Abstract
We evaluated the symptoms, changes in laboratory findings during the novel coronavirus disease (COVID‐19) pandemic, and the effect of depression in patients with peritoneal dialysis (PD). This is an observational and cross‐sectional study. All patients were asked to fill the clinical assessment form and Beck depression and anxiety inventory. Also, the last two laboratory evaluations during this period were examined. A total of 123 patients performing PD were included. None of the patients were diagnosed with COVID‐19. In the total study population, parathyroid hormone (PTH), serum albumin, phosphorus and ferritin levels significantly elevated at the end of 97 ± 31 days. PTH and phosphorus levels remained stable in remote monitoring automated PD (RM‐APD) group (p = 0.4 and p = 0.5), they tended to increase in continuous ambulatory PD group and significantly increased in automated PD group (p = 0.09 and p = 0.01 for PTH and p = 0.06 and p = 0.001 for phosphorus, respectively). Moderate to severe depression was associated with dyspnoea, weight gain more than 5 kg, fatigue, palpitation and increased anxiety. PD is a reliable and successful form of dialysis and can be safely administered even if hospital access is restricted. Also, RM‐APD may be a better choice because of providing more stable bone‐mineral metabolism. Moreover, evaluating depression and anxiety is essential for the accurate clinical assessment.
Keywords: Beck inventory, COVID‐19, depression, peritoneal dialysis, remote patient management
1. INTRODUCTION
Novel coronavirus disease (COVID‐19) caused by severe acute respiratory syndrome coronavirus 2 was declared as a pandemic on 11 March 2020 by the World Health Organization. 1 The disease, which primarily manifests as an acute upper and lower respiratory tract infection, could affect multiple organs and systems, including the heart, intestine, kidneys, blood and nervous system. 1 , 2 The overall estimated frequency of severe cases and mortality reported was 25% (17.4–34.9) and 3.6% (1.1–7.2) respectively. 3
The patient population with chronic disease was the most affected group by this pandemic. One of these frail groups consisted of patients with end‐stage renal disease (ESRD). Patients on maintenance dialysis are likely to be at increased risk of COVID‐19 and its complications due to older age, having multiple co‐morbid conditions, and suppressed immune system. 1 , 2 , 4 Also, the need for travel thrice weekly to the dialysis centre, clustering of patients in dialysis units, and contact of dialysis staff member with more than one patient also restricts the physical isolation of haemodialysis patients, which is necessary for protection from the virus. Hereby, frequency and mortality are detected as 16% and 16.2% in this patient population. 5
On the other hand, peritoneal dialysis (PD) is the most frequent home‐based dialysis treatment and could provide physical isolation of dialysis patients. The International Society of Peritoneal Dialysis (ISPD) provided practical guidelines that encourage clinicians to choose PD as maintenance dialysis modality during this pandemic. 6 Technological advances, such as remote access modules in the PD field, made it easier to manage patients' dialysis prescriptions for physicians and provided increased patient treatment compliance. 7 , 8 , 9 However, it is known that there is a strong relationship between depression and non‐compliance. 10 Patients were constantly at home during this pandemic, and this social isolation may lead to anxiety and depression. 11 Also, the anxiety that the disease has caused may affect dialysis treatment compliance.
However, there is no literature about how successful PD was as a maintenance dialysis treatment without regular clinic visits. Also, it is not known how often the patients experienced the important consequences of ESRD, including itching, fatigue, palpitation, loss of appetite, muscle‐joint pain, hypervolemia and difficulty in blood pressure control, and how did they solve the problem during the COVID‐19 pandemic. Besides, the effect of anxiety and depression level on symptoms associated with ESRD is unknown.
The aim of this study was to evaluate the symptoms experienced by patients during this period. Also, change in laboratory findings when they could not admit to hospital visits is analysed. Besides, the depression and anxiety levels of patients who underwent PD during pandemic were questioned, and the effects of depression on symptoms associated with ESRD were examined.
2. METHODS
2.1. Study population
This study was an observational and cross‐sectional study investigating the effects of COVID‐19 pandemic on patients performing PD. The study was conducted in the PD units of Gazi University, Ankara, and Diskapi education and research hospitals. All patients were asked about symptoms related to ESRD, problems specific to PD (e.g., catheter outflow problems and peritonitis), and patients were asked to fill Beck depression and anxiety scale. Health Ministry of Turkey Republic and the local Ethics Committee of Gazi University approved the study's design and procedures in agreement with the principles of the Declaration of Helsinki and ethical standards for human experimentation. Written informed consent was obtained from all participants. The inclusion criteria of the present study were as follows: treatment by maintenance PD for at least 6 months, age>18 years, and patients who agreed to participate.
2.2. PD modalities, clinical assessment, beck depression, and anxiety scales
The patients included in this study performed three different PD modalities. Continuous ambulatory PD (CAPD) consisted of multiple exchanges (generally three or four) during the day by the patient or caregiver. Automated peritoneal dialysis (APD) uses a cycler device to perform multiple overnight exchanges with short dwells. Remote monitoring automated peritoneal dialysis (RM‐APD) is working on the same principle as APD, additionally, transmitting relevant dialysis session data including missing a treatment, lost connectivity, bypass drain through PD centre via cloud‐based software. The same PD nurse checked the record of the patients with performing RM‐APD included in the study on the ShareSource connectivity platform daily (Homechoice Claria; Baxter Healthcare Corporation). Patients were contacted when a problem was detected.
