Abstract
Background
Hypercoagulability and thromboembolism are prominent features of severe COVID‐19, and ongoing anticoagulant use might be protective.
Methods
We conducted a nationwide register‐based cohort study in Sweden, February through May, 2020, to assess whether ongoing direct oral anticoagulant (DOAC) use was associated with reduced risk of hospital admission for laboratory‐confirmed COVID‐19, or a composite of intensive care unit (ICU) admission or death due to laboratory‐confirmed COVID‐19.
Results
DOAC use (n = 103 703) was not associated with reduced risk of hospital admission for COVID‐19 (adjusted hazard ratio [aHR] [95% confidence interval] 1.00 [0.75–1.33] vs. nonuse atrial fibrillation comparator [n = 36 875]; and aHR 0.94 [0.80–1.10] vs. nonuse cardiovascular disease comparator [n = 355 699]), or ICU admission or death due to COVID‐19 (aHRs 0.76 [0.51–1.12], and 0.90 [0.71–1.15], respectively).
Conclusion
Ongoing DOAC use was not associated with reduced risk of severe COVID‐19, indicating that prognosis would not be modified by early outpatient DOAC initiation.
Keywords: anticoagulants, atrial fibrillation, COVID‐19, direct‐acting oral anticoagulants, SARS‐CoV‐2
Introduction
Coronavirus disease 2019 (COVID‐19), caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), is associated with hypercoagulability. Thrombosis is common in patients hospitalized with COVID‐19 [1] and seems to play a key role both in the pathophysiology [2], and as incident complications [3].
Given the thrombogenic features of COVID‐19 potentially predisposing for a progressive disease course, it is plausible that anticoagulation may protect against severe disease. Currently, pharmacological parenteral thromboprophylaxis is widely advocated for patients hospitalized for COVID‐19 [4, 5], although the efficacy of this intervention on further disease progression and prognosis is unknown and the subject of ongoing trials. Whether long‐term anticoagulation with therapeutic doses initiated before SARS‐CoV‐2 infection influences COVID‐19 progression is unclear. To date, eight mostly small regional or single‐centre studies of a total of 434 patients with preexisting anticoagulant use have been reported, presenting inconclusive and inconsistent results [6, 7, 8, 9, 10, 11, 12, 13].
We conducted a nationwide register‐based cohort study in Sweden to investigate whether ongoing direct oral anticoagulant (DOAC) use was associated with reduced risk of severe COVID‐19.
Materials and methods
This cohort study was based on nationwide Swedish registers. Patients with DOAC use and nonvalvular atrial fibrillation or flutter (AF) were compared with two nonuse comparator groups: patients with AF, and patients with major cardiovascular disease (CVD). We assessed the risk of two co‐primary outcomes reflecting severe forms of COVID‐19: hospital admission for COVID‐19, and the composite of intensive care unit (ICU) admission or death due to COVID‐19. The Swedish Ethical Review Authority approved the study (number 2020‐02536).
Data sources
Data sources included the National Patient Register, which accumulates data on all hospital admissions and outpatient and emergency department visits in Sweden, including physician‐assigned diagnoses coded according to the International Classification of Diseases, 10th Revision, Swedish Edition (ICD‐10‐SE) and procedure codes (positive predictive value of AF diagnosis record, 97%) [14]; the Swedish Prescribed Drug Register, which captures detailed information on all prescriptions filled at all Swedish pharmacies; Statistics Sweden’s sociodemographic data; the Cause of Death Register, which captures dates and causes of death based on death certificates; and the Swedish Intensive Care Registry, which is a national registry that captures close to all adult ICU admissions in the country.
