Abstract
Introduction
Severe COVID‐19 is often compounded by a prothrombotic state that is associated with poor outcomes. In this investigation, we aimed to evaluate ADAMTS13 activity, von Willebrand factor level (VWF:Ag), and the corresponding ADAMTS13 activity/VWF:Ag ratio, in patients with COVID‐19 and for associations with disease progression and acute kidney injury (AKI).
Methods
Patients presenting to the emergency department (ED) with COVID‐19 were enrolled in this prospective, observational study. ADAMTS13 activity and VWF:Ag were measured at index ED visit. The primary endpoint was severe AKI defined by KDIGO stage 2 + 3 criteria, while the secondary endpoint was peak 30‐day COVID‐19 severity.
Results
A total of 52 adult COVID‐19 patients were enrolled. Overall, we observed that 23.1% of the cohort had a relative deficiency in ADAMTS13 activity, while 80.8% had elevated VWF:Ag. The ADAMTS13 activity/VWF:Ag ratio was significantly lower in patients with severe AKI (P = .002) and those who developed the severe form of COVID‐19 (P = .020). The ADAMTS13 activity/VWF:Ag ratio was negatively correlated with age (P < .001) and LDH (P < .001), while positively correlated with hemoglobin (P = .041). After controlling for confounders, a one‐unit increase in ADAMTS13/VWF:Ag ratio was associated with 20% decreased odds of severe AKI.
Conclusion
A low ADAMTS13 activity:VWF:Ag ratio at ED presentation is associated with progression to severe COVID‐19 disease and severe AKI, with a pattern suggestive of a secondary microangiopathy. Further interventional studies should be conducted to assess the restoration of ADAMTS13:VWF:Ag ratio in hospitalized patients with COVID‐19.
Keywords: acute kidney injury, ADAMTS13, coagulopathy, outcome, thrombosis, von Willebrand factor
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19), the respiratory and systemic illness caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), is clearly associated with coagulopathy, now termed COVID‐19‐associated coagulopathy (CAC). 1 The severity of CAC correlates with severity of COVID‐19 illness and manifests clinically as arterial and venous thromboembolism, especially in critically ill patients. 1 On routine laboratory assessment, CAC is typically associated with moderately increased D‐dimer values, mild thrombocytopenia, and modestly prolonged prothrombin time (PT). 2 , 3 CAC is thought to be sustained via a combination of direct cytopathic endothelial injury by SARS‐CoV‐2, as well as hyperinflammation leading to immunothrombosis. 4 However, the exact mechanisms underlying CAC have yet to be fully elucidated.
Recent evidence has pointed to the substantial possibility of a thrombotic microangiopathy (TMA)‐like phenomenon, secondary to SARS‐CoV‐2 infection. 5 Recent laboratory studies have reported a relative ADAMTS13 deficiency in patients hospitalized with COVID‐19, along with elevations in lactate dehydrogenase (LDH) and serum creatinine (SCr), with decreases in hemoglobin and platelets. 2 , 5 When combined with clinical and pathologic findings of acute kidney injury (AKI), lung injury, and cardiac injury, this becomes suggestive of a TMA‐like picture, as opposed to disseminated intravascular coagulopathy (DIC). 6 , 7 , 8
ADAMTS13, also known as “a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13” or “von Willebrand factor‐cleaving protease,” is a liver‐generated enzyme, which cleaves large von Willebrand factor (VWF) multimers in blood. 9 In COVID‐19, elevated levels of VWF have been reported, which is likely due to a combination of endothelial injury and its release as an acute phase reactant. 9 , 10 Low ADAMTS13 activity, due to consumption or inflammation‐mediated degradation, may result in accumulation of ultralarge VWF multimers that may then interplay with platelets to cause microthrombosis and trigger immunothrombosis. 4 , 8 Thus, the presence of low ADAMTS13 coupled with high VWF may represent a “double insult” leading to a heightened state of hypercoagulability, and also to a TMA‐like phenomenon, first localized to lung, but eventually spreading systematically, leading to end‐organ damage.
Huisman et al 9 reported a disproportionately high level of VWF antigen (VWF:Ag) to ADAMTS13 level in a case series of 12 COVID‐19 patients with suspected TMA, while Bazzan et al, 11 in a series of 88 COVID‐19 patients, found low ADAMTS13 levels to be a predictor of mortality.
However, to the best of our knowledge, no study has investigated the direct relationship between the ADAMTS13 and VWF axis with respect to outcomes in COVID‐19 patients. Therefore, the aim of this investigation was to study this relationship, exploring ADAMTS13 activity/VWF:Ag ratio measured at emergency department presentation and COVID‐19 clinical outcomes, specifically severe AKI and COVID‐19 severity, as well as to correlate this ratio with other biochemical markers of TMA. Given that renal injury is the most common manifestation of TMA, as commonly associated with multi‐organ dysfunction syndrome, impairment of innate immunity, and increased mortality risk among critically ill patients, 12 severe AKI was chosen as the primary endpoint of this study.
