Abstract
Since the emergence of the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) at the end of 2019, no vaccine has been approved to counter this infection and the available treatments are mainly directed against the immune pathology caused by the infection. The coronavirus disease 2019 (COVID‐19) is currently causing a worldwide pandemic, pointing the urgent need for effective treatment. In such emergency, drug repurposing presents the best option for a rapid antiviral response. We assess here the in vitro activity of nilotinib, imatinib and dasatinib, three Abl tyrosine kinase inhibitors, against SARS‐CoV‐2. Although the last two compounds do not show antiviral efficacy, we observe inhibition with nilotinib in Vero‐E6 cells and Calu‐3 cells with EC50s of 1.44 μM and 3.06 μM, respectively. These values are close to the mean peak concentration of nilotinib observed at steady state in serum, making this compound a potential candidate for treatment of COVID‐19 in vivo.
Keywords: COVID‐19, nilotinib, SARS‐CoV‐2, tyrosine kinases inhibitors
1. INTRODUCTION
At the end of 2019, severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), an enveloped positive‐sense RNA virus, was identified for the first time in Wuhan, a city in the Chinese province of Hubei, as the cause of a new pathology, which was later named coronavirus disease 2019 (COVID‐19). 1 SARS‐CoV‐2 belongs to the family Coronaviridae and shares 79% nucleotide sequence identity with SARS‐CoV and 96% with bat coronavirus RatG13. 1 , 2 The most frequent symptoms of COVID‐19 are cough, fever and weakness, but it can lead to severe and potentially fatal forms of pneumonia. Additionally, cytokine storm associated with SARS‐CoV‐2 infection (ie massive release of cytokines from the immune system) and immune pathology can lead to acute respiratory distress syndrome, responsible for a considerable number of deaths among infected patients together with coagulopathy. 1
Remdesivir, a nucleoside analogue that blocks the RNA polymerase of several coronaviruses, was the first antiviral drug with emergency use authorized in the United States. 3 A randomized double‐blind clinical trial showed a reduction in the length of hospitalization (12 vs 17 days) and a reduction in mortality (11.4% vs 15.2%) by day 29 in the remdesivir group compared with the placebo group. 3
Drug repurposing offers an optimal strategy to reprofile existing drugs, thereby reducing the time and minimizing the cost necessary for the development of an entirely new drug. In this context, imatinib and dasatinib, two Bcr‐Abl tyrosine kinase inhibitors, have been identified as inhibitors of SARS‐CoV and MERS‐CoV, and nilotinib of SARS‐CoV only. 4 , 5 , 6 The antiviral mechanism of action appears to involve the inhibition of virus‐cell fusion in vitro by blocking of the Abelson (Abl) kinases, Abl1 and Abl2, likely involved in coronavirus infection. 4 , 5
Based on these results, we sought to evaluate the in vitro antiviral activity of three tyrosine kinase inhibitors, imatinib, dasatinib and nilotinib, commonly prescribed for chronic myeloid leukaemia. Here, we show that nilotinib displays promising antiviral activity in two different cell lines and can be of interest for further investigation in clinical trials.
2. MATERIALS AND METHODS
The study was conducted in accordance with the Basic & Clinical Pharmacology & Toxicology policy for experimental and clinical studies. 7
2.1. Compounds
Nilotinib, dasatinib and imatinib were purchased from Alsachim (Illkirch‐Graffenstaden, France). The compounds were resuspended in DMSO at a concentration of 10 mM.
2.2. Cells and virus
Vero C1008 (clone E6) (ATCC CRL‐1586) were a kind gift from Prof Kobinger from University of Laval and were propagated in DMEM High Glucose + Glutamax supplemented with 10% foetal bovine serum (FBS) and 1% penicillin/streptavidin (pen/strep). Calu‐3 (ATCC HTB‐55) were a kind gift of Prof Chanson from the University of Geneva and were propagated in MEM + Glutamax supplemented with 10% FBS 1% pen/strep, non‐essential amino acids, HEPES and sodium pyruvate.