Study cohort
We constructed a cohort consisting of all individuals in Sweden aged 45 to 84 years and alive through 31 January 2020 (which was the date of the first confirmed COVID‐19 case in the country), with a recorded diagnosis of AF, ischaemic heart disease, heart failure or cardiomyopathy, stroke or transient ischaemic attack, systemic thromboembolism, or vascular disease (Table S1), who had resided in Sweden throughout the last year and had at least one drug prescription or specialist care contact within the previous three years (to ensure healthcare system activity). To minimize bias, including exposure misclassification, patients were excluded in case of DOAC use with non‐AF indication; recent but ceased DOAC use; possible DOAC contraindication; recent warfarin use or likely warfarin indication, including mechanical heart valve or mitral stenosis; and severe illness. Further exclusion criteria were recent hospital admission; recent venous thromboembolism; and recent use of heparins. Exclusion criteria are specified in Table S2.
DOAC use and comparator groups
Ongoing use of a DOAC (dabigatran, apixaban, rivaroxaban or edoxaban) was defined as a prescription for any of these drugs that was filled before the index date of 1 February 2020 and whose duration overlapped the index date (Table S3). The estimated duration of filled prescriptions was defined based on the number of tablets dispensed, allowing for a gap of 30 days. Nonuse was defined as no DOAC use within 12 months before the index date. Once defined as a DOAC user or nonuser on the index date, a patient was considered to belong to that group throughout follow‐up.
The exposed group consisted of patients with nonvalvular AF with ongoing DOAC use. An active comparator design was unfeasible in this setting. In the interest of robustness and consistency of study findings, two different nonuse comparator groups were used: patients with AF who were nonusers of DOAC, and patients with CVD (AF, ischaemic heart disease, heart failure/cardiomyopathy, stroke, transient ischaemic attack, systemic thromboembolism, or vascular disease) who were nonusers of DOAC. By design, the nonuse AF comparator group was thus a subgroup of the nonuse CVD comparator group.
Outcomes
The two co‐primary outcomes were hospital admission for laboratory‐confirmed COVID‐19, defined as the first hospital admission with primary diagnosis ICD‐10‐SE code U07.1 (COVID‐19, virus identified), and the composite of ICU admission or death due to laboratory‐confirmed COVID‐19, which was defined as an ICU admission during a hospitalization with a primary diagnosis U07.1, or death where U07.1 was recorded as the underlying cause or death of any cause within 30 days of a hospital admission with a primary diagnosis U07.1.
Prespecified secondary analyses assessed both co‐primary outcomes according to DOAC subtype (direct thrombin inhibitor [dabigatran], or direct factor Xa inhibitor [apixaban, rivaroxaban or edoxaban]), and the individual components of the second co‐primary outcome (ICU admission and death due to COVID‐19, respectively). In a sensitivity analysis, we assessed the risk of all‐cause mortality to examine the potential for residual confounding.
Statistical analysis
We used Cox proportional‐hazards regression, estimating the following models: unadjusted; adjusted for age and sex; and fully adjusted. The fully adjusted multivariable models included 42 potential confounders including age, sex, sociodemographic factors, comorbidities, medications, and healthcare utilization (Table S4). Hazard ratios (HRs) were considered statistically significant if the 95% confidence intervals (CIs) did not contain 1.0. Adjusted absolute risk differences were calculated as [adjusted HR (aHR)–1]×crude risk amongst the unexposed. In the analysis of hospital admission for COVID‐19, patients were followed from cohort entry until outcome event, end of study period or any‐cause death, whichever occurred first. In the analysis of ICU admission or death due to COVID‐19, patients were followed from cohort entry until outcome event, end of study period or other‐cause death, whichever occurred first. All analyses were performed using SAS, version 9.4 (SAS Institute Inc.).
Results
Cohort and exposure/comparator groups
The study cohort eligibility criteria were met in 459 402 patients (Fig. 1). Of these, 103 703 were patients with nonvalvular AF who were DOAC users (93 354 [90.0%] using direct factor Xa inhibitors). The comparator groups included 36 875 patients with AF with no DOAC use and 355 699 patients with CVD with no DOAC use.
Fig. 1.
