Abstract
Although healthcare providers are actively involved in offering education, information and interventions for asthmatic patients, medication and therapeutic adherence remain low in the paediatric population, with estimates suggesting that adherence rates hover below 50%. A range of available digital health interventions has been explored in paediatric asthma with promising but variable results, limiting their widespread adoption in clinical practice. They include emerging technologies that yield the advantage of tracking asthma symptoms and medications, setting drug reminders, improving inhaler technique and delivering asthma education, such as serious games (video games designed for medical‐ or health‐related purposes), electronic monitoring devices, speech recognition calls, text messaging, mobile apps and interactive websites. Some of the proposed digital interventions have used multiple components, including educational and behavioural strategies and interactions with medical professionals. Overall, the implementation of such interventions may offer the opportunity to improve adherence and asthma control. In a state of emergency as the COVID‐19 pandemic, telemedicine can also play a central role in supporting physicians in managing children with asthma. This review evaluates the published literature examining digital health interventions for paediatric asthma and explores the most relevant issues affecting their implementation in practice and the associated evidence gaps, research limitations and future research perspectives.
Keywords: adherence, asthma, children, control, digital health, intervention, serious games, telemedicine
1. INTRODUCTION
Asthma is a common chronic disease in childhood, affecting approximately 10% of children worldwide. 1 The management of the disease is primarily aimed at maintaining symptoms control and reducing the risk of exacerbations. 2 Although most children achieve good control with standard therapies, such as inhaled corticosteroids (ICS) and/or one or more controllers, asthma still imposes a high burden, especially in children with uncontrolled symptoms. 3 , 4 A major cause of uncontrolled asthma is poor adherence to treatment, which has been described in 49%‐71% of paediatric patients, resulting in an increased risk of missed days of school, decline in lung function, emergency department visits, hospitalizations and even death. 5 , 6 , 7 , 8 , 9 , 10 Therefore, it is important to take into account treatment adherence, inhaler technique and self‐management education in the management of children with asthma . However, asthma management can be hampered by several factors related to individual, family, community, healthcare system and patient‐provider interaction domains. 11
Identifying interventions for promoting asthma treatment adherence and self‐management is essential to obtain and maintain symptoms control and, finally, improve disease outcomes. These interventions may take many forms, including patient/caregiver education, simplified drug regimens, school nursing. Nonetheless, most of the studies performed to date have not shown significant self‐management changes or better health outcomes due to improved adherence, mainly because these interventions are labour‐intensive and not easily transferable to daily clinical practice. 12 , 13 , 14 , 15
Over the last years, digital health emerged as a promising research area for achieving optimal and personalized asthma management. The so‐called electronic‐Health (e‐Health) solutions encompass various tools for self‐monitoring of symptoms, self‐management action plans, and patient education materials to improve treatment adherence and disease control. 16 They include emerging technologies that yield the advantage of tracking asthma symptoms and medications, setting drug reminders, improving inhaler technique and delivering asthma education, such as serious games (video games designed for medical or health‐related purposes), electronic monitoring devices, speech recognition calls, text messaging, mobile‐Health (m‐Health) apps and interactive websites. 17 Of note, in the times of the pandemic, telemedicine would allow for quick contact and maintain continuity of care, especially for patients with chronic diseases.
A range of digital health interventions has been tested in paediatric asthma with variable results, which have currently limited their widespread adoption in real life, mainly due to heterogeneity of trials and lack of long‐term effects. 18
This review evaluates the published literature examining digital health interventions for paediatric asthma and exploring the most relevant issues affecting their implementation in practice and the associated evidence gaps, research limitations and future research perspectives.
2. SERIOUS GAMES: AN INNOVATIVE APPROACH TO CHILDREN WITH ASTHMA
‘Serious games’, (SGs) that are games that do not have entertainment, enjoyment or fun as their primary purpose, have emerged as a new generation of videogames that offer the opportunity for constructive learning and training. 18 , 19 Unlike traditional video games, SGs are built to convey meaningful information through interactive backgrounds similar to real‐life situations.
One of the earliest SGs was an educational software program called ‘Asthma Control’: players could help Spacer, a superhero with asthma, manage his asthma by making decisions about taking rescue medication for acute symptoms and preventive medications regularly and consulting physicians for advice. Also, maintaining of normal activity, such as school attendance, was one of the proposed educational objectives. 20 This SG was tested in a randomized controlled trial (RCT) of 148 ‘inner‐city’ children, aged 7 to 12 years. All children in the intervention group enjoyed playing the game and reached higher knowledge on asthma than the control group; however, no significant differences in parents’ knowledge, children's behaviour related to asthma care and asthma severity were noted. 20
In a recent systematic review, Drummond et al reported the impact of 10 SGs focusing on asthma education targeting children and adolescents (Table 1). 21 The most used console or gaming systems were computers, and various games were used in each study, such as management simulation, mini‐games and quiz, and adventure games. 21 The 10 SGs also include: ‘Asthma Files’, where players are ‘secret agents’ encouraged to explore and find out as much about asthma self‐management as possible; ‘Bronkie's Asthma Adventure’, where children improve their self‐management strategies and receive feedback on their performance; ‘Wee Willie Wheezie’, where patients guide an asthmatic boy to navigate a home setting while avoiding various allergens and taking his medications to prevent asthma symptoms, exacerbations and trips to the hospital. 22 , 23 , 24 , 25
Table 1.
