Abstract
Aims
Renin–angiotensin–aldosterone system inhibitors (RAASi) improve outcomes in cardiorenal disease but concerns have been raised over increased risk of incident hospitalization and death from coronavirus disease 2019 (COVID‐19). We investigated the association between use of angiotensin‐converting enzyme inhibitors (ACEi), angiotensin receptor blockers (ARBs) or mineralocorticoid receptor antagonists (MRAs) and COVID‐19 hospitalization/death in a large nationwide population.
Methods and results
Patients with hypertension, heart failure, diabetes, kidney disease, or ischaemic heart disease registered in the Swedish National Patient Registry until 1 February 2020 were included and followed until 31 May 2020. COVID‐19 cases were defined based on hospitalization/death for COVID‐19. Multivariable logistic and Cox regressions were fitted to investigate the association between ACEi/ARB and MRA and risk of hospitalization/death for COVID‐19 in the overall population, and of all‐cause mortality in COVID‐19 cases. We performed consistency analysis to quantify the impact of potential unmeasured confounding. Of 1 387 746 patients (60% receiving ACEi/ARB and 5.8% MRA), 7146 (0.51%) had incident hospitalization/death from COVID‐19. After adjustment for 45 variables, ACEi/ARB use was associated with a reduced risk of hospitalization/death for COVID‐19 (odds ratio 0.86, 95% confidence interval 0.81–0.91) in the overall population, and with reduced mortality in COVID‐19 cases (hazard ratio 0.89, 95% confidence interval 0.82–0.96). MRA use was not associated with risk of any outcome. Consistency analysis showed that unmeasured confounding would need to be large for there to be harmful signals associated with RAASi use.
Conclusions
In a 1.4 million nationwide cohort, use of RAASi was not associated with increased risk of hospitalization for or death from COVID‐19.
Keywords: Coronavirus, COVID‐19, SARS‐CoV‐2, Renin–angiotensin–aldosterone system inhibitors, Angiotensin‐converting enzyme inhibitors, Angiotensin receptor blockers, Mineralocorticoid receptor antagonists, Registry, Sweden
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) attaches to its target cells by its outer ‘spike’ protein binding to the angiotensin‐converting enzyme 2 (ACE2) receptor, with ACE2 levels being increased by renin–angiotensin system inhibitors. 1 Therefore, major concerns regarding the use of this treatment during the COVID‐19 pandemic have been raised since it may favour infection, impact disease severity and worsen prognosis. Conversely, once cells have become infected, the ACE2 expression is down‐regulated, which stimulates neutrophil infiltration, unopposed angiotensin II accumulation and renin–angiotensin system activation, promoting acute lung injury. 1 Based on these findings, renin–angiotensin system inhibitors [i.e. angiotensin‐converting enzyme inhibitors (ACEi) and angiotensin receptor blockers (ARBs)] might be beneficial in patients with coronavirus disease 2019 (COVID‐19) by reducing the severity of acute lung injury. 1
Due to the lack of convincing studies, major international scientific associations have warned against the discontinuation of ACEi/ARB in patients with cardiovascular disease but also called for large and rigorous studies to address this issue. 2 , 3 , 4
Results from studies to date on the interaction between ACEi/ARB use and the risk for and prognosis with COVID‐19 have been conflicting, providing safety signals, 5 , 6 , 7 , 8 , 9 or potential harm. 10 Due to the observational setting, the use of small and/or selected cohorts, and the limited adjustment for confounders in most of these analyses, more evidence from large and unselected real‐world populations and from randomized controlled trials is needed.
Furthermore, mineralocorticoid receptor antagonists (MRAs) have not been widely considered relevant for COVID‐19. MRAs may upregulate ACE2 even more strongly than ACEi and ARBs, 11 potentially increasing COVID‐19 risk, but may also increase circulating ACE2 which may act as a competitive interceptor of the virus, 12 and has anti‐androgen effects that may reduce the risk for and/or severity of COVID‐19. 13
Therefore, we aimed to investigate in patients with heart failure (HF), hypertension, kidney disease, ischaemic heart disease (IHD) or diabetes in a full national coverage registry, the association of renin–angiotensin–aldosterone system inhibitors (RAASi) with (i) incident hospitalization/death for COVID‐19, and (ii) mortality in COVID‐19 cases.
Methods
Data sources
All analyses were performed using the Swedish National Patient Registry (NPR) linked to the Cause of Death Registry, the Dispensed Drug Registry, and Statistics Sweden by the personal identification number. 14
The NPR, the Cause of Death Registry and the Dispensed Drug Registry are administered by the Swedish Board of Health and Welfare (www.socialstyrelsen.se). They have national complete coverage and collect International Classification of Diseases, Tenth Revision (ICD‐10) diagnoses from all residents in Sweden at hospitalizations and at visits to outpatient non‐primary care clinics. The Dispensed Drug Registry contains data for all dispensed prescriptions in Sweden since 2005. Statistics Sweden collects socioeconomic data from all residents in Sweden (www.scb.se).
The current analysis requiring the linkage of the above reported registries was approved by the Swedish Ethics Review Authority and was conducted in accordance with the Declaration of Helsinki. Patient consent is not required for registration in these registries and was not required for this study.
Patients, diagnoses and outcomes
The study population consisted of all patients in Sweden with a diagnosis of HF, hypertension, kidney disease, IHD, or diabetes in the NPR since 1997 (when ICD‐10 coding was implemented) and who were alive on 1 February 2020. Further exclusion criteria are reported in online supplementary Table S1 .
Outcomes were incident COVID‐19 in the overall population and all‐cause mortality in patients with COVID‐19. Incident COVID‐19 cases were defined based on incident hospitalization/death (without prior hospitalization) for confirmed COVID‐19 (as main diagnosis in the inpatient setting recorded in the NPR or as underlying cause of death in the Cause of Death Registry, respectively) between 2 February 2020 (the first case of COVID‐19 was recorded in Sweden in late January) and 31 May 2020. Death included both in‐hospital and out‐of‐hospital deaths.
Definitions for exposures, comorbidities, COVID‐19 disease, other treatments and variables are reported in online supplementary Table S2 .
Statistical analyses
Baseline characteristics of patients receiving vs. not receiving ACEi/ARB and MRA were reported as frequencies (percentages) for categorical variables and as medians (interquartile range) for continuous variables, and compared by chi‐square and Mann–Whitney U tests, respectively. Differences in baseline characteristics across study groups were further evaluated by calculating standardized mean differences, where we considered a value <0.1 as non‐relevant.
Multivariable logistic regression models were fitted to investigate the association between ACEi/ARB and MRA and incident hospitalization/death for COVID‐19, which was expressed as odds ratio (OR) and 95% confidence interval (CI).
In patients with COVID‐19, unadjusted survivor functions using the Kaplan–Meier method and multivariable Cox regression models were performed to assess the association between ACEi/ARB and MRA use and the risk for all‐cause death over the entire follow‐up, and to calculate the proportional hazard ratios (HR) with 95% CI. The proportional hazards assumption was assessed based on the scaled Schoenfeld residuals, with potential outliers visually inspected by plotting the dfbetas. In addition, differences in 30‐day mortality across the study arms were assessed by logistic regression models, where patients without 30‐day follow‐up were excluded from the analysis.