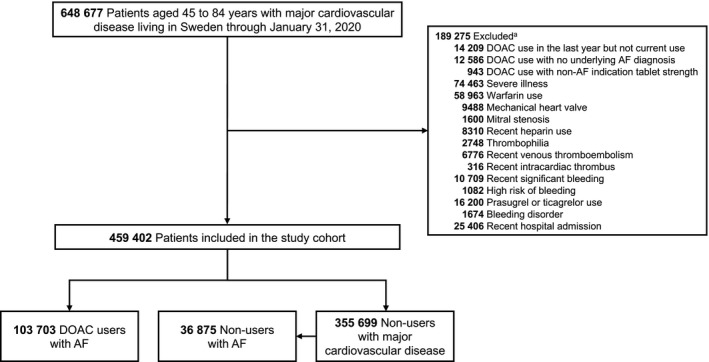
Cohort construction flow chart. AF, atrial fibrillation; DOAC, direct oral anticoagulant. a Numbers of excluded patients do not accumulate to the total sum, since some patients were excluded for more than one reason.
Baseline patient characteristics are shown in Table 1. Compared with AF patients with no DOAC use, DOAC users were older and more often female, had more somatic comorbidities, specialist care consumption, and medications except for antiplatelet agents and nonsteroidal anti‐inflammatory drugs. When compared with the CVD group with no DOAC use, DOAC users were older, but had a lower prevalence of ischaemic heart disease and cerebrovascular disease/systemic thromboembolism, whilst having higher specialist care consumption and medication use except for antiplatelet agents, statins, and nonsteroidal anti‐inflammatory drugs. Overall, the median follow‐up time during the four‐month study period was 121.0 days (interquartile range, 121.0–121.0 days).
Table 1.
Baseline patient characteristics a
| Characteristic | DOAC use, AF (n = 103 703) | Comparator groups | |
|---|---|---|---|
| No DOAC use, AF (n = 36 875) | No DOAC use, major CVD (n = 355 699) | ||
| Male sex, n (%) | 62 488 (60.3) | 25 020 (67.9) | 214 041 (60.2) |
| Age, mean (SD), years | 73.6 (7.6) | 66.4 (10.5) | 69.3 (9.6) |
| Age group, n (%) | |||
| 45–49 years | 739 (0.7) | 2648 (7.2) | 12 872 (3.6) |
| 50–54 years | 1918 (1.8) | 3785 (10.3) | 22 531 (6.3) |
| 55–59 years | 3747 (3.6) | 4723 (12.8) | 32 991 (9.3) |
| 60–64 years | 7500 (7.2) | 5832 (15.8) | 44 929 (12.6) |
| 65–69 years | 14 974 (14.4) | 4987 (13.5) | 56 036 (15.8) |
| 70–74 years | 24 900 (24.0) | 5677 (15.4) | 72 449 (20.4) |
| 75–79 years | 27 557 (26.6) | 5149 (14.0) | 66 033 (18.6) |
| 80–84 years | 22 368 (21.6) | 4074 (11.0) | 47 858 (13.5) |
| DOAC, n (%) | |||
| Dabigatran | 10 349 (10.0) | NA | NA |
| Apixaban | 72 347 (69.8) | NA | NA |
| Rivaroxaban | 18 781 (18.1) | NA | NA |
| Edoxaban | 2226 (2.1) | NA | NA |
| Time since first DOAC dispensing, n (%) | |||
| 0–6 months | 7394 (7.1) | NA | NA |
| 7–24 months | 29 012 (28.0) | NA | NA |
| >24 months | 67 245 (64.8) | NA | NA |
| Place of birth, n (%) | |||
| Scandinavia | 96 685 (93.2) | 33 410 (90.6) | 313 295 (88.1) |
| Rest of Europe | 4922 (4.7) | 2008 (5.4) | 21 939 (6.2) |
| Outside Europe | 2095 (2.0) | 1455 (3.9) | 20 448 (5.7) |
| Civil status, n (%) | |||
| Married/living with partner | 57 437 (55.4) | 19 593 (53.1) | 185 901 (52.3) |
| Single | 46 266 (44.6) | 17 282 (46.9) | 169 798 (47.7) |
| Education, n (%) | |||
| Primary/secondary school, vocational training | 73 138 (70.5) | 24 208 (65.6) | 259 527 (73.0) |
| Short tertiary education | 12 582 (12.1) | 5035 (13.7) | 41 077 (11.5) |
| Medium or long tertiary education | 17 047 (16.4) | 7333 (19.9) | 51 774 (14.6) |
| Comorbidities, n (%) | |||
| Ischaemic heart disease | 24 519 (23.6) | 7320 (19.9) | 167 019 (47.0) |
