Abstract
Background
It has been suggested that women admitted for delivery should have universal PCR testing for SARS‐CoV‐2. Yet, the considerable difference in the incidence of COVID‐19 between different geographic regions may affect screening strategies. Therefore, we aimed to compare questionnaire‐based testing versus universal PCR testing for SARS‐CoV‐2 in women admitted for delivery.
Methods
A prospective cohort study of women admitted for delivery at a single center during a four‐week period (April 22‐May 25, 2020). All women completed a questionnaire about COVID‐19 signs, symptoms, or risk factors, and a nasopharyngeal swab for PCR for SARS‐CoV‐2. Women who were flagged as suspected COVID‐19 by the questionnaire (questionnaire‐positive) were compared with women who were not flagged by the questionnaire (questionnaire‐negative).
Results
Overall, 446 women were eligible for analysis, of which 54 (12.1%) were questionnaire‐positive. PCR swab detected SARS‐CoV‐2 in four (0.9%) women: 3 of 392 (0.8%) in the questionnaire‐negative group, and 1 of 54 (1.9%) in the questionnaire‐positive group (P = .43), yielding a number needed to screen of 92 (95% CI 62‐177). In 96% of the cases, the PCR results were obtained only in the postpartum period. No positive PCR results were obtained from neonatal testing for SARS‐CoV‐2. The sensitivity of the questionnaire was 75.0%, and the negative predictive value was 99.7%.
Conclusions
Although the rate of positive PCR results was not significantly different between the groups, the number needed to screen is considerably high. The use of questionnaire‐based PCR testing in areas with low incidence of COVID‐19 allows for a reasonable allocation of resources and is easy to implement.
Keywords: SARS‐CoV‐2, COVID‐19, screening, pregnancy, labor and delivery, swab
1. INTRODUCTION
On March 11, 2020, the World Health Organization declared the rapid outbreak of COVID‐19 as a worldwide pandemic. 1 , 2 , 3 Early identification of infected individuals, social distancing, adoption of isolation practices, and appropriate use of personal protective equipment (PPE) emerged as crucial keystones necessary to control this pandemic. 4
The optimal screening strategy for the virus causing the COVID‐19 disease (SARS‐CoV‐2) is unclear. Screening patients based on symptoms and risk factors is clearly indicated, yet the natural history of the disease, which in many cases is infectious in the asymptomatic latent stage, questions this approach. 5 , 6 On the other hand, universal testing using nasopharyngeal (NP) swabs for polymerase chain reaction (PCR) for every admitted patient may potentially lead to misuse of limited resources and create unwarranted stress.
Pregnant women are a special population. They are usually young and healthy, and thus are likely to be asymptomatic even if infected with SARS‐CoV‐2. 6 They also require multiple interactions with numerous care practitioners during pregnancy, and mainly during labor and delivery. As such, the potential impact of even a single silent carrier to infect multiple health care professionals during one delivery, and by proxy, cause a widespread infection among mothers and newborns, is significant.
Recently, a universal testing approach for SARS‐CoV‐2 of women admitted for delivery was recommended by hospitals in New York, as the test‐positive rate among asymptomatic laboring women was approximately 13%. 6 , 7 Although this approach may be justified in areas with high prevalence of COVID‐19, it may not be efficient in areas with low prevalence. 8 , 9
As per June 14th, 2020, there were 31,992 confirmed cases in Ontario (~219 cases per 100 000), almost ninefold lower than the incidence in New York (383 325 cases, ~1970 cases per 100 000). 3 , 10 , 11 As the incidence of the COVID‐19 disease in ours, and in other areas, is considerably lower compared with the aforementioned reports, we aimed to compare the policy of a questionnaire‐based PCR testing versus universal PCR testing of SARS‐CoV‐2 for patients admitted to labor and delivery.
2. METHODS
This was a prospective cohort study of all women admitted to the labor and delivery unit at a single center, between April 22 and May 25, 2020. The annual number of births in the center is approximately 5100 deliveries. All women admitted for delivery were asked to complete a questionnaire about COVID‐19 signs, symptoms, and risk factors, and also having a nasopharyngeal swab for RT‐PCR testing for SARS‐CoV‐2. Women who declined the swab or did not complete the screening tool were excluded. The study protocol was approved by the local institutional review board.