As main method for adjusting for potential confounders, a propensity score for treatment with ACEi/ARB and one for MRA was separately calculated for each patient by a logistic regression model including the 45 variables marked with a in Table 1 . The inverse of the propensity score was then incorporated in the multivariable models as weights, truncated at 10, i.e. inverse probability weighting, where the sandwich variance estimators were applied to calculate P‐values and 95% CI. We also performed multivariable models only including the 45 variables marked with a in Table 1 , whose results are reported in the online supplementary material. Continuous variables (i.e. age) were modelled using cubic splines with four degrees of freedom.
Table 1.
Baseline characteristics according to use of renin–angiotensin system inhibitors and mineralocorticoid receptor antagonists in the overall study population
| Variables | ACEi/ARB No | ACEi/ARB Yes | P‐value | SMD | MRA No | MRA Yes | P‐value | SMD |
|---|---|---|---|---|---|---|---|---|
| Patients, n (%) | 549 480 (39.6) | 838 266 (60.4) | 1 306 958 (94.2) | 80 788 (5.8) | ||||
| Demographic/socioeconomic characteristics | ||||||||
| Male sex a , n (%) | 264 907 (48.2) | 457 993 (54.6) | <0.001 | 0.129 | 679 978 (52.0) | 42 922 (53.1) | <0.001 | 0.022 |
| Age (years) a , median [IQR] | 71.0 [59.0–80.0] | 72.0 [64.0–79.0] | <0.001 | 0.204 | 72.0 [62.0–79.0] | 75.0 [67.0–82.0] | <0.001 | 0.325 |
| Marital status (single/widowed/divorced), n (%) | 307 423 (56.0) | 412 423 (49.2) | <0.001 | 0.137 | 676 076 (51.8) | 43 770 (54.2) | <0.001 | 0.049 |
| Living alone a , n (%) | 262 628 (47.8) | 365 960 (43.7) | <0.001 | 0.084 | 588 335 (45.0) | 40 253 (49.8) | <0.001 | 0.096 |
| Education level a , n (%) | <0.001 | 0.024 | <0.001 | 0.122 | ||||
| Compulsory school | 162 549 (30.0) | 252 626 (30.4) | 387 173 (29.9) | 28 002 (35.0) | ||||
| Secondary school | 239 874 (44.2) | 372 882 (44.8) | 577 911 (44.7) | 348,45 (43.5) | ||||
| University | 140 045 (25.8) | 206 079 (24.8) | 328 952 (25.4) | 17 172 (21.5) | ||||
| ≥1 Children a , n (%) | 442 671 (80.6) | 709 774 (84.7) | <0.001 | 0.109 | 1 084 896 (83.0) | 67 549 (83.6) | <0.001 | 0.016 |
| Income, tertiles a , n (%) | <0.001 | 0.087 | <0.001 | 0.176 | ||||
| Low | 194 386 (35.4) | 263 356 (31.4) | 427 796 (32.7) | 29 946 (37.1) | ||||
| Medium | 177 208 (32.3) | 280 320 (33.4) | 428 023 (32.8) | 29 505 (36.5) | ||||
| High | 177 363 (32.3) | 294 573 (35.1) | 450 600 (34.5) | 21 336 (26.4) | ||||
| Resident in region Stockholm a , n (%) | 107 904 (19.7) | 157 639 (18.8) | <0.001 | 0.022 | 252 568 (19.3) | 12 975 (16.1) | <0.001 | 0.086 |
| Country of birth a , n (%) | <0.001 | 0.098 | <0.001 | 0.112 | ||||
| Sweden | 457 829 (83.4) | 718 509 (85.7) | 1 106 318 (84.7) | 70 020 (86.7) | ||||
| Europe | 56 991 (10.4) | 85 281 (10.2) | 133 847 (10.2) | 8425 (10.4) | ||||
| Other | 34 332 (6.3) | 34 438 (4.1) | 66 429 (5.1) | 2341 (2.9) | ||||
| Treatment, n (%) | ||||||||
| ACEi/ARB a | 0 (0.0) | 838 266 (100.0) | <0.001 | ‐ | 779 611 (59.7) | 58 655 (72.6) | <0.001 | 0.276 |
| ACEi | 0 (0.0) | 374 776 (44.7) | <0.001 | 1.272 | 347 830 (26.6) | 26 946 (33.4) | <0.001 | 0.148 |
| ARB | 0 (0.0) | 473 003 (56.4) | <0.001 | 1.609 | 440 394 (33.7) | 32 609 (40.4) | <0.001 | 0.138 |
| MRA a | 22 133 (4.0) | 58 655 (7.0) | <0.001 | 0.130 | 0 (0.0) | 80 788 (100.0) | <0.001 | ‐ |
| Diuretics a | 99 437 (18.1) | 344 320 (41.1) | <0.001 | 0.520 | 400 930 (30.7) | 42 827 (53.0) | <0.001 | 0.465 |
| Beta‐blockers a | 201 399 (36.7) | 423 032 (50.5) | <0.001 | 0.281 | 563 096 (43.1) | 61 335 (75.9) | <0.001 | 0.710 |
| Calcium channel blockers a | 139 964 (25.5) | 324 027 (38.7) | <0.001 | 0.285 | 439 782 (33.6) | 24 209 (30.0) | <0.001 | 0.079 |
| Antiplatelet a | 136 357 (24.8) | 289 981 (34.6) | <0.001 | 0.215 | 401 352 (30.7) | 24 986 (30.9) | 0.192 | 0.005 |
| Anticoagulant a | 86 138 (15.7) | 170 077 (20.3) | <0.001 | 0.120 | 221 246 (16.9) | 34 969 (43.3) | <0.001 | 0.600 |
| Insulin a | 69 242 (12.6) | 91 544 (10.9) | <0.001 | 0.052 | 150 816 (11.5) | 9970 (12.3) | <0.001 | 0.025 |
| Non‐insulin anti‐hyperglycaemic agents a | 84 214 (15.3) | 189 163 (22.6) | <0.001 | 0.186 | 255 128 (19.5) | 18 249 (22.6) | <0.001 | 0.075 |
| Lipid‐lowering agents a | 184 685 (33.6) | 446 772 (53.3) | <0.001 | 0.405 | 588 505 (45.0) | 42 952 (53.2) | <0.001 | 0.163 |
| Digoxin a | 8025 (1.5) | 17 649 (2.1) | <0.001 | 0.049 | 19 482 (1.5) | 6192 (7.7) | <0.001 | 0.299 |
| Nitrate a | 31 848 (5.8) | 64 398 (7.7) | <0.001 | 0.075 | 86 114 (6.6) | 10 132 (12.5) | <0.001 | 0.203 |
| Anti‐arrhythmic agents a | 4042 (0.7) | 8615 (1.0) | <0.001 | 0.031 | 10 900 (0.8) | 1757 (2.2) | <0.001 | 0.110 |
| ICD/CRT a | 2393 (0.4) | 9583 (1.1) | <0.001 | 0.080 | 7086 (0.5) | 4890 (6.1) | <0.001 | 0.312 |
| Comorbidities, n (%) | ||||||||
| Hypertension a | 366 947 (66.8) | 740 121 (88.3) | <0.001 | 0.533 | 1 038 603 (79.5) | 68 465 (84.7) | <0.001 | 0.138 |
| Heart failure a | 58 097 (10.6) | 127 820 (15.2) | <0.001 | 0.140 | 141 256 (10.8) | 44 661 (55.3) | <0.001 | 1.073 |
| Diabetes a | 163 726 (29.8) | 234 808 (28.0) | <0.001 | 0.039 | 374 127 (28.6) | 24 407 (30.2) | <0.001 | 0.035 |
| Renal disease a | 56 018 (10.2) | 59 744 (7.1) | <0.001 | 0.109 | 105 376 (8.1) | 10 386 (12.9) | <0.001 | 0.157 |
| Ischaemic heart disease a | 144 642 (26.3) | 241 327 (28.8) | <0.001 | 0.055 | 353 731 (27.1) | 32 238 (39.9) | <0.001 | 0.275 |