| Heart failure/cardiomyopathy | 26 544 (25.6) | 4442 (12.0) | 46 889 (13.2) |
| Valve disorder | 11 456 (11.0) | 3611 (9.8) | 20 249 (5.7) |
| Ischaemic stroke/TIA/systemic thromboembolism | 17 650 (17.0) | 2853 (7.7) | 102 916 (28.9) |
| Haemorrhagic/unspecified stroke | 6451 (6.2) | 1849 (5.0) | 35 789 (10.1) |
| Other vascular disease | 8112 (7.8) | 2540 (6.9) | 59 007 (16.6) |
| Arrhythmia (other than AF/flutter) | 18 264 (17.6) | 7357 (20.0) | 27 735 (7.8) |
| Lung disease | 21 762 (21.0) | 6093 (16.5) | 61 856 (17.4) |
| Renal disease | 6082 (5.9) | 1741 (4.7) | 14 354 (4.0) |
| Liver disease | 1445 (1.4) | 832 (2.3) | 6613 (1.9) |
| Venous thromboembolism (>1 year prior) | 5692 (5.5) | 1390 (3.8) | 12 196 (3.4) |
| Malignancy (>1 year prior) | 10 826 (10.4) | 3210 (8.7) | 30 589 (8.6) |
| Peptic ulcer disease (>90 days prior) | 2550 (2.5) | 1001 (2.7) | 9316 (2.6) |
| Psychiatric disorder/substance abuse | 14 249 (13.7) | 6994 (19.0) | 68 861 (19.4) |
| Prescription drug use, n (%) | |||
| Aspirin | 3514 (3.4) | 9211 (25.0) | 198 617 (55.8) |
| P2Y12 inhibitor (excl. prasugrel/ticagrelor) | 1576 (1.5) | 1104 (3.0) | 48 929 (13.8) |
| ACE inhibitor/ARB | 65 066 (62.7) | 13 357 (36.2) | 203 516 (57.2) |
| Calcium‐channel blocker | 28 913 (27.9) | 6571 (17.8) | 103 639 (29.1) |
| Loop diuretic | 21 484 (20.7) | 2961 (8.0) | 29 834 (8.4) |
| Other diuretic | 19 880 (19.2) | 2972 (8.1) | 41 863 (11.8) |
| Beta‐blocker | 83 454 (80.5) | 19 020 (51.6) | 170 370 (47.9) |
| Statin | 48 104 (46.4) | 10 297 (27.9) | 219 559 (61.7) |
| Metformin | 13 974 (13.5) | 2848 (7.7) | 50 626 (14.2) |
| Insulin | 6687 (6.4) | 1552 (4.2) | 26 906 (7.6) |
| Other glucose‐lowering drug | 8634 (8.3) | 1678 (4.6) | 30 435 (8.6) |
| Antidepressant/antipsychotic | 14 888 (14.4) | 5205 (14.1) | 60 606 (17.0) |
| Beta2‐agonist inhalant | 7828 (7.5) | 2099 (5.7) | 24 769 (7.0) |
| Anticholinergic inhalant | 6354 (6.1) | 1444 (3.9) | 18 944 (5.3) |
| Glucocorticoid inhalant | 9827 (9.5) | 2647 (7.2) | 28 930 (8.1) |
| Oral glucocorticoid | 8959 (8.6) | 2071 (5.6) | 22 792 (6.4) |
| NSAID | 3563 (3.4) | 3277 (8.9) | 29 036 (8.2) |
| Opioid | 12 137 (11.7) | 3458 (9.4) | 38 406 (10.8) |
| Healthcare utilization in the last year, n (%) | |||
| Specialist care outpatient visits | |||
| 0 | 23 507 (22.7) | 13 728 (37.2) | 128 877 (36.2) |
| 1–3 | 47 814 (46.1) | 16 038 (43.5) | 153 267 (43.1) |
| >3 | 32 382 (31.2) | 7109 (19.3) | 73 555 (20.7) |
| Hospital admissions | |||
| 0 | 75 301 (72.6) | 31 192 (84.6) | 293 656 (82.6) |
| 1 | 17 366 (16.7) | 3581 (9.7) | 41 528 (11.7) |
| >1 | 11 036 (10.6) | 2102 (5.7) | 20 515 (5.8) |
| Prescription drugs | |||
| 0–5 | 21 999 (21.2) | 19 121 (51.9) | 112 239 (31.6) |
| 6–10 | 42 370 (40.9) | 10 371 (28.1) | 137 551 (38.7) |
| 11–15 | 24 638 (23.8) | 4771 (12.9) | 68 478 (19.3) |
| >15 | 14 696 (14.2) | 2612 (7.1) | 37 431 (10.5) |
Percentages may not total 100 because of rounding.
