantitative variables. A P‐value of less than .05 was considered statistically significant.
2.6. Derivation of the risk model
Logistic regression was applied to derive the risk model. The candidate predictors for the model were selected based on their clinical relevance, absence (predictors with <20% of missing data were selected), and a P‐value of .20 or less in the univariate logistic regression analysis. The stepwise backward elimination method was applied on candidate predictors to identify predictors for developing the final model. Only those predictors with P‐values of .10 or less were retained in the final model. In addition, the interaction effects of various predictors were evaluated in the model.
The risk equation was derived from the final model to predict the log‐odds of in‐hospital mortality of COVID‐19 by adding the product of the constant (β0) to the product of β coefficients and the values of each predictor included in the model. The resulting log‐odds were then converted to the probability value of in‐hospital mortality.
2.7. Performance of the risk model
The predictive performance of the risk model was assessed in terms of discrimination and calibration. Discrimination was assessed by calculating the C statistics and calibration was assessed by performing the Hosmer–Lameshaw goodness‐of‐fit test and fitting calibration plots of observed versus expected probability of the in‐hospital mortality.
2.8. Validation of the risk model
The bootstrap method was used for internal validation of the risk model. The bootstrap samples (n = 1000) were drawn from the whole derivation cohort and the model was developed in each bootstrap sample, adopting the same methodology of the derivation cohort and using the Stata swboot package. The predictive performance of the risk model was evaluated on bootstrap samples then on the derivation cohort to achieve optimism‐corrected estimates of the performance of the risk model.
2.9. Development of the nomogram
After generating and validating the risk model, a nomogram was generated from the multivariable logistic regression model using the Stata nomolog package.
3. RESULTS
3.1. Demographics and clinical characteristics
In total, 247 people with diabetes or prediabetes who had tested positive for SARS‐CoV‐2 in the participating hospitals were recorded in the registry; 238 were admitted to inpatient wards and constitute the current analysis set.
The mean age of participants was 71.1 ± 12.9 years and 152 were male (63.9%); 75.6% (n = 180) had established type 2 diabetes, 4.6% (n = 11) had type 1 diabetes and 19.8% (n = 47) had prediabetes (Table 1). People with prediabetes were older compared with people with established diabetes (71.9 ± 12.5 vs. 67.6 ± 14.0 years; P = .043) (Table 2). Median HbA1c upon admission was 5.9% (IQR 0.3%) and median fasting glucose was 107 (IQR 93) mg/dL in people with prediabetes.
TABLE 1.
Comparison and unadjusted odds ratios (95% confidence interval) of characteristics, anthropometric indices, co‐morbidities, medications and laboratory variables with in‐hospital mortality in patients hospitalized with COVID‐19
| Characteristics | All | In‐hospital mortality | ||||
|---|---|---|---|---|---|---|
| N | Statistics | Yes | No | Unadjusted OR (95% CI) | P‐value | |
| All, n (%) | 238 | – | 58 (24.4) | 180 (76.6) | – | ‐ |
| Characteristics | ||||||