Serious Games developed and tested for asthma (modified from Drummond et al 21
| Type of Serious Game | Game Genre | Targeted age | Primary Learning Objectives | Reference |
|---|---|---|---|---|
| Asthma Command | Management simulation | 7‐12 y |
Knowledge of the disease, Medication: adherence, education and safety |
Rubin et al 26 |
| Watch, Discover, Think and Act | Training simulation |
6‐17 y; 9‐13 y |
Knowledge of the disease, Medication: adherence, education and safety |
Bartholomew et al 27 ; Shegog et al 28 |
| Air Academy: The Quest for Airtopia | Adventure | 6‐12 y |
Knowledge of the disease, Medication: adherence, education and safety |
Yawn et al 29 |
| Asthma control | Adventure | 3‐12 y |
Knowledge of the disease, Medication: adherence, education and safety |
Homer et al 20 |
| The Asthma Files | mini‐games, quiz | 7‐14 y |
Knowledge of the disease, Medication: adherence, education and safety |
McPherson et al 22 ; McPherson et al 23 |
| Wee Willie Wheezie | Platform | 7‐12 y |
Knowledge of the disease, Medication: adherence, education and safety |
Huss et al 24 |
| Bronkie's Asthma Adventure | Adventure | 5‐12 y |
Knowledge of the disease, Inhaler technique, Medication: adherence, education and safety |
Shames et al 24 |
| Quest for the code | Mini‐games, quiz, adventure | 8‐11 y |
Knowledge of the disease, Medication: adherence, education and safety |
Howell et al 30 |
| Asthma: 1,2,3… Breath! | Board game | 14‐18 y |
Knowledge of the disease, Medication: adherence, education and safety |
Kaufmann et al 31 |
| Lungtropolis | Mini‐games, quiz, puzzle | 5‐10 y |
Knowledge of the disease, Medication: adherence, education and safety |
Schroeder et al 32 |
Learning objectives of the SGs mentioned above widely vary from teaching the pathophysiology of asthma to the recognizing of triggers and symptoms and the appropriateness of inhaler techniques, therapeutic adherence and use of health services. Besides reporting a high level of satisfaction and fun associated with SGs, the modification of both children's and parents’ self‐efficacy and knowledge were evaluated, as well as changes in behaviour and clinical outcomes. 20 , 32 Most of the SGs were associated with improving children's knowledge while assessing the impact of SGs on clinical asthma outcomes led to mixed results. In this context, no significant differences were noted in acute visits and/or hospital admissions for asthma between the intervention (SGs) and the control group in the study evaluating clinical outcomes. 21 This can be explained by the fact that improving children's knowledge of the disease alone is insufficient to change patients’ behaviours and positively influence asthma burden. Thus, educational interventions in the form of SGs should be extended to involve parents and school together and include all the asthma control elements. 3
The role of SGs has also been investigated in teaching how to perform lung function tests, namely spirometry. Among others, ‘SpiroGame’ is an interactive respiratory game developed to teach spirometry. 33 Through a computer‐animated program, children are facilitated to perform forced spirometry manoeuvers using multiple targets in a step‐by‐step manner. In the first step, the game teaches the child to differentiate between inhalation and exhalation by simulating a caterpillar crawling on a window to an apple for 30s of tidal breathing. In the second step, the game teaches performance of forced vital capacity by simulating a bee flying from flower to flower. 34 This approach has been evaluated both in healthy children and in children with asthma. Many children performed reliable forced expiratory flow‐volume curves, with an overall success rate increasing with age. These were consistent with most of the established criteria by the American Thoracic Society/European Respiratory Society. 34 In comparison with verbal coaching techniques, ‘SpiroGame’ yielded comparable results and was also sensitive in detecting airway obstruction in children with moderate and severe symptoms.
Education delivered through SGs may facilitate healthcare professionals' specific tasks. Being child‐centred and actively involving the child in his/her care, SGs may also enhance communication between children, their parents and clinicians by discussing progress achieved on a particular game. Also, including parents and caregivers in the learning process may positively impact family health behaviours.
Although SGs have shown good profile acceptability and feasibility and are effective in gaining knowledge, their use in paediatric asthma requires further studies on clinical outcomes.
3. DIGITAL HEALTH TOOLS FOR ASTHMA ADHERENCE
3.1. Digital health tools available to monitor adherence
Electronic monitoring devices (EMDs): used in conjunction with inhalers, EMDs measure date/time of drug actuations even though they currently are not suitable for checking whether patients are inhaling correctly. 35 EMDs are useful for identifying children with poor symptoms control, and their use could be particularly relevant in those with severe and/or difficult to treat asthma. 36 EMDs may promote the control of asthma symptoms and medication adherence and may provide data not biased by patient self‐reporting. Among the commercial devices, the Doser CT™ is the cheapest EMD for ICS as it uses older technology than some of the newer EMDs; it can be used with Meter Dose Inhalers (MDIs) to count the number of doses used within a day, but it does not reveal if the amounts of doses activated are due to dose dumping and its memory is only 30 days long. 37 The gold standard among commercially available tools are currently considered Smartinhalers; they can be fitted to many different types of inhalers and contain a microchip that objectively detects and stores the date and time of each doses used, revealing if the patient dumps doses just before clinical visit. 38 In a study on twenty‐six children aged between 6 and 14 years, adherence was measured using a Smartinhaler™ (Nexus 6, Auckland, NZ). Subjects were randomized to either be informed of their adherence during medical consultations or remained unknown to the parent, child and physician. Adherence was significantly higher in the intervention group (79% vs 58%, P < .01); however, a significant difference between groups was not found for asthma control and lung function, which may be because Smartinhalers do not provide information about inhalation technique. 39 Newer versions of Smartinhaler are Bluetooth enabled so that patients and their physicians can download an app to monitor adherence to therapy. 40
3.2. Digital health tools available as reminders of adherence