ACE, angiotensin‐converting enzyme; AF, atrial fibrillation; ARB, angiotensin‐receptor blocker; CVD, cardiovascular disease; DOAC, direct oral anticoagulant; NA, not applicable; NSAID, nonsteroidal antiinflammatory drug; SD, standard deviation; TIA, transient ischaemic attack.
Geographic baseline patient characteristics are shown in Table S5.
Risk of severe COVID‐19
Table 2 shows results of analyses of the co‐primary outcomes. There were 360 hospital admissions for COVID‐19 amongst the DOAC users (crude risk, 0.35%), vs. 95 amongst nonusers with AF (0.26%) and 1119 amongst nonusers with CVD (0.31%). In the fully adjusted multivariable analysis, DOAC use, as compared with nonuse, was not associated with reduced risk of hospital admission for COVID‐19 (aHR, 1.00; 95% CI, 0.75–1.33 vs. nonuse AF comparator, and 0.94; 95% CI, 0.80–1.10 vs. nonuse CVD comparator).
Table 2.
Risk of severe COVID‐19 amongst DOAC users vs. nonuse comparators
| Outcome | Patients, n | Events, n (%) | Unadjusted hazard ratio (95% CI) | Hazard ratio (95% CI) adjusted for age and sex | Fully adjusted a hazard ratio (95% CI) | Fully adjusted a absolute risk difference, % (95% CI) |
|---|---|---|---|---|---|---|
| Hospital admission for COVID‐19 | ||||||
| DOAC use | 103 703 | 360 (0.35) | — | — | — | — |
| vs. nonuse AF comparator | 36 875 | 95 (0.26) | 1.35 (1.07–1.69) | 1.14 (0.89–1.45) | 1.00 (0.75–1.33) | 0.00 (–0.07–0.09) |
| vs. nonuse CVD comparator | 355 699 | 1119 (0.31) | 1.10 (0.98–1.24) | 1.04 (0.92–1.17) | 0.94 (0.80–1.10) | –0.02 (–0.06–0.03) |
| ICU admission or death due to COVID‐19 | ||||||
| DOAC use | 103 703 | 161 (0.16) | — | — | — | — |
| vs. nonuse AF comparator | 36 875 | 55 (0.15) | 1.04 (0.77–1.41) | 0.71 (0.52–0.98) | 0.76 (0.51–1.12) | –0.04 (–0.07–0.02) |
| vs. nonuse CVD comparator | 355 699 | 473 (0.13) | 1.17 (0.98–1.40) | 0.97 (0.81–1.17) | 0.90 (0.71–1.15) | –0.01 (–0.04–0.02) |
AF, atrial fibrillation; CI, confidence interval; COVID‐19, coronavirus disease 2019; CVD, cardiovascular disease; DOAC, direct oral anticoagulant; ICU, intensive care unit.