| Obesity a | 44 130 (8.0) | 71 544 (8.5) | <0.001 | 0.018 | 105 019 (8.0) | 10 655 (13.2) | <0.001 | 0.168 |
| Anaemia a | 72 469 (13.2) | 89 775 (10.7) | <0.001 | 0.076 | 147 623 (11.3) | 14 621 (18.1) | <0.001 | 0.193 |
| Previous myocardial infarction a | 70 690 (12.9) | 152 013 (18.1) | <0.001 | 0.146 | 201 840 (15.4) | 20 863 (25.8) | <0.001 | 0.259 |
| PCI a | 49 593 (9.0) | 122 234 (14.6) | <0.001 | 0.173 | 157 062 (12.0) | 14 765 (18.3) | <0.001 | 0.175 |
| CABG a | 57 868 (10.5) | 133 802 (16.0) | <0.001 | 0.161 | 173 694 (13.3) | 17 976 (22.3) | <0.001 | 0.236 |
| Peripheral artery disease a | 32 024 (5.8) | 60 782 (7.3) | <0.001 | 0.058 | 83 994 (6.4) | 8812 (10.9) | <0.001 | 0.160 |
| Atrial fibrillation a | 89 396 (16.3) | 163 756 (19.5) | <0.001 | 0.085 | 217 387 (16.6) | 35 765 (44.3) | <0.001 | 0.630 |
| Stroke/TIA a | 81 386 (14.8) | 139 884 (16.7) | <0.001 | 0.052 | 205 587 (15.7) | 15 683 (19.4) | <0.001 | 0.097 |
| Valvular heart disease a | 34 019 (6.2) | 65 761 (7.8) | <0.001 | 0.065 | 85 455 (6.5) | 14 325 (17.7) | <0.001 | 0.348 |
| Hyperkalaemia a | 5139 (0.9) | 5822 (0.7) | <0.001 | 0.027 | 9494 (0.7) | 1467 (1.8) | <0.001 | 0.097 |
| Hypokalaemia a | 16 243 (3.0) | 18 582 (2.2) | <0.001 | 0.047 | 29 313 (2.2) | 5512 (6.8) | <0.001 | 0.222 |
| Dialysis a | 8116 (1.5) | 5845 (0.7) | <0.001 | 0.075 | 13 125 (1.0) | 836 (1.0) | 0.408 | 0.003 |
| COPD a | 39 889 (7.3) | 55 010 (6.6) | <0.001 | 0.027 | 84 676 (6.5) | 10 223 (12.7) | <0.001 | 0.211 |
| Liver disease a | 18 987 (3.5) | 19 023 (2.3) | <0.001 | 0.071 | 33 853 (2.6) | 4157 (5.1) | <0.001 | 0.133 |
| Dementia a | 23 071 (4.2) | 19 136 (2.3) | <0.001 | 0.108 | 39 847 (3.0) | 2360 (2.9) | 0.041 | 0.007 |
| Malignancy (within 3 years) a | 68 312 (12.4) | 100 949 (12.0) | <0.001 | 0.012 | 158 345 (12.1) | 10 916 (13.5) | <0.001 | 0.042 |
| Musculoskeletal disease a | 162 601 (29.6) | 246 438 (29.4) | 0.015 | 0.004 | 382 561 (29.3) | 26 478 (32.8) | <0.001 | 0.076 |
| Alcohol abuse a | 35 322 (6.4) | 35 555 (4.2) | <0.001 | 0.097 | 65 838 (5.0) | 5039 (6.2) | <0.001 | 0.052 |
| Major bleeding a | 124 075 (22.6) | 175 233 (20.9) | <0.001 | 0.041 | 275 441 (21.1) | 23 867 (29.5) | <0.001 | 0.196 |
Missing data: 1.0% for education level, <0.01% for marital status, living alone, income and country of birth.
ACEi, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; CABG, coronary artery bypass graft; COPD, chronic obstructive pulmonary disease; CRT, cardiac resynchronization therapy; ICD, implantable cardioverter defibrillator; IQR, interquartile range; MRA, mineralocorticoid receptor antagonist; PCI, percutaneous coronary intervention; SMD, standardized mean difference; TIA, transient ischaemic attack.
Variables included in multivariable models.
All the analyses were performed in the overall population, in six pre‐specified non‐mutually exclusive subgroups of patients (i.e. with HF, hypertension, kidney disease, IHD, diabetes and inpatients living in the Stockholm region where a majority of COVID‐19 cases have been reported in Sweden). Consistency analyses were performed separately in patients receiving ACEi and ARB (patients receiving both treatments were excluded from this analysis), and in those treated vs. not treated with the combination of ACEi or ARB and MRA.
To assess the impact of potential unmeasured confounders on the association between ACEi/ARB and risk of incident hospitalization/death for COVID‐19 in the overall population, and for death in patients with COVID‐19, we introduced in the main analysis a hypothetical unmeasured confounder for which we set a prevalence of 35% in the ACEi/ARB group and 70% in the non‐ACEi/ARB group, and for which we defined different strengths for the associations (OR/HRs) between the potential unmeasured confounder and the risk of incident hospitalization/death for COVID‐19 in the overall population, or for death in patients with COVID‐19. 15 The same approach was used for assessing potential confounding for the association between MRA use and outcomes.
The amount of missing data in the current analysis was very limited and thus patients with missing data for variables considered for adjustments were excluded from the multivariable analyses.
In all the analyses, a P‐value <0.05 (two‐tailed) was considered statistically significant. No adjustment for multiple comparison was performed. R software version 3.6.2 was used for all the calculations and data management. The R code is available on https://github.com/KIHeartFailure/covid19raasi.
Results
Of 1 451 271 patients with a hospital diagnosis of hypertension, HF, kidney disease, IHD or diabetes recorded in the NPR between 1997 and 1 February 2020, 1 387 746 were included in our analysis after applying the exclusion criteria (online supplementary Table S1 ). Of these, 185 917 (13%) had HF (69% ACEi/ARB and 24% MRA users), 1 107 068 (80%) hypertension (67%% ACEi/ARB and 6% MRA users), 115 762 (8%) kidney disease (52% ACEi/ARB and 9% MRA users), 385 969 (28%) IHD (63% ACEi/ARB and 8% MRA users), and 398 534 (29%) diabetes (59% ACEi/ARB and 6% MRA users), with 838 266 (60%) using ACEi/ARB and 80 788 (5.8%) using MRA in the overall study population (online supplementary Figure S1 ). As many as 58 655 patients (4%) received a combination of ACEi/ARB and MRA. Length of treatment with study drugs is reported in online supplementary Table S3 .
Baseline characteristics
In the overall cohort, median age was 72 years (interquartile range 62–80) and 48% were female.