2. METHODS
2.1. Study design
This prospective, observational investigation was performed on the Cincinnati Emergency Department (ED) COVID‐19 cohort. Adults (≥18 years) presenting to the ED of the University of Cincinnati Medical Center (UCMC) between April and May 2020 with symptoms clinically suspicious for COVID‐19 and receiving a clinically indicated blood draw were initially enrolled. Only patients displaying a positive test result for SARS‐CoV‐2 on standard‐of‐care reverse transcriptase‐polymerase chain reaction (RT‐PCR) on nasopharyngeal swab were included. Patients with a negative RT‐PCR results, or who were <18 years of age at time of ED visit, or who were known prisoners, were excluded. This study was approved by the Institutional Review Board of the University of Cincinnati and performed under a waiver of informed consent. This study was performed in compliance with the Declaration of Helsinki and in accordance with local and national regulations.
2.2. Sample collection and processing
Patient blood samples were collected during the index ED visit via a clinically routine blood collection. All laboratory analyses were performed either at the UCMC Clinical Laboratory or the Clinical Nephrology Lab of the Cincinnati Children's Hospital Medical Center. Samples for routine standard‐of‐care laboratories were processed immediately, per standard laboratory protocols. Samples for assessing ADAMTS13 activity and VWF:Ag were centrifuged at 2000 g for 15 minutes at 4°C within 3 hours of collection and subsequently frozen at −80°C until analysis.
2.3. Measurements
The complete blood cell count (CBC) with differential was performed using a Beckman Coulter UniCel DxH 800 Cellular Analysis System on EDTA‐collected blood. Serum creatinine was measured using a kinetic alkaline picrate (modified Jaffe) method on either a Beckman Coulter AU480 Chemistry Analyzer or a Beckman Coulter AU5822 Chemistry Analyzer. Plasma aspartate transaminase (AST), alanine aminotransferase (ALT), total bilirubin, and albumin were analyzed on a Beckman Coulter AU5822 Chemistry Analyzer. Plasma concentrations of ferritin, fibrinogen, myoglobin, haptoglobin, and C‐reactive protein were measured with a Behring Nephelometer II System (BN II, Siemens Medical Solutions USA, Inc). Lactate dehydrogenase was assayed with Dimension RxL Max Integrated Chemistry System (Siemens Medical Solutions USA, Inc), while procalcitonin was measured with a chemiluminescent immunoassay (CLIA) on a Diasorin Liaison XL (DiaSorin S.p.A.). Measurements of plasma prothrombin time (PT), activated partial thromboplastin time (APTT), and D‐dimer were carried out with Stago STA‐R Evolution or Stago STA R MAX (Diagnostica Stago). VWF:Ag was measured using a Technozym enzyme‐linked immunosorbent assay kit (ELISA; DiaPharma Group Inc). A VWF:Ag level of >215 U/dL was considered elevated. 13 ADAMTS13 activity was measured using a fluorescence resonance energy transfer (FRET) assay (Immucor, Inc). An ADAMTS13 activity level of >67% was defined as normal activity. All assays were performed according to manufacturers' instructions and guidance.
2.4. Data collection
Patient data were extracted from electronic medical records (EMR) and recorded into a REDCap (Research Electronic Data Capture) database. Variables extracted included patient demographics, past medical history, clinical course, and COVID‐19 complications. Data extraction was performed by an ED physician, with select records checked for accuracy by a second ED physician. Data on the clinical course of patients hospitalized at index ED visit were recorded through discharge, while data on the clinical course of patients discharged at the index ED visit were monitored for 30 days.
2.5. Outcomes and study definitions
The primary outcome was development of AKI during the COVID‐19 course of illness defined as Kidney Disease Improving Global Outcomes (KDIGO) Stage 2 and 3 evaluated using SCr criteria. 14 When a previous SCr value prior to COVID‐19 illness was not available, the lowest SCr during the 30‐day follow‐up was used for evaluating AKI over the course of illness.
The secondary outcome was progression to severe COVID‐19 during hospitalization or within 30 days of index ED presentation. Patient severity was first evaluated using an 8‐point ordinal scale as outlined by the World Health Organization (WHO) R&D Blueprint, 15 which allowed to classify patients into two severity groups: mild/moderate (ordinal scale 1‐4) or severe (ordinal scale 5‐8) illness.
2.6. Statistical analysis
Categorical variables were reported as absolute number (n) and relative frequency (%), while continuous variables were reported as median and interquartile range (IQR). Categorical variables were compared using the chi‐squared test (χ2) or Fisher's exact test, as appropriate, based on expected accounts. Continuous variables were compared using the nonparametric Mann‐Whitney U test, while comparisons of multiple continuous variables were analyzed using Kruskal‐Wallis one‐way analysis of variance. Correlations between variables were performed using Spearman's correlation. Multivariable logistic regression was employed to identify if ADAMTS13/VWF:Ag ratio and baseline variables were independent predictors of the primary and secondary outcomes. All baseline patient characteristics (excluding laboratory values) from Table 1 with a P‐value of <.10 were included in the model, and relevant variable selection was performed using a bidirectional stepwise algorithm. Adjusted odds ratios (ORs) and 95% confidence intervals (95% CIs) were computed. Statistical analysis was performed using Prism 8 (GraphPad Software) and R (version 4.0.2; R Foundation for Statistical Computing) with a P < .05 considered statistically significant.