| Age – years, mean ± SD | 238 | 71.1 ± 12.9 | 79.8 ± 8.8 | 68.3 ± 12.8 | 1.66 (1.38‐1.98) a | <.001 |
| Sex, n (%) | 238 | |||||
| Male | 152 (63.9) | 36 (62.1) | 116 (64.4) | 1 | ||
| Female | 86 (36.1) | 22 (37.9) | 64 (35.6) | 1.10 (0.56‐2.16) | .787 | |
| Smoking status, n (%) | 238 | |||||
| Non‐smoker | 196 (82.3) | 46 (79.3) | 150 (83.3) | 1 | ||
| Former smoker | 38 (16.0) | 12 (20.7) | 26 (14.4) | 1.30 (0.62‐2.75) | .485 | |
| Current smoker | 4 (1.7) | 0 (0.0) | 4 (2.2) | |||
| Body mass index – kg/m2, mean ± SD | 114 | 29.1 ± 5.7 | 29.2 ± 5.8 | 29.0 ± 5.7 | 1.01 (0.94‐1.08) | .847 |
| Vital signs | ||||||
| Systolic BP – mmHg, mean ± SD | 154 | 132.8 ± 21.9 | 134.1 ± 25.5 | 132.4 ± 20.5 | 1.02 (0.94‐1.10) b | .670 |
| Diastolic BP – mmHg, mean ± SD | 154 | 76.6 ± 16.2 | 77.1 ± 21.9 | 76.4 ± 13.6 | 1.01 (0.91‐1.03) b | .790 |
| Pulse – beats/min, mean ± SD | 137 | 87.3 ± 17.4 | 88.2 ± 20.9 | 87.0 ± 16.1 | 1.02 (0.92‐1.14) c | . 717 |
| Diabetes | ||||||
| Type of diabetes, n (%) | 238 | |||||
| Prediabetes | 47 (19.8) | 7 (12.1) | 40 (22.2) | 1 | ||
| Type 1 diabetes | 11 (4.6) | 1 (1.7) | 10 (5.6) | 0.46 (0.44‐4.90) | .522 | |
| Type 2 diabetes | 180 (75.6) | 50 (86.2) | 130 (72.2) | 1.85 (0.66‐5.16) | .240 | |
| Co‐morbidities | ||||||
| Hypertension, n (%) | 238 | 169 (71.0) | 50 (86.2) | 119 (66.1) | 2.93 (1.25‐6.90) | .014 |
| CHD, n (%) | 238 | 63 (26.5) | 23 (39.7 | 40 (22.2) | 2.14 (1.08‐4.23) | .028 |
| Myocardial infarction, n (%) | 238 | 29 (12.2) | 12 (20.7) | 17 (9.4) | 2.12 (0.87‐5.13) | .097 |
| Heart failure, n (%) | 238 | 30 (12.6) | 15 (25.9) | 15 (8.3) | 3.87 (1.64‐9.13) | .002 |
| Arterial occlusive disease, n (%) | 238 | 38 (16.0) | 18 (31.0) | 20 (11.1) | 4.08 (1.79‐9.29) | .001 |
| Stroke, n (%) | 238 | 20 (8.4) | 8 (13.8) | 12 (6.7) | 2.58 (0.91‐7.31) | .074 |
| Chronic kidney disease, n (%) | 238 | 55 (23.1) | 24 (41.4) | 31 (17.2) | 3.18 (1.55‐6.54) | .002 |
| Cancer, n (%) | 238 | 37 (15.5) | 12 (20.7) | 25 (13.9) | 1.32 (0.58‐2.99) | .503 |
| Respiratory disease, n (%) | 238 | 48 (20.2) | 15 (25.9) | 33 (18.3) | 1.40 (0.65‐3.01) | .387 |
| Liver disease, n (%) | 238 | 12 (5.0) | 6 (10.3) | 6 (3.3) | 5.11 (1.32‐19.70) | .018 |
| Medication | ||||||
| Insulin, n (%) | 238 | 52 (21.9) | 12(20.7) | 40 (22.2) | 0.83 (0.37‐1.82) | .636 |
| Other glucose‐lowering drugs, n (%) | 238 | 111 (46.6) | 31 (53.5) | 80 (44.4) | 1.39 (0.71‐2.70) | .338 |
| Metformin, n (%) | 238 | 77 (32.3) | 14 (24.1) | 63 (35.0) | 0.55 (0.26‐1.14) | .111 |
| Sulphonylurea, n (%) | 238 | 14 (5.9) | 6 (10.3) | 8 (4.4) | 2.13 (0.63‐7.15) | .220 |
| DPP‐4 inhibitors, n (%) | 238 | 42 (17.7) | 15 (25.9) | 27 (15.0) | 1.84 (0.85‐3.97) | .121 |
| SGLT‐2 inhibitors, n (%) | 238 | 24 (10.1) | 3 (5.2) | 21 (11.7) | 0.38 (0.10‐1.46) | .158 |
| GLP‐1 agonists, n (%) | 238 | 3 (1.3) | 0 (0.0) | 3 (1.7) | ||
| Antihypertensive drugs, n (%) | 238 | 169 (71.0) | 46 (79.3) | 125 (69.4) | 1.49 (0.70‐.20) | .303 |
| ACE inhibitors, n (%) | 238 | 72 (30.3) | 21 (36.2) | 51 (28.3) | 1.47 (0.75‐2.88) | .262 |
| AR blockers, n (%) | 238 | 49 (20.6) | 10 (17.2) | 39 (21.7) | 0.66 (0.29‐1.50) | .319 |
| Beta blockers, n (%) | 238 | 95 (39.9) | 30 (51.7) | 65 (36.1) | 1.81 (0.95‐3.45) | .071 |
| Ca+ channel blockers, n (%) | 238 | 73 (30.7) | 18 (31.0) | 55 (30.6) | 1.09 (0.55‐2.15) | .811 |
| Central antihypertensive drugs, n (%) | 238 | 10 (4.2) | 3 (5.2) | 7 (3.9) | 1.65 (0.38‐7.02) | .501 |