The questionnaire was based on a questionnaire developed by the province of Ontario, which included the reported signs and symptoms of COVID‐19. In our department, we added additional signs and symptoms to the questionnaire in order to increase its sensitivity. Some of these additions were later introduced into the provincial questionnaire as well. The modified questionnaire was used for the entire duration of the study. The questionnaire was completed by the patient and was verified by a care practitioner, and included typical and atypical signs and symptoms and risk factors for COVID‐19 (Appendix). Typical signs and symptoms of COVID‐19 included the following: fever (temperature of 37.8°C or greater), new or worsening acute respiratory illness symptom (eg, cough, dyspnea, sore throat, runny nose or sneezing, nasal congestion, hoarse voice, or difficulty swallowing), and clinical or radiological evidence of pneumonia. Atypical signs and symptoms included the following: loss of taste or smell sensations, unexplained fatigue/malaise, vertigo/syncope, delirium, exacerbation of chronic medical conditions, digestive symptoms (eg, including nausea, vomiting, diarrhea, or abdominal pain not attributed to pregnancy), chills, headaches, unexplained tachycardia or hypotension, and hypoxia (defined as saturation levels in room air of <90%). O2 saturation level was routinely measured for all of the women who presented to the labor and delivery unit. Risk factors for COVID‐19 included use of cough or antipyretic medication in the last 48 hours before admission and travel out of Canada or contact with an ill individual in the 21 days before admission. Patients were considered positive if any one or more sign, symptom, or risk factor was reported as positive. Health care practitioners who treated questionnaire‐positive women used appropriate PPE until the results of the SARS‐CoV‐2 PCR testing were obtained.
Labor and delivery care practitioners underwent a designated swabbing education to reduce false‐negative rates of the test. PCR testing was done using Universal Transport Media Kit (Thermo Fisher) or Eswab Kit (Copan, Italy). Turnaround time for SARS‐CoV‐2 PCR testing in our center was between 8 and 24 hours. Newborns of PCR‐positive mothers underwent a SARS‐CoV‐2 testing as well.
Our primary objective was to compare two approaches: questionnaire‐based versus universal testing for the detection of SARS‐CoV‐2 in women admitted for delivery. Our secondary objective was to test the performance of the questionnaire in our population.
Statistical analysis was performed using SPSS version 25 (IBM, Armonk). Positive SARS‐CoV‐2 PCR rates were compared between women screened negative and women who screened positive by the screening tool. Continuous variables were tested for normal distribution using the Shapiro‐Wilk test. Categorical and continuous variables were compared using the chi‐square or Fisher exact test and Mann‐Whitney U test, respectively. Patients with missing data were excluded from the study and the final analysis. Sensitivity, specificity, positive and negative predictive values, and their correspondent 95% confidence intervals (CI) were calculated. Number needed to screen (NNS) was also calculated/ 12 Significance level was determined at P < .05.
3. Results
Overall, out of 456 women who were eligible to be included in the study, eight did not complete the questionnaire, one declined testing, and one swab was deemed technically inadequate, leaving a total of 446 women for analysis (Figure 1). Of the 446 women for analysis, one was admitted to the labor and delivery ward because of symptoms of preterm birth, which subsequently resolved, and was discharged home. Three other women were admitted for symptoms of preeclampsia and subsequently delivered. The rest of the cohort were admitted for delivery.
Figure 1.
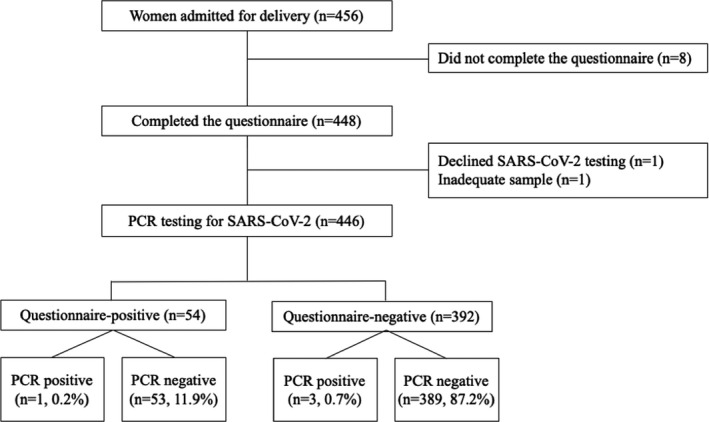
Study population
Of all women, 54 (12.1%) were questionnaire‐positive for COVID‐19 based on the screening tool, and the remainder were questionnaire‐negative. There were no significant differences in demographics, gestational age at delivery, mode of delivery, or neonatal birthweight between the questionnaire‐positive and questionnaire‐negative groups (Table 1).