TABLE 1.
Patient demographics and baseline characteristics at index ED visit by presence or absence of severe acute kidney injury
| Variable | All patients (n = 52) | Severe acute kidney injury | ||
|---|---|---|---|---|
| No (n = 40) | Yes (n = 12) | P‐value | ||
| Age (y): median (IQR) | 51 (39‐66) | 47 (37‐64) | 66 (51‐71) | .003 |
| Sex (male): n (%) | 30 (57.7%) | 23 (57.5%) | 7 (58.3%) | 1.000 |
| Race: n (%) | ||||
| Black | 22 (42.3%) | 13 (32.5%) | 9 (75.0%) | .042 |
| Hispanic | 18 (34.6%) | 17 (42.5%) | 1 (8.3%) | |
| White | 7 (17.3%) | 7 (17.5%) | 2 (16.7%) | |
| Other | 3 (5.8%) | 3 (7.5%) | 0 | |
| Comorbidities: n (%) | ||||
| Coronary artery disease | 8 (15.4%) | 3 (7.5%) | 5 (41.7%) | .015 |
| Heart failure | 9 (17.3%) | 3 (7.5%) | 6 (50%) | .003 |
| Hypertension | 26 (50%) | 15 (37.5%) | 11 (91.7%) | .002 |
| Hyperlipidemia | 15 (28.8%) | 11 (27.5%) | 4 (33.3%) | .726 |
| Diabetes | 21 (40.4%) | 15 (37.5%) | 6 (50%) | .518 |
| Chronic obstructive pulmonary disease | 8 (15.4%) | 4 (10%) | 4 (33.3%) | .072 |
| Asthma | 8 (15.4%) | 6 (15%) | 2 (16.7%) | 1.000 |
| Chronic kidney disease | 6 (11.5%) | 1 (2.5%) | 5 (41.7%) | .002 |
| Chronic liver disease | 7 (13.5%) | 3 (7.5%) | 4 (33.3%) | .041 |
| Cerebrovascular disease | 1 (1.9%) | 0 | 1 (8.3%) | .375 |
| Cancer | 4 (7.7%) | 1 (2.5%) | 3 (25%) | .034 |
| Obesity | 18 (34.6%) | 16 (40%) | 2 (16.7%) | .179 |
| Inherited immunodeficiency | 0 (0%) | 0 (0%) | 0 (0%) | — |
| Acquired immunodeficiency (HIV, transplant) | 3 (5.8%) | 2 (5%) | 1 (8.3%) | .561 |
| Autoimmune disease | 2 (3.8%) | 2 (5%) | 0 (0%) | 1.000 |
| Current smoker | 12 (23.1%) | 5 (12.5%) | 7 (58.3%) | .003 |
| Former smoker | 11 (21.2%) | 10 (25%) | 1 (8.3%) | .421 |
| Laboratories at ED visit: n (%) | ||||
| White blood cell count (×103/mm3) | 6.7 (5.2‐9.5) | 5.7 (5.1‐9.3) | 7.8 (5.4‐12.5) | .372 |
| Absolute neutrophil count (×103/mm3) | 4.5 (3.7‐7.2) | 4.5 (3.7‐6.8) | 5.6 (4.2‐9.5) | .170 |
| Absolute lymphocyte count (×103/mm3) | 1.0 (0.7‐1.4) | 01.0 (0.7‐1.4) | 0.9 (0.6‐1.2) | .555 |
| Platelet count (×103/mm3) | 208.5 (163.8‐253) | 206 (166.2‐246.2) | 211 (140.5‐265) | .924 |
| Hemoglobin (g/dL) | 13.3 (11.4‐14.3) | 14 (12.3‐15.1) | 12 (10.9‐12.9) | .013 |
| C‐reactive protein (mg/dL) | 4.8 (1‐12.1) | 4.7 (0.7‐10.8) | 7.9 (4.2‐8.8) | .131 |
| Procalcitonin (ng/mL) | 0.11 (0.05‐0.33) | 0.07 (0.05‐0.17) | 1.02 (0.38‐2.55) | <.001 |
| Ferritin (μg/L) | 366.5 (121‐1285) | 288 (108‐1062.5) | 995.5 (368.8‐2610) | .017 |
| Lactate dehydrogenase (U/L) | 310 (248‐448.8) | 298.5 (237.8‐394.2) | 404 (297.8‐554.5) | .039 |
| Aspartate transaminase (U/L) | 52.5 (34.5‐71.5) | 53 (34.5‐70) | 52 (35‐82) | .917 |
| Alanine aminotransferase (U/L) | 31.5 (17.8‐53.3) | 42 (20‐57.5) | 25 (6‐36) | .056 |
| Total bilirubin (mg/dL) | 0.6 (0.4‐0.8) | 0.6 (0.4‐0.75) | 0.6 (0.5‐0.8) | .568 |
| Albumin (g/dL) | 3.7 (3.4‐ 4.1) | 3.8 (3.6‐4.2) | 3.4 (3.3‐3.8) | .064 |
| Fibrinogen (g/L) | 5.4 (3.4‐6.9) | 5.2 (3.1‐6.6) | 5.6 (4.5‐8.5) | .18 |
| Haptoglobin (mg/dL) | 254 (162.8‐332.2) | 254 (168.8‐330.5) | 249.5 (162.8‐389) | .836 |