Speech recognition (SR) calls: SR uses software that creates computer‐generated telephone conversations. The software can tailor each call with medical and demographic information from the electronic health record database to support patients and/or caregivers who desire help with their medication plan. In a 24‐month clinical trial, 1187 children with persistent asthma were randomized to a computerized SR intervention or usual care. SR telephone calls to parents were triggered when an ICS refill was due or overdue. In the intention‐to‐treat analysis, ICS adherence was 25.4% higher in the intervention group than in the usual care group (24‐month mean adherence: 44.5% vs 35.5%, respectively; P < .001), suggesting the potential for such a digital intervention in children with persistent asthma. 41
Text messages: personalized text messaging include automated messages (ie standardized messages sent at the same time every day regardless of whether medication had already been taken), or text messages delivered in response to missed doses. 42 Personalized text message reminders were used in a 6‐month longitudinal cross‐over study in 64 adolescents with poorly controlled asthma. Adherence increased by 2.75% each month relative to no intervention, along with modest improvement in primary outcome measures of self‐reported asthma control and QoL. Although the acceptability of the text messaging system was high among participants, controller adherence declined over time. 43
Recently, a real‐time medication monitoring device recording date and time of actuations was given in a 12‐month RCT to 209 children with persistent asthma. In the intervention group, tailored text message reminders sent when an ICS dose was at risk of omission allowed a higher mean adherence than the control group: 69.3% vs 57.3% (difference 12.0%, 95% CI 6.7%–17.7%). No differences were found for the secondary outcomes of as symptoms control, QoL and asthma exacerbations. This suggests that the observed improvement in ICS adherence is not likely to be sufficient for gaining a clinically relevant improvement. 44
Electronic reminders: electronic tools allowing the patient to remember each medication dose through audio‐visual devices. Reminder systems are usually designed to ring only when an actuation/dose has been missed. 45 They can be connected to mobile devices and take advantage of the calendar of events/appointments with schedules configured based on the treatment plan. The Smartinhaler technology platform has recently been combined with audio‐visual alerts on the sensor device to remind patients if any medication is missed, and data on inhaler use can be uploaded using the Smartinhaler app or the Smartinhaler Connection Centre for sharing with clinicians via the software. 46
The effect of an EMD (SmartTrack; Nexus6, Auckland, New Zealand) recording time and number of actuations was investigated in a 6‐month RCT on 220 schoolchildren with persistent asthma. Children in the intervention group were also provided with an audio‐visual reminder function and showed a median percentage adherence of 84% compared with 30% in the control group (P < .0001). Moreover, they showed significantly higher symptoms control from baseline to 6 months than the control group (P = .008). However, it should be noted the lack of significant difference between groups for the primary outcome (number of days absent from school), likely because the study was underpowered to detect differences or because children with good adherence were using their devices incorrectly. 47
Larger and rigorous RCTs with post‐intervention assessments are required to confirm the long‐term efficacy of digital interventions in assessing adherence and other asthma‐related outcomes, especially in managing children with persistent asthma.
4. DIGITAL MULTICOMPONENT INTERVENTIONS AND MOBILE‐HEALTH
4.1. Digital health tools for delivering multicomponent interventions
Some of the proposed digital interventions have used multiple components, including educational and behavioural strategies and interactions with medical professionals.
Peer support group meetings and peer asthma messages delivered via mp3 players improved self‐reported adherence to ICS in a 10‐week RCT on 68 low‐income adolescents with persistent asthma and poor adherence. No significant difference in objectively measured adherence was observed in comparison with controls. Of note, adherence declined in both groups over the study period, even though self‐reported adherence resulted significantly higher than objectively measured adherence at the end of the study (P < .0001). 48 This finding suggests that healthcare professionals should be aware that there may be different priorities for adolescents and their caregivers for the management of asthma. About digital health, the use of electronic monitoring tools should be considered on a case‐by‐case basis, also taking into account patients’ preferences in relation to asthma management within a context of shared decision‐making. Actively engaging patients and their caregivers in this process may reinforce the relationship with healthcare providers and ultimately improve self‐management and disease outcomes. 49
An RCT testing the use of a website and text message reminders found a positive effect on medication adherence in adolescents with asthma. Forty‐six subjects randomized to the intervention were asked to create a medication schedule and receive text message reminders at designated medication administration times for 3 weeks. Controls received action lists as a part of their usual care. Although the authors did not perform an objective evaluation of treatment adherence, the intervention was associated with significant improvements in self‐reported adherence (P = .011), QoL (P = .037) and self‐efficacy (P = .016). Interestingly, no significant improvement of asthma control was observed likely because most subjects in this study had good asthma control at baseline or because self‐reported adherence was overestimated. 50
The use of a multidimensional web platform, including educational activities and interactive communication with healthcare providers, provided promising results in children with low adherence to asthma medication. An interactive website designed for education, monitoring and communication with primary care providers was tested in comparison with a usual care in a 6‐month randomized pilot study on 58 children with persistent asthma, resulting in significantly improved adherence (objectively monitored by electronic device or dose counter) to preventer medications in the intervention group, even if only among the subgroup of children with low adherence (<75%) at baseline (P < .01). Noteworthy, asthma knowledge significantly increased in the intervention group (P = .03). However, no significant differences were found with regard to clinical outcomes (eg days of wheezing, nights woke‐up, days of limited activity from asthma, days missed school for asthma, emergency room or acute visits to a physician for asthma) likely due to the small sample size which resulted in limited statistical power. 51