Table 1.
Women’s characteristics
| Variable | Questionnaire positive (n = 54) | Questionnaire negative (n = 392) | P value |
|---|---|---|---|
| Positive PCR testing, n (%) | 1 (1.9) | 3 (0.8) | 43 |
| Results of PCR testing obtained postpartum, n (%) | 52 (96.3) | 380 (96.9) | .79 |
| Maternal age, years | 32 (30‐35) | 33 (30‐35) | .82 |
| Gravidity, n | 2 [1‐3] | 2 [1‐3] | .73 |
| Parity, n | 0 [0‐1] | 0 [0‐1] | .31 |
| Nulliparity, n (%) | 31 (57.4) | 204 (50.9) | .37 |
| Gestational age, weeks | 39 [38‐40] | 39 [38‐40] | .25 |
| Cesarean, n (%) | 18 (32.7) | 133 (33.2) | .95 |
| Birthweight, grams | 3344 [3030‐3652] | 3340 [3023‐3619] | .87 |
| Positive neonatal PCR testing, n (%) | 0 (0) | 0 (0) | NA |
Data are presented as n (%) or median [25th‐75th percentiles].
In approximately 96%‐97% of women, the results of the PCR testing were obtained only in the postpartum period (Table 1). Out of the cohort, four women were tested positive to SARS‐CoV‐2 (4/446, 0.9%).
Out of the four PCR‐positive women, one was in the questionnaire‐positive group and three were in the questionnaire‐negative group (1.9% vs 0.8%, P = .43). The results of the four positive PCR patients were available only after delivery. No positive PCR results were obtained from neonatal testing. These results yielded a number needed to screen (NNS) of 92 patients per 4‐week period (95% CI 62‐177).
The sensitivity, specificity, and positive and negative predictive values of the questionnaire are presented in Table 2. The sensitivity of the questionnaire for positive PCR testing was 75.0%, and the negative predictive values were 99.7%.
Table 2.
Questionnaire’s performance
| Variable | |
|---|---|
| Sensitivity, % (95% CI) | 75.0 (73.6‐76.4) |
| Specificity, % (95% CI) | 88.5 (88.4‐88.6) |
| Positive predictive value, % (95% CI) | 5.6 (5.4‐5.7) |
| Negative predictive value, % (95% CI) | 99.7 (99.7‐99.8) |
3.1. Interpretation
In this study, we aimed to compare two approaches for SARS‐CoV‐2 PCR testing in women admitted for labor and delivery: questionnaire‐based PCR testing versus universal PCR testing. Although the rate of positive swabs was higher in the questionnaire‐positive group, the difference did not reach statistical significance. The negative predictive value of the questionnaire was 99.7%.
Although previous studies have reported higher incidence of positive SARS‐CoV‐2 PCR in asymptomatic women, these results emerged from a population with a high incidence of COVID‐19. 6 , 7 Thus, these findings may not be applicable to a population with lower incidence, as in ours. Currently (June 14th, 2020), the incidence of COVID‐19 confirmed cases in Ontario is 219 cases per 100 000, almost ninefold lower than the incidence in New York (1970 cases per 100 000). 3 , 10 , 11 Correspondently, the detection rate of SARS‐CoV‐2–positive cases in asymptomatic patients in our obstetric population was only 0.8%.
In the New York–based study, Vintzileos 6 et al reported that 20% (32/161) of women admitted for delivery were SARS‐CoV‐2–positive, of whom 66% were asymptomatic. In our cohort, only 0.9% (4/446) of women were positive, of which three patients (75%) were asymptomatic. Although the proportion of asymptomatic patients out of the total positive patients is similar in our cohort, the absolute numbers are considerably lower than the report from New York, and similar to reports from other areas with low incidence of COVID‐19. 8 , 9
The NNS in our cohort was 92 per 4‐weeks period, which means that 92 patients would have to have swabs in order to detect one patient with positive SARS‐CoV‐2 swab. When a similar approach was tested in Milan, Italy, the NNS was only 3 per week (or 12 per 4 weeks), which is considerably lower. 8 Yet, out study differ both by the time period in which it was performed and by the larger sample size. As the screening period prolongs, and the sampled population size increases, the higher the number of women who will screen negative using the questionnaire, thus the higher the NNS.