Also, all patients were evaluated by phone if they had any problems (e.g. constipation, discharge problem, abdominal pain, blurred dialysate or high blood pressure), and the problem was tried to be solved without a hospital visit. If there was a problem that could not be solved by phone, the patient was called to the clinic visit. In addition, all patients, regardless of the problem, were called by phone once a month for clinical evaluation.
After the 3 months (between March and June 2020) of limited hospital access, patients who referred to routine clinic visits were asked to fill a ‘clinical evaluation form’ (Supporting Information S1). This form was prepared mainly to investigate symptoms, evaluate problems specific to PD, and also evaluate the effects of COVID‐19. Also, patients were asked to fill Beck's depression and anxiety inventories. Beck depression inventory is a widely used measure of depression and validated in the Turkish population. 12 The survey consisted of 21 items, and scores are in the range 0–63. Higher scores indicating severe depression (0–13: minimal; 14–19:mild; 20–28:moderate; 29–63:severe). Beck anxiety inventory with 21 items is a severity indicator of anxiety and validated in the Turkish population. 13 Scores are in the range 0–63 and higher scores indicating severe anxiety (0–21:low, 22–35:moderate; and >36:concerning levels of anxiety).
2.3. Clinical outcomes
Hospital electronic medical records system was used for baseline information such as sex, age, PD modality, education level, laboratory parameters including haemoglobin, ferritin, blood urea nitrogen, creatinine, total protein, albumin, calcium, phosphorus and parathyroid hormone (PTH). Data were collected at the last clinical visit, and also data of patients, which were obtained 3 months ago, were also recorded. More than 100 ml of urine per day was considered residual renal function (RRF). The patients were asked to perform blood pressure measurement at home, with regularly calibrated and validated automatic devices following the 10‐minute rest period. Smoking, drinking tea, or coffee and exercise for at least 30 minutes were prohibited. We determined target blood pressure as <140/90 mm/Hg for all patients. Dialysis interruption refers to skipping a session.
2.4. Statistical analysis
Analyses were performed with SPSS 21.0.0.1 (SPSS; IBM) software for Windows. Data distribution was determined using the Kolmogorov‐Smirnov test. The homogeneity of variables was determined using the one‐way ANOVA homogeneity of variance test. Continuous variables are reported as means and standard deviation or as median and minimum‐maximum according to data distribution. Categorical variables are reported by percentages. We used the paired sample T‐test or Wilcoxon test according to data distribution when we compared changes in laboratory parameters within groups. Chi‐square test was used to compare categorical variables between two groups. When we compared more than two groups, the Kruskal–Wallis test was used for numerical variables, and the Chi‐square trend test was used for categorical variables. Post hoc analysis was used to determine the statistical difference between more than two groups. A value of p < 0.05 was considered statistically significant.
3. RESULTS
3.1. Demographic and laboratory evaluation
We analysed a total of 123 patients performing PD. Fifty‐nine (48%) of total patients were female and the mean age of the study population was 51 ± 14 years. The most common cause of CKD was hypertension (54/123, 44%). While the median dialysis vintage time was 41 (8–120) months, 80% (98/123) of patients had RRF. The distribution of demographic characteristics was similar between the three groups, except the education level. Twenty‐five percentage of patients who performed RM‐APD were university graduates, and the difference was significantly higher than groups performing CAPD and APD (p < 0.001) (Table 1).
TABLE 1.
Demographic characteristics of study population
|
Total n = 123 |
CAPD n = 53 (43%) |
RM‐APD n = 40 (33%) |
APD n = 30 (24%) |
p value | |
|---|---|---|---|---|---|
| Age, (years) | 51 ± 14 | 51 ± 12 | 49 ± 15 | 53 ± 17 | .6 |
| Gender, n (%) | .5 | ||||
| Female | 59 (48) | 29 (55) | 20 (50) | 10 (33) | |
| Male | 64 (52) | 24 (45) | 20 (50) | 20 (67) | |
| Aetiology of CKD, n (%) | |||||
| Diabetes | 24 (20) | 10 (19) | 8 (20) | 6 (20) | .9 |
| Hypertension | 54 (44) | 27 (51) | 17 (43) | 10 (33) | .3 |
| Glomerulonephritis | 16 (13) | 5 (9) | 7 (17) | 4 (13) | .5 |
| Others | 20 (16) | 5 (9) | 7 (17) | 8 (27) | .1 |
| Unknown | 9 (7) | 6 (12) | 1 (3) | 2 (7) | .2 |
| School level, n (%) | |||||
| Illiteracy or reading | 11 (9) | 6 (12) | 3 (8) | 2 (7) | .7 |
| Elementary | 46 (37) | 24 (45) | 9 (22) | 13 (43) | .06 |
| High school | 55 (45) | 23 (43) | 18 (45) | 14 (47) | .9 |
| University | 11 (9) | 0 | 10 (25) | 1 (3) | <.001 |
| Smoking, n (%) | 11 (9) | 3 (6) | 5 (13) | 3 (10) | .3 |
| RRF, n (%) | 98 (80) | 46 (87) | 30 (75) | 22 (73) | .2 |
| People living together, n | 3 (1–7) | 2 (1–7) | 3 (2–5) | 3 (2–7) | .06 |
| Dialysis vintage, (months) | 41 (8–120) | 34 (12–118) | 48 (12–120) | 48 (8–120) | .2 |
Abbreviations: APD, automatized peritoneal dialysis; CAPD, continuous ambulatory peritoneal dialysis; CKD; chronic kidney disease; RM‐APD, remote monitoring automatized peritoneal dialysis, RRF, residue renal function.