| Myoglobin (μg/L) | 32.1 (18‐184.2) | 23.8 (16.5‐43.3) | 351.5 (186.8‐1064.8) | <.001 |
| Prothrombin time (s) | 16.1 (13.9‐17.2) | 16 (13.5‐17.2) | 16.2 (15‐17.1) | .672 |
| D‐dimer (μg/mL FEU) | 1.19 (0.71‐1.72) | 1.25 (0.62‐1.59) | 1.11 (0.93‐2.4) | .289 |
| Days since symptom onset: median (IQR) | 7 (3‐10) | 7 (3‐10) | 4 (2‐9) | .459 |
3. RESULTS
3.1. Patient characteristics and outcomes
A total of 52 adult patients with laboratory‐confirmed COVID‐19 were enrolled, 12 (23.1%) of whom reached the primary outcome of severe AKI, 2 with stage 2 KDIGO AKI and 10 with stage 3 KDIGO AKI. Eight (15.4%) patients needed renal replacement therapy. A total of 16 (30.8%) patients reached the secondary endpoint of severe COVID‐19 within 30 days of index ED visit, while 36 (69.2%) had mild/moderate COVID‐19. Among the 12 severe AKI patients, 5 (41.7%) had mild/moderate COVID‐19 and 7 (58.3%) had severe COVID‐19. Three (25%) of the patients with severe AKI died during hospitalization. No major arterial or venous thrombotic events were observed. The baseline characteristics of our cohort at index ED visit according to the primary outcome are shown in Table 1. Patients who developed severe AKI were older (P = .003) and disproportionality black as opposed to white or his panic (P = .042). Moreover, higher rates of coronary artery disease (P = .015), heart failure (P = .003), hypertension (P = .002), chronic kidney disease (P = .002), chronic liver disease (P = .041), and cancer (P = .034) were observed in those who developed severe AKI. Interestingly, patients who were current smokers (P = .003) but not former smokers (P = .421) were observed to have higher rates of severe AKI during course of illness. In patients who developed severe AKI, procalcitonin (P < .001), ferritin (P = .017), myoglobin (P < .001), and LDH (P = .039) were significantly elevated, while hemoglobin was significantly lower (P = .013). No difference was found for D‐dimer (P = .289), PT (P = .672) or platelet count (P = .924).
3.2. ADAMTS13 and VWF:Ag
The levels of ADAMTS13 and VWF:Ag and their ratio stratified by AKI are shown in Table 2. ADAMTS activity was found to be 35% lower in patients with severe AKI (P = .005), while VWF:Ag was 67.5 U/dL higher in patients with severe AKI, albeit this difference did not reach statistical significance (P = .062). A total of 12 patients (23.1%) have low ADAMTS13 levels (<67%). The majority of patients (80.8%) had elevated VWF, while 10 (19.2%) patients had levels within the normal range. The corresponding ADAMTS13/VWF:Ag ratio was significantly lower in patients with severe AKI (P = .002) (Figure 1A), as well as in those needing renal replacement therapy (P = .039).
TABLE 2.
ADAMTS13 and VWF:Ag by presence of severe acute kidney injury (AKI)
| Severe AKI | |||
|---|---|---|---|
| No (n = 40) | Yes (n = 12) | P‐value | |
| ADAMTS13 (% activity) | 106 (81‐107) | 71 (56‐89) | .005 |
| VWF:Ag (U/dL) | 243 (205.9‐328.5) | 310.5 (277.9‐353.6) | .062 |
| ADAMTS13/VWF:Ag ratio | 0.36 (0.28‐0.48) | 0.21 (0.18‐0.33) | .002 |
Results presented as median (IQR).
FIGURE 1.