SARS‐CoV‐2/Switzerland/GE9586/2020 was isolated from a clinical specimen in the University Hospital of Geneva in Vero‐E6. Cells were infected, and the supernatant was collected 3 days post‐infection, clarified, aliquoted and frozen at −80°C and subsequently titrated by plaque assay in Vero‐E6.
2.3. Toxicity assay
Vero‐E6 (13 000 cells per well) were seeded in 96‐well plate. Nilotinib, dasatinib and imatinib were serially diluted in DMEM supplemented with 5% FBS and added on cells for 48 hours. Thiazolyl blue tetrazolium bromide solution (0.5 mg/mL) was added on cells for 3 hours at 37°C; subsequently, cells were lysed with pure DMSO, and absorbance was read at 570 nm. Percentages of viability were calculated by comparing the absorbance in treated wells and wells treated with DMSO in equal volume of the drugs. 50% cytotoxic concentration (CC50) was calculated with Prism 8 (GraphPad).
2.4. Inhibition assay on Vero‐E6 cells
Vero‐E6 cells (100 000 cells per well) were seeded in 24‐well plate. Nilotinib, dasatinib and imatinib were serially diluted in DMEM and added on cells for 1 hour at 37°C; subsequently, cells were infected with SARS‐CoV‐2 (MOI, 0.005 PFU/cell) for 1 hour at 37°C. The monolayers were then washed and overlaid with 0.8% avicel rc581 in medium supplemented with 5% FBS containing serial dilutions of compounds. Alternatively, to assess post‐infection efficacy, the cells were only treated with nilotinib at the time of addition of the medium containing avicel. Two days after infection, cells were fixed with 4% paraformaldehyde and stained with crystal violet solution containing ethanol. Plaques were counted, and the per cent inhibition of virus infectivity was determined by comparing the number of plaques in treated wells with the number in untreated control wells. 50% effective concentration (EC50) was calculated with Prism 8 (GraphPad).
2.5. Inhibition assay on Calu‐3 cells
Calu‐3 cells (25 000 cells per well) were seeded in 96‐well plate. Cells were infected with SARS‐CoV‐2 (MOI 0.02 PFU/cell) for 1 hour at 37°C. The monolayers were then washed and overlaid with medium containing serial dilutions of nilotinib. At 24 hpi, cells were lysed, and viral RNA was extracted with EZNA viral RNA kit (Omega Bio‐tek). SARS‐CoV‐2 RNA was quantified by qPCR with the QuantiTect Kit (Qiagen, 204443) with Sarbeco E gene primers and probe in a StepOne thermocycler (Applied Biosystems). Per cent inhibition of virus infectivity was determined by comparing viral load in treated wells with the viral load in untreated control wells. EC50 was calculated with Prism 8 (GraphPad).
3. RESULTS
Abl kinase inhibitors were previously reported to exert inhibitory activities against different viruses, including SARS‐CoV and MERS‐CoV by blocking the fusion between viral envelope and endosomal membrane. 4 , 5 , 6 Therefore, at first, we tested three Abl kinase inhibitors, namely nilotinib, imatinib and dasatinib, at non‐toxic concentration in Vero‐E6 cells by treating the cells starting 1h before infection. The only compound showing inhibitory activity is nilotinib with an EC50 of 1.88 µM (Table 1).
Table 1.
Antiviral activity of Abl kinase inhibitors in Vero‐E6 cells
| EC50 (µM) ± 95% CI | CC50 (µM) ± 95% CI | SI | |
|---|---|---|---|
| Dasatinib | >0.1 | 0.23 (0.21‐0.26) | n.a. |
| Imatinib | >5 | 18.4 (16.8‐20.0) | n.a. |
| Nilotinib | 1.88 (1.56‐2.27) | 29.7 (19.1‐50.0) | 15.8 |
95% CI, 95% confidence interval; CC50, 50% cytotoxic concentration; EC50, 50% effective concentration; SI, selectivity index.