ACEi/ARB vs. non‐ACEi/ARB‐treated patients were more likely to be male and older, to be married and have children, to suffer from hypertension and HF, to have history of myocardial infarction and coronary revascularization, and to receive other cardiovascular treatments. Conversely, they were less likely to have renal disease and dementia (Table 1 ).
Patients receiving vs. not receiving MRA were older were more likely to suffer from cardiovascular comorbidities such as obesity, hypertension, HF, atrial fibrillation, valvular heart disease, peripheral artery disease, to have history of myocardial infarction and coronary revascularization, and to receive other cardiovascular treatments. They were also more likely to report non‐cardiovascular comorbidities such as kidney disease, anaemia, chronic obstructive pulmonary disease, liver disease, and to have history of major bleeding (Table 1 ).
Baseline characteristics of patients hospitalized for or died from COVID‐19 according to ACEi/ARB or MRA use are shown in online supplementary Table S4 .
Association between renin–angiotensin–aldosterone system inhibitor use and risk of incident hospitalization/death for COVID‐19
Of 1 387 746 patients included in our analyses, 3909 (0.47%) were hospitalized for or died from COVID‐19 in the ACEi/ARB group and 3237 (0.59%) in the non‐ACEi/ARB group. Therefore, ACEi/ARB use was associated with reduced risk of incident hospitalization/death for COVID‐19 in crude analysis (OR 0.79, 95% CI 0.75–0.83). The association was weaker but remained significant in adjusted analysis (OR 0.86, 95% CI 0.81–0.91) (Table 2 ). A similar association was observed when ARB use was analysed separately (OR 0.88, 95% CI 0.83–0.94), whereas ACEi use was not significantly associated with the risk of the outcome (OR 0.97, 95% CI 0.92–1.03)(online supplementary Table S5 ).
Table 2.
Main and subgroup analyses for the association between use of renin–angiotensin–system inhibitors and mineralocorticoid receptor antagonists and outcomes
| Outcome/subgroup | Risk of hospitalization/ death for COVID‐19 in the overall population | Risk of all‐cause death in patients with COVID‐19 | ||||||
|---|---|---|---|---|---|---|---|---|
| ACEi/ ARB No | ACEi/ ARB Yes | MRA No | MRA Yes | ACEi/ ARB No | ACEi/ ARB Yes | MRA No | MRA Yes | |
| All patients | ||||||||
|
n (%) events b Event rate (95% CI) as PY c |
3237 (0.59) | 3909 (0.47) | 6580 (0.50) | 566 (0.70) | 8.5 (8.1–8.9) | 6.1 (5.8–6.4) | 7.0 (6.8–7.3) | 8.2 (7.3–9.3) |
| Crude OR b /HR c (95% CI) | Ref. | 0.79 (0.75–0.83) a | Ref. | 1.39 (1.28–1.52) a | Ref. | 0.76 (0.71–0.81) a | Ref. | 1.11 (0.98–1.26) |
| Adjusted (individual variables) OR b /HR c (95% CI) | Ref. | 0.86 (0.82–0.91) a | Ref. | 0.98 (0.90–1.08) | Ref. | 0.90 (0.83–0.97) a | Ref. | 0.96 (0.84–1.09) |
| Adjusted (IPW) OR/HR c (95% CI) | Ref. | 0.86 (0.81–0.91) a | Ref. | 0.97 (0.87–1.08) | Ref. | 0.89 (0.82–0.96) a | Ref. | 0.97 (0.84–1.12) |
| Patients with HF | ||||||||
|
n (%) events b Event rate (95% CI) as PY c |
766 (1.32) | 1192 (0.93) | 1563 (1.11) | 395 (0.88) | 13.1 (12.0–14.3) | 9.6 (8.9–10.4) | 11.2 (10.5–11.9) | 9.6 (8.4–11.0) |
| Crude OR b /HR c (95% CI) | Ref. | 0.70 (0.64–0.77) a | Ref. | 0.80 (0.71–0.89) a | Ref. | 0.77 (0.68–0.86) a | Ref. | 0.89 (0.77–1.03) |
| Adjusted (individual variables) OR b /HR c (95% CI) | Ref. | 0.90 (0.81–1.00) a | Ref. | 0.93 (0.83–1.04) | Ref. | 0.87 (0.77–0.99) a | Ref. | 0.94 (0.80–1.10) |
| Adjusted (IPW) OR/HR c (95% CI) | Ref. | 0.88 (0.79–0.98) a | Ref. | 0.91 (0.80–1.03) | Ref. | 0.86 (0.75–0.98) a | Ref. | 0.98 (0.83–1.15) |
| Patients with hypertension | ||||||||
|
n (%) events b Event rate (95% CI) as PY c |
2467 (0.67) | 3502 (0.47) | 5467 (0.53) | 502 (0.73) | 9.8 (9.3–10.4) | 6.3 (6.0–6.6) | 7.5 (7.2–7.8) | 7.9 (7.0–9.0) |
| Crude OR b /HR c (95% CI) | Ref. | 0.70 (0.67–0.74) a | Ref. | 1.40 (1.27–1.53) a | Ref. | 0.71 (0.66–0.76) a | Ref. | 1.02 (0.89–1.16) |
| Adjusted (individual variables) OR b /HR c (95% CI) | Ref. | 0.84 (0.79–0.89) a | Ref. | 0.99 (0.90–1.10) | Ref. | 0.89 (0.82–0.97) a | Ref. | 0.93 (0.80–1.07) |
| Adjusted (IPW) OR/HR c (95% CI) | Ref. | 0.84 (0.79–0.89) a | Ref. | 0.98 (0.87–1.09) | Ref. | 0.88 (0.81–0.96) a | Ref. | 0.95 (0.81–1.11) |
| Patients with renal disease | ||||||||
|
n (%) events b Event rate (95% CI) as PY c |
711 (1.27) | 711 (1.19) | 1277 (1.21) | 145 (1.40) | 10.7 (9.7–11.8) | 8.1 (7.2–8.9) | 9.3 (8.7–10.1) | 8.7 (6.9–11.0) |
| Crude OR b /HR c (95% CI) | Ref. | 0.94 (0.84–1.04) | Ref. | 1.15 (0.97–1.37) | Ref. | 0.78 (0.67–0.90) a | Ref. | 0.90 (0.71–1.14) |
| Adjusted (individual variables) OR b /HR c (95% CI) | Ref. | 0.90 (0.80–1.01) | Ref. | 0.91 (0.75–1.09) | Ref. | 0.91 (0.78–1.07) | Ref. | 0.91 (0.69–1.20) |
| Adjusted (IPW) OR/HR c (95% CI) | Ref. | 0.90 (0.80–1.01) | Ref. | 0.76 (0.61–0.93) a | Ref. | 0.88 (0.75–1.04) | Ref. | 1.01 (0.76–1.35) |
| Patients with IHD | ||||||||
|
n (%) events b Event rate (95% CI) as PY c |
1076 (0.74) | 1409 (0.58) | 2215 (0.63) | 270 (0.84) | 9.9 (9.1–10.7) | 7.1 (6.6–7.7) | 8.1 (7.6–8.6) | 9.6 (8.1–11.3) |