| Thiazide diuretics, n (%) | 238 | 36 (15.1) | 6 (10.3) | 30 (16.7) | 0.58 (0.23‐1.46) | .247 |
| Loop diuretics, n (%) | 238 | 38 (16.0) | 15 (25.9) | 23 (12.8) | 2.38 (1.14‐4.95) | .020 |
| Mineralocorticoid receptor blockers, n (%) | 238 | 20 (8.4) | 7 (12.1) | 13 (7.2) | 4.47 (1.11‐17.89) | .034 |
| Sacubitril, n (%) | 238 | 3 (1.3) | 1 (1.7) | 2 (1.1) | 1.56 (0.14‐17.54) | .718 |
| Glucocorticoid therapy, n (%) | 238 | 10 (4.2) | 5 (8.6) | 5 (2.8) | 3.30 (0.92‐11.84) | .067 |
| Immuno‐suppressive therapy, n (%) | 238 | 2 (0.8) | 0 (0.0) | 2 (1.1) | 0.61 (0.03‐12.89) | .751 |
| Oral anticoagulants, n (%) | 238 | 32 (13.5) | 12 (20.7) | 20 (11.1) | 1.64 (0.77‐3.47) | .198 |
| Non‐vitamin K oral anticoagulants, n (%) | 238 | 30 (12.6) | 7 (12.1) | 23 (12.8) | 0.94 (038‐2.31) | .888 |
| Ibuprofen therapy, n (%) | 238 | 3 (1.3) | 1 (1.7) | 2 (1.1) | 1.56 (0.14‐17.54) | .718 |
| Laboratory variables | ||||||
| Leukocytes – 109/L, mean ± SD | 236 | 7.2 ± 3.2 | 8.1 ± 4.3 | 6.9 ± 2.7 | 1.07 (0.97‐1.18) | .178 |
| Haemoglobin – mg/dL, mean ± SD | 238 | 13.3 ± 2.2 | 12.9 ± 1.9 | 13.4 ± 2.3 | 0.90 (0.76‐1.07) | .225 |
| eGFR – mL/min/1.73m2, mean ± SD | 233 | 66.3 ± 26.9 | 45.0 ± 21.2 | 73.1 ± 25.0 | 0.95 (0.94‐0.97) | <.001 |
| Lactate dehydrogenase – U/L, mean ± SD | 215 | 331.3 ± 188.9 | 330.4 ± 169.2 | 331.6 ± 194.9 | 0.89 (0.39‐2.06) | .786 |
| AST – U/L, median (IQR) | 220 | 37.5 (27.5) | 40.0 (31.0) | 37.0 (26.0) | 1.01 (1.00‐1.02) | .029 |
| ALT – U/L, median (IQR) | 233 | 29.0 (23.0) | 27.0 (23.0) | 31.5 (23.0) | 0.80 (0.48‐1.34) | .396 |
| CRP – mg/dL, median (IQR) | 232 | 9.9 (14.8) | 16.5 (57.9) | 9.0 (11.6) | 1.35 (1.07‐1.71) | .012 |
| Ferritin – ng/mL, median (IQR) | 193 | 572.0 (938.0) | 590.0 (897.0) | 568.0 (1019.0) | 0.99 (0.88‐1.10) | .792 |
| Procalcitonin – ng/mL, median (IQR) | 153 | 0.1 (0.2) | 0.3 (0.5) | 0.1 (0.1) | 1.37 (1.06‐1.76) | .016 |
| IL6 – pg/mL, median (IQR) | 169 | 50.6 (75.8) | 90.5 (149.4) | 41.8 (57.4) | 2.27 (1.37‐3.75) | .001 |
| Fasting plasma glucose – mg/dL, median (IQR) | 187 | 127.0 (83.0) | 148.5 (63.0) | 121.0 (88.0) | 0.99 (0.99‐1.01) | .464 |
| HbA1c – %, median (IQR) | 174 | 6.4 (1.4) | 6.6 (0.8) | 6.4 (1.5) | 0.92 (0.69‐1.22) | .554 |
| HDL cholesterol – mg/dL, mean ± SD | 124 | 34.1 ± 13.5 | 31.9 ± 14.1 | 34.8 ± 11.7 | 0.98 (0.94‐1.02) | .312 |
| LDL cholesterol – mg/dL, mean ± SD | 123 | 72.4 ± 30.8 | 65.1 ± 31.6 | 74.8 ± 30.3 | 0.98 (0.97‐1.00) | .076 |
| Triglycerides – mg/dL, median (IQR) | 135 | 116.0 (62.0) | 118.0 (66.0) | 115.0 (63.0) | 1.00 (0.99‐1.01) | .354 |
| NT‐proBNP – pg/mL, median (IQR) | 86 | 542.0 (1408.0) | 1250.0 (2646.0) | 253.0 (471.0) | 1.97 (1.25‐3.08) | .003 |
| TroponinT – pg/mL, median (IQR) | 126 | 20.0 (32.0) | 42.0 (52.0) | 16.0 (21.0) | 3.63 (1.60‐8.24) | .002 |
Abbreviations: ACE, angiotensin‐converting enzyme; ALT, alanine aminotransferase; AST, aspartate aminotransferase; AR, angiotensin receptor; BP, blood pressure; CI, confidence interval; CHD, coronary heart disease; CRP, C‐reactive protein; HDL, high density lipoprotein; DPP‐4, dipeptidyl peptidase‐4; eGFR, estimated glomerular filtration rate; GLP‐1, glucagon‐like peptide‐1; IL6, interleukin 6; LDL, low density lipoprotein; NT‐proBNP, n‐terminal pro brain natriuretic peptide; OR, odds ratio; SGLT‐2, sodium‐dependent glucose co‐transporter‐2.
