Abstract
The effects of the clinically used protease inhibitor nafamostat on influenza virus replication have not been well studied. Primary human tracheal (HTE) and nasal (HNE) epithelial cells were pretreated with nafamostat and infected with the 2009 pandemic [A/Sendai‐H/108/2009/(H1N1) pdm09] or seasonal [A/New York/55/2004(H3N2)] influenza virus. Pretreatment with nafamostat reduced the titers of the pandemic and seasonal influenza viruses and the secretion of inflammatory cytokines, including interleukin‐6 and tumor necrosis factor‐α, in the supernatants of the cells infected with the pandemic influenza virus. HTE and HNE cells exhibited mRNA and/or protein expression of transmembrane protease serine 2 (TMPRSS2), TMPRSS4, and TMPRSS11D. Pretreatment with nafamostat reduced cleavage of the precursor protein HA0 of the pandemic influenza virus into subunit HA1 in HTE cells and reduced the number of acidic endosomes in HTE and HNE cells where influenza virus RNA enters the cytoplasm. Additionally, nafamostat (30 mg/kg/day, intraperitoneal administration) reduced the levels of the pandemic influenza virus [A/Hyogo/YS/2011 (H1N1) pdm09] in mouse lung washes. These findings suggest that nafamostat may inhibit influenza virus replication in human airway epithelial cells and mouse lungs and reduce infection‐induced airway inflammation by modulating cytokine production.
Keywords: antiviral agents, cell cultures, cytokines, influenza virus, protease inhibitor, respiratory tract
1. INTRODUCTION
Clinically used anti‐influenza drugs, such as the neuraminidase inhibitors oseltamivir and zanamivir and the newly developed anti‐influenza drug baloxavir, are beneficial in the context of pandemic and/or seasonal human influenza virus infection. 1 , 2 , 3 However, several patients with pandemic influenza virus infection have died despite intensive drug treatment, including neuraminidase inhibitor treatment. 4 Oseltamivir‐resistant influenza A (A/H1N1) virus can cause severe disease in immunocompromised patients, and influenza viruses resistant to baloxavir have been frequently identified. 5 , 6 The use of the polymerase inhibitor favipiravir, which was approved in Japan, is highly restricted. Furthermore, because of the worldwide outbreak of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection, treatment of patients with coronavirus disease 2019 (COVID‐19) also infected with influenza virus will be required during the winter season. Therefore, further development of anti‐influenza drugs is needed.
Host proteases, including trypsin, furins, transmembrane protease serine S1 member (TMPRSS) 2, and human trypsin‐like protease (HAT; also known as TMPRSS11D), activate the influenza virus hemagglutinin (HA) protein, which is essential for viral gene entry into a cell and the start of viral replication. 7 , 8 , 9 , 10 TMPRSS2 also mediates the entry of SARS‐CoV‐2. 11
Serine protease inhibitors, including aprotinin, gabexate, and camostat, suppress viral HA cleavage and reduce influenza virus replication. 12 , 13 , 14 , 15 , 16 The serine protease inhibitor nafamostat, which has been clinically used to treat patients with acute pancreatitis and disseminated intravascular coagulation, 17 , 18 also reduces influenza virus replication in MDCK cells. 12 However, the effects of nafamostat have not been studied in human airway epithelial cells.
In this study, primary cultures of human tracheal epithelial (HTE) cells, which retain the functions of the original tissue, 19 and human nasal epithelial (HNE) cells 20 were infected with the 2009 pandemic or a seasonal influenza virus, and the effects of nafamostat on viral replication and release of interleukin‐6 (IL‐6) and tumor necrosis factor‐α (TNF‐α), which are associated with disease severity, 21 were examined. An animal study using mice was also utilized to examine the effects of nafamostat on viral replication, survival, and body weight loss after influenza virus infection.
2. METHODS
2.1. Human tracheal and nasal epithelial cell culture
HTE and HNE cells were isolated as described previously 16 , 20 and cells were cultured in 24‐well plates in a mixture of Dulbecco's modified Eagle's medium (DMEM)‐Ham's F‐12 (DF‐12) (Life Technologies) medium containing 2% Ultroser G (USG) serum substitute. The tracheas used for cell cultures were obtained from 9 patients after death (age: 55 ± 6 years; mean ± SEM; 3 females, 6 males). HNE cells were obtained excised from the nasal polyps of subjects undergoing endoscopic surgery (n = 21; age: 60 ± 3 years; 7 females, 14 males). The cause of death or reason for surgery and statuses for allergic rhinitis and bronchial asthma are shown in Table 1. None of the patients were being treated with nafamostat at the time of death or surgery. This study was approved by the Tohoku University Ethics Committee.