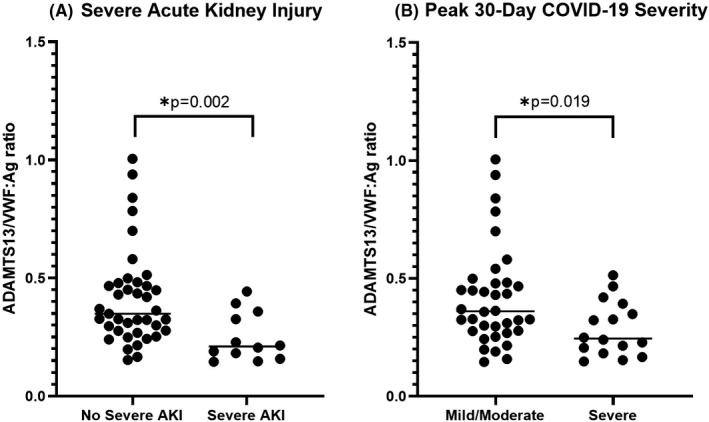
ADAMTS13/VWF:ratio measured at emergency department presentation in patients with and without severe acute kidney injury (AKI) (A) and according to peak 30‐d COVID‐19 severity (B)
Levels of ADAMTS13 and VWF:Ag and their ratio by peak 30‐day COVID‐19 severity are shown in Table 3. ADAMTS13 trended lower in patients progressing to severe disease (P = .005), while VWF:Ag was not significantly different between groups (P = .463). The ADAMTS13/VWF:Ag ratio trended lower with disease severity, with patients with severe disease having a significantly lower ratio compared to those with mild/moderate disease (P = .020). (Figure 1B). A further analysis comparing these variables between a more detailed clinically defined cohort (mild vs moderate vs severe) is available in Table S1.
TABLE 3.
ADAMTS13 and VWF:Ag according to peak 30‐d COVID‐19 severity
| Peak 30‐d COVID‐19 severity | P‐value | ||
|---|---|---|---|
| Mild/Moderate | Severe | ||
| ADAMTS13 (% activity) | 107 (83.5‐107) | 72 (55.8‐95.3) | .005 |
| VWF‐1 U/dL (or %) | 267 (210.4‐328.9) | 307.5 (226.9‐329.2) | .463 |
| ADAMTS13/VWF ratio | 0.36 (0.28‐0.48) | 0.24 (0.20‐0.36) | .020 |
Results presented as median (IQR).
The ADAMTS13/VWF:Ag ratio correlated with age and other laboratory values associated with TMA (Figure 2). Both age (r = −.50 [95% CI: −0.68, −0.25]; P < .001) and LDH (r = −.44 [95% CI: −0.63, −0.18]; P < .001) were negatively correlated with ADAMTS13/VWF:Ag, while hemoglobin positively correlated with ADAMTS13/VWF:Ag (r = .29 [95% CI: 0.01, 0.54]; P = .041). No correlation was found between platelet count and ADAMTS13/VWF:Ag (r = .03 [95% CI: −0.26, 0.31]; P = .838). Myoglobin was substantially elevated in patients with severe AKI, and also negatively correlated with ADAMTS13/VWF:Ag (r = −.59 [95% CI: −0.87, −0.03]; P = .046).
FIGURE 2.
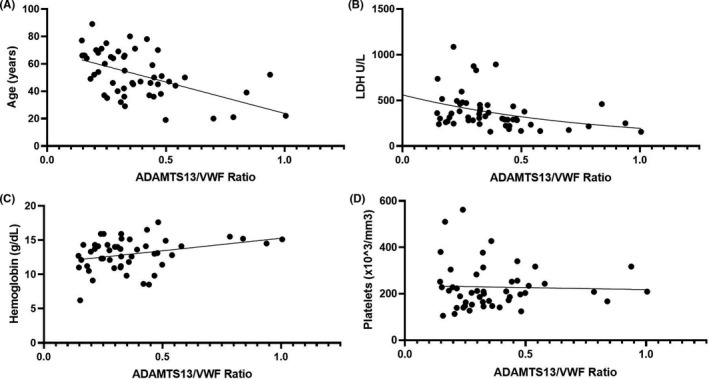
Correlation of ADAMTS13/VWF:Ag ratio with age (A), lactate dehydrogenase (LDH) (B), hemoglobin (C), and platelet count (D)
3.3. Multivariate analysis
The results of multivariate logistic regression are presented in Table S2. After controlling for confounders, such as advanced age and presence of comorbidities, a one‐unit increase in ADAMTS13/VWF:Ag ratio was associated with 20% decreased odds of severe AKI (OR: 0.8 [95% CI: 0.7, 0.9]; P = .018). In contrast, history of hypertension (OR: 16.3 [95% CI: 1.2, 220.0]; P = .036) or chronic kidney disease (OR: 63.8 [95% CI: 1.8, 2253.5]; P = .022) was associated with significant increases in the odds of severe AKI. Stepwise algorithm variable selection did not find advanced age or smoking to be relevant in this analysis.
4. DISCUSSION
In this prospective, observational study of 52 laboratory‐confirmed COVID‐19 patients we found that low ADAMTS13/VWF:Ag ratio was associated with poor clinical outcomes, including disease severity and severe AKI. This finding, combined with laboratory patterns observed in these patients consisting of elevated LDH, low hemoglobin but with nonsignificant changes in D‐dimer and prothrombin time, are suggestive of a TMA‐like phenomenon, as opposed to an infection‐driven coagulopathy.