odds for change of 5 years.
odds for change of 5 mmHg.
odds for change of 5 beats/min.
TABLE 2.
Comparison of characteristics, anthropometric indices, co‐morbidities and laboratory variables by diabetes and prediabetes in patients hospitalized with COVID‐19
| Variables | N | Prediabetes | Diabetes | P‐value |
|---|---|---|---|---|
| All, n (%) | 238 | 47 (19.7) | 191 (80.3) | – |
| Characteristics | ||||
| Age – years, mean ± SD | 238 | 71.9 ± 12.5 | 67.6 ± 14.0 | .043 |
| Sex, n (%) | 238 | |||
| Male | 47 | 35 (74.5) | 117 (61.3) | |
| Female | 191 | 12 (25.5) | 74 (38.7) | .126 |
| Body mass index – kg/m2, mean ± SD | 114 | 26.4 ± 4.3 | 29.4 ± 5.7 | .104 |
| Vital signs | ||||
| Systolic BP – mmHg, mean ± SD | 154 | 139 ± 21 | 132 ± 22 | .334 |
| Diastolic BP – mmHg, mean ± SD | 154 | 79 ± 13 | 76 ± 16 | .617 |
| Pulse – beats/min, mean ± SD | 137 | 84 ± 11 | 88 ± 18 | .469 |
| Co‐morbidities | ||||
| Hypertension, n (%) | 238 | 22 (46.8) | 147 (77.0) | <.001 |
| CHD, n (%) | 238 | 3 (6.4) | 60 (31.4) | <.001 |
| Myocardial infarction, n (%) | 238 | 0 (0.0) | 29 (15.2) | .002 |
| Heart failure, n (%) | 238 | 2 (4.3) | 28 (14.7) | .082 |
| Arterial occlusive disease, n (%) | 238 | 8 (17.0) | 41 (21.5) | .553 |
| Stroke, n (%) | 238 | 5 (10.6) | 15 (7.9) | .559 |
| Chronic kidney disease, n (%) | 238 | 6 (12.8) | 49 (25.7) | .081 |
| Cancer, n (%) | 238 | 4 (8.5) | 33 (17.3) | .179 |
| Respiratory disease, n (%) | 238 | 5 (10.6) | 43 (22.5) | .103 |
| Liver disease, n (%) | 238 | 0 (0.0) | 12 (6.3) | .131 |
| Artificial respiration, n (%) | 238 | 7 (14.9) | 50 (26.2) | .128 |
| ICU admission, n (%) | 238 | 8 (17.0) | 51 (26.7) | .191 |
| In‐hospital death, n (%) | 238 | 7 (14.9) | 51 (26.7) | .128 |
| Laboratory variables | ||||
| Fasting plasma glucose – mg/dL, median (IQR) | 187 | 107 (93.0) | 149 (91.0) | <.001 |
| HbA1c – %, median (IQR) | 174 | 5.9 (0.3) | 6.7 (1.9) | <.001 |
Abbreviations: BP, blood pressure; CHD, coronary heart disease; ICU, intensive care unit; IQR, interquartile range.
The median duration of hospital stay was 12 (IQR 14) days, whereby almost 25% of the cohort was admitted to an intensive care unit (ICU) for the mean stay of 19 ± 17 days. Ventilation therapy was needed in 23.9% and in‐hospital mortality was 24.4%. Table 1 lists detailed clinical characteristics of all participants and compares those characteristics in people who died in the hospital versus those who were discharged alive. People who died had greater than 4‐fold known co‐morbidities upon admission (48.3%) compared with those who survived (12.2%; P < .001).