A multicomponent intervention based on the use of a smart nebulizer connected to smartphones via a mobile app and an interactive website through which the paediatrician reminded the parents of drug doses missed was tested in 65 preschool wheezers participating in a 12‐week study, vs conventional nebulization. The smart nebulizer significantly improved the rate of objective adherence to ICS in the intervention group vs the control group after 4, 8 and 12 weeks (86.67% vs 62.86%, 76.67% vs 51.42% and 67.33% vs 40.00%, respectively, P < .05), along with day‐ and night‐time wheezing scores (P < .05). Additional outcomes such as frequency of emergency visits, frequency of respiratory infections, antibiotics or systemic steroid usage, and additional treatment cost during the study period were significantly lower in the smart nebulization group with respect to the conventional nebulization group (P < .05). However, the lack of significant differences was noted between the two groups about the frequency and severity of recurrent wheezing or hospitalization frequency. This might suggest that either ICS treatment was not appropriate for many of the children or that clinical application of such digital device in preschool children may need further improvement. 52
4.2. Mobile‐health
The widespread use of smartphones contributed to the recent advancements in m‐Health, an emerging healthcare model that is achievable through mobile devices. More than 500 asthma‐related apps were reported in 2019, mainly providing health education, compliance to therapies, symptom tracking and environmental alerts. However, despite the significant number of available mobile apps for asthma, their use in clinical settings is not validated yet. 53 An electronic medication monitor integrated with a smart app has been tested in a small proof‐of‐concept 8‐week study including underserved minority adolescents with asthma: this intervention based on low‐literacy design strategies and basic principles of behaviour change demonstrated an improvement in ICS adherence and asthma control among the study population, showing a good profile of acceptability and feasibility. 54 More recently, a 3‐month study evaluating the effect of an education program (MyTherapeutic Education Program—MyTEP) coupling multidisciplinary TEP with a mHealth Program (mHP—smartphone app) for 50 children with mild‐moderate asthma failed to show significant differences in self‐reported adherence to medication between children randomized to receive MyTEP vs mHP alone, despite significant gains in asthma control (P = .03) and QoL (P = .05). This may be related to the high levels of self‐reported adherence in both groups at baseline, probably due to children's awareness of taking part in a trial evaluating asthma outcomes, including adherence, which might have enhanced their motivation in following the medication prescription. 55
A summary of emerging e‐Health solutions for asthma management is provided in Table 2. 53 , 56 , 57 , 58 , 59 , 60 , 61 , 62 Overall, both digital stand‐alone and combined multicomponent interventions showed promising results on treatment adherence and clinical outcomes. Nevertheless, inconsistencies between published results exist and might be mainly ascribed to different study populations and design.
Table 2.
Emerging e‐Health solutions for asthma management
| e‐Health solutions | Measured parameters | Comments | References |
|---|---|---|---|
| Electronic monitoring devices | |||
|
Digihaler™ |
Time of inhaler use, the peak of inspiratory flow rate (PIFR), time of PIFR, inspiratory volume and duration. | Its efficacy has not been evaluated; one pilot study in adults has shown its ability to predict asthmatic exacerbations. | Safioti et al, 2019. 56 |
| Propeller Health System | The inhaler sensor measures date, time, and the number of doses taken. Asthma Health Platform App assesses: the location of inhaler uses with GPS technology, current weather/pollen counts/air pollution and self‐report asthma symptoms/triggers. | This technology is portable and showed high acceptability among patients (children and adults), and improvements in asthma control. | Merchant et al, 2016. 57 |
| Hailie™ solution, previously known as SmartInhaler™ platform | The inhaler sensor measures dates, time, number of inhaler actuation, and missed doses. Hailie™ App assesses medication adherence and reminds daily medication. | This sensor has demonstrated high user acceptability and efficacy in increasing medication adherence in children and adults. |
Charles et al, 2007. 58 Foster et al, 2012. 59 |
| Mobile‐based applications | |||
| Mobile‐based Apps | The vast majority of Apps provide self‐monitoring of asthma symptoms, triggers and medication use. | Apps showed the low quality of evidence in improving asthma control, lung function, and quality of life both in children and in adults. Apps have not been validated for clinical use and may show a high risk of lost private health information. |
Wu et al, 2015. 60 Ramsey et al, 2019. 61 |
| Wearable technologies | |||
| Fitbit™ | Fitbit™ measures heart rate, steps/day, physical activity, sedentary time, sleep efficiency and wake counts. | Fitbit™ is portable and commercially available. It has been tested for monitoring activity and sleep in children. Fitbit‐derived sleep quality correlates with PROMIS paediatric asthma impact score. |
Bian et al, 2017. 62 Jaimini et al, 2018. 53 |
Abbreviations: Apps, applications. GPS, Global Positioning System. PIFR, Peak of Inspiratory Flow Rate. PROMIS, Patient‐Reported Outcomes Measurement Information System.
5. THE POTENTIAL ROLE OF TELEMEDICINE IN THE MANAGEMENT OF PAEDIATRIC ASTHMA
Telemedicine involves the use of information and communication technologies to improve patient outcomes by increasing access to care and medical information. 63 Previous data on telemedicine in paediatric asthma management are available. Trials of telemedicine interventions were mainly applied in school settings, providing counselling services and managing exacerbations. The impact of telemedicine on asthma‐related clinical outcomes, such as symptoms, pulmonary function, healthcare utilization and medication use, was also investigated, as well as patient/parent satisfaction and QoL (Figure 1). 64 , 65 As reported by recent systematic reviews, the available evidence supporting the introduction of telemedicine for asthma management showed conflicting data, but none of the included studies indicated adverse effects. 64 , 65
Figure 1.
