Abstract
Background
The onset of the COVID19 pandemic drove the rapid development and adoption of physical barriers intended to protect providers from aerosols generated during airway management. We report our initial experience with aerosol barrier devices in pediatric patients and raise concerns that they may increase risk to patients.
Methods
In March 2020, we developed and implemented simulation training and use of plastic aerosol barrier devices as a component of our perioperative COVID‐19 workflow. As part of our quality improvement process, we obtained detailed feedback via a web‐based survey after cases were performed while using these aerosol barriers.
Results
Between March and June 2020, 36 pediatric patients age 1mo‐18years with anatomically normal airways and either PCR confirmed or suspected COVID‐19 were intubated under an aerosol barrier as part of urgent or emergent anesthetic care at our institution. Experienced providers had more difficulty than expected in six (16.7%) of the cases with four cases requiring multiple attempts to secure the airway and two cases involving pronounced difficulty in a single attempt. The aerosol barrier was perceived as a contributing factor to difficulty in all cases.
Conclusion
The use of barriers may result in unanticipated difficulties with airway management, particularly in pediatric patients, which could lead to hypoxemia or other patient harm. Our initial experience in pediatric patients is the first such report in patients and provides clinical data which corroborates the simulation data prompting the FDA to withdraw support of barriers.
What is already known about this topic?
The COVID‐19 pandemic prompted rapid development and deployment of a variety of unproven physical barriers intended to protect providers from aerosols generated during airway management. The US Food and Drug Administration initially granted an umbrella emergency use authorization for passive protective barrier enclosures which was later withdrawn on the basis of simulation studies raising concerns about safety and efficacy.
What new information this study adds?
Experienced providers had more difficulty than expected intubating one in six (16.7%) patients using aerosol barriers. This is the first data on clinical use of physical barriers for airway management, and provides clinical data which corroborates the simulation data prompting the FDA to withdraw support of barriers.
The COVID‐19 pandemic has prompted rapid development and deployment of a variety of unproven physical barriers intended to protect providers from aerosols generated during airway management. 1 , 2 The US Food and Drug Administration (FDA) initially granted an umbrella emergency use authorization (EUA) for passive protective barrier enclosures on May 1, 2020. Although anecdotal evidence drove widespread adoption of these devices, there are no data we are aware of demonstrating they protect providers from infection with the SARS‐CoV‐2 virus. Furthermore, some have expressed concern about their safety and efficacy for both patients and providers, 3 with particular concern that barriers may prolong intubation times and increase the risk of hypoxemia. 4 We report our initial experience with aerosol barrier devices in pediatric patients and raise concerns that they may increase risk to patients.
In March 2020, our airway group developed and implemented plastic aerosol barrier devices as a component of our perioperative COVID‐19 workflow. 3 Simulation sessions using supporting documentation including videos for donning/doffing personal protective equipment, and intubating patients with suspected and confirmed COVID‐19 were provided to all clinicians starting in mid‐March prior to the care of any known COVID‐19 cases at our institution. Aerosol barriers were used at the discretion of the attending anesthesiologist and clinical team and consisted of plastic sheets draped over the patient with or without a transparent plexiglass shield (Figure 1). Barriers provided ample working space around the patient's head to allow unencumbered preoxygenation. As part of our quality improvement process, we obtained detailed feedback via a web‐based survey after cases were performed while using these aerosol barriers. This review of our quality assurance data was deemed exempt by our institutional review board.
Figure 1.
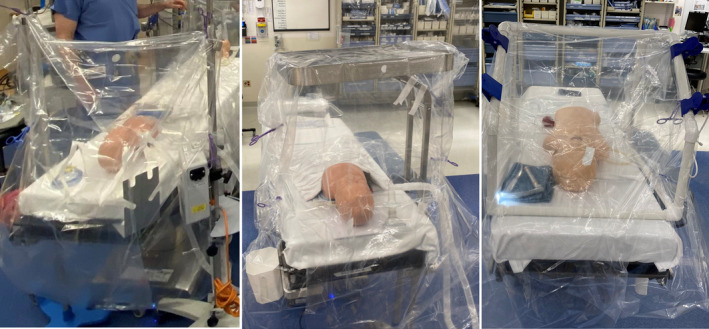
Aerosol barrier used in our clinical practice. Barriers consisted of plastic sheets draped over the patient with or without a transparent plexiglass shield. Barriers provided ample working space around the patient's head to allow unencumbered preoxygenation [Colour figure can be viewed at wileyonlinelibrary.com]
As of June 30, 2020, 49 pediatric patients age 1 month–18 years with anatomically normal airways and either PCR confirmed or suspected COVID‐19 have received anesthesia care at our institution. Of these, 36 were intubated using an aerosol barrier and standard (i.e., Macintosh or Miller) blade video laryngoscopy, 5 were intubated without aerosol barriers, and 8 were maintained with spontaneous respirations and face mask oxygen.