We observed that nearly a fourth (23.1%) of our COVID‐19 patients had relative ADAMTS13 deficiency, while ~80% had elevated VWF:Ag. These results are consistent with early studies in COVID‐19 patients. Martinelli et al 5 reported mild relative deficiency of ADAMTS13 activity in a sample of 13 COVID‐19 patients with schistocytes confirmed by microscopy, while Morici et al 16 reported in a case series of critically ill COVID‐19 patients that 5 of 6 had low ADAMTS13 activity accompanied by high VWF:Ag. Huisman et al 9 similarly reported low ADAMTS13 activity in the presence of high VWF:Ag in COVID‐19 patients with suspected TMA, while Ladikou et al 10 described high levels of VWF:Ag in critically ill COVID‐19 patients similar to that seen in critically ill patients with severe sepsis. Finally, Bazzan et al 11 observed high VWF:Ag and low ADAMTS13 activity in 9 fatal cases of COVID‐19 as opposed to values in 79 survivors.
Interestingly, ADAMTS13:VWF:Ag ratio was negatively correlated with age, which may be partly explained by expected trends observed with advancing age. Kokame et al 17 reported that ADAMTS13 activity decreases with age, especially after 60 years, while plasma VWF:Ag levels increase in the elderly, thus resulting in significant changes in their ratio (increase in VWF/ADAMTS13 ratio, or decrease in ADAMTS13/VWF ratio). Such changes likely contribute to a prothrombotic state with advancing age, potentially contributing to pathologies such as myocardial infarction and stroke. 17 Similarly, we hypothesize that such changes may contribute to COVID‐19 severity and in part explain higher morbidity and mortality in elderly patients. Thus, elderly patients with lower baseline ADAMTS13 activity, but higher VWF levels, may not be able to compensate for the inflammation‐driven release of large VWF multimers, leading to faster and/or enhanced accumulation of microthrombi within the lungs. Given the possibility for age to confound the primary analysis due to multiple factors, we explored advancing age as an independent predictor of severe AKI in multivariate analysis; however, it was not significant when controlling for other variables.
The ADAMTS13:VWF:Ag ratio was also found to be negatively correlated with LDH, while positively correlated with hemoglobin. These correlations further paint a picture of a secondary microangiopathy, with progressive tissue death and erythrocyte injury accompanying decreases in the ratio driven by low ADAMTS13 activity and accumulation of large VWF multimers. However, no correlation could be observed between platelet count and ADAMTS13:VWF:Ag ratio. Previous investigations in patients with consumptive coagulopathies showed that decreased ADAMTS13 activity does not necessarily correlate with platelet count, but correlated with dynamic changes in platelet count. 18 This could explain the observations in our study, where measures from only a single early time point were used. Serial measurements of platelets should be further investigated in patients with COVID‐19. Mild thrombocytopenia appears to be most common finding in CAC, and a low platelet count has been clearly established as unfavorable prognostic factor in COVID‐19. 19
In multivariable logistic regression, we observed that a low ADAMTS13:VWF:Ag ratio at ED presentation independently predicted severe AKI in patients with COVID‐19. We chose severe AKI as the primary outcome as this is a typical feature of TMA. Moreover, a recent meta‐analysis in COVID‐19 patients found that AKI was associated with 18‐fold increase in odds of severe disease and 23‐fold increase in odds of mortality. 20 While we provide substantial evidence of a TMA‐like phenomenon, it is important to consider that the observed AKI is likely a combination of multiple factors. As observed in this analysis, we found very high myoglobin values in patients with severe AKI, and a significant negative correlation was also noted between ADAMTS13:VWF:Ag ratio and myoglobin. Thus, high myoglobin driven by muscle cell death may contribute to AKI. Moreover, renal tropism of SARS‐CoV‐2 has now been clearly described in patients with COVID‐19. 21 Braun et al 22 recently demonstrated that SARS‐CoV‐2 RNA was found in the kidneys of 60% of autopsied patients, and that this renal tropism was associated with cytopathic injury, ischemia, disease severity, and outcomes, including development of AKI. Thus, the etiology of AKI is likely complex in COVID‐19, and further exacerbated by prerenal insults in patients with critical illness.
Despite our observations, it is difficult to ascertain to what extent ADAMTS13:VWF:Ag imbalance contributes to CAC. Low ADMATS13 levels have been associated with poor outcomes in patients with sepsis. 23 , 24 In this study, we found that low ADAMTS13:VWF:Ag was associated with poor outcomes in COVID‐19. Nonetheless, whether mild ADAMTS13 deficiency, as seen in COVID‐19 or otherwise, can significantly contribute to driving a TMA‐like phenomenon is unknown. 16 However, low ADAMTS13 activity is still likely to substantially contribute to the development of thrombosis, especially with concomitant high VWF levels. Here, we would expect an interruption of the normal equilibrium and interplay between ADAMTS13 and VWF. Further investigations are needed to elucidate the mechanism of CAC, including further evaluation of VWF, as well as evaluation of complement pathways, which may significantly contribute to TMA.