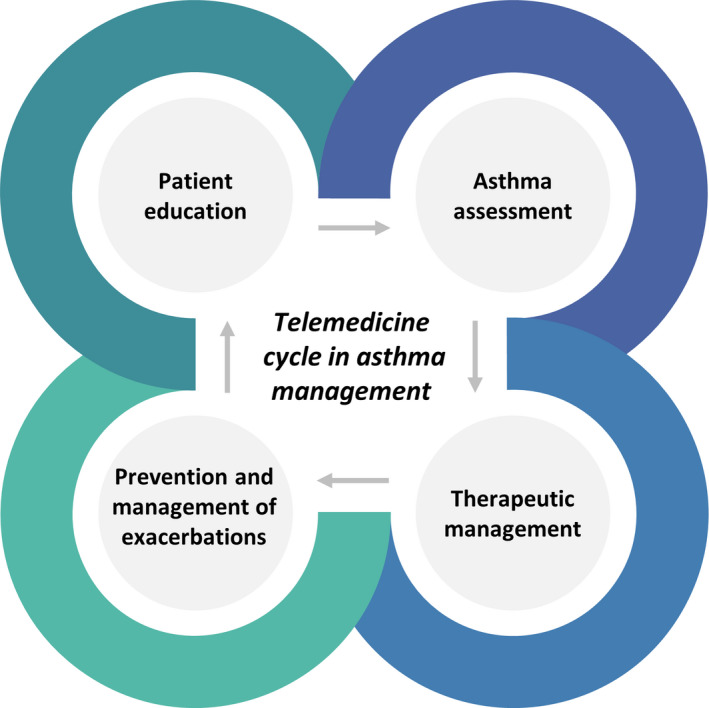
Telemedicine cycle in asthma management
In the context of the rapid worldwide spread of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), telemedicine might provide an additional and essential resource for managing children with asthma, replacing in‐person visits. 66 , 67 , 68 , 69 Telemedicine may also be helpful to patients with severe or uncontrolled asthma who might be at increased risk per se, due to lack of monitoring caused by social distancing and lockdowns. 70 , 71
In order to prevent the risk of SARS‐CoV‐2 transmission, as well as gain and maintain asthma control, national and international guidelines were promptly published to provide new and essential recommendations for the management of paediatric asthma during the pandemic. 70 , 72 , 73 , 74 , 75 Firstly, telemedicine promotes social distancing. 70 Particularly, telemedicine may (a) limit the exposure of healthcare providers to potentially infected patients, (b) avoid the patient‐to‐patient viral transmission, protecting children with immunodeficiencies or other chronic comorbidities and (c) provide a rapid evaluation for potential viral infection. 76 Secondly, the availability of online communication platforms may easily allow virtual consultations for first and follow‐up visits of asthmatic children and share clinical data (investigations, imaging and laboratory results). In this context, the suspension of spirometry during the COVID‐19 pandemic (as it is a possible aerosol‐generating procedure) represents a barrier to proper asthma diagnosis and monitoring. 73 Thus, telemedicine should also be considered for remote lung function testing, as many innovative and emerging approaches are becoming available. They include peak expiratory flow devices, portable electronic spirometers, portable exhaled nitric oxide measurement and novel digital health tools such as smartphone microphone spirometers. 77 Most of these devices that are commercially available are also designed to download results onto mobile devices or computers, facilitating transmission to and monitoring by healthcare professionals. While considering the limitations, including cost, lack of technique feedback and variable accuracy, some of these devices, such as portable spirometers, may be valuable in‐home monitoring in some settings, integrating virtual care with critical physiological data. Finally, telemedicine may help paediatricians manage mild‐moderate asthmatic exacerbations that do not show ‘red flags’, requiring urgent care or tests for COVID‐19.
However, although telemedicine's potential, no studies have been realized to assess its real benefits and efficacy in managing asthma in children during this period of health emergency; thus, extensive and multicentric studies are truly indispensable.
6. FUTURE DIRECTIONS AND CONCLUSION
The increased use of health digital devices will likely become a relevant aspect of a proactive asthma care model in the next few years (Figure 2). Extrapolated data will allow physicians to provide personalized tools, tailored solutions to improve child health, improve symptom reports and appropriate specialty referrals. Before such an approach can be widely integrated in routine clinical practice, the acceptability and feasibility should be ascertained among all patients, including those disproportionately affected by the disease. Indeed, most tools have not been designed to address barriers faced by racial/ethnic minority groups or those of low socioeconomic status and poor health literacy. 78
Figure 2.
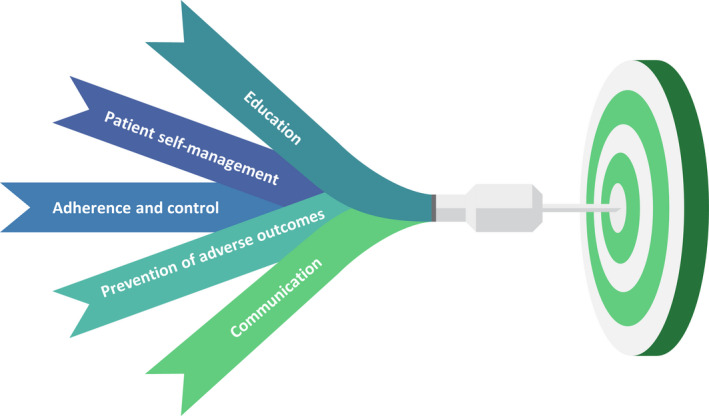
Future goals of digital health interventions in paediatric asthma
Overall, future research in this field should be based on larger and rigorous controlled trials with objective adherence assessment and post‐intervention evaluation of long‐term efficacy. 42 Finally, unique regulatory concerns need to be addressed with the increasing use of these new technologies. Not all devices require approval by regulatory bodies or need an evidence‐based background to be marketed.
The increasing availability of technology‐based solutions, only if combined with the clinician's equipment, will offer more successful opportunities in reaching the full benefit for patients and health systems alike.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
Ferrante G, Licari A, Marseglia GL, La Grutta S. Digital health interventions in children with asthma. Clin Exp Allergy.2021;51:212–220. 10.1111/cea.13793
Giuliana Ferrante and Amelia Licari are equally contributing co‐first authors.
Contributor Information
Giuliana Ferrante, Email: amelia.licari@unipv.it.
Amelia Licari, Email: amelia.licari@unipv.it.