With regard to medication use, no difference was observed for angiotensin‐converting enzyme inhibitors, angiotensin receptor blockers or any other glucose‐lowering medication between people who survived or died. Loop diuretics and mineralocorticoid receptor blockers were used more frequently in those who died in the hospital.
3.2. Laboratory findings
eGFR was significantly lower in people who died in the hospital (45.0 ± 21.2 vs. 73.1 ± 25.0; P < .001). Moreover, CRP levels were significantly higher in those people admitted to the ICU who died, as were procalcitonin, interleukin‐6 levels and NT‐proBNP levels.
3.3. Outcomes analyses and risk model
Table 2 displays the adjusted odds ratios of significant predictors of in‐hospital mortality identified within our cohort. Besides age, the presence of arterial occlusive disease, CRP levels, eGFR and aspartate aminotransferase (AST) levels were significant predictors of in‐hospital mortality (Table 3). No significant differences in the odds of in‐hospital mortality were observed with respect to prediabetes and type 1 and type 2 diabetes.
Derived from the logistic regression model and the aforementioned variables, we developed a risk score for in‐hospital mortality. The nomogram in Figure 1 displays the variables included and assigns a score to each one of them, either in a categorical or continuous manner. In the derivation cohort, the model achieved an area under the curve (AUC) or C‐statistic of 0.889 (95% CI: 0.837‐0.941) and calibration of 1.000 (Hosmer–Lameshow test, P = .909). In internal validation using the bootstrapping method, the C‐statistic was 0.893 (95% CI: 0.801‐0.959) and calibration was 0.930 (Hosmer–Lameshow test, P = .918) (Figure S1, Figure S2).
FIGURE 1.
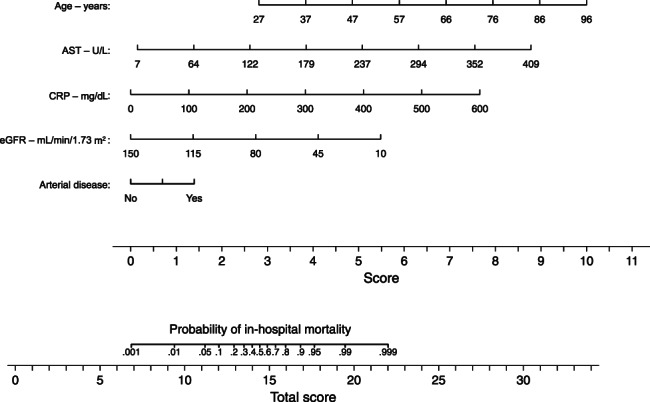
Nomogram for predicting in‐hospital mortality in patients hospitalized with COVID‐19. AST, aspartate aminotransferase; CRP, C‐reactive protein; eGFR, estimated glomerular filtration rate. Risk is given as a probability, and to improve readability we have omitted the second decimal place (e.g. 0.90 is written as 0.9). In order to estimate the fatality risk for a patient with diabetes or prediabetes upon hospital admission, one needs to find the corresponding score of points for each of the five clinical characteristics then add them together. The scale at the bottom gives the probability of in‐hospital mortality corresponding to the calculated score
4. DISCUSSION
In our cohort of people with established diabetes and prediabetes hospitalized with COVID‐19 in Austria, in‐hospital mortality was as high as 24.4% (Table 1). We did not observe a statistically significant difference for mortality between people with type 1 diabetes and type 2 diabetes, although the number of people with type 1 diabetes was only 11. Interestingly, the mortality rate in those with prediabetes was numerically lower (14.9%), albeit this was not statistically significant in comparison with people with type 2 diabetes. With the identified predictors for in‐hospital mortality, namely, age, the presence of arterial occlusive disease, AST, eGFR and CRP levels upon admission, we developed a simple clinical score to identify those people at the highest risk of a fatal outcome.
TABLE 3.
Adjusted coefficients and odds ratios of significant predictors with in‐hospital mortality in patients hospitalized for COVID‐19
| Predictor | Adjusted coefficient (95% CI) | Adjusted OR | P‐value |
|---|---|---|---|
| Constant | −7.80 | 0.0004 | |
| Age – years | 0.095 (0.047‐0.143) | 1.099 (1.048‐1.153) | <.001 |
| CRP – mg/dL (log) | 0.012 (0.003‐0.020) | 1.012 (1.003‐1.020) | .007 |
| eGFR – mL/min/1.73m2 | −0.036 (−0.055 to −0.0166) | 0.965 (0.947‐0.983) | <.001 |
| AST – U/L | 0.020 (0.002‐0.037) | 1.020 (1.002‐1.038) | .031 |
| Arterial occlusive disease | |||
| No | 1 | 1 | .016 |
| Yes | 1.269 (0.234‐2.305) | 3.558 (1.264‐10.022) | |
Abbreviations: AST, aspartate aminotransferase; CI, confidence interval; CRP, C‐reactive protein; eGFR, estimated glomerular filtration rate; OR, odds ratio.