Experienced providers had more difficulty than expected in six (16.7%) of the 36 cases using aerosol barriers (Table 1). Four cases required multiple attempts to secure the airway and two cases involved pronounced difficulty in a single attempt. All cases with reported difficulty were managed by experienced anesthesia providers (senior anesthesia trainee, CRNA, or attending anesthesiologist). This difficulty occurred in otherwise healthy (ASA 1 or 2) children age 2–18 years with anatomically normal airways presenting for routine emergency procedures. Subjective feedback solicited from the providers indicated that the aerosol barrier was seen as a contributing factor to the difficulty in all six of these cases. The patients in these cases underwent rapid sequence inductions followed by standard (i.e., Macintosh or Miller) blade video laryngoscopy in accordance with published guidelines. 5 The airway was ultimately secured by orotracheal intubation with a reported grade 1 Cormack‐Lehane view on video laryngoscopy in all cases. Although difficulty was encountered, none of these patients suffered any apparent complications from airway management.
Table 1.
Description of patients in whom providers experienced more difficulty than expected in airway management using an aerosol barrier
| Age | Weight | ASA | COVID status | Surgical Procedure | Barrier type | Induction | Clinician | PPE | Airway Management | Final Cormack‐Lehane View | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient 1 | 14 years | 72 kg | 2E | Test pending, febrile | Laparoscopic appendectomy | Plastic drapes | RSI | CRNA with >5 years experience | N95 and face shield | Single attempt with difficulty with optimizing laryngeal view with video laryngoscopy, with this issue, the patent had a minor drop in saturation to low 90's | Grade 1 view with standard video laryngoscope |
| Patient 2 | 15 years | 70 kg | 1E | PCR positive, febrile | Laparoscopic appendectomy | Plastic drapes | RSI | CRNA with >5 years experience for 2 attempts, Attending anesthesiologist with >10 years experience for third attempt | N95 and face shield | 3 attempts with standard blade video laryngoscope | Grade 1 view with standard video laryngoscope |
| Patient 3 | 14 years | 48 kg | 1E | Test pending, febrile | Laparoscopic appendectomy | Plastic drapes | RSI | CRNA with >5 years experience | Powered Air Purifying Respirator (PAPR) | Single attempt with difficulty, tube was caught in the drape and there was difficulty maneuvering the tube into the glottis | Grade 1 view with standard video laryngoscope |
| Patient 4 | 17 years | 68 kg | 1 | PCR positive, asymptomatic | Hernia repair | Plastic drapes | RSI | PGY4 anesthesia resident | N95 and face shield | 2 attempts with standard blade video laryngoscope | Grade 1 view with standard video laryngoscope |
| Patient 5 | 3 years | 15 kg | 2E | Test pending, asymptomatic | Craniotomy | Plastic drapes | RSI | Pediatric anesthesia fellow | N95 and face shield | 2 attempts with standard blade video laryngoscope | Grade 1 view with standard video laryngoscope |
| Patient 6 | 14 years | 63 kg | 1 | Test pending, asymptomatic | Diagnostic laparoscopy (ovarian torsion) | Plastic drapes + clear shield | RSI | CRNA with >5 years experience | N95 and face shield | 2 attempts with standard blade video laryngoscope | Grade 1 view with standard video laryngoscope |
Abbreviations: CRNA, certified registered nurse anesthetist; PGY, postgraduate year; RSI, rapid sequence induction.
As part of a separate quality improvement effort, we collected data on the airway management and airway‐related adverse events for every patient cared for at our institution over a two‐week period in May and June 2020. The same clinicians were involved in the care of these patients and the patients with suspected or PCR confirmed COVID‐19. Per institutional policy during this time period, standard surgical mask, gloves, and eye protection were worn for COVID negative patients, but not N95s or PAPRs. In this audit, we identified 173 patients who were ASA physical status 1 or 2, ages 2–18 years, COVID‐19 negative, asymptomatic, and did not have a history of difficult intubation who were intubated without the use of an aerosol barrier. 4 of 173 patients (2.3%) required more than one attempt to intubate by an experienced provider. Of these 4 patients, one was designated as an emergency case and none underwent rapid sequence induction. Rapid sequence induction and intubation without an aerosol barrier was performed in 9 ASA 1 and 2 patients age 2–18 years in the COVID negative, asymptomatic group during this 2‐week audit. Clinicians performed direct laryngoscopy for 8 patients and standard blade video laryngoscopy in 1 patient. All were successfully intubated on the first attempt, and none experienced adverse events associated with intubation.