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
REFERENCES
- 1. Ferrante G, La Grutta S. The burden of pediatric asthma. Front Pediatr. 2018;6:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pocket Guide for Asthma Management and Prevention. Global Initiative for Asthma; 2019. Available at: https://ginasthma.org
- 3. Licari A, Brambilla I, Marseglia A, De Filippo M, Paganelli V, Marseglia GL. Difficult vs. severe asthma: definition and limits of asthma control in the pediatric population. Front Pediatr. 2018;6:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fleming L, Murray C, Bansal AT, et al. The burden of severe asthma in childhood and adolescence: results from the paediatric U‐BIOPRED cohorts. Eur Respir J. 2015;46:1322‐1333. [DOI] [PubMed] [Google Scholar]
- 5. Desager K, Vermeulen F, Bodart E. Adherence to asthma treatment in childhood and adolescence – a narrative literature review. Acta Clin Belg. 2018;73:348‐355. [DOI] [PubMed] [Google Scholar]
- 6. Sullivan PW, Ghushchyan V, Navaratnam P, et al. National prevalence of poor asthma control and associated outcomes among school‐aged children in the United States. J Allergy Clin Immunol Pract. 2018;6(536–544):e1. [DOI] [PubMed] [Google Scholar]
- 7. Jentzsch NS, Camargos P, Sarinho ES, Bousquet J. Adherence rate to beclomethasone dipropionate and the level of asthma control. Respir Med. 2012;106:338‐343. [DOI] [PubMed] [Google Scholar]
- 8. Klok T, Kaptein AA, Duiverman EJ, Brand PL. It's the adherence, stupid (that determines asthma control in preschool children)!. Eur Respir J. 2014;43:783‐791. [DOI] [PubMed] [Google Scholar]
- 9. Herndon JB, Mattke S, Evans Cuellar A, Hong SY, Shenkman EA. Anti‐inflammatory medication adherence, healthcare utilization and expenditures among Medicaid and children's health insurance program enrollees with asthma. Pharmacoeconomics. 2012;30:397‐412. [DOI] [PubMed] [Google Scholar]
- 10. Suissa S, Ernst P, Benayoun S, Baltzan M, Cai B. Low‐dose inhaled corticosteroids and the prevention of death from asthma. N Engl J Med. 2000;343:332‐336. [DOI] [PubMed] [Google Scholar]
- 11. McQuaid EL. Barriers to medication adherence in asthma: The importance of culture and context. Ann Allergy Asthma Immunol. 2018;121:37‐42. [DOI] [PubMed] [Google Scholar]
- 12. Normansell R, Kew KM, Stovold E. Interventions to improve adherence to inhaled steroids for asthma. Cochrane Database Syst Rev. 2017;4:CD012226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mosnaim GS, Pappalardo AA, Resnick SE, et al. Behavioral interventions to improve asthma outcomes for adolescents: a systematic review. J Allergy Clin Immunol Pract. 2016;4:130‐141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Salazar G, Tarwala G, Reznik M. School‐based supervised therapy programs to improve asthma outcomes: current perspectives. J Asthma Allergy. 2018;11:205‐215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Harrington CB, Langhans E, Shelef DQ, Savitz M, Whitmore C, Teach SJ. A pilot randomized trial of school‐based administration of inhaled corticosteroids for at‐risk children with asthma. J Asthma. 2018;55:145‐151. [DOI] [PubMed] [Google Scholar]
- 16. Bonini M. Electronic health (e‐Health): emerging role in asthma. Curr Opin Pulm Med. 2017;23:21‐26. [DOI] [PubMed] [Google Scholar]
- 17. Bonini M, Usmani OS. Novel methods for device and adherence monitoring in asthma. Curr Opin Pulm Med. 2018;24:63‐69. [DOI] [PubMed] [Google Scholar]
- 18. Licari A, Ferrante G, Marseglia GL, et al. What is the impact of innovative electronic health interventions in improving treatment adherence in asthma? The pediatric perspective. J Allergy Clin Immunol Pract. 2019;7:2574‐2579. [DOI] [PubMed] [Google Scholar]
- 19. Michael DR, Chen SL.Serious games: games that educate, train, and inform. Muska & Lipman/Premier‐Trade. 2005. Available from: https://trove.nla.gov.au/work/20014497. Accessed July 12, 2019.
- 20. Homer C, Susskind O, Alpert HR, et al. An evaluation of an innovative multimedia educational software program for asthma management: report of a randomized, controlled trial. Pediatrics. 2000;106:210‐215. [PubMed] [Google Scholar]
- 21. Drummond D, Monnier D, Tesnière A, et al. A systematic review of serious games in asthma education. Pediatr Allergy Immunol. 2017;28:257‐265. [DOI] [PubMed] [Google Scholar]
- 22. McPherson A, Forster D, Glazebrook C, et al. The asthma files: evaluation of a multimedia package for children's asthma education. Paediatr Nurs. 2002;14:32‐35. [DOI] [PubMed] [Google Scholar]
- 23. McPherson AC, Glazebrook C, Forster D, et al. A randomized, controlled trial of an interactive educational computer package for children with asthma. Pediatrics. 2006;117:1046‐1054. [DOI] [PubMed] [Google Scholar]
- 24. Shames RS, Sharek P, Mayer M, et al. Effectiveness of a multicomponent self‐management program in at‐risk, school‐aged children with asthma. Ann Allergy Asthma Immunol. 2004;92:611‐618. [DOI] [PubMed] [Google Scholar]
- 25. Huss K, Winkelstein ML, Crosbie K, et al.‘Backpack adventures in asthma’: Interactive multimedia computer game piques childrens’ interest in asthma. In: Journal of Allergy and Clinical Immunology. Mosby, Inc 11830 Westline Industrial Dr, St Louis, MO 63146–3318 USA 2001: S71.