No positive SARS‐CoV‐2 PCR results were obtained from newborns of PCR‐positive mothers, likely ruling out vertical transmission in these cases. Nevertheless, the question of vertical transmission in COVID‐19 cases is still under extensive research worldwide and cannot be assumed based on these two cases. 13 , 14 , 15
On the one hand, these results may seem to be in favor of universal PCR testing on admission to labor and delivery, as no differences were found between the groups. One might assume that admission to labor and delivery is similar to admission for elective surgical procedures; thus, a practice of universal screening may be reasonable. 16 On the other hand, in the obstetrical population, the odds of having an aerosol‐producing procedure is low, and the timing of which is quite uncertain. Although the goal of identifying all SARS‐CoV‐2–positive mothers is reasonable, the effort and cost invested in the detection of a single asymptomatic case is questionable, given the low incidence in our population and the calculated NNS (92, 95% CI 62‐177). As such, the use of questionnaire allows for a reasonable allocation of resources and efforts when coping with the COVID‐19 pandemic.
Regardless of the test result, all patients must be treated as if they may be carriers of SARS‐CoV‐2 given the community spread and nature of this disease. When the local prevalence of COVID‐19 is low, the effectiveness (both clinical and financial) of a universal SARS‐CoV‐2 PCR testing is doubtful. The relatively low positivity rate of PCR testing in a low prevalence setting combined with long turnaround time and limited health care resources questions the utility of universal PCR testing.
Testing pregnant women is important to flatten the curve of transmission in society. The impact to identifying a positive mother who has the potential of transmitting it to numerous others is profound. Although it is a worthy cause, we are not sure that universal PCR testing for laboring women is the correct answer to this challenge. In a perfect world with unlimited resources and high sensitivity, low‐turnaround time testing, universal SARS‐CoV‐2 testing might have been the right strategy. A rapid SARS‐CoV‐2 test will help to administer isolation practices and appropriate use of PPE and will help in counseling patients in a shared decision‐making process about separating a newborn from a SARS‐CoV‐2–positive mother. 17 , 18
With current laboratory technology and PCR testing performance, and in a low‐incidence population, several, potentially more cost‐effective measures may be sufficient alternatives to universal PCR testing. Universal use of droplet PPE in all deliveries, good hand‐hygiene practices, social distancing, and limiting the number of support persons in labor can be used to minimize the risk of spread. We believe that the use of these measures is superior to universal PCR testing of laboring women in areas with low incidence of COVID‐19. Of note, this approach is also supported by the Center for Disease Control and Prevention, 19 which advocates that the decision of who should be tested, should consider the occurrence of COVID‐19 in the community.
Our study is not without limitations. The low incidence of SARS‐CoV‐2 in our area limits the interpretation of the results. Notwithstanding, to the best of our knowledge this is one of the only studies comparing two approaches for SARS‐CoV‐2 testing and offering the use of a screening tool in order to guide PCR testing in a low‐prevalence setting. Although results from other geographic areas are may defer, we should remember that in most parts of the world, the prevalence of SARS‐CoV‐2 is still relatively low, resembling the prevalence in Ontario or lower, and does not approximate the numbers seen from the United States, and several areas in Italy or Spain. In addition, our results stem from a single, tertiary center. The resource allocation and efforts diverted to manage the COVID‐19 pandemic that are available in tertiary centers differ substantially from other, smaller centers. As such, the results may not be applicable to other centers with lesser resources. On the other hand, the use of questionnaire may prove to be rather a cost‐effective tool, especially in those centers.
To conclude, our results suggest that a questionnaire‐based PCR testing for SARS‐CoV‐2 resulted in higher rate of positive PCR but is not more effective than universal PCR screening in women admitted for delivery. Nonetheless, given the current laboratory technology and specimen turnaround time, the use of questionnaire in areas with low incidence of COVID‐19 allows for a reasonable allocation of resources and is easy to implement.
Acknowledgment
The authors would like to extend their gratitude to Andrea Hollingshead, MHM, Christie Vermeiren, PhD, and Sheri Ferkl, RN (EC), for their significant contribution and assistance.