Adjusted for 42 potential confounders, including age, sex, sociodemographic factors, comorbidities, medications and healthcare utilization (Table S4).
One hundred sixty‐one composite ICU admission or death due to COVID‐19 outcome events occurred amongst the DOAC users (crude risk, 0.16%), vs. 55 amongst nonusers with AF (0.15%) and 473 amongst nonusers with CVD (0.13%). In the fully adjusted multivariable analysis, DOAC use was not associated with a reduced risk of ICU admission or death due to COVID‐19 (aHR, 0.76; 95% CI, 0.51–1.12 vs. nonuse AF comparator, and 0.90; 95% CI, 0.71–1.15 vs. nonuse CVD comparator).
Additional analyses
Estimates for both co‐primary outcomes were similar for both DOAC subtypes (direct thrombin inhibitor and direct factor Xa inhibitors) (Table S6). Analyses of the individual components of the second co‐primary outcome yielded aHRs of 1.06; 95% CI, 0.48–2.35 vs. nonuse AF comparator, and 0.86; 95% CI, 0.55–1.34 vs. nonuse CVD comparator for ICU admission due to COVID‐19; and 0.72; 95% CI, 0.47–1.10 vs. nonuse AF comparator, and 0.91; 95% CI, 0.70–1.17 vs. nonuse CVD comparator for death due to COVID‐19 (Table S7). In the sensitivity analysis of all‐cause mortality, the aHRs were 0.62; 95% CI, 0.53–0.73 vs. nonuse AF comparator, and 0.79; 95% CI, 0.71–0.87 vs. nonuse CVD comparator (Table S8).
Discussion
In this nationwide register‐based cohort study including more than 100 000 DOAC users, ongoing use of this class of anticoagulants was not associated with a reduced risk of the two co‐primary outcomes hospital admission for COVID‐19 and a composite of ICU admission or death due to COVID‐19. These findings were consistent in analyses with two different comparator groups, as well as across DOAC subtypes.
In the light of the current pandemic, measures to prevent related morbidity and mortality are much looked‐for. Identified as a key feature of severe COVID‐19, large focus has been put on managing hypercoagulability in hospitalized patients, with interim guidelines supportive of anticoagulation [4, 5]. Preliminary retrospective data ratify these recommendations [15], although results may be biased [16]. Seemingly, the thrombotic disease processes could have commenced already prior to hospital admission, as studies have found early event occurrence (at or within 24 hours of admission) in approximately half of COVID‐19 cases with associated venous thromboembolism [17, 18]. Pulmonary and extra‐pulmonary microvascular thrombosis may considerably contribute to the acute lung injury and multiple organ dysfunction that leads to disease progression and ensuing hospitalization, critical illness, and death. Preemptive anticoagulant treatment before or at the time of SARS‐CoV‐2 infection to protect against severe disease is theoretically appealing but real‐world data have been lacking. Previously, only small studies of COVID‐19 patient cohorts have been conducted showing mixed results [6, 7, 8, 9, 10, 11, 12, 13]. An additional case–control study aimed at investigating the association between renin–angiotensin–aldosterone system blockers and COVID‐19 reported also on oral anticoagulant use (odds ratio, 1.16; 95% CI 1.04–1.30) [19]. The results from the present large observational study do not support that DOAC administration reduces the risk of severe COVID‐19. Rather than against secondary hypercoagulability, therapies may be better directed against thrombogenic inflammation or vasculopathy, but further investigation is required.