- 26. Rubin DH, Leventhal JM, Sadock RT, et al. Educational intervention by computer in childhood asthma: a randomized clinical trial testing the use of a new teaching intervention in childhood asthma. Pediatrics. 1986;77:1‐10. [PubMed] [Google Scholar]
- 27. Bartholomew LK, Gold RS, Parcel GS, et al. Watch, Discover, Think, and Act: evaluation of computer‐assisted instruction to improve asthma self‐management in inner‐city children. Patient Educ Couns. 2000;39:269‐280. [DOI] [PubMed] [Google Scholar]
- 28. Shegog R, Bartholomew LK, Parcel GS, et al. Impact of a computer‐assisted education program on factors related to asthma self‐management behavior. J Am Med Inform Assoc. 2001;8:49‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yawn BP, Algatt‐Bergstrom PJ, Yawn RA, et al. An in‐school CD‐ROM asthma education program. J Sch Health. 2000;70:153‐159. [DOI] [PubMed] [Google Scholar]
- 30. Howell K.‘Quest for the Code’: a study of a computer based education program for children with asthma. Psychol – Diss Published Online First: 1 January 2005. http://surface.syr.edu/psy_etd/32
- 31. Kaufman D, Sauve L, Renaud L. Enhancing learning through an online secondary school educational game. J Educ Comput Res. 2011;44:409‐428. [Google Scholar]
- 32. Schroeder S, Swartz L, Gau J, et al.A randomized, controlled trial of LungtropolisTM: An internet asthma game for children and companion website for parents. Unpubl. Manuscr.
- 33. Vilozni D, Barak A, Efrati O, et al. The role of computer games in measuring spirometry in healthy and "asthmatic" preschool children. Chest. 2005;128:1146‐1155. [DOI] [PubMed] [Google Scholar]
- 34. Vilozni D, Barker M, Jellouschek H, et al. An interactive computer‐animated system (SpiroGame) facilitates spirometry in preschool children. Am J Respir Crit Care Med. 2001;164:2200‐2205. [DOI] [PubMed] [Google Scholar]
- 35. Chan AH, Reddel HK, Apter A, Eakin M, Riekert K, Foster JM. Adherence monitoring and e‐health: how clinicians and researchers can use technology to promote inhaler adherence for asthma. J Allergy Clin Immunol Pract. 2013;1:446‐454. [DOI] [PubMed] [Google Scholar]
- 36. Jochmann A, Artusio L, Jamalzadeh A, et al. Electronic monitoring of adherence to inhaled corticosteroids: an essential tool in identifying severe asthma in children. Eur Respir J. 2017;50:pii1700910. [DOI] [PubMed] [Google Scholar]
- 37. Simmons MS, Nides MA, Kleerup EC, et al. Validation of the Doser, a new device for monitoring metered‐dose inhaler use. J Allergy Clin Immunol. 1998;102:409‐413. [DOI] [PubMed] [Google Scholar]
- 38. Vrijens B, Dima AL, Van Ganse E, et al. What we mean when we talk about adherence in respiratory medicine. J Allergy Clin Immunol Pract. 2016;4:802‐812. [DOI] [PubMed] [Google Scholar]
- 39. Burgess SW, Sly PD, Devadason SG. Providing feedback on adherence increases use of preventive medication by asthmatic children. J Asthma. 2010;47:198‐201. [DOI] [PubMed] [Google Scholar]
- 40. Pearce CJ, Fleming L. Adherence to medication in children and adolescents with asthma: methods for monitoring and intervention. Expert Rev Clin Immunol. 2018;14:1055‐1063. [DOI] [PubMed] [Google Scholar]
- 41. Bender BG, Cvietusa PJ, Goodrich GK, et al. Pragmatic trial of health care technologies to improve adherence to pediatric asthma treatment: a randomized clinical trial. JAMA Pediatr. 2015;169:317‐323. [DOI] [PubMed] [Google Scholar]
- 42. Ramsey RR, Plevinsky JM, Kollin SR, Gibler RC, Guilbert TW, Hommel KA. Systematic review of digital interventions for pediatric asthma management. J Allergy Clin Immunol Pract. 2020;8:1284‐1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Britto MT, Rohan JM, Dodds CM, et al. A randomized trial of user‐controlled text messaging to improve asthma outcomes: a pilot study. Clin Pediatr (Phila). 2017;56:1336‐1344. [DOI] [PubMed] [Google Scholar]
- 44. Vasbinder EC, Goossens LM, Rutten‐van Mölken MP, et al. e‐Monitoring of Asthma Therapy to Improve Compliance in children (e‐MATIC): a randomised controlled trial. Eur Respir J. 2016;48:758‐767. [DOI] [PubMed] [Google Scholar]
- 45. Chan AH, Harrison J, Black PN, Mitchell EA, Foster JM. Using electronic monitoring devices to measure inhaler adherence: a practical guide for clinicians. J Allergy Clin Immunol Pract. 2015;3(335–49):e495. [DOI] [PubMed] [Google Scholar]
- 46. Smartinhaler for asthma. Available at. https://www.nice.org.uk/advice/mib90/resources/smartinhaler‐for‐asthma‐pdf‐63499461673669
- 47. Chan AH, Stewart AW, Harrison J, et al. The effect of an electronic monitoring device with audiovisual reminder function on adherence to inhaled corticosteroids and school attendance in children with asthma: a randomised controlled trial. Lancet Respir Med. 2015;3:210‐219. [DOI] [PubMed] [Google Scholar]
- 48. Mosnaim G, Li H, Martin M, et al. The Impact of Peer Support and mp3 Messaging on Adherence to Inhaled Corticosteroids in Minority Adolescents with Asthma: A Randomized, Controlled Trial. J Allergy Clin Immunol Pract. 2013;1:485‐493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Stewart AC, Gannon KN, Beresford F, Fleming L. Adolescent and caregivers' experiences of electronic adherence assessment in paediatric problematic severe asthma. J Child Health Care. 2018;22:238‐250. [DOI] [PubMed] [Google Scholar]