Following implementation of plastic aerosol barrier devices into our clinical workflow, we found that experienced providers had difficulty during intubations of patients with anatomically straightforward airways. This finding drove us to share our early quality assurance data with the wider anesthesia community as use of barriers may result in unanticipated difficulties with airway management, particularly in pediatric patients. This difficulty may arise from the visual and physical interference caused by the barrier, which is further exacerbated by the stress associated with caring for potentially infectious patients. Such difficulty could lead to hypoxemia or other patient harm, although no patient in this cohort experienced intubation‐related adverse events.
The nature of the COVID‐19 pandemic led to rapid development and implementation of new clinical workflows. We implemented training and simulation prior to their clinical use; still, our preliminary experience revealed important challenges. In response to these observations, we set up a dedicated operating room for Just‐in‐Time simulation training 6 with the tools and aerosol barriers, which has subjectively improved the comfort of staff using these new techniques. It is notable that five of the cases discussed above occurred prior to instituting the Just‐in Time training, and only one occurred since that time. We are conducting ongoing monitoring for adverse events through our quality improvement program.
On August 21, 2020, the FDA withdrew the umbrella EUA and advised against the use of protective barrier enclosures without negative pressure due to simulations demonstrating increased exposure to airborne particles to healthcare provider as well as concerns about risk to patients. 4 , 7 Our initial experience in pediatric patients is the first such report in patients and is consistent with the simulation data cited in the FDA letter raising concern about potential harm to patients. We have stopped using aerosol barriers in the care of patients with suspected or confirmed COVID‐19 at our institution. There are important limitations of this observational report of subjective difficulty with intubation based on quality assurance data from a small sample at single institution without a defined research protocol or control group. Nevertheless, our experience highlights the need for a careful analysis of the risks and benefits of using an aerosol barrier for airway management and provides clinical data which corroborates the simulation data prompting the FDA to withdraw support of barriers.
CONFLICT OF INTEREST
None to report.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author, MLS, upon reasonable request.
ACKNOWLEDGMENTS
Shannon McAvoy, Mark Breibart, and the Boston Children's Hospital Anesthesia Information Systems team.
Tighe NTG, McClain CD, Vlassakova BG, et al. Aerosol barriers in pediatric anesthesiology: Clinical data supports FDA caution. Pediatr Anaesth.2021;31:461–464. 10.1111/pan.14091
Funding information
Support was provided solely from institutional and/or departmental sources.
REFERENCES
- 1. Canelli R, Connor CW, Gonzalez M, Nozari A, Ortega R. Barrier enclosure during endotracheal intubation. N Engl J Med. 2020;382:1957‐1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shaw R, Tighe N, Odegard KC, Alexander P, Emani S, Yuki K. Intubation precautions in a pediatric patient with severe COVID‐19. J Pediatr Surg Case Rep. 2020;58:101495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kovatsis PG, Matava CT, Peyton JM. More on barrier enclosure during endotracheal intubation. N Engl J Med. 2020;382:e69. [DOI] [PubMed] [Google Scholar]
- 4. Begley JL, Lavery KE, Nickson CP, Brewster DJ. The aerosol box for intubation in coronavirus disease 2019 patients: an in‐situ simulation crossover study. Anaesthesia. 2020;75:1014‐1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Matava CT, Kovatsis PG, Summers JL, et al. Pediatric airway management in COVID‐19 patients ‐ consensus guidelines from the society for pediatric anesthesia's pediatric difficult intubation collaborative and the Canadian pediatric anesthesia society. Anesth Analg. 2020;131:61‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Braga M, Tyler M, Rhoads J, et al. Effect of just‐in‐time simulation training on provider performance and patient outcomes for clinical procedures: a systematic review. BMJ Simul Technol Enhanc Learn. 2015;1:94‐102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Simpson JP, Wong DN, Verco L, Carter R, Dzidowski M, Chan PY. Measurement of airborne particle exposure during simulated tracheal intubation using various proposed aerosol containment devices during the COVID‐19 pandemic. Anaesthesia. 2020;75:1587‐1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, MLS, upon reasonable request.