Change of laboratory parameters of study population are shown in Table 2. The mean time between the last two laboratory evaluations during this period, when the patients had limited hospital access, was 97 ± 31 days. In this time interval, we found that serum ferritin, creatinine, phosphorus, albumin and PTH levels were significantly elevated in the total study population (p = 0.03, p = 0.01, p = 0.02, p = 0.02, and p = 0.048, respectively). The serum ferritin level of patients performing RM‐APD was significantly elevated during this period (from 387 ± 208 to 460 ± 237 ng/ml, p = 0.02). On the other hand, there was no significant change in laboratory parameters in patients performing RM‐APD. While serum creatinine was significantly elevated in patients with CAPD (from 8.42 to 9.09 ± 3.05 mg/dl, p = 0.03), serum calcium, phosphorus and PTH levels were tended to be significantly elevated during this time interval (p = 0.08, p = 0.06, and p = 0.09, respectively). In the group of patients performing APD, serum phosphorus, albumin and PTH levels were found to be significantly elevated [from 4.66 ± 1 to 5.05 ± 1.27 mg/dl, from 3.43 ± 0.52 to 3.49 ± 0.48 g/dl and from 297 (7–1956) to 398 (9–808) pg/ml; p = 0.001, p = 0.04 and p = 0.01, respectively].
TABLE 2.
Change of laboratory parameters of study population within 3 months
|
Total n = 123 |
CAPD n = 53 (43%) |
RM‐APD n = 40 (33%) |
APD n = 30 (24%) |
p value | |
|---|---|---|---|---|---|
| Interval between laboratory tests (days) | 97 ± 31 | 92 ± 29 | 96 ± 28 | 102 ± 37 | .2 |
| Haemoglobin, g/dl | |||||
| First | 10.7 ± 1.83 | 10.64 ± 1.86 | 10.61 ± 1.62 | 10.9 ± 2.12 | .8 |
| Last | 10.5 ± 2.01 | 10.34 ± 2 | 10.45 ± 1.74 | 10.88 ± 2.4 | .6 |
| p value | 0.1 | 0.3 | 0.6 | 0.3 | |
| Ferritin, ng/ml | |||||
| First | 372 ± 249 | 385 ± 277 | 387 ± 208 | 338 ± 233 | .7 |
| Last | 404 ± 299 | 398 ± 335 | 460 ± 237 | 375 ± 278 | .2 |
| p value | 0.03 | 0.6 | 0.02 | 0.1 | |
| BUN, mg/dl | |||||
| First | 47.7 ± 13.5 | 52.5 ± 15 | 47 ± 11.6 | 41.4 ± 11.2 | .002 |
| Last | 48.4 ± 13 | 51.6 ± 11.8 | 46.7 ± 11.2 | 44.7 ± 16.45 | .07 |
| p value | 0.8 | 0.5 | 0.4 | 0.1 | |
| Creatinine, mg/dl | |||||
| First | 8.38 ± 3.1 | 8.42 ± 2.82 | 9.1 ± 3.07 | 7.37 ± 3.4 | .07 |
| Last | 8.62 ± 3.3 | 9.09 ± 3.05 | 9.18 ± 3.3 | 6.97 ± 3.37 | .02 |
| p value | 0.01 | 0.03 | 0.9 | 0.09 | |
| Calcium, mg/dl | |||||
| First | 8.85 ± 0.86 | 8.65 ± 0.85 | 9.2 ± 0.62 | 8.77 ± 1.08 | .02 |
| Last | 8.88 ± 0.98 | 8.83 ± 1.17 | 8.98 ± 0.63 | 8.83 ± 1.01 | .8 |
| p value | 0.08 | 0.08 | 0.5 | 0.1 | |
| Phosphorus, mg/dl | |||||
| First | 4.98 ± 1.27 | 5.2 ± 1.34 | 4.97 ± 1.34 | 4.66 ± 1 | .2 |
| Last | 5.24 ± 1.39 | 5.53 ± 1.22 | 4.99 ± 1.65 | 5.05 ± 1.27 | .2 |
| p value | 0.02 | 0.06 | 0.5 | 0.001 | |
| Albumin, g/dl | |||||
| First | 3.64 ± 0.44 | 3.75 ± 0.47 | 3.69 ± 0.28 | 3.43 ± 0.52 | .007 |
| Last | 3.71 ± 0.44 | 3.79 ± 0.49 | 3.76 ± 0.3 | 3.49 ± 0.48 | .02 |
| p value | 0.02 | 0.3 | 0.2 | 0.04 | |
| Alkaline phosphatase, U/L | |||||
| First | 118 (59–862) | 117 (59–368) | 119 (62–862) | 118 (42–628) | .9 |
| Last | 125 (51–962) | 129 (51–355) | 120 (63–962) | 125 (48–330) | .8 |
| p value | 0.1 | 0.2 | 0.3 | 0.8 | |
| Parathyroid hormone, pg/ml | |||||
| First | 367 (7–2348) | 330 (42–2348) | 413 (94–2100) | 297 (7–1956) | .2 |
| Last | 395 (9–2340) | 386 (14–2340) | 426 (115–2260) | 398 (9–808) | .2 |
| p value | 0.048 | 0.09 | 0.4 | 0.01 | |
Abbreviations: APD, automatized peritoneal dialysis; BUN, blood urea nitrogen; CAPD, continuous ambulatory peritoneal dialysis; RM‐APD, remote monitoring automatized peritoneal dialysis.