Earlier this year, data on the high prevalence of diabetes in people hospitalized with COVID‐19, and in particular severe episodes of the disease, emerged worldwide. 2 , 3 , 4 , 5 Later, more thorough analyses adjusting the results for covariates still identified diabetes as a significant risk factor for fatal outcomes. 7
The CORONADO study included the first large dataset investigating people with diabetes hospitalized for COVID‐19 in France. 11 Similar to our study, the authors showed that HbA1c upon admission was not a significant predictor of outcomes in this patient cohort. Meanwhile, a UK analysis reported a higher mortality rate in people with higher HbA1c levels, both in type 1 and type 2 diabetes. 13 A recent Italian study showed glucose upon admission as a predictor of disease severity and prognosis; however, admission glucose mainly appears to reflect the inflammatory response, rather than the quality of pre‐COVID‐19 glycaemic control. 14 While other studies have examined recommendations for glucose lowering at home, in the hospital setting or around surgery, our data do not cover this important aspect. 15 , 16
In contrast to our data, body mass index was a predictor of mortality in both the British and French studies; hence, this might reflect the issue of sample size in our study. In addition, the French cohort identified age, obstructive sleep apnoea syndrome, and microvascular and macrovascular complications, as predictors of adverse outcomes. In terms of the laboratory variables, similar to our findings, AST and CRP were directly, and eGFR inversely, related to mortality. In addition, a recent Chinese study reported CRP as a major predictor of mortality in people with diabetes. 17 In line with the CORONADO data, we also did not find a difference in mortality between people with type 1 and type 2 diabetes who were hospitalized. In a UK NHS dataset, the adjusted odds ratios (for age, sex, deprivation, ethnicity and geographical region) for in‐hospital‐related COVID‐19 mortality were higher in people with type 1 diabetes than in people with type 2 diabetes. 18 However, these data are difficult to interpret, as the analyses were not adjusted for additional confounding factors such as diabetes duration or the presence of co‐morbidities.
Recently, Klein et al. analysed data from an intensive care unit in Austria, where they identified previously undiagnosed prediabetes in 36.3% of patients. 8 Also, in our cohort, 47 people (19.7%) had prediabetes diagnosed according to their admission HbA1c, and their outcomes were no different compared with those with manifest diabetes, suggesting that the prediabetic state also has an adverse impact on the progress of COVID‐19 disease.
Because countries have adopted different approaches to tackle the SARS‐CoV‐2 pandemic using various hospitalization strategies, outcomes data vary considerably among them. While the in‐hospital mortality rate in the CORONADO study was 10.6%, 9 the mortality rate in people with diabetes hospitalized with COVID‐19 was almost 25% in our study. Hence, we believe it is important to study country‐based mortality rates in greater depth and put them into context when discussing each country's COVID‐19 strategy.
Also, in our database, no specific glucose‐lowering drug was associated with an increased or reduced risk of in‐hospital death. For a clinician, simple and easily applicable risk stratification for patients admitted to the emergency room is helpful for triage and planning further care. Moreover, this risk stratification is also an important tool with which to design clinical trials for therapeutic agents, as it is probable that these will have different effects across different at‐risk groups. Therefore, we propose a simple risk score based on age, the presence of arterial occlusive disease, as well as CRP, AST and eGFR levels. With an AUC of more than 0.8, this score looks promising; however, we were only able to validate it internally by using the bootstrapping technique, and it clearly needs external validation before clinical applications can be considered.
One limitation of our study is the sample size of 238 subjects. However, given that the total population of Austria is less than 9 million people, and the importance of making in‐hospital mortality data for people with diabetes accessible to as many countries as possible, these findings are of value. Another limitation is the lack of comparison data for people without diabetes in Austria who were hospitalized with COVID‐19. In addition, because of the pragmatic design, we do not have a full dataset consisting of all laboratory variables of interest available in this registry. Hence, we decided to only use those laboratory variables in the risk score model that were available for more than 80% of participants. Sensitivity analyses including further laboratory variables (even where the frequency was <80%) did not substantially change the predictive performance of the score. Given that HbA1c is not routinely measured in all people admitted to hospital, prediabetes was probably underdiagnosed in the overall cohort of people with COVID‐19, a matter which requires further investigation.