This study has strengths and limitations. The nationwide register‐based design enabled the inclusion of over 100 000 DOAC‐exposed individuals and complete follow‐up. The analyses controlled for a large number of potential confounders. Still, pharmacoepidemiologic study designs that utilize a nonuser comparator may be sensitive to confounding. This is however less likely. First, the results were consistent across analyses with two disparate comparator groups exhibiting different characteristics, why confounding would have had to act similarly in both analyses. Second, for confounding to lead to the observation of false‐neutral results in a scenario where a true protective association exists, confounding would have to skew HRs upwards. Thus, it would have to be an unmeasured factor that was more common amongst DOAC users and that led to increased risk of severe COVID‐19 outcomes. Such a factor could be frailty and would be expected to also lead to increased risk of all‐cause mortality. This was not the case; in a sensitivity analysis, DOAC use was associated with a reduced risk of all‐cause mortality. The estimated aHRs for all‐cause mortality are well in line with those from previous real‐world studies comparing DOACs with vitamin K antagonists [20]. Last, thromboprophylaxis administered during hospitalization could not be assessed and may have introduced bias towards the null. This is less likely since it cannot have affected the outcome of hospital admission for COVID‐19.
Conclusion
In this large nationwide cohort study, there was no significant association between ongoing DOAC use and risk of severe COVID‐19. In search of therapeutics, these findings indicate that COVID‐19 prognosis would not be modified by early outpatient DOAC initiation.
Author contribution
Benjamin Flam: Conceptualization (equal); Investigation (equal); Methodology (equal); Project administration (equal); Writing‐original draft (lead); Writing‐review & editing (equal). Viktor Wintzell: Conceptualization (equal); Data curation (lead); Formal analysis (lead); Investigation (equal); Methodology (equal); Writing‐review & editing (equal). Jonas Ludvigsson: Conceptualization (equal); Investigation (equal); Methodology (equal); Writing‐original draft (supporting); Writing‐review & editing (equal). Johan Mårtensson: Conceptualization (equal); Funding acquisition (equal); Investigation (equal); Methodology (equal); Writing‐review & editing (equal). Björn Pasternak: Conceptualization (equal); Funding acquisition (equal); Investigation (equal); Methodology (equal); Project administration (equal); Supervision (lead); Writing‐original draft (supporting); Writing‐review & editing (equal).
Conflicts of interest
Dr. Ludvigsson coordinates a study on behalf of the Swedish Inflammatory Bowel Disease Register (SWIBREG). This study has received funding from Janssen Corporation. The remaining authors declare no competing financial interests.
Supporting information
Table S1. Study cohort inclusion diagnoses.
Table S2. Study cohort exclusion criteria.
Table S3. Study drugs.
Table S4. Covariates included in multivariable analyses.
Table S5. Geographic baseline patient characteristics.
Table S6. Risk of severe COVID‐19 among DOAC users vs. non‐use comparators according to DOAC subtype.
Table S7. Risk of ICU admission and death due to COVID‐19 analyzed separately.
Table S8. All‐cause mortality among DOAC users vs. non‐use comparator groups.
Acknowledgements
The authors wish to acknowledge the Swedish National Board of Health and Welfare, Statistics Sweden and the Swedish Intensive Care Registry for efficient data provision. This study was conducted with support by a grant from the Swedish Government Funds for Clinical Research (ALF). Dr. Pasternak is supported by investigator grants from the Karolinska Institutet Strategic Research Area Epidemiology programme and the Swedish Research Council.