When we compared the PD modalities, we found that serum BUN and albumin levels were significantly higher in patients with CAPD (p = 0.002 and p = 0.007) at baseline. Post‐hoc analysis revealed that CAPD and APD (p = 0.001) caused a significant difference. During this period, while the serum albumin level difference between groups remained significant (p = 0.02), serum BUN level lost the significance (p = 0.07). On the other hand, serum calcium level was significantly higher in patients with RM‐APD (p = 0.02), and post‐hoc analysis showed that the difference was caused by RM‐APD and APD (p = 0.02). However, the difference between the three groups lost its significance during this time interval (p = 0.8).
3.2. Clinical assessment
Table 3 is designed for the evaluation of the clinical assessment of patients with PD. While 67% (82/123) of total patients did not describe dyspnoea, only 2% (3/123) of them had dyspnoea at rest. Fifty‐one percentage (63/123) of patients did not experience pitting oedema as evidence of hypervolemia. During this period, only 10 patients (8%) did not measure blood pressure in any way, while 52% (64/123) of total patients measured blood pressure daily by following instructions. Although 69% (78/113) of patients had blood pressure within the target range, two (2%) of the patients had to refer to the E.R due to uncontrolled hypertension. While PD prescription changes were not required in most of the patients due to hypervolemia (109/123, 88%), PD prescription was changed more than once in 7% (2/30) of patients who performed APD. While fatigue was the most common complaint of patients, 32% of them complained of bone‐muscle pain, 20% complained of loss of appetite and 19% complained of itching. The distribution of complaints was similar in patients performing all three PD modalities.
TABLE 3.
Clinical assessment related to end stage renal disease of study population for the last 3 months
|
Total n = 123 |
CAPD n = 53 (43%) |
RM‐APD n = 40 (33%) |
APD n = 30 (24%) |
p value | |
|---|---|---|---|---|---|
| Dyspnoea, n (%) | |||||
| No | 82 (67) | 38 (72) | 23 (58) | 21 (70) | .3 |
| Exercise | 12 (10) | 4 (8) | 7 (18) | 1 (3) | .1 |
| Walking | 26 (21) | 10 (19) | 9 (23) | 7 (23) | .8 |
| Resting | 3 (2) | 1 (3) | 1 (3) | 1 (3) | .9 |
| Pitting oedema, n (%) | |||||
| No | 63 (51) | 32 (60) | 18 (45) | 13 (43) | .2 |
| 1–3 times | 35 (29) | 12 (23) | 12 (30) | 11 (37) | .4 |
| More often | 14 (11) | 5 (9) | 5 (13) | 4 (13) | .5 |
| Always | 11 (9) | 4 (8) | 5 (12) | 2 (7) | .1 |
| BP measurement, n (%) | |||||
| No | 10 (8) | 5 (9) | 2 (5) | 3 (10) | .7 |
| Everyday | 64 (52) | 20 (38) | 25 (62) | 19 (63) | .02 |
| Once a week | 36 (29) | 21 (40) | 9 (23) | 6 (20) | .08 |
| Once a month | 13 (11) | 7 (13) | 4 (10) | 2 (7) | .7 |
| Difficult in BP control, n (%) a | |||||
| No | 78 (69) | 36 (75) | 25 (66) | 17 (63) | .8 |
| Reducing salt consumption | 14 (12) | 4 (8) | 6 (16) | 4 (15) | .5 |
| Increasing the dose of drug | 9 (8) | 2 (4) | 5 (13) | 2 (7) | .3 |
| Add new antihypertensive drug | 12 (11) | 6 (13) | 2 (5) | 4 (15) | .4 |
| Problem due to high BP, n (%) a | |||||
| No | 87 (77) | 38 (79) | 28 (73) | 21 (78) | .8 |
| Headache | 10 (9) | 6 (13) | 4 (11) | 3 (11) | |
| Nausea | 5 (4) | 1 (2) | 2 (5) | 2 (7) | |
| Fatigue | 4 (3) | 1 (2) | 4 (11) | 1 (4) | |
| Referring E.R. | 2 (2) | 2 (4) | 0 | 0 | |
| RRF change, n (%) b | |||||
| No change | 85 (87) | 37 (80) | 27 (90) | 21 (96) | .2 |
| Increased RRF | 3 (3) | 1 (2) | 1 (3) | 1 (4) | .9 |
| Extra diuretic for preservation | 10 (11) | 8 (17) | 2 (7) | 0 | .06 |
| PD prescription change due to hypervolemia | |||||
| No | 109 (88) | 46 (87) | 39 (98) | 24 (80) | .06 |
| Once | 12 (10) | 7 (13) | 1 (2) | 4 (13) | .2 |
| More than once | 2 (2) | 0 | 0 | 2 (7) | .03 |
| Gain weight, n (%) | |||||
| No | 70 (57) | 30 (57) | 20 (50) | 20 (67) | .4 |
| 1–3 kg | 41 (33) | 20 (38) | 13 (32) | 8 (27) | .6 |
| 3–5 kg | 9 (7) | 3 (6) | 5 (13) | 1 (3) | .3 |
| More than 5 kg | 3 (3) | 0 | 2 (5) | 1 (3) | .3 |
| Fatigue, n (%) | |||||
| Frequently (more than once per weak) | 58 (47) | 26 (39) | 19 (48) | 13 (43) | .9 |
| Palpitation, n (%) | |||||
| Frequently (more than once per weak) | 14 (11) | 4 (8) | 6 (15) | 4 (13) | .5 |
| Itching, n (%) | |||||
| Frequently (more than once per weak) | 23 (19) | 8 (15) | 10 (25) | 5 (17) | .5 |
| Loss of appetite, n (%) | |||||
| Frequently (more than once per weak) | 24 (20) | 10 (19) | 9 (23) | 5 (17) | .8 |
| Bone‐muscle pain, n (%) | |||||
| Frequently (more than once per weak) | 39 (32) | 16 (30) | 14 (35) | 9 (30) | .8 |
Abbreviations: APD, automatized peritoneal dialysis; BP, blood pressure; CAPD, continuous ambulatory peritoneal dialysis; PD, peritoneal dialysis; RM‐APD, remote monitoring automatized peritoneal dialysis; RRF, residue renal function
113 patients who measured blood pressure during this period.