Although heparin may be a prophylaxis option for preventing thromboembolism in COVID‐19, it is unlikely to be effective at preventing or treating microthrombi in the lung and other tissues, especially that driven by VWF. Treatment could be aimed at restoring the balance in the ADAMTS13:VWF:Ag ratio. 11 , 16 , 25 Thus, it seems reasonable to conclude that fresh frozen plasma, plasma exchange, recombinant ADAMTS13, caplacizumab (anti‐VWF), and antiplatelet agents may reveal more beneficial effects at preventing severe AKI and other TMA insults in patients with COVID‐19. 16 , 25 In fact, observational data from multiple case series in critically ill COVID‐19 patients have demonstrated a potential improvement in outcomes with therapeutic plasma exchange that should be further evaluated in randomized controlled trials. 26 , 27
This study was limited by a relatively small sample size; however, to the best of our knowledge, is the largest study to date measuring ADAMTS13 and VWF:Ag in patients with COVID‐19, and the only study to have investigated these values with respect to outcomes. The cohort in our study did not experience major thromboembolic events and thus our results should be confirmed in other cohorts with potentially worse outcomes. However, given the timeline of the pandemic, the presence of a high‐risk prothrombotic state was well known by the time the first wave hit our institution; thus, appropriate countermeasures were prepared for hospitalized patients, with the majority receiving heparin. Also, not all patients had a pre‐COVID SCr value available to define a baseline. It is hence possible that AKI may be underrepresented in our cohort if patients presented with AKI that did not improve during the observation period and were thus classified as unchanged from baseline. Finally, though informative from pathophysiologic standpoint, the clinical value of ADAMTS13:VWF:Ag ratio remains unclear and requires further investigation.
5. CONCLUSION
We found that low ADAMTS13:VWF:Ag ratio at ED presentation is associated with an increased risk of progressing to critical COVID‐19 illness and severe AKI, with a pattern suggestive of a secondary microangiopathy. Nearly, a fourth of our patients presented with relative deficiency in ADAMTS13 activity, while 80.8% had elevated levels of VWF:Ag. The ADAMTS13:VWF:Ag ratio correlated with age, reinforcing evidence that patients with advanced age may be more susceptible to thrombotic complications and unfavorable outcome in COVID‐19. Interventional studies should be conducted to assess the benefit of restoring ADAMTS13:VWF:Ag ratios in hospitalized patients with COVID‐19.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
BMH, EJF, and GL conceived and designed the study. SWB and JLB collected samples and ran experiments. JLB and MHSO performed data acquisition and collection. BMH and MHSO did data analysis. BMH, EJF, GL, SWB, and JLB interpreted the data. BMH prepared the first draft. BMH, JLB, MHSO, EJF, GL, and SWB critically revised the manuscript for important intellectual content.
Supporting information
Table S1
Table S2
Henry BM, Benoit SW, de Oliveira MHS, Lippi G, Favaloro EJ, Benoit JL. ADAMTS13 activity to von Willebrand factor antigen ratio predicts acute kidney injury in patients with COVID‐19: Evidence of SARS‐CoV‐2 induced secondary thrombotic microangiopathy. Int J Lab Hematol.2021;43:129–136. 10.1111/ijlh.13415
Emmanuel J. Favaloro and Justin L. Benoit share senior authorship of this article.
Funding information
This study was funded by the University of Cincinnati College of Medicine Special Coronavirus (COVID‐19) Research Pilot Grant Program.
DATA AVAILABILITY STATEMENT
Data available on reasonable request from the authors.
REFERENCES
- 1. Lippi G, Sanchis‐Gomar F, Henry BM. COVID‐19: unravelling the clinical progression of nature's virtually perfect biological weapon. Ann Transl Med. 2020;8(11):693. 10.21037/atm-20-3989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Henry BM, de Oliveira MHS, Benoit S, Plebani M, Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID‐19): a meta‐analysis. Clin Chem Lab Med. 2020;58(7):1021‐1028. 10.1515/cclm-2020-0369 [DOI] [PubMed] [Google Scholar]
- 3. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054‐1062. 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Henry BM, Vikse J, Benoit S, Favaloro EJ, Lippi G. Hyperinflammation and derangement of renin‐angiotensin‐aldosterone system in COVID‐19: a novel hypothesis for clinically suspected hypercoagulopathy and microvascular immunothrombosis. Clin Chim Acta. 2020;507:167‐173. 10.1016/j.cca.2020.04.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Martinelli N, Montagnana M, Pizzolo F, et al. A relative ADAMTS13 deficiency supports the presence of a secondary microangiopathy in COVID 19. Thromb Res. 2020;193:170‐172. 10.1016/j.thromres.2020.07.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid‐19. N Engl J Med. 2020;383(2):120‐128. 10.1056/NEJMoa2015432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gavriilaki E, Brodsky RA. Severe COVID‐19 infection and thrombotic microangiopathy: success does not come easily. Br J Haematol. 2020;189(6):e227‐e230. 10.1111/bjh.16783 [DOI] [PubMed] [Google Scholar]
- 8. Levi M, Thachil J. Coronavirus disease 2019 coagulopathy: disseminated intravascular coagulation and thrombotic microangiopathy‐either, neither, or both. Semin Thromb Hemost. 2020;46:781–784. 10.1055/s-0040-1712156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Huisman A, Beun R, Sikma M, Westerink J, Kusadasi N. Involvement of ADAMTS13 and von Willebrand factor in thromboembolic events in patients infected with SARS‐CoV‐2. Int J Lab Hematol. 2020;42. 10.1111/ijlh.13244. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ladikou EE, Sivaloganathan H, Milne KM, et al. Von Willebrand factor (vWF): marker of endothelial damage and thrombotic risk in COVID‐19. Clin Med (Lond). 2020;20:e178–e182. 10.7861/clinmed.2020-0346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bazzan M, Montaruli B, Sciascia S, Cosseddu D, Norbiato C, Roccatello D. Low ADAMTS 13 plasma levels are predictors of mortality in COVID‐19 patients. Intern Emerg Med. 2020;15(5):861‐863. 10.1007/s11739-020-02394-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Singbartl K, Kellum JA. AKI in the ICU: definition, epidemiology, risk stratification, and outcomes. Kidney Int. 2012;81(9):819‐825. 10.1038/ki.2011.339 [DOI] [PubMed] [Google Scholar]
- 13. ARUP Laboratories . von Willebrand factor antigen reference interval. https://ltd.aruplab.com/Tests/Pub/0030285. Accessed August 21, 2020.