| Crude OR b /HR c (95% CI) | Ref. | 0.78 (0.72–0.85) a | Ref. | 1.34 (1.18–1.52) a | Ref. | 0.75 (0.68–0.84) a | Ref. | 1.09 (0.91–1.29) |
| Adjusted (individual variables) OR b /HR c (95% CI) | Ref. | 0.84 (0.77–0.92) a | Ref. | 0.96 (0.84–1.11) | Ref. | 0.88 (0.78–1.00) a | Ref. | 1.09 (0.91–1.32) |
| Adjusted (IPW) OR/HR c (95% CI) | Ref. | 0.83 (0.76–0.91) a | Ref. | 0.92 (0.79–1.08) | Ref. | 0.86 (0.75–0.98) a | Ref. | 1.09 (0.89–1.33) |
| Patients with diabetes | ||||||||
|
n (%) events b Event rate (95% CI) as PY c |
1093 (0.67) | 1599 (0.68) | 2454 (0.66) | 238 (0.98) | 6.8 (6.2–7.4) | 5.7 (5.2–6.1) | 6.0 (5.6–6.4) | 7.3 (6.0–8.8) |
| Crude OR b /HR c (95% CI) | Ref. | 1.02 (0.94–1.10) | Ref. | 1.49 (1.30–1.70) a | Ref. | 0.84 (0.75–0.95) a | Ref. | 1.09 (0.89–1.33) |
| Adjusted (individual variables) OR b /HR c (95% CI) | Ref. | 0.93 (0.85–1.01) | Ref. | 1.00 (0.86–1.16) | Ref. | 0.89 (0.78–1.02) | Ref. | 0.91 (0.73–1.14) |
| Adjusted (IPW) OR/HR c (95% CI) | Ref. | 0.92 (0.84–1.01) | Ref. | 0.96 (0.81–1.13) | Ref. | 0.86 (0.75–0.98) a | Ref. | 0.93 (0.73–1.19) |
| Patients in Stockholm region | ||||||||
|
n (%) events b Event rate (95% CI) as PY c |
1506 (1.40) | 1733 (1.10) | 3030 (1.20) | 209 (1.61) | 8.3 (7.7–8.9) | 5.4 (5.0–5.8) | 6.7 (6.3–7.0) | 5.8 (4.7–7.1) |
| Crude OR b /HR c (95% CI) | Ref. | 0.79 (0.73–0.84) a | Ref. | 1.35 (1.17–1.55) a | Ref. | 0.71 (0.64–0.78) a | Ref. | 0.89 (0.72–1.10) |
| Adjusted (individual variables) OR b /HR c (95% CI) | Ref. | 0.84 (0.78–0.91) a | Ref. | 0.89 (0.77–1.04) | Ref. | 0.87 (0.78–0.98) a | Ref. | 0.80 (0.63–1.00) |
| Adjusted (IPW) OR/HR c (95% CI) | Ref. | 0.83 (0.76–0.90) a | Ref. | 0.83 (0.70–0.98) a | Ref. | 0.85 (0.76–0.96) a | Ref. | 0.83 (0.65–1.06) |
ACEi, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; CI, confidence interval; COVID‐19, coronavirus disease 2019; HF, heart failure; HR, hazard ratio; IHD, ischaemic heart disease; IPW, inverse probability weighting; MRA, mineralocorticoid receptor antagonist; OR, odds ratio; PY, patient‐years.
P <0.05.
Estimates provided for the analyses assessing the risk of hospitalization/death for COVID‐19 in the overall population.
Estimates provided for the analysis assessing the risk of all‐cause death in patients with COVID‐19.
As many as 566 (0.70%) patients in the MRA group and 6580 (0.50%) in the non‐MRA group experienced incident hospitalization/death for COVID‐19, translating into an increased risk of incident hospitalization or death from COVID‐19 associated with MRA use in crude analyses (OR 1.39, 95% CI 1.28–1.52). However, after adjustments, no association between MRA and outcome was observed (OR 0.97, 95% CI 0.87–1.08).
In patients receiving a combination of ACEi/ARB and MRA vs. those receiving none or only one of these drugs, 362 (0.62%) vs. 6784 (0.51%) were hospitalized for or died from COVID‐19. Thus, the combined use of ACEi/ARB and MRA was significantly associated with an increased crude risk of outcome (OR 1.21, 95% CI 1.09–1.35), whereas no statistically significant association was found after adjustments (OR 0.91, 95% CI 0.80–1.03) (online supplementary Table S5 ).
Association between renin–angiotensin–aldosterone system inhibitor use and all‐cause mortality in COVID‐19 cases
Of 7146 (0.51%) COVID‐19 cases, 3909 (55%) were treated with ACEi/ARB and 566 (7.9%) with MRA, whereas 362 (5.1%) received a combination of ACEi/ARB and MRA.
Over the entire follow‐up, crude event rates for all‐cause mortality in patients with COVID‐19 receiving vs. not receiving ACEi/ARB were 6.1 (95% CI 5.8–6.4) vs. 8.5 (95% CI 8.1–8.9) patient‐years, respectively, which translated into a reduced risk of all‐cause death associated with ACEi/ARB use in crude (HR 0.76, 95% CI 0.71–0.81) and adjusted (HR 0.89, 95% CI 0.82–0.96) analyses (Figure 1 and Table 2 ). A similar association was observed for ARB use (HR 0.89, 95% CI 0.81–0.97), whereas ACEi use was not associated with risk of all‐cause mortality (HR 1.00, 95% CI 0.92–1.09) (online supplementary Table S5 ). Thirty‐day risk of all‐cause mortality was not significantly different in patients treated vs. not treated with ACEi/ARB, or with ACEi or ARB individually (online supplementary Tables S5 and S6 ).
Figure 1.
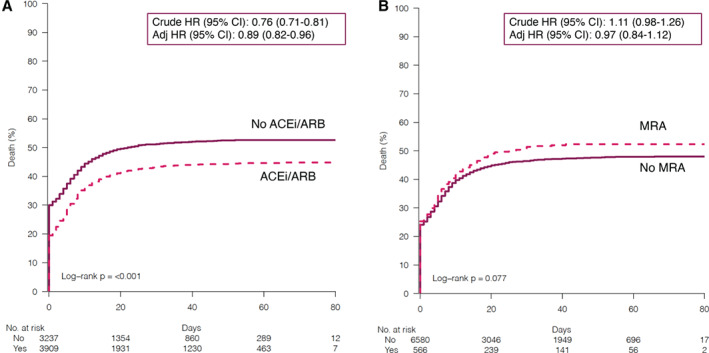
Kaplan–Meier curves for risk of incident hospitalization/death for COVID‐19 in patients receiving vs. not receiving (A) angiotensin converting enzyme inhibitors (ACEi) or angiotensin receptor blockers (ARB) and (B) mineralocorticoid receptor antagonists (MRA). CI, confidence interval; HR, hazard ratio.
Over the entire follow‐up, in COVID‐19 cases treated vs. not treated with MRA, event rates for all‐cause mortality were 8.2 (95% CI 7.3–9.3) vs. 7.0 (95% CI 6.8–7.3) patient‐years, respectively. No significant association between MRA use and risk of all‐cause death was observed in crude (HR 1.11, 95% CI 0.98–1.26) or adjusted (HR 0.97, 95% CI 0.84–1.12) analyses. Similar results were observed when 30‐day risk of mortality was considered (online supplementary Table S6 ).