Subsequently, nilotinib was also tested by only adding the compound 1h after inoculation. Nilotinib showed a comparable inhibitory activity in this condition, with an EC50 of 1.44 µM (Figure 1). Finally, we also confirmed the antiviral activity in the human respiratory cell line Calu‐3 (EC50 3.06 µM), to exclude any bias related to the green monkey cell line Vero‐E6 (Figure 1). In this cell line, the percentages of infection were derived from qPCR measurements, possibly causing the variation of the EC50 observed.
Figure 1.
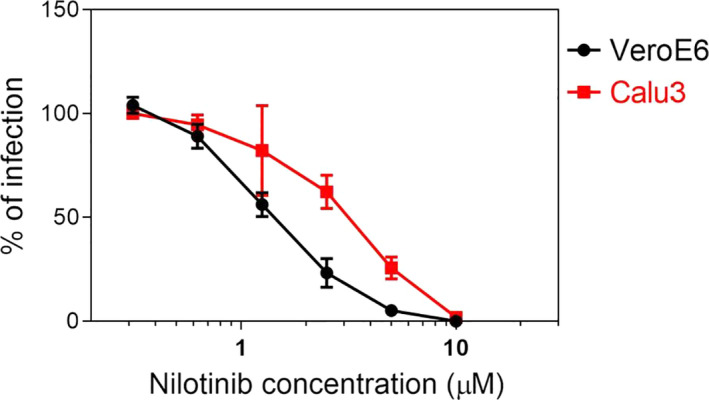
Inhibitory activity of nilotinib in Vero‐E6 and Calu‐3 cells. Cells were infected with SARS‐CoV‐2 and 1 h post‐inoculation treated with serial dilutions of nilotinib. The infection rate was evaluated at 48 hpi for Vero‐E6 and at 24 hpi for Calu‐3 cells. Results are mean and SEM of 3 independent experiments performed in duplicate
4. DISCUSSION
The current strategy to combat SARS‐CoV‐2 consists of supportive care with combination therapy using antiviral and anti‐inflammatory drugs, while many repurposed therapeutic drugs are undergoing clinical trials. In our work, imatinib and dasatinib were both inactive against SARS‐CoV‐2. These results contrast with previous publications in which these two drugs were active against both SARS‐CoV and MERS‐CoV, In addition to the difference in the targeted virus, the discrepancy could be related to the different toxicity observed in our cells, especially for dasatinib. Indeed, due to the toxicity of the compound, only low concentrations of the drug could be tested against the virus. The difference in the read‐out of the infection may also play a role: in our settings, the percentages of inhibition were calculated by plaque assays or qPCR, while in the cited work, it was measured by ELISA for MERS and cell viability for SARS. 6 Additionally, in parallel with the submission process of our manuscript, imatinib was shown to have no significant in vitro impact on SARS‐CoV‐2 infection and replication by Zhao et al, which supports our findings. 8
In contrast, nilotinib was previously reported to inhibit SARS‐CoV but not MERS‐CoV. 6 In our study, this drug is capable of interfering with the replication of SARS‐CoV‐2 in vitro in both Vero‐E6 and Calu‐3 cells, with an EC50 evaluated at 1.44 μM and 3.06 μM, respectively. In patients receiving nilotinib 400 mg twice daily, the mean peak concentration in plasma was 3.6 μM at steady state. 9 Therefore, at therapeutic plasma concentrations, nilotinib appears to reduce SARS‐CoV‐2 infection by approximately 50%, which is noteworthy. Furthermore, nilotinib accumulates in all body tissues in rat with tissue/blood ratios ranging between 10 and 40. 10 Therefore, expected concentrations in human lung epithelia should be much higher than measured EC50 in vitro. In addition, it is worth noting that nilotinib has an established safety profile for human use at therapeutic doses and is relatively well tolerated. 9 Moreover, in silico repurposing studies showed that nilotinib appears to bind the receptor‐binding domain of SARS‐CoV‐2 spike protein, which could prevent entry into host cells via the ACE2 receptor. Additional in silico studies are ongoing to understand the interaction between tyrosine kinase inhibitors and SARS‐CoV‐2 in order to elucidate the mechanism of action. 11
In conclusion, although preliminary, the results of this in vitro study demonstrate the promising antiviral activity of nilotinib, a Bcr‐Abl tyrosine kinase inhibitor, not previously investigated to combat SARS‐CoV‐2. Determining the mechanism of action of nilotinib's antiviral activity must be considered for future studies, and clinical studies have to be performed to confirm these promising data.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
ACKNOWLEDGEMENTS
The study was funded thanks to the financial support of the “Fondation privée des HUG” and the Carigest Foundation to CT.