Table 1.
Characteristics of the subjects
| HTE cell donors | ||||||||
|---|---|---|---|---|---|---|---|---|
| Noa | Sex | Cause of death | Allergic rhinitis or asthma | Noa | Sex | Cause of death | Allergic rhinitis or asthma | |
| 1 | M | AMI | ND | 6 | M | DCM | ND | |
| 2 | M | MOF | ND | 7 | M | DCM | ND | |
| 3 | M | CHF | ND | 8 | F | CS | ND | |
| 4 | F | MCTD | ND | 9 | F | IIP | ND | |
| 5 | M | PC | ND | ‒ | ‒ | ‒ | ‒ | |
| HNE cell donors | ||||||||
|---|---|---|---|---|---|---|---|---|
| No a | Sex | Reason for surgery | Allergic rhinitis or asthma | No a | Sex | Reason for surgery | Allergic rhinitis or asthma | |
| 1 | F | SM | ND | 11 | M | ECS | BA | |
| 2 | F | SM | ND | 12 | M | SC | ND | |
| 3 | F | CRS | ND | 13 | M | SM | ND | |
| 4 | M | SM | ND | 14 | M | CRS | BA | |
| 5 | M | CRS | ND | 15 | M | CRS | BA | |
| 6 | M | CRS | ND | 16 | F | PNS | ND | |
| 7 | M | PNS | ND | 17 | M | SC | ND | |
| 8 | F | CRS | ND | 18 | M | NPC | ND | |
| 9 | F | PNS | ND | 19 | F | CRS | BA | |
| 10 | M | CRS | ND | 20 | M | PNS | ND | |
| ‒ | ‒ | ‒ | ‒ | 21 | M | SC | ND | |
Abbreviations: AMI, acute myocardial infarction; BA, bronchial asthma; CHF, chronic heart failure; CRS: chronic rhinosinusitis; CS, cardiac sarcoidosis; DCM, dilated cardiomyopathy; ECS, eosinophilic chronic sinusitis; IIP, idiopathic interstitial pneumonia; MCTD, mixed connective tissue disease; MOF, multiple organ failure; ND, not determined, the presence of allergic rhinitis and bronchial asthma was assessed but not found; NPC, nasopharyngeal cancer; PC, pancreatic cancer; PNS, papilloma in the nasal cavity; SC, sinus.
No, subject number.
2.2. Culture of Madin‐Darby Canine kidney (MDCK) cells
MDCK cells were cultured in T25 flasks in Eagle's minimum essential medium (MEM) supplemented with 10% fetal calf serum. 16 The cells were then cultured in 96‐well plates.
2.3. Viral stocks
To prepare the pandemic [A/Sendai‐H/N0633/2009 (H1N1) pdm09 and A/Hyogo/YS/2011 (H1N1) pdm09] and seasonal [A/New York/55/2004(H3N2)] influenza viruses, nasal swabs were collected from patients and suspended in MEM. 22 To study the effects of nafamostat on viral release from human airways, stocks of the 2009 pandemic [A/Sendai‐H/N0633/2009 (H1N1) pdm09] and seasonal influenza viruses were generated by infecting HTE or HNE cells with the viruses for 1 h. 16 The cells were then cultured in a DF‐12 medium containing 2% USG at 37°C in 5% CO2–95% air. Stocks of the pandemic [A/Hyogo/YS/2011 (H1N1) pdm09] influenza virus were generated by infecting MDCK cells with the virus in MEM containing trypsin. To obtain an influenza virus solution, the supernatants were collected and snap‐frozen in ethanol at −80°C.