- 14. KDIGO AKI Working Group . KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2(1):1. [Google Scholar]
- 15. World Health Organization . WHO R&D Blueprint novel coronavirus: COVID‐19 theapeutic trial synposis. 2020. https://www.who.int/blueprint/priority‐diseases/key‐action/COVID‐19_Treatment_Trial_Design_Master_Protocol_synopsis_Final_18022020.pdf. Accessed June 1, 2020.
- 16. Morici N, Bottiroli M, Fumagalli R, Marini C, Cattaneo M. Role of von Willebrand factor and ADAMTS‐13 in the pathogenesis of thrombi in SARS‐CoV‐2 infection: time to rethink. Thromb Haemost. 2020;120:1339‐1342. 10.1055/s-0040-1713400 [DOI] [PubMed] [Google Scholar]
- 17. Kokame K, Sakata T, Kokubo Y, Miyata T. von Willebrand factor‐to‐ADAMTS13 ratio increases with age in a Japanese population. J Thromb Haemost. 2011;9(7):1426‐1428. 10.1111/j.1538-7836.2011.04333.x [DOI] [PubMed] [Google Scholar]
- 18. Song J, Lee KA, Park TS, Park R, Choi JR. Linear relationship between ADAMTS13 activity and platelet dynamics even before severe thrombocytopenia. Ann Clin Lab Sci. 2008;38(4):368‐375. [PubMed] [Google Scholar]
- 19. Lippi G, Plebani M, Henry BM. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID‐19) infections: a meta‐analysis. Clin Chim Acta. 2020;506:145‐148. 10.1016/j.cca.2020.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cheruiyot I, Henry B, Lippi G, et al. Acute kidney injury is associated with worse prognosis in COVID‐19 patients: a systematic review and meta‐analysis. Acta Biomed. 2020;91(3):e2020029. 10.23750/abm.v91i3.10222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Puelles VG, Lütgehetmann M, Lindenmeyer MT, et al. Multiorgan and renal tropism of SARS‐CoV‐2. N Engl J Med. 2020;383(6):590‐592. 10.1056/NEJMc2011400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Braun F, Lütgehetmann M, Pfefferle S, et al. SARS‐CoV‐2 renal tropism associates with acute kidney injury. Lancet. 2020;396:597‐598. 10.1016/S0140-6736(20)31759-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lin J‐J, Chan O‐W, Hsiao H‐J, Wang Y, Hsia S‐H, Chiu C‐H. Decreased ADAMTS 13 activity is associated with disease severity and outcome in pediatric severe sepsis. Medicine (Baltimore). 2016;95(16):e3374. 10.1097/MD.0000000000003374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kremer Hovinga JA, Zeerleder S, Kessler P, et al. ADAMTS‐13, von Willebrand factor and related parameters in severe sepsis and septic shock. J Thromb Haemost. 2007;5(11):2284‐2290. 10.1111/j.1538-7836.2007.02743.x [DOI] [PubMed] [Google Scholar]
- 25. Cattaneo M, Bertinato EM, Birocchi S, et al. Pulmonary embolism or pulmonary thrombosis in COVID‐19? Is the recommendation to use high‐dose heparin for thromboprophylaxis justified? Thromb Haemost. 2020;120(8):1230‐1232. 10.1055/s-0040-1712097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Morath C, Weigand MA, Zeier M, Speer C, Tiwari‐Heckler S, Merle U. Plasma exchange in critically ill COVID‐19 patients. Crit Care. 2020;24(1):481. 10.1186/s13054-020-03171-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Khamis F, Al‐Zakwani I, Al Hashmi S, et al. Therapeutic plasma exchange in adults with severe COVID‐19 infection. Int J Infect Dis. 2020;99:214‐218. 10.1016/j.ijid.2020.06.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Table S2
Data Availability Statement
Data available on reasonable request from the authors.