Cagno V, Magliocco G, Tapparel C, Daali Y. The tyrosine kinase inhibitor nilotinib inhibits SARS‐CoV‐2 in vitro. Basic Clin Pharmacol Toxicol. 2021;128:621–624. 10.1111/bcpt.13537
Caroline Tapparel and Youssef Daali contributed equally to this work
REFERENCES
- 1. Lisi L, Lacal PM, Barbaccia ML, Approaching GG, Disease C. mechanisms of action of repurposed drugs with potential activity against SARS‐CoV‐2. Biochem Pharmacol. 2020;180:114169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Boni MF, Lemey P, Jiang X, et al. Evolutionary origins of the SARS‐CoV‐2 sarbecovirus lineage responsible for the COVID‐19 pandemic. Nat. Microbiol. 2020;5(11):1408‐1417. 10.1038/s41564-020-0771-4 [DOI] [PubMed] [Google Scholar]
- 3. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of covid‐19 — Final report. N Engl J Med 2020;383(19):1813‐1826. 10.1056/NEJMoa2007764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sisk JM, Frieman MB, Machamer CE. Coronavirus S protein‐induced fusion is blocked prior to hemifusion by Abl kinase inhibitors. J Gen Virol 2018;99(5):619‐630. 10.1099/jgv.0.001047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Coleman CM, Sisk JM, Mingo RM, Nelson EA, White JM, Frieman MB. Abelson kinase inhibitors are potent inhibitors of severe acute respiratory syndrome coronavirus and middle east respiratory syndrome coronavirus fusion. J Virol. 2016;90(19):8924‐8933. 10.1128/JVI.01429-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dyall J, Coleman CM, Hart BJ, et al. Repurposing of clinically developed drugs for treatment of Middle East respiratory syndrome coronavirus infection. Antimicrob Agents Chemother. 2014;58(8):4885‐4893. 10.1128/AAC.03036-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tveden‐Nyborg P, Bergmann TK, Lykkesfeldt J. Basic & Clinical Pharmacology & Toxicology Policy for Experimental and Clinical studies. Basic Clin Pharmacol Toxicol. 2018;123(3):233‐235. 10.1111/bcpt.13059 [DOI] [PubMed] [Google Scholar]
- 8. Zhao H, Mendenhall M, Deininger MW. Imatinib is not a potent anti‐SARS‐CoV‐2 drug. Leukemia. 2020;34(11):3085‐3087. 10.1038/s41375-020-01045-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kantarjian H, Giles F, Wunderle L, et al. Nilotinib in imatinib‐resistant CML and Philadelphia chromosome‐positive ALL. N Engl J Med. 2006;354(24):2542‐2551. 10.1056/NEJMoa055104 [DOI] [PubMed] [Google Scholar]
- 10. European Medicines Agency, Tasigna. https://www.ema.europa.eu/en/medicines/human/EPAR/tasigna
- 11. Deganutti G, Prischi F, Reynolds CA. Supervised molecular dynamics for exploring the druggability of the SARS‐CoV‐2 spike protein. J Comput Aided Mol Des. 2020. 10.1007/s10822-020-00356-4 [DOI] [PMC free article] [PubMed] [Google Scholar]