The strengths of the current study are the data on people with prediabetes and COVID‐19, and the idea of summarizing the risk variables into a simple clinical score; however, a limitation related to this is the lack of external validation concerning this score, which is key regarding its potential utility in routine care.
Our data show high in‐hospital mortality in people with diabetes and prediabetes in Austria. A simple five‐variable risk score could help to identify patients at the greatest risk of fatal outcomes, but this needs further validation in other cohorts.
CONFLICT OF INTEREST
H. Sourij received unrestricted research grants from AstraZeneca, Boehringer Ingelheim, Eli Lilly, MSD, NovoNordisk and Sanofi; and received speaker's honoraria from Amgen, AstraZeneca, BMS, Boehringer Ingelheim, Eli Lilly, MSD, NovoNordisk and Sanofi. SK received unrestricted research grants from Boehringer Ingelheim and MSD (CD Laboratory for Metabolic Crosstalk). SK received speaker's honoraria from AstraZeneca, Boehringer Ingelheim, Eli Lilly, MSD, NovoNordisk and Sanofi. CC received speaker's honoraria from AstraZeneca, Boehringer Ingelheim, Eli Lilly, MSD, NovoNordisk and Sanofi. H. Stingl received an unresctricted research grant from Boehringer Ingelheim; and received speaker's honoraria from Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Merck Sharp & Dohme, NovoNordisk, Novartis, and Sanofi Aventis and Servier. CR received speaker's honoraria and congress support from AstraZeneca, NovoNordisk and Sanofi. All the other authors declare no conflicts of interest with regard to this manuscript.
AUTHOR CONTRIBUTIONS
H. Sourij and SK conceived the study, H. Sourij, NT and CS wrote the protocol and designed the eCRF. H. Sourij, AB, CC, MC, PF, MK, AKW, CK, OM, EP, SP, CR, CS, LS, H. Stingl, TS, NT, PW, AZ and SK collected the data. FA and AO performed the statistical analyses. H. Sourij, CS, NT and FA wrote the first draft of the manuscript and all the authors revised the manuscript. H. Sourij and FA are the guarantors of the data.
COVID‐19 IN DIABETES IN AUSTRIA STUDY GROUP
Medical University Graz, Austria:
Harald Sourij, Norbert J. Tripolt, Caren Sourij, Farah Abbas, Oliver Malle, Julia Mader.
Medical Division for Endocrinology, Rheumatology and Acute Geriatrics, Wilhelminen Hospital Vienna, Austria:
Peter Fasching, Gersina Rega‐Kaun, Kadriye Aydinkov‐Tuzcu, Alexander Bräuer, Brigitte Bernhardt.
Clinical Division for Internal Medicine, Endocrinology, Diabetology and Metabolic Diseases St. Vinzenz Hospital Zams, Austria:
Christian Ciardi, Marc Schaber, Anna Schapfl, David Fiegl.
Clinical Division for Internal Medicine, Konventhospital Barmherzige Brüder Linz, Austria:
Martin Clodi, Carmen Klammer, Michael Resl, Matthias Heinzl, Roland Feldbauer, Johannes Pohlhammer.
4th Medical Division with Infectiology, SMZ Süd – KFJ‐Hospital, Vienna, Austria:
Mario Karolyi, Erich Pawelka.
Clinical Division for Endocrinology and Diabetology and Metabolic Diseases, AKH Vienna, Austria:
Alexandra Kautzky‐Willer, Peter Wolf.
Department for Internal Medicine I, Paracelsus Medical University, Salzburg, Austria:
Lars Stechemesser, Michael Schranz.
Clinical Division for Internal Medicine, Hospital Melk, Austria:
Harald Stingl, Michael Wagner, Reinhard Würfel.
3rd Medical Department and Karl Landsteiner Institute for Metabolic Diseases and Nephrology, Clinic Hietzing, Vienna Health Care Group, Austria:
Thomas M. Stulnig, Slobodan Peric, Andreas Zitterl.
Department for Internal Medicine I, Medical University Innsbruck, Austria:
Susanne Kaser, Claudia Ress.
PREPRINT PUBLICATION
This study will be published on https://www.medrxiv.org as a preprint.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14256.
Supporting information
Figure S1 – ROC curves: A – Derivation cohort, B – Internal bootstrap validation sample
Figure S2 – Calibration plots: A – Derivation cohort, B – Internal bootstrap validation sample H‐L p: Hosmer‐Lameshow test p‐value
ACKNOWLEDGEMENTS
We would like to thank Kapsch Austria, Icomedias, and Microsoft Austria for programming the electronic case report form and the provision of secure data storage space. We thank Andrew Spencer for proofreading the manuscript. The study was supported by unrestricted research grants to the Austrian Diabetes Association from NovoNordisk, Novartis, Sanofi, AstraZeneca and Boehringer Ingelheim. The study funder was not involved in the design of the study; the collection, analysis and interpretation of data; writing the report; and did not impose any restrictions regarding the publication of the report.