Questionnaire

Mei‐Dan E, Satkunaratnam A, Cahan T, Leung M, Katz K, Aviram A. Questionnaire‐based vs universal PCR testing for SARS‐CoV‐2 in women admitted for delivery. Birth.2021;48:96–103. 10.1111/birt.12520
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization (WHO) . Director‐General’s opening remarks at the media briefing on COVID‐19. May 11, 2020. Geneva: World Health Organization. [Google Scholar]
- 3. Johns Hopkins University . Coronavirus COVID‐19 global cases by Johns Hopkins CSSE. Baltimore: Johns Hopkins University. [Google Scholar]
- 4. World Health Organization . Rational use of personal protective equipment (PPE) for coronavirus disease (COVID‐19). Interim Guidance. Geneva: World Health Organization (WHO). March 19, 2020. World Health Organization. [Google Scholar]
- 5. World Health Organization (WHO) . Coronavirus disease 2019 (COVID‐19), Situation Report 73. Geneva: World Health Organization (WHO). [Google Scholar]
- 6. Vintzileos WS, Muscat J, Hoffmann E, et al. Screening all pregnant women admitted to Labor and Delivery for the virus responsible for COVID‐19. Am J Obstet Gynecol. 2020;223:P284–P286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sutton D, Fuchs K, D’Alton M, Goffman D. Universal screening for SARS‐CoV‐2 in women admitted for delivery. N Engl J Med. 2020:382(22):2163–2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tassis B, Lunghi G, Frattaruolo MP, Ruggiero M, Somigliana E, Ferrazzi E. Effectiveness of a COVID‐19 screening questionnaire for pregnant women at admission to an obstetric unit in Milan. Int J Gynecol Obstet. 150(1):124‐126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Campbell KH, Tornatore JM, Lawrence KE, et al. Prevalence of SARS‐CoV‐2 among patients admitted for childbirth in Southern Connecticut. JAMA. 2020; 23(24):2520–2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Government of Canada. Coronavirus disease (Covid‐19): Outbreak update – current situation. https://www.canada.ca/en/public-health/services/diseases/2019-novel-coronavirus-infection.html#a1. Accessed June 14, 2020. [Google Scholar]
- 11. NYSDOH COVID‐19 Tracker, Department of Health, New‐York State. https://covid19tracker.health.ny.gov/views/NYS-COVID19-Tracker/NYSDOHCOVID-19Tracker-Map?%3Aembed=yes&%3Atoolbar=no&%3Atabs=n. Accessed May 10, 2020. Accessed June 14, 2020. [Google Scholar]
- 12. Rembold CM. Number needed to screen: development of a statistic for disease screening. BMJ. 1998;317(7154):307–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lamouroux A, Attie‐Bitach T, Martinovic J, Leruez‐Ville M, Ville Y. Evidence for and against vertical transmission for SARS‐CoV‐2 (COVID‐19). Am J Obtet Gynecol. 2020; 223(1):e1‐91.e4. 10.1016/j.ajog.2020.04.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hu X, Gao J, Luo X, et al. Severe acute respiratory syndrome coronavirus 2 (sars‐cov‐2) vertical transmission in neonates born to mothers with coronavirus disease 2019 (covid‐19) pneumonia. Obstet Gynecol. 2020:136(1):65–67. 10.1097/AOG.0000000000003926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cheruiyot I, Henry BM, Lippi G. Is there evidence of intra‐uterine vertical transmission potential of COVID‐19 infection in samples tested by quantitative RT‐PCR? Eur J Obstet Gynecol Reprod Biol. 2020;249:100‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tenenbein P, Riazi S, Johnstone J, Keshavjee S, Karkouti K. The case for routine screening for SARS‐CoV‐2 before surgery. Can J Anesth Can d’anesthésie. 2020;67(10):1315–1320. 10.1007/s12630-020-01730-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nguyen T, Duong Bang D, Wolff A. 2019 Novel coronavirus disease (COVID‐19): paving the road for rapid detection and point‐of‐care diagnostics. Micromachines. 2020;11(3):306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dotters‐Katz SK, Behal HB. Coronavirus (COVID‐19) and Pregnancy: What Maternal‐Fetal Medicine Subspecialists Need to Know. The Society for Maternal‐Fetal Medicine (SMFM). https://s3.amazonaws.com/cdn.smfm.org/media/2322/COVID19-What_MFMs_need_to_know_revision_4-11-20_(final)_highlighted_changes_PDF.pdf. Accessed May 7, 2020. [Google Scholar]
- 19. Centers for Disease Control and Prevention . Evaluating and Reporting Persons Under Investigation (PUI) . Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-criteria.html#foot3. Published 2020. Accessed May 12, 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