- 50. Johnson KB, Patterson BL, Ho YX, et al. The feasibility of text reminders to improve medication adherence in adolescents with asthma. J Am Med Inform Assoc. 2016;23:449‐455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wiecha JM, Adams WG, Rybin D, et al. Evaluation of a web‐based asthma self‐management system: a randomised controlled pilot trial. BMC Pulm Med. 2015;15:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhou Y, Lu Y, Zhu H, et al. Short‐term effect of a smart nebulizing device on adherence to inhaled corticosteroid therapy in Asthma Predictive Index‐positive wheezing children. Patient Prefer Adherence. 2018;12:861‐868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Jaimini U, Thirunarayan K, Kalra M, et al. "How Is My Child's Asthma?" Digital Phenotype and Actionable Insights for Pediatric Asthma. JMIR Pediatr Parent. 2018;1:e11988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mosnaim G, Li H, Martin M, et al. A tailored mobile health intervention to improve adherence and asthma control in minority adolescents. J Allergy Clin Immunol Pract. 2015;3(288–90):e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Montalbano L, Ferrante G, Cilluffo G, et al. Targeting quality of life in asthmatic children: The MyTEP pilot randomized trial. Respir Med. 2019;153:14‐19. [DOI] [PubMed] [Google Scholar]
- 56. Safioti G, Granovsky L, Li T, et al. A predictive model for clinical asthma exacerbations using albuterol eMDPI (ProAir Digihaler): a 12‐week, open‐label study. Am Thoracic Soc. 2019;A7307‐A7317. [Google Scholar]
- 57. Merchant RK, Inamdar R, Quade RC. Effectiveness of population health management using the propeller health asthma platform: a randomized clinical trial. J Allergy Clin Immunol Pract. 2016;4:455‐463. [DOI] [PubMed] [Google Scholar]
- 58. Charles T, Quinn D, Weatherall M, Aldington S, Beasley R, Holt S. An audiovisual reminder function improves adherence with inhaled corticosteroid therapy in asthma. J Allergy Clin Immunol. 2007;119:811‐816. [DOI] [PubMed] [Google Scholar]
- 59. Foster JM, Smith L, Usherwood T, et al. The reliability and patient acceptability of the SmartTrack device: a new electronic monitor and reminder device for metered dose inhalers. J Asthma. 2012;49:657‐662. [DOI] [PubMed] [Google Scholar]
- 60. Wu AC, Carpenter JF, Himes BE. Mobile health applications for asthma. J Allergy Clin Immunol Pract. 2015;3:446‐448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ramsey RR, Caromody JK, Voorhees SE, et al. A systematic evaluation of asthma management apps examining behavior change techniques. J Allergy Clin Immunol Pract. 2019;7:2583‐2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bian J, Guo Y, Xie M, et al. Exploring the Association Between Self‐Reported Asthma Impact and Fitbit‐Derived Sleep Quality and Physical Activity Measures in Adolescents. JMIR Mhealth Uhealth. 2017;5:e105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Telemedicine ‐ World Health Organization. https://www.who.int/goe/publications/goe_telemedicine_2010.pdf
- 64. Kim CH, Lieng MK, Rylee TL, et al. School‐based telemedicine interventions for asthma: a systematic review. Acad Pediatr. 2020;S1876–2859(20):30186‐30188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Culmer N, Smith T, Stager C, et al. Telemedical asthma education and health care outcomes for school‐age children: a systematic review. J Allergy Clin Immunol Pract. 2020;8:1908‐1918. [DOI] [PubMed] [Google Scholar]
- 66. Blakey JD, Bender BG, Dima AL, et al. Digital technologies and adherence in respiratory diseases: the road ahead. Eur Respir J. 2018;52:1801147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hamine S, Gerth‐Guyette E, Faulx D, et al. Impact of mHealth chronic disease management on treatment adherence and patient outcomes: a systematic review. J Med Internet Res. 2015;17:e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Orozco‐Beltran D, Sánchez‐Molla M, Sanchez JJ, et al. Telemedicine in primary care for patients with chronic conditions: the ValCrònic Quasi‐experimental study. J Med Internet Res. 2017;19:e400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Brophy PD. Overview on the challenges and benefits of using telehealth tools in a pediatric population. Adv Chronic Kidney Dis. 2017;24:17‐21. [DOI] [PubMed] [Google Scholar]
- 70. Shaker MS, Oppenheimer J, Grayson M, et al. COVID‐19: Pandemic Contingency Planning for the Allergy and Immunology Clinic. J Allergy Clin Immunol Pract. 2020;8(1477–1488):e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Brough HA, Kalayci O, Sediva A, et al. Managing childhood allergies and immunodeficiencies during respiratory virus epidemics ‐ The 2020 COVID‐19 pandemic: A statement from the EAACI‐section on pediatrics [published online ahead of print, 2020 Apr 22]. Pediatr Allergy Immunol. 2020;31(5):442‐448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Cardinale F, Ciprandi G, Barberi S, et al. Consensus statement of the Italian society of pediatric allergy and immunology for the pragmatic management of children and adolescents with allergic or immunological diseases during the COVID‐19 pandemic. Ital J Pediatr. 2020;46:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Bignamini E, Cazzato S, Cutrera R, et al. Italian pediatric respiratory society recommendations on pediatric pulmonary function testing during COVID‐19 pandemic. Version 2. Ital J Pediatr. 2020;46:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Papadopoulos NG, Custovic A, Deschildre A, et al. Impact of COVID‐19 on Pediatric Asthma: Practice Adjustments and Disease Burden. J Allergy Clin Immunol Pract. 2020;8(2592–2599):e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Licari A, Votto M, Brambilla I, et al. Allergy and asthma in children and adolescents during the COVID outbreak: What we know and how we could prevent allergy and asthma flares [published online ahead of print, 2020 May 17]. Allergy. 2020;75(9):2402‐2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Bokolo A Jr. Use of telemedicine and virtual care for remote treatment in response to COVID‐19 Pandemic. J Med Syst. 2020;44:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kouri A, Gupta S, Yadollahi A, et al. Addressing reduced laboratory‐based pulmonary function testing during a pandemic. Chest. 2020;S0012–3692(20):31867‐31875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Himes BE, Weitzman ER. Innovations in health information technologies for chronic pulmonary diseases. Respir Res. 2016;17:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.