Flam B, Wintzell V, Ludvigsson JF, Mårtensson J, Pasternak B (Karolinska University Hospital, Stockholm; Karolinska Institutet, Stockholm; Örebro University Hospital, Örebro, Sweden; University of Nottingham, Nottingham, UK; Columbia University College of Physicians and Surgeons, New York, NY, USA; and Statens Serum Institut, Copenhagen, Denmark). Direct oral anticoagulant use and risk of severe COVID‐19 (Brief Report). J Intern Med 2021; 289: 411–419. 10.1111/joim.13205
References
- 1. Kunutsor SK, Laukkanen JA. Incidence of venous and arterial thromboembolic complications in COVID‐19: A systematic review and meta‐analysis. Thromb Res 2021; 196: 27–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ackermann M, Verleden SE, Kuehnel M et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in covid‐19. N Engl J Med 2020; 383: 120–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bikdeli B, Madhavan MV, Jimenez D et al. COVID‐19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow‐up: JACC state‐of‐the‐art review. J Am Coll Cardiol 2020; 75: 2950–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Thachil J, Tang N, Gando S et al. ISTH interim guidance on recognition and management of coagulopathy in COVID‐19. J Thromb Haem 2020; 18: 1023–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Marietta M, Coluccio V, Luppi M. COVID‐19, coagulopathy and venous thromboembolism: more questions than answers. Intern Emerg Med 2020; 15: 1375–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Russo V, Di Maio M, Attena E et al. Clinical impact of pre‐admission antithrombotic therapy in hospitalized patients with COVID‐19: A multicenter observational study. Pharmacol Res 2020; 159: 104965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tremblay D, van Gerwen M, Alsen M et al. Impact of anticoagulation prior to COVID‐19 infection: a propensity score‐matched cohort study. Blood 2020; 136: 144–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rossi R, Coppi F, Talarico M, Boriani G. Protective role of chronic treatment with direct oral anticoagulants in elderly patients affected by interstitial pneumonia in COVID‐19 era. Eur J Intern Med 2020; 77: 158–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sivaloganathan H, Ladikou EE, Chevassut T. COVID‐19 mortality in patients on anticoagulants and antiplatelet agents. Br J Haematol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brouns SH, Brüggemann R, Linkens AEMJH et al. Mortality and the Use of Antithrombotic Therapies Among Nursing Home Residents with COVID‐19. J Am Geriatr Soc 2020; 68: 1647–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Klok FA, Kruip M, van der Meer NJM et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID‐19: An updated analysis. Thromb Res 2020; 191: 148–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schiavone M, Gasperetti A, Mancone M et al. Oral anticoagulation and clinical outcomes in COVID‐19: An Italian multicenter experience. Int J Cardiol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rivera‐Caravaca JM, Nunez‐Gil IJ, Vivas D et al. Clinical profile and prognosis in patients on oral anticoagulation before admission for COVID‐19. Eur J Clin Invest 2020; e13436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Smith JG, Platonov PG, Hedblad B, Engstrom G, Melander O. Atrial fibrillation in the Malmo Diet and Cancer study: a study of occurrence, risk factors and diagnostic validity. Eur J Epidemiol 2010; 25: 95–102. [DOI] [PubMed] [Google Scholar]
- 15. Kamel AM, Sobhy M, Magdy N, Sabry N, Farid S. Anticoagulation outcomes in hospitalized Covid‐19 patients: A systematic review and meta‐analysis of case‐control and cohort studies. Rev Med Virol 2020; e2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Maley JH, Petri CR, Brenner LN et al. Anticoagulation, immortality, and observations of COVID‐19. Res Pract Thromb Haemostasis 2020; 4: 674–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lodigiani C, Iapichino G, Carenzo L et al. Venous and arterial thromboembolic complications in COVID‐19 patients admitted to an academic hospital in Milan. Italy. Thromb Res 2020; 191: 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Whyte MB, Kelly PA, Gonzalez E, Arya R, Roberts LN. Pulmonary embolism in hospitalised patients with COVID‐19. Thromb Res 2020; 195: 95–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mancia G, Rea F, Ludergnani M, Apolone G, Corrao G. Renin‐angiotensin‐aldosterone system blockers and the risk of covid‐19. N Engl J Med 2020; 382: 2431–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ntaios G, Papavasileiou V, Makaritsis K, Vemmos K, Michel P, Lip GYH. Real‐world setting comparison of nonvitamin‐K antagonist oral anticoagulants versus vitamin‐K antagonists for stroke prevention in atrial fibrillation: a systematic review and meta‐analysis. Stroke 2017; 48: 2494–503. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Study cohort inclusion diagnoses.
Table S2. Study cohort exclusion criteria.
Table S3. Study drugs.
Table S4. Covariates included in multivariable analyses.
Table S5. Geographic baseline patient characteristics.
Table S6. Risk of severe COVID‐19 among DOAC users vs. non‐use comparators according to DOAC subtype.
Table S7. Risk of ICU admission and death due to COVID‐19 analyzed separately.
Table S8. All‐cause mortality among DOAC users vs. non‐use comparator groups.