Rates for all‐cause mortality were 6.6 (95% CI 5.6–7.7) vs. 7.1 (95% CI 6.9–7.4) patient‐years with combined vs. not combined use of ACEi/ARB and MRA, respectively. Thus, combined treatment was not associated with crude (HR 0.91, 95% CI 0.77–1.07) or adjusted (HR 0.91, 95% CI 0.76–1.10) risk of all‐cause death (online supplementary Table S6 ). Similar results were observed for 30‐day mortality (online supplementary Table S5 ).
Residual confounding analysis
In anticipation of potential unmeasured confounding, we introduced a simulated confounder hypothetically present in 35% of patients receiving ACEi/ARB and in 70% not receiving ACEi/ARB. For the association between ACEi/ARB use and outcomes (i.e. risk of incident hospitalization/death for COVID‐19 in the overall population and risk of all‐cause mortality in COVID‐19 cases), if the simulated potential confounder was associated with outcomes with an OR/HR = 1.4, the association between ACEi/ARB use and outcomes would be neutral; if the OR/HR were 2.0 there would be a potential signal of a weak harmful association between ACEi/ARB use and outcomes (i.e. OR/HR for the association with outcomes ≈1.1); if the OR/HR were as high as 15 and 10, respectively, there would be a potentially stronger signal of harm (i.e. OR/HR for the association with outcomes ≈1.6) (Figure 2 ). For the analyses focusing on MRA, in a similar setting as above for ACEi/ARB, a potential confounder with an OR/HR = 1.8 would lead to a potentially weak harmful association between MRA and outcomes (i.e. OR/HR for the association with outcomes ≈1.2), and an OR/HR = 5 to lead to a potentially stronger signal of harm (i.e. OR/HR for the association with outcomes ≈1.5 ) (Figure 3 ).
Figure 2.
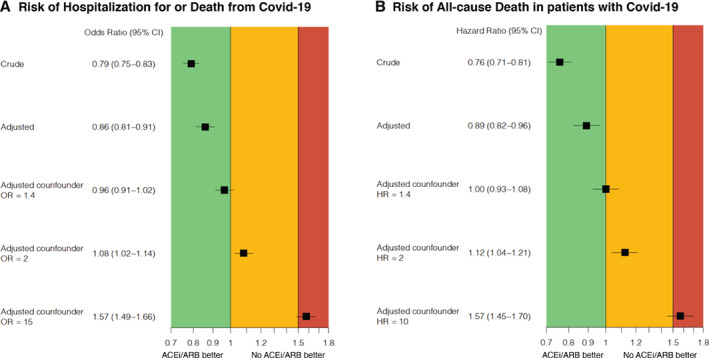
Impact of potential unmeasured confounders on the association between use of renin–angiotensin system inhibitors and risk of (A) incident hospitalization/death for COVID‐19 and (B) all‐cause death in COVID‐19 cases. ACEi, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; CI, confidence interval; HR, hazard ratio; OR, odds ratio.
Figure 3.
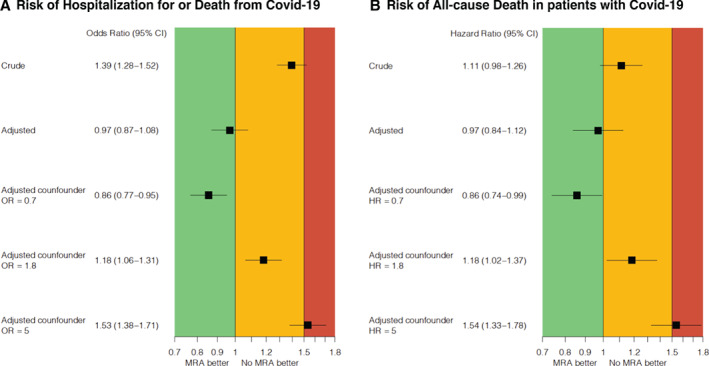
Impact of potential unmeasured confounders on the association between use of mineralocorticoid receptor antagonists (MRA) and risk of (A) incident hospitalization/death for COVID‐19 and (B) all‐cause death in COVID‐19 cases. CI, confidence interval; HR, hazard ratio; OR, odds ratio.
Subgroup analysis
The results of our analyses were overall consistent across the explored subgroups. However, we observed a statistically significant reduction in risk of incident hospitalization/death for COVID‐19 associated with MRA use in patients with kidney disease (Table 2 , Figure 4 , and online supplementary Table S6 ).
Figure 4.
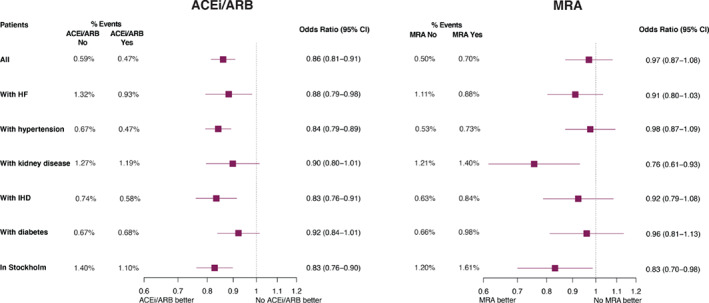
Subgroup analyses for the association between use of renin–angiotensin system inhibitors/mineralocorticoid receptor antagonists (MRA) and risk of hospitalization/death for COVID‐19. ACEi, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; CI, confidence interval; HF, heart failure; IHD, ischaemic heart disease.
Discussion
In a nationwide registry including patients with hypertension, HF, kidney disease, IHD or diabetes, we observed an association between ACEi/ARB use and reduced risk of hospitalization/mortality for COVID‐19 and all‐cause mortality in patients with COVID‐19, whereas MRA use was not associated with outcomes. Therefore, we could not confirm previous concerns regarding a harmful association between ACEi/ARB or MRA and COVID‐19 severity/prognosis. Although the analysis included extensive covariate adjustments, residual unmeasured confounding might have been present. However, in order to observe a meaningful harmful association between RAASi and COVID‐19, such a confounder would need to be both much more common in patients not receiving RAASi and independently associated with unrealistically high ORs and HRs.
Our analysis confirms previous studies suggesting absent harmful associations between use of RAASi and COVID‐19 severity and mortality. 5 , 6 , 8 , 9 , 16 A potential ‘beneficial’ association between ACEi/ARB use and outcomes observed in our and previous studies might be explained by the link between a reduction of ACE2 (and subsequent higher circulating angiotensin II) and increased lung injury. 8 , 17 Additionally, we showed that ARB but not ACEi use was significantly associated with reduced risk of outcomes. It might be speculated that the increased stimulation of the angiotensin II type 2 receptor secondary to the use of ARB, which has been shown to alleviate inflammatory stress in the lungs in experimental models, might play a role to explain this finding. 18 We also observed a high mortality rate on day 1, which consisted mostly of out‐of‐hospital deaths. This represented deaths in assisted living, elderly care and rehabilitation facilities, as well as in nursing homes, where the number of deaths from COVID‐19 during the spring of 2020 was especially high.