2.4. Detection and titration of viruses
The detection and titration of influenza viruses in culture supernatants were performed using the endpoint method involving infection of MDCK cell replicates in plastic 96‐well plates with 10‐fold dilutions of virus‐containing supernatants as previously described. 16 After exposing MDCK cells to the virus‐containing supernatants, the supernatants were aspirated, and the cells were rinsed with phosphate‐buffered saline (PBS); fresh MEM containing trypsin was then added. The presence of the characteristic cytopathic effects of the influenza virus was then determined. The TCID50 (TCID, tissue culture infective dose) was calculated, and viral titers in supernatants are expressed as TCID50/ml. 16
2.5. Viral infection of HTE or HNE cells
Infection of cells with influenza virus was performed using previously described methods. 16 A stock solution of influenza virus was added to HTE or HNE cells in 24‐well plates (400 μl per well, 1.0 × 103 TCID50/ml, a multiplicity of infection [MOI] of 0.8 × 10−3 TCID50/cell). After a 1‐h incubation, the viral solution was removed, and the cells were rinsed with PBS and cultured in 1 ml of fresh medium without trypsin at 37°C in 5% CO2−95% air.
2.6. Treatment of cells with nafamostat
The treatment of cells with 10 μg/ml (20 μM) nafamostat 12 was started 30 before infection and continued during infection and after infection until the end of the experimental period. 16 To examine the concentration‐dependent effects of nafamostat, the cells were treated with nafamostat at concentrations ranging from 0.001 to 10 μg/ml using the same methods previously used for camostat studies. 16
2.7. Collection of supernatants
A portion of supernatant (300 μl) was collected 24 and 72 h after infection, and an equal volume (300 μl) of fresh medium supplemented with nafamostat or vehicle was added to the cell culture. 16 The entire supernatant volume (1 ml) was collected 120 h after infection.
2.8. Quantification of influenza virus RNA levels
Viral RNA in cells was measured to confirm the differences in the magnitude of viral replication. A two‐step real‐time quantitative reverse‐transcription PCR (RT‐PCR) assay was performed using TaqMan® Gene Expression Master Mix (Applied Biosystems) as described previously. 16 The primers and TaqMan probe used for each virus were designed as previously reported. 16 , 23 The expression of viral RNA was normalized to the constitutive mRNA expression of β‐actin.
2.9. Western blot analysis
The inhibitory effects of nafamostat on HA cleavage by serine proteases in HTE cells were examined as previously described. 16 , 24 Cells were infected with the 2009 pandemic [A/Sendai‐H/N0633/2009 (H1N1) pdm09] influenza virus at an MOI of 10 for 60 min, rinsed with PBS, and cultured for 48 h at 37°C without or with 0.1, 1.0, 3.0, or 10.0 μg/ml nafamostat. Then, the supernatants were collected and the proteins of the progeny virus that were released from HTE cells into the supernatants were lysed and analyzed by immunoblotting with an anti‐HA monoclonal antibody that recognizes HA0 and HA1. The proteins were detected with ECL Prime Western Blotting Detection Reagent (GE Healthcare) according to the manufacturer's instructions.
2.10. Expression of transmembrane protease serine 2, transmembrane protease serine 4 and transmembrane protease serine 11D
The mRNA expression of TMPRSS2, TMPRSS4, and TMPRSS11D was measured using the RT‐PCR methods described above (Quantification of influenza virus RNA levels) by utilizing primers that were designed previously. 8 , 16 , 25 The protein concentrations of TMPRSS2 in supernatants were measured using a human transmembrane protease 2 TMPRSS2 ELISA kit (MYBioSourse).
2.11. Indirect immunofluorescence assay for TMPRSS2
An indirect immunofluorescence assay was performed as reported previously. 16 Cells were fixed with 4% paraformaldehyde in PBS for 10 min at room temperature. The fixed cells were incubated with a monoclonal anti‐TMPRSS2 antibody (GeneTex) as the primary antibody for 1 h at room temperature and then incubated with an Alexa Fluor 488‐conjugated goat anti‐mouse IgG (HþL) antibody (Molecular Probes) as the secondary antibody. Nuclei were stained with Hoechst 33342 (Molecular Probes). The cells were observed under an LSM700 laser scanning confocal microscope (Carl Zeiss). Image capture, analysis, and processing were performed using Zen2011 software (Carl Zeiss) and Photoshop CS5 (Adobe).
2.12. Measurement of changes in acidic endosomes
The distribution and fluorescence intensity of acidic endosomes in cells were measured with the LysoSensor DND‐189 dye (Molecular Probes) as previously described. 20 The fluorescence intensity was calculated using a fluorescence image analyzer system (Lumina Vision®; Mitani).