Sourij H, Aziz F, Bräuer A, et al. COVID‐19 fatality prediction in people with diabetes and prediabetes using a simple score upon hospital admission. Diabetes Obes Metab. 2021;23:589–598. 10.1111/dom.14256
Funding information This study was supported by unrestricted research grants to the Austrian Diabetes Association from NovoNordisk, Novartis, Sanofi, AstraZeneca and Boehringer Ingelheim.
DATA AVAILABILITY STATEMENT
The Austrian Diabetes Association has full access to the dataset and access can be granted upon request.
REFERENCES
- 1. Abbasi‐Oshaghi E, Mirzaei F, Farahani F, Khodadadi I, Tayebinia H. Diagnosis and treatment of coronavirus disease 2019 (COVID‐19): Laboratory, PCR, and chest CT imaging findings. Int J Surg. 2020;79:143‐153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the New York City Area. JAMA. 2020;323:2052‐2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS‐CoV‐2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323:1574‐1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: Summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239‐1242. [DOI] [PubMed] [Google Scholar]
- 7. Shi Q, Zhang X, Jiang F, et al. Clinical characteristics and risk factors for mortality of COVID‐19 patients with diabetes in Wuhan, China: a two‐center, retrospective study. Diabetes Care. 2020;43(7):1382‐1391. [DOI] [PubMed] [Google Scholar]
- 8. Klein SJ, Fries D, Kaser S, Mathis S, Thome C, Joannidis M. Unrecognized diabetes in critically ill COVID‐19 patients. Crit Care. 2020;24(1):406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pearson‐Stuttard J, Blundell S, Harris T, Cook DG, Critchley J. Diabetes and infection: assessing the association with glycaemic control in population‐based studies. Lancet Diabetes Endocrinol. 2016;4(2):148‐158. [DOI] [PubMed] [Google Scholar]
- 10. Joshi N, Caputo GM, Weitekamp MR, Karchmer AW. Infections in patients with diabetes mellitus. N Engl J Med. 1999;341(25):1906‐1912. [DOI] [PubMed] [Google Scholar]
- 11. Cariou B, Hadjadj S, Wargny M, et al. Phenotypic characteristics and prognosis of inpatients with COVID‐19 and diabetes: the CORONADO study. Diabetologia. 2020;63(8):1500‐1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Harreiter J, Roden M. Diabetes mellitus ‐ definition, classification, diagnosis, screening and prevention (update 2019). Wien Klin Wochenschr. 2019;131(suppl 1):6‐15. [DOI] [PubMed] [Google Scholar]
- 13. Holman N, Knighton P, Kar P, et al. Risk factors for COVID‐19‐related mortality in people with type 1 and type 2 diabetes in England: a population‐based cohort study. Lancet Diabetes Endocrinol. 2020;8:823‐833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Coppelli A, Giannarelli R, Aragona M, et al. Hyperglycemia at hospital admission is associated with severity of the prognosis in patients hospitalized for COVID‐19: The Pisa COVID‐19 Study. Diabetes Care. 2020;43:2345‐2348. [DOI] [PubMed] [Google Scholar]
- 15. Farahani F, Mirzaei F, Khodadadi I, Abbasi‐Oshaghi E. Importance of hyperglycemia in preoperative, intraoperative and postoperative periods in COVID‐19 patients. Int J Surg. 2020;83:1‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Katulanda P, Dissanayake HA, Ranathunga I, et al. Prevention and management of COVID‐19 among patients with diabetes: an appraisal of the literature. Diabetologia. 2020;63(8):1440‐1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen Y, Yang D, Cheng B, et al. Clinical characteristics and outcomes of patients with diabetes and COVID‐19 in association with glucose‐lowering medication. Diabetes Care. 2020;43(7):1399‐1407. [DOI] [PubMed] [Google Scholar]
- 18. Barron E, Bakhai C, Kar P, et al. Associations of type 1 and type 2 diabetes with COVID‐19‐related mortality in England: a whole‐population study. Lancet Diabetes Endocrinol. 2020;8:813‐822. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 – ROC curves: A – Derivation cohort, B – Internal bootstrap validation sample
Figure S2 – Calibration plots: A – Derivation cohort, B – Internal bootstrap validation sample H‐L p: Hosmer‐Lameshow test p‐value
Data Availability Statement
The Austrian Diabetes Association has full access to the dataset and access can be granted upon request.