One strength of our study is the inclusion of a large nationwide registry population, with full coverage, leading to high generalizability of our findings and extensive adjustments for confounders. However, there are still major limitations in the form of potential unmeasured confounding, which is inherent to observational designs and precludes establishing any causal relationship between exposures and outcomes. 19 Important clinical variables, such as blood pressure, estimated glomerular filtration rate, glycaemic control, and variables linked with disease severity, which may affect treatment decisions and the outcomes of our study, were not available. In order to try to quantify the impact of potential residual confounding on our findings, we simulated how our results would have changed whether we had omitted to adjust for an important confounder. Therefore, we included in our regression models a simulated variable with different prevalence across the study groups and associated with our outcomes with specific simulated HRs/ORs (i.e. a confounder). 20 We found that such a variable/confounder would need to be twofold more prevalent in untreated patients (35% and 70% of the ACEi/ARB and non‐ACEi/ARB population, respectively), and would also need to be associated with our study outcomes with an OR/HR of around 1.4 in order to be able to show a non‐significant association between ACEi/ARB and reduced risk of COVID‐19/mortality, with OR/HR of 2 to show a weak signal of harm, and with an OR/HR of 10–15 to show a stronger signal of harm. Thus, such confounder would need to be unrealistically differently distributed, and unrealistically strongly associated with outcomes, for ACEi/ARB drugs to be meaningfully associated with increased risk of COVID‐19 hospitalization/death in the overall population, or death in patients with COVID‐19. For example, chronic kidney disease and anaemia in HF have been shown to be associated with all‐cause mortality with HRs ranging 1.2–1.5. 21 , 22 In a previous study assessing patient characteristics independently associated with COVID‐19, the most important predictor was the highest chronic disease score category (i.e. severe multimorbidity status), with an OR of 1.6. 6 Therefore, our study strongly suggests, but does not conclusively prove, that ACEi/ARB drugs are not associated with an increased risk of severe COVID‐19 (i.e. hospitalization/death for COVID‐19) or death in patients with COVID‐19.
To conclusively determine the role of RAASi in COVID‐19, evidence from randomized controlled trials is key. The recently presented BRACE CORONA trial showed similar short‐term mortality rates in 659 patients with confirmed mild or moderate COVID‐19 randomized to ACEi/ARB discontinuation vs. continued use. 23 However, a randomized trial may be less generalizable than a nationwide complete‐coverage registry and underpowering might lead to no statistically significant effects but a failure to rule out harm (or benefit) with 95% certainty. 24 Therefore, our analysis provides evidence complementary to that provided by available and potential future trials in this setting. Our study does not conclude that RAASi are effective with regard to COVID‐19, but strongly suggests that they are safe, which may be helpful for patients with conditions such as HF, hypertension, diabetes, IHD and kidney disease, who benefit from use of, and would suffer harm from withdrawal of, RAASi drugs.
Our study has limitations beyond the observational design. First, based on the available data, COVID‐19 patients were defined based on the occurrence of a hospitalization or death for COVID‐19. Therefore, generalizability of our findings to asymptomatic or symptomatic COVID‐19 patients who were not hospitalized may be limited. Second, use of RAASi was defined at the index date and therefore whether they were discontinued following hospital admission for COVID‐19 cannot be excluded. Third, the data released by the Swedish Board of Health and Welfare usually undergo several rounds of quality checks, the number of which were reduced to meet the urgent need of data for COVID‐19‐related research. Fourth, data on race/ethnicity were not available, and therefore we could not investigate race/ethnic‐specific differences in this setting. However, to limit confounding we adjusted for country of birth which might represent a surrogate of race/ethnicity. Based on the limited number of patients collected in our cohort who were born in non‐European countries, we might assume that black patients, where ACEi/ARB effect is potentially reduced, were also scarcely represented in our study population. Fifth, our data might not be completely updated in terms of ICD‐10 diagnoses for COVID‐19 till 31 May 2020 but this is unlikely to be differently distributed across the study arms. Finally, this 1.4 million patient cohort had only 7146 COVID‐19 cases. The cases were used to study mortality from COVID‐19, which has been done in other studies. However, the 1.4 million population was used to study incidence of hospitalization or death from COVID‐19, equally important but less studied.
In conclusion, in a large national cohort we did not observe any signal of a harmful association between RAASi use and incident hospitalization/death for COVID‐19, or risk of all‐cause death in patients with COVID‐19. An unmeasured confounder would need to be both substantially more common in non‐RAASi users, and more strongly associated with COVID‐19 than is clinically realistic, for our analyses to fail to demonstrate such harmful associations.
Funding
This study received support from the EU/EFPIA Innovative Medicines Initiative 2 Joint Undertaking BigData@Heart grant [no 116074]; the Swedish Research Council [grant 523–2014‐2336]; and the Swedish Heart Lung Foundation [grants 20150557 and 20170841].
Conflict of interest: G.S. reports grants and personal fees from AstraZeneca, Vifor, grants and non‐financial support from Boehringer Ingelheim, personal fees from Società Prodotti Antibiotici, Roche, Servier, GENESIS, Cytokinetics, Medtronic, grants from Novartis, Boston Scientific, outside the submitted work. L.B. has nothing to disclose. J.S. reports stock ownership in companies with consultancy fees from Itrim, Amgen, Janssen, Novo Nordisk, Eli Lilly, Boehringer, Bayer and AstraZeneca, outside the submitted work. L.H.L. reports research grants from AstraZeneca, Novartis, Boerhinger Ingelheim, Vifor‐Fresenius, and Boston Scientific, and consulting or speaker's honoraria from AstraZeneca, Novartis, Boehringer Ingelheim, Vifor‐Fresenius, Bayer, Sanofi, Merck, Myokardia, Orion Pharma, MedScape, Radcliffe Cardiology, Lexicon, and Respicardia, and stock ownership in AnaCardio, outside the submitted work.
Supporting information
Table S1. Patient selection.
Table S2. Variable definitions.
Table S3. Length of treatment with study drugs.
Table S4. Baseline characteristics of patients hospitalized or died for COVID‐19 (i.e. COVID‐19 cases) according to use of renin–angiotensin system inhibitors and mineralocorticoid receptor antagonists.
Table S5. Outcome analysis according to use of angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers, individually, and to the combination of angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers and mineralocorticoid receptor antagonists.
Table S6. Thirty‐day all‐cause mortality outcome analysis in patients with COVID‐19.
Figure S1. Prevalence of hypertension, diabetes, kidney disease, heart failure and ischaemic heart disease in the study population.
References
- 1. Vaduganathan M, Vardeny O, Michel T, McMurray JJ, Pfeffer MA, Solomon SD. Renin‐angiotensin‐aldosterone system inhibitors in patients with Covid‐19. N Engl J Med 2020;382:1653–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. European Society of Cardiology . Position Statement of the ESC Council on Hypertension on ACE‐inhibitors and Angiotensin Receptor Blockers. https://www.escardio.org/Councils/Council‐on‐Hypertension‐(CHT)/News/position‐statement‐of‐the‐esc‐council‐on‐hypertension‐on‐ace‐inhibitors‐and‐ang (8 July 2020).
- 3. American College of Cardiology . HFSA/ACC/AHA Statement Addresses Concerns Re: Using RAAS Antagonists in COVID‐19. https://www.acc.org/latest‐in‐cardiology/articles/2020/03/17/08/59/hfsa‐acc‐aha‐statement‐addresses‐concerns‐re‐using‐raas‐antagonists‐in‐covid‐19 (8 July 2020).