2.13. Measurement of cytokine production
IL‐6 and TNF‐α levels in supernatants were measured using a solid‐phase chemiluminescent enzyme‐linked immunosorbent assay (ELISA) kit (QuantiGlo® ELISA, R&D Systems) for the measurement of IL‐6 concentrations and an ELISA kit (QuantiGlo ELISA Human TNF‐α Immunoassay, R&D Systems) for the measurement of TNF‐α concentrations.
2.14. Measurement of airway epithelial cell damage
To examine uninfected HTE and HNE cell damage after treatment with nafamostat, the number of floating cells in supernatants that were detached from the cell sheets that adhered to the 24‐well plates, and the number and viability of the adhered cells were measured. Lactate dehydrogenase (LDH) concentrations in the supernatant were also measured.
To measure the number of detached cells in the supernatants, the supernatants were collected from the wells of 24‐well plates and floating cells were collected by centrifugation of the supernatants. Then, the cell number was measured using a hemocytometer.
For the cytotoxicity assay, adhered cells were collected by treating cell sheets with trypsin, and the live cells were quantified by the exclusion of trypan blue using a hemocytometer. 16
2.15. Experimental infection of mice
To confirm the effects of nafamostat on animal models, 2‐week‐old female BALB/c mice were intranasally inoculated under isoflurane anesthesia with 104 TCID50/0.03 ml/head of the pandemic [A/Hyogo/YS/2011 (H1N1) pdm09] influenza virus. The mice were intraperitoneally injected with 30 mg/kg/day nafamostat (AY Pharmaceuticals) or camostat (Ono Pharmaceutical Co. Ltd.), or the same dose of oral oseltamivir or PBS, according to previous reports on the intraperitoneal injection of camostat 13 or intravenous injection of peramivir. 26 Furthermore, because 30 mg/kg/day nafamostat reduced lung viral levels but did not improve body weight reductions in preliminary experiments, the effects of a lower amount (2 mg/kg/day) of nafamostat, which does not affect renal function, 27 were also examined. Lung viral titers were measured 72 h after infection, and mortality and/or bodyweight were monitored for 14 days.
2.16. Statistical analysis
Results are expressed as the mean ± SEM. For the comparison of viral titers, RNA levels, cytokine release, TMPRSS expression, and body weight between two groups, Student's t‐test or the Mann‐Whitney U‐test was performed. Subsequent post hoc analyses were performed using Bonferroni's method. Fisher's test was performed to assess differences in survival rates. For all analyses, values of p < .05 were considered significant. In the experiments using cultures of HTE or HNE cells, n refers to the number of donors (tracheae or nasal polyps) from which the cultured epithelial cells were obtained. All analyses were performed using SPSS version 21 (IBM Japan).
3. RESULTS
3.1. Release of influenza viruses and effects of nafamostat
The pandemic [A/Sendai‐H/N0633/2009 (H1N1) pdm09] influenza virus was detected in supernatants at 24 h, and the viral titer progressively increased between 24 and 72 h after infection in HTE and HNE cells (Figure 1A,B). The viral titer increased over 72 h of observation and was consistent across all culture replicates at 120 h (Figure 1A,B).
Figure 1.