98 patients who had RRF were used for analysis.
Table 4 shows the PD‐related clinical assessment of patients. Eighty‐three percentage of the total patients did not experience any PD solution outflow problem during this period. More than half of the outflow problem has been solved by changing the position (12/21, 57%). PD nurses have solved nine of the 21 outflow problems, and five episodes need to add heparin to the PD solution. All of the PD solution discharge problems have been solved via phone call. While 12% (15/123) of total patients experienced erythema at the catheter exit site, most of the episodes have been treated by daily catheter care (11/15, 69%). Like the PD solution outflow problem, all of the catheter exit site problems have been solved via phone call. In general, problems related to PD and also related to ESRD had been solved without clinical visits via phone call. However, 12 (10%) of the total patients had to be hospitalized during this period. Four of them (3%) had peritonitis, and two of them (2%) were hospitalized due to cardiovascular problems. None of the patients, who needed to be hospitalization, died during this period. Although the caregiver of one patient and first degree relative of two patients were diagnosed with COVID‐19, patients who were included in this study were not infected. Caregiver and relatives, who were diagnosed with COVID‐19, had mild to moderate symptoms and hospitalization was not required. All of them were isolated.
TABLE 4.
Clinical assessment related to peritoneal dialysis of study population for the last 3 months.
|
Total n = 123 |
CAPD n = 53 (43%) |
RM‐APD n = 40 (33%) |
APD n = 30 (24%) |
p value | |
|---|---|---|---|---|---|
| Constipation, n (%) | |||||
| Frequently (more than once per weak) | 16 (13) | 3 (6) | 10 (25) | 3 (10) | |
| PD solution discharge problem, n (%) | .3 | ||||
| No | 102 (83) | 46 (87) | 34 (85) | 22 (73) | |
| Frequently (more than once per month) | 21 (17) | 7 (13) | 6 (15) | 8 (27) | |
| How the problem was solved, n (%) | |||||
| Change position | 12 (57) | 3 (42) | 4 (66) | 5 (62) | |
| PD nurse call | 9 (43) | 4 (58) | 2 (33) | 3 (38) | |
| Add heparin to PD solution | 5 (23) | 2 (29) | 0 | 3 (38) | |
| Erythema at the PD catheter exit site, n (%) | .9 | ||||
| No | 108 (88) | 47 (89) | 35 (87) | 26 (87) | |
| Yes | 15 (12) | 6 (11) | 5 (13) | 4 (13) | |
| How the problem is solved, n (%) | |||||
| Daily care | 11 (69) | 5 (83) | 2 (40) | 4 (100) | |
| Topical antibiotic | 4 (31) | 1 (17) | 3 (60) | 0 | |
| Abdominal or groin hernia, n (%) | |||||
| Yes | 15 (12) | 4 (8) | 6 (15) | 5 (17) | .4 |
| Increase in size | 3 (20) | 1 (25) | 2 (33) | 0 | .3 |
| Cause to pain | 4 (27) | 1 (25) | 2 (33) | 1 (20) | .9 |
| Dialysis interruption, n (%) | |||||
| Frequently (more than once per month) | 10 (8) | 3 (6) | 2 (5) | 5 (17) | .04 |
| Why was the dialysis interrupted, n (%) | |||||
| Fatigue | 5 (50) | 1 (33) | 1 (50) | 3 (60) | |
| Not affect treatment adequacy | 5 (50) | 2 (66) | 1 (50) | 2 (40) | |
| Hospitalization, n (%) | |||||
| Peritonitis | 12 (10) | 6 (11) | 2 (5) | 4 (13) | .1 |
| COVID‐19 | 4 (3) | 2 (4) | 0 | 2 (6) | |
| CVD | 0 | 0 | 0 | 0 | |
| Others | 2 (2) | 2 (4) | 0 | 0 | |
| Hospitalization time | 6 (5) | 2 (4) | 2 (5) | 2 (6) | |
Abbreviations: APD, automatized peritoneal dialysis; CAPD, continuous ambulatory peritoneal dialysis; PD, peritoneal dialysis; COVID‐19, novel coronavirus disease; RM‐APD, remote monitoring automatized peritoneal dialysis.
Peritoneal dialysis‐related clinical assessment of patients was similar among three PD modalities, except dialysis interruption. Ten patients interrupted their dialysis sessions more than once per month. Five of them (17%) were performing APD, and the frequency was significantly higher than patients with CAPD and performing RM‐APD [5 (17%) vs. 3 (6%) vs. 2 (5%), p = 0.04].