- 4. Lipsitch M, Swerdlow DL, Finelli L. Defining the epidemiology of Covid‐19 – studies needed. N Engl J Med 2020;382:1194–1196. [DOI] [PubMed] [Google Scholar]
- 5. Reynolds HR, Adhikari S, Pulgarin C, Troxel AB, Iturrate E, Johnson SB, Hausvater A, Newman JD, Berger JS, Bangalore S, Katz SD, Fishman GI, Kunichoff D, Chen Y, Ogedegbe G, Hochman JS. Renin‐angiotensin‐aldosterone system inhibitors and risk of Covid‐19. N Engl J Med 2020;382:2441–2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mancia G, Rea F, Ludergnani M, Apolone G, Corrao G. Renin‐angiotensin‐aldosterone system blockers and the risk of Covid‐19. N Engl J Med 2020;382:2431–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fosbol EL, Butt JH, Ostergaard L, Andersson C, Selmer C, Kragholm K, Schou M, Phelps M, Gislason GH, Gerds TA, Torp‐Pedersen C, Kober L. Association of angiotensin‐converting enzyme Inhibitor or angiotensin receptor blocker use with COVID‐19 diagnosis and mortality. JAMA 2020;324:168–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang P, Zhu L, Cai J, Lei F, Qin JJ, Xie J, Liu YM, Zhao YC, Huang X, Lin L, Xia M, Chen MM, Cheng X, Zhang X, Guo D, Peng Y, Ji YX, Chen J, She ZG, Wang Y, Xu Q, Tan R, Wang H, Lin J, Luo P, Fu S, Cai H, Ye P, Xiao B, Mao W, Liu L, Yan Y, Liu M, Chen M, Zhang XJ, Wang X, Touyz RM, Xia J, Zhang BH, Huang X, Yuan Y, Rohit L, Liu PP, Li H. Association of inpatient use of angiotensin‐converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID‐19. Circ Res 2020;126:1671–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hippisley‐Cox J, Young D, Coupland C, Channon KM, Tan PS, Harrison DA, Rowan K, Aveyard P, Pavord ID, Watkinson PJ. Risk of severe COVID‐19 disease with ACE inhibitors and angiotensin receptor blockers: cohort study including 8.3 million people. Heart 2020;106:1503–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liabeuf S, Moragny J, Bennis Y, Batteux B, Brochot E, Schmit JL, Lanoix JP, Andrejak C, Ganry O, Slama M, Maizel J, Mahjoub Y, Masmoudi K, Gras‐Champel V. Association between renin‐angiotensin system inhibitors and COVID‐19 complications. Eur Heart J Cardiovasc Pharmacother 2020. Jun 12. 10.1093/ehjcvp/pvaa062 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Keidar S, Gamliel‐Lazarovich A, Kaplan M, Pavlotzky E, Hamoud S, Hayek T, Karry R, Abassi Z. Mineralocorticoid receptor blocker increases angiotensin‐converting enzyme 2 activity in congestive heart failure patients. Circ Res 2005;97:946–953. [DOI] [PubMed] [Google Scholar]
- 12. Batlle D, Wysocki J, Satchell K. Soluble angiotensin‐converting enzyme 2: a potential approach for coronavirus infection therapy? Clin Sci (Lond) 2020;134:543–545. [DOI] [PubMed] [Google Scholar]
- 13. Heurich A, Hofmann‐Winkler H, Gierer S, Liepold T, Jahn O, Pohlmann S. TMPRSS2 and ADAM17 cleave ACE2 differentially and only proteolysis by TMPRSS2 augments entry driven by the severe acute respiratory syndrome coronavirus spike protein. J Virol 2014;88:1293–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ludvigsson JF, Almqvist C, Bonamy AK, Ljung R, Michaelsson K, Neovius M, Stephansson O, Ye W. Registers of the Swedish total population and their use in medical research. Eur J Epidemiol 2016;31:125–136. [DOI] [PubMed] [Google Scholar]
- 15. Lin DY, Psaty BM, Kronmal RA. Assessing the sensitivity of regression results to unmeasured confounders in observational studies. Biometrics 1998;54:948–963. [PubMed] [Google Scholar]
- 16. de Abajo FJ, Rodríguez‐Martín S, Lerma V, Mejía‐Abril G, Aguilar M, García‐Luque A, Laredo L, Laosa O, Centeno‐Soto GA, Ángeles Gálvez M, Puerro M, González‐Rojano E, Pedraza L, de Pablo I, Abad‐Santos F, Rodríguez‐Mañas L, Gil M, Tobías A, Rodríguez‐Miguel A, Rodríguez‐Puyol D, Barreira‐Hernandez D, Zubiaur P, Santos‐Molina E, Pintos‐Sánchez E, Navares‐Gómez M, Aparicio RM, García‐Rosado V, Gutiérrez‐Ortega C, Pérez C, Ascaso A, Elvira C. Use of renin‐angiotensin‐aldosterone system inhibitors and risk of COVID‐19 requiring admission to hospital: a case‐population study. Lancet 2020;395:1705–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Murthy VL, Koupenova M, Shah RV. ACEing COVID‐19: a role for angiotensin axis inhibition in SARS‐CoV‐2 infection? Circ Res 2020;126:1682–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Menk M, Graw JA, von Haefen C, Steinkraus H, Lachmann B, Spies CD, Schwaiberger D. Angiotensin II type 2 receptor agonist compound 21 attenuates pulmonary inflammation in a model of acute lung injury. J Inflamm Res 2018;11:169–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. D'Agostino RB Jr, D'Agostino RB Sr. Estimating treatment effects using observational data. JAMA 2007;297:314–316. [DOI] [PubMed] [Google Scholar]
- 20. Lund LH, Benson L, Dahlstrom U, Edner M. Association between use of renin‐angiotensin system antagonists and mortality in patients with heart failure and preserved ejection fraction. JAMA 2012;308:2108–2117. [DOI] [PubMed] [Google Scholar]
- 21. Savarese G, Jonsson A, Hallberg AC, Dahlstrom U, Edner M, Lund LH. Prevalence of, associations with, and prognostic role of anemia in heart failure across the ejection fraction spectrum. Int J Cardiol 2020;298:59–65. [DOI] [PubMed] [Google Scholar]
- 22. Savarese G, Settergren C, Schrage B, Thorvaldsen T, Lofman I, Sartipy U, Mellbin L, Meyers A, Fazeli Farsani S, Brueckmann M, Brodovicz KG, Vedin O, Asselbergs FW, Dahlstrom U, Cosentino F, Lund LH. Comorbidities and cause‐specific outcomes in heart failure across the ejection fraction spectrum: a blueprint for clinical trial design. Int J Cardiol 2020;313:76–82. [DOI] [PubMed] [Google Scholar]
- 23. European Society of Cardiology . First randomised trial backs safety of common heart drugs in COVID‐19 patients. https://www.escardio.org/The‐ESC/Press‐Office/Press‐releases/LOPES (25 November 2020).
- 24. Frieden TR. Evidence for health decision making – beyond randomized, controlled trials. N Engl J Med 2017;377:465–475. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Patient selection.
Table S2. Variable definitions.
Table S3. Length of treatment with study drugs.
Table S4. Baseline characteristics of patients hospitalized or died for COVID‐19 (i.e. COVID‐19 cases) according to use of renin–angiotensin system inhibitors and mineralocorticoid receptor antagonists.
Table S5. Outcome analysis according to use of angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers, individually, and to the combination of angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers and mineralocorticoid receptor antagonists.
Table S6. Thirty‐day all‐cause mortality outcome analysis in patients with COVID‐19.
Figure S1. Prevalence of hypertension, diabetes, kidney disease, heart failure and ischaemic heart disease in the study population.