3.3. Assessment of depression
Eighty‐five of the total patients were analysed for depression evaluation (Table 5). Twenty‐two percentage (19/85) of them had moderate to severe depression. Patients, who had moderate to severe depression, complain more dyspnoea, fatigue and palpitation when compared to patients who had minimal to mild depression [11 (58%) vs. 20 (30%), 14 (74%) vs. 28 (42%) and 6 (32%) vs. 3 (5%); p = 0.03, p = 0.01 and p = 0.003, respectively]. Also, the frequency of patients who gained more than 5 kg was significantly higher in patients with moderate to severe depression than those with minimal to mild depression [2 (11%) vs. 0, p = 0.008]. None of the patients had a concerning‐level anxiety in our study population. However, moderate degree anxiety was significantly higher in patients who also had moderate to severe depression [3 (16%) vs. 2 (3%), p = 0.03]. When we asked patients whether COVID‐19 affected their lives, 38% (47/123) of patients stated that they were affected (Figure 1). The most common condition that patients complained about was the restriction of their activity (23/123, 49%). Also, 30% of the patients stated that they felt fear and panic, while 17% of them stated that the restriction of access to the hospital was the most important effect of the pandemic.
TABLE 5.
Assessment of patients' depression
| Severity of depression | p value | ||
|---|---|---|---|
|
Minimal to mild n = 66 (78%) |
Moderate to severe n = 19 (22%) |
||
| Gender, n (%) | .3 | ||
| Female | 29 (43) | 10 (53) | |
| Male | 37 (56) | 9 (47) | |
| PD modality, n (%) | |||
| CAPD | 29 (44) | 4 (21) | .06 |
| RM‐APD | 28 (42) | 10 (53) | .3 |
| APD | 9 (14) | 5 (26) | .2 |
| Dyspnoea, n (%) | |||
| Yes | 20 (30) | 11 (58) | .03 |
| Difficult in BP control, n (%) | |||
| Yes | 20 (32) | 7 (37) | .5 |
| Reducing salt consumption | |||
| Increasing the dose of drug | |||
| Add new antihypertensive drug | |||
| Gain weight, n (%) | |||
| Yes | 27 (41) | 10 (53) | .3 |
| 1–3 kg | 22 (33) | 6 (32) | .6 |
| 3–5 kg | 5 (8) | 2 (11) | .5 |
| More than 5 kg | 0 | 2 (11) | .008 |
| Fatigue (frequently), n (%) | 28 (42) | 14 (74) | .01 |
| Palpitation (frequently), n (%) | 3 (5) | 6 (32) | .003 |
| Loss of appetite (frequently), n (%) | 10 (15) | 4 (21) | .4 |
| Bone‐muscle pain (frequently), n (%) | 16 (24) | 7 (37) | .2 |
| Constipation (frequently), n (%) | 7 (11) | 4 (21) | .2 |
| Peritonitis, n (%) | 2 (3) | 2 (11) | .2 |
| Did COVID‐19 effected your life, n (%) | |||
| Yes | 25 (38) | 7 (37) | .9 |
| Severity of anxiety, n (%) | .03 | ||
| Low | 64 (97) | 16 (84) | |
| Moderate | 2 (3) | 3 (16) | |
| Concerning level | 0 | 0 | |
Abbreviations: APD, automatized peritoneal dialysis; CAPD, continuous ambulatory peritoneal dialysis; PD, peritoneal dialysis; COVID‐19, novel coronavirus disease; RM‐APD, remote monitoring automatized peritoneal dialysis.
FIGURE 1.
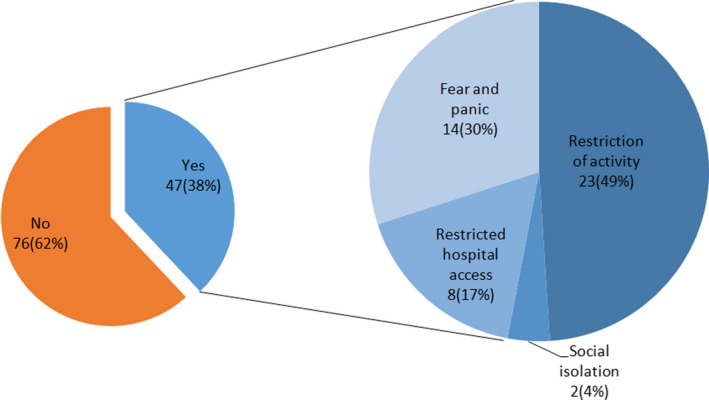
Effects of novel coronavirus disease pandemic on patients with peritoneal dialysis [Color figure can be viewed at wileyonlinelibrary.com]
4. DISCUSSION
The COVID‐19 pandemic is a global crisis that affected and changed the world order and caused hundreds of thousands of deaths. In this period, people's social lives have been restricted, and perhaps most importantly, there have been delays in the diagnosis and treatment of chronic diseases that need regular follow‐up. Our results suggested that PD, which is a home‐based dialysis modality, is a reliable and successful form of dialysis and can be safely administered even if hospital access is restricted. Most of the problems were solved without clinical visits via phone calls with patients. However, we found that depression and anxiety can mimic the symptoms, which could be seen in conditions such as renal anaemia and dialysis insufficiency.
Direct person‐to‐person transmission is the primary way of transmission of COVID‐19. It is well known that close‐range contact contributes mainly via respiratory droplets, which spreads while coughing, sneezing or even talking. 14 Therefore, the primary way of preventing disease is social isolation and protection from droplets. However, this is a relatively difficult situation to follow for patients with chronic diseases, especially ESRD. Although outpatient haemodialysis facilities have taken the necessary precautions and try to maintain the distance between patients, this could not be entirely achieved due to the need for travel thrice weekly to the dialysis centre, clustering of patients, and contact of dialysis staff members. These limitations combined with older age, impaired immune system and multiple co‐morbid conditions and resulted in increased mortality. In the literature, the mortality rate of patients with maintenance centre haemodialysis due to COVID‐19 reported between 16% and 30%. 4 , 5
During the pandemic, home‐based dialysis modalities such as PD become prominent, and it is recommended to be preferred as the first‐line treatment option if possible by the ISPD. 6 There are limited data on the frequency and mortality of COVID‐19 in patients performing PD. Ronco et al. reported that the frequency of COVID‐19 is 0.7% (1/130) in Vicenza and 0.6% (3/497) in the Venoto region, and none of the patients died. 15 Their results also showed that PD have a significantly lower rate of COVID‐19 and all‐cause hospitalization when compared to HD. In our study population, we did not observe any PD patient with COVID‐19. On the other hand, in the three centres included in the study, there were 289 patients who received HD in centre and five (2%) of these patients were diagnosed with COVID‐19. One of these patients died due to COVID‐19. Similarly, Valeri et al. conducted a study in the USA with 59 COVID‐19‐infected patients on maintenance dialysis. Only two of them performed PD, and they did not observe mortality in patients with PD. 16 The reason why the frequency of COVID‐19 is lower in patients with PD than those with centre haemodialysis may be that patients apply hygiene rules as well as isolation. These patients are regularly trained about hand hygiene and the correct way of wearing face masks by PD nurses to prevent peritonitis. Although there is limited study in the literature on the safety of PD during the COVID‐19 pandemic, the data obtained support the reliability of PD based on decreasing the frequency of the disease transmission and mortality.
Although PD is a safe way of maintaining dialysis during the pandemic, it is necessary to clarify several important points, including renal anaemia, bone mineral disease, phosphorus balance and compliance with dialysis treatment to prove the success of PD. To the best of our knowledge, no previous studies have examined the clinical and laboratory assessment of patients with PD during the limited access to the hospital.
In this study, we found that the mean haemoglobin value of patients remained stable during the average of 3 months. We think that the most critical contributor factor for remaining haemoglobin stable is RRF. It is well known that the decline of RRF contributes significantly to anaemia and also resistance to erythropoietin‐stimulating agents. 17 , 18 In our study population, 80% of them had RRF, and we observed that 90% of them preserved RRF without increased diuretic needs during this period. PD provides better long‐term preservation of RRF compared to HD patients, and it makes the PD more prominent and successful in this period. 19 It is important to note that it is necessary to be careful in the clinical evaluation of patients via phone calls without examining laboratory values. Because based on our results, some findings, including fatigue, palpitation and dyspnoea, suggesting inadequate dialysis or deep anaemia, may be misleading. In our study, moderate to severe depression was observed in 22% of patients, and it was also associated with increased anxiety. It is known that fatigue and increased appetite are well described symptoms of depression, and increased anxiety could lead to dyspnoea and palpitation. 20
The other points to consider when evaluating the success of PD in this period are bone mineral disease, and hypervolemia. Based on our results, 80% of total patients complained little or no peripheral oedema as hypervolemia finding. Also, the fact that more than 90% of patients did not have serious adverse events due to increased blood pressure, and 80% of them could control blood pressure within normal limits also supported our results. On the other hand, it was found that patients were adversely affected in terms of bone mineral metabolism. At the end of three months, serum calcium, phosphorus, and PTH levels tended to increase. Many factors during this period may have affected bone mineral metabolism. A constant stay of patients at home may have affected their eating habits (increased frequency or amount of meals or junk food consumption) or increased immobilization or medical and dialysis treatment compliance could lead this condition. 21 , 22 However, this trend in the total study population was not detected in patients performing RM‐APD, and it was found that bone mineral metabolism of these patients was similar compared to baseline. We think that PD treatment compliance is one of the reasons which could explain why bone mineral metabolism remained stable in patients with performing RM‐APD while it was trended to increase in other PD modalities.
The frequency of dialysis interruption was 8% based on our results, and it was mostly observed in patients with performing APD. However, the overall non‐adherence rates to PD prescription were 2.6% to 85% in the literature, and non‐adherence to APD prescription was reported to be 5%–%20. 23 , 24 It has been shown that dialysis prescription adherence became more than 90% with the use of RM‐APD. 7 , 25 Because this platform enables patients treatment data including peritoneal volume, alerts during treatment, drainage problems, interruption of therapy, loss of dwell time, loss of therapy time to receive and transmit to PD centre. 7 , 9 It has provided many opportunities such as instant monitoring of treatment adherence, early detection of problems and resolving most of the problems remotely without admitting to the hospital. 9 , 26 On the other hand, our clinical assessment was only about whether patients skipped the PD session, and other possible, which were mentioned above, PD treatment incompatibilities did not indicate.
In conclusion, PD is a safe way of renal replacement therapy to protect patients from COVID‐19 pandemic. The data obtained support that PD is successful and safe during the pandemic. Also, RM‐APD may be a better choice in patients with PD because bone mineral metabolism seems to remain more stable. Moreover, evaluating depression and anxiety at the phone visits may be important for the accurate clinical assessment.
CONFLICT OF INTEREST
None declared.
Supporting information
Supplementary Material S1
ACKNOWLEDGEMENT
This article has a preprint form (https://doi.org/10.21203/rs.3.rs‐70335/v1).
REFERENCES
- 1. Ikizler TA, Kliger AS. Minimizing the risk of COVID‐19 among patients on dialysis. Nat Rev Nephrol. 2020;16:311‐313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ikizler TA. COVID‐19 and dialysis units: what do we know now and what should we do? Am J Kidney Dis. 2020;76:1‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fu L, Wang B, Yuan T, et al. Clinical characteristics of coronavirus disease 2019 (COVID‐19) in China: a systematic review and meta‐analysis. J Infect. 2020;80:656‐665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Goicoechea M, Cámara LAS, Macías N, et al. COVID‐19: clinical course and outcomes of 36 maintenance hemodialysis patients from a single center in Spain. Kidney Int. 2020;98:27‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rombolà G, Brunini F. COVID‐19 and dialysis: why we should be worried. J Nephrol. 2020;33:401‐403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wilkie M, Davies S. Peritoneal dialysis in the time of COVID‐19. Perit Dial Int. 2020;40(4):357‐358. [DOI] [PubMed] [Google Scholar]
- 7. Yeter HH, Akcay OF, Ronco C, Derici U. Automated remote monitoring for peritoneal dialysis and its impact on blood pressure. Cardiorenal Med. 2020;10:198‐208. [DOI] [PubMed] [Google Scholar]
- 8. Blaauw M. Use of sharesource in remote patient management in peritoneal dialysis: a UK nurse's perspective. In Ronco C. (Vicenza), Crepaldi C. (Vicenza) (Eds.) Remote Patient Management in Peritoneal Dialysis. Vol 197. Basel: Karger Publishers; 2019:73‐83. [DOI] [PubMed] [Google Scholar]
- 9. Manani SM, Rosner M, Virzì G, et al. Longitudinal experience with remote monitoring for automated peritoneal dialysis patients. Nephron. 2019;142(1):1‐9. [DOI] [PubMed] [Google Scholar]
- 10. DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta‐analysis of the effects of anxiety and depression on patient adherence. Arch InternMed. 2000;160:2101‐2107. [DOI] [PubMed] [Google Scholar]
- 11. Choi EPH, Hui BPH, Wan EYF. Depression and anxiety in Hong Kong during COVID‐19. Int J Environ Res Public health. 2020;17:3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hisli N. Beck Depresyon Envanterinin gecerliligi uzerine bit calisma (A study on the validity of Beck Depression Inventory.). Psikoloji Dergisi. 1988;6:118‐122. [Google Scholar]
- 13. Ulusoy M, Sahin NH, Erkmen H. The Beck anxiety inventory: psychometric properties. J Cogn Psychother. 1998;12(2):163‐172. [Google Scholar]
- 14. Lai C‐C, Liu YH, Wang C‐Y, et al. Asymptomatic carrier state, acute respiratory disease, and pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARSCoV‐2): facts and myths. J Microbiol Immunol Infect. 2020;53:404‐412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ronco C, Manani SM, Giuliani A, Tantillo I, Reis T, Brown EA. Remote patient management of peritoneal dialysis during COVID‐19 pandemic. Perit Dial Int. 2020;40(4):363‐367. [DOI] [PubMed] [Google Scholar]
- 16. Valeri AM, Robbins‐Juarez SY, Stevens JS, et al. Presentation and outcomes of patients with ESKD and COVID‐19. J Am Soc Nephrol. 2020;31:1409‐1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang A‐M, Lai K‐N. The importance of residual renal function in dialysis patients. Kidney Int. 2006;69:1726‐1732. [DOI] [PubMed] [Google Scholar]
- 18. Pérez‐Flores I, Coronel F, Cigarrán S, Herrero JA, Calvo N. Relationship between residual renal function, inflammation, and anemia in peritoneal dialysis. Adv Perit Dial. 2007;23:140‐143. [PubMed] [Google Scholar]
- 19. Misra M, Vonesh E, Van Stone JC, Moore HL, Prowant B, Nolph KD. Effect of cause and time of dropout on the residual GFR: a comparative analysis of the decline of GFR on dialysis. Kidney Int. 2001;59:754‐763. [DOI] [PubMed] [Google Scholar]
- 20. Perlis RH, Fava M, Trivedi MH, et al. Irritability is associated with anxiety and greater severity, but not bipolar spectrum features, in major depressive disorder. Acta Psychiatr Scand. 2009;119(4):282‐289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Taketani Y, Koiwa F, Yokoyama K. Management of phosphorus load in CKD patients. Clin Exp Nephrol. 2017;21(1):27‐36. [DOI] [PubMed] [Google Scholar]
- 22. Yamamoto S, Fukagawa M. Uremic toxicity and bone in CKD. J Nephrol. 2017;30:623‐627. [DOI] [PubMed] [Google Scholar]
- 23. Griva K, Lai AY, Lim HA, Yu Z, Foo MWY, Newman SP. Non‐adherence in patients on peritoneal dialysis: a systematic review. PLoS One. 2014;9(2):e89001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bernardini J. Measuring compliance with prescribed exchanges in CAPD and CCPD patients. Perit Dial Int. 1997;17:338‐342. [PubMed] [Google Scholar]
- 25. Sanabria M, Rosner M, Vesga J, et al. A remote management program in automated peritoneal dialysis patients in Colombia. Nefro Latinoam. 2018;15(2):48‐51. [Google Scholar]
- 26. Sanabria M, Buitrago G, Lindholm B, et al. Remote patient monitoring program in automated peritoneal dialysis: impact on hospitalizations. Perit Dial Int. 2019;39:472‐478. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material S1