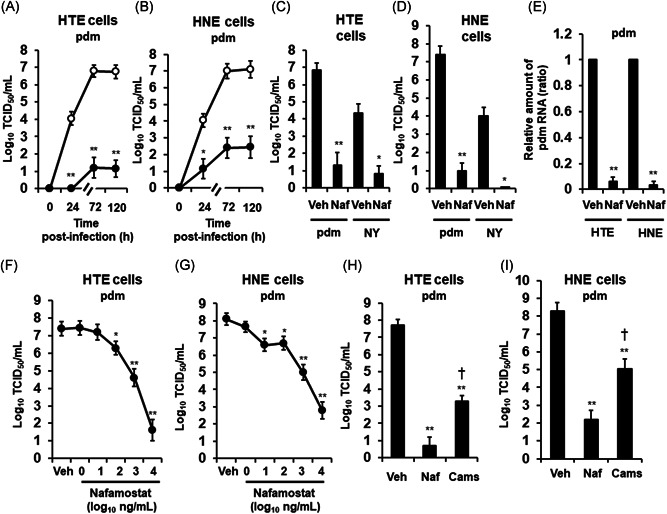
A and B, Time course of viral release into supernatants of primary cultures of human tracheal (HTE) (A) or nasal (HNE) (B) epithelial cells showing levels at different times after exposure to the 2009 pandemic [A/Sendai‐H/108/2009/(H1N1) pdm09] influenza virus (pdm) in the presence of nafamostat (10 μg/ml) (closed circles) or vehicle (0.1% water) (control, open circles). C and D, The viral titers in supernatants collected between 24 h and 72 h after infection of HTE (C) or HNE (D) cells with the pdm or the seasonal [A/New York/55/2004 (H3N2)] (NY) influenza virus in the presence of nafamostat (Naf) or vehicle (0.1% water) (Veh). E, RNA levels of the pdm in HTE or HNE cells at 72 h after infection in the presence of nafamostat (Naf) or vehicle (Veh). The results are expressed as the relative amount of RNA (ratio) compared to the influenza virus RNA level in the vehicle‐pretreated cells. F and G, Concentration‐dependent effects of nafamostat on the release of the pandemic influenza virus (pdm) in the supernatants of HTE (F) or HNE (G) cells collected between 24 and 72 h after infection. H and I, Titers of the pandemic influenza virus (pdm) in supernatants collected between 24 and 72 h after infection of HTE (H) or HNE (I) cells pretreated with nafamostat (Naf, 10 μg/ml), camostat (Camos, 10 μg/ml) or vehicle (Veh). A‐I: Treatment with nafamostat (A–I) or camostat (H, I) was initiated 30 min before infection and continued during infection and after infection until the end of the experiments. Changes in the viral titers in supernatants are expressed as log10TCID50/ml (A–D, F–I). The results are expressed as the mean ± SEM of five (A–D, F, G) or four (E, H, I) different tracheal or nasal samples. Camos, camostat; HNE, human nasal; HTE, human tracheal; Naf, nafamostat; Veh, vehicle. Significant differences versus the vehicle alone group are indicated by *p < .05 and **p < .01. Significant differences versus the nafamostat group are indicated by † p < .05
Pretreatment of HTE or HNE cells with nafamostat (10 μg/ml) significantly reduced the titers of the pandemic influenza virus in the supernatants at 24, 72 h, and 120 h after infection (Figure 1A–D).
The seasonal [A/New York/55/2004(H3N2)] influenza virus was also detected in the supernatants of HTE and HNE cells (Figure 1C,D). Viral titers were detected 24 h after infection and increased over the 3 days of observation, and the viral titers were consistent across all culture replicates at 120 h (data not shown). Pretreatment of HTE or HNE cells with nafamostat also reduced the titers of the seasonal influenza virus in the supernatants collected between 24 and 72 h after infection (Figure 1C,D).
The possible cytotoxicity of nafamostat toward HTE and HNE cells was also examined. The number of detached cells in the supernatants of wells of 24‐well plates treated with nafamostat (10 μg/ml) for 72 h did not differ from that of the wells treated with vehicle (water) (0.39 ± 0.12 × 104 in the nafamostat group vs. 0.42 ± 0.11 × 104 in the vehicle group, number/well, n = 4, p > .20 for HTE cells; 0.68 ± 0.12 × 104 in the nafamostat group vs. 0.71 ± 0.14 × 104 in the vehicle group, number/well, n = 4, p > .20 for HNE cells). Likewise, treating cells with nafamostat did not decrease the proportion of dead cells among the attached cells (95% ± 2% in the nafamostat group vs. 96 ± 1% in the vehicle group, n = 4, p > .20 for HTE cells; 96% ± 2% in the nafamostat group vs. 97% ± 1% in the vehicle group, n = 4, p > .20 for HNE cells). Pretreatment with nafamostat did not increase the LDH concentrations in the supernatants (34 ± 3 U/L in the nafamostat group vs. 32 ± 3 U/L in the vehicle group, n = 3, p > .20 for HTE cells; 18 ± 1 U/L in the nafamostat group vs. 19 ± 2 U/L in the vehicle group, n = 3, p > .20 for HNE cells).
3.2. Effects of nafamostat on the RNA replication of influenza viruses
Replication of the RNA of the pandemic influenza virus in HTE and HNE cells increased with time, and maximum viral RNA expression in the cells was observed 72–120 h after infection, as previously described (data not shown). 16
Pretreatment of HTE cells or HNE cells with nafamostat (10 μg/ml) significantly reduced the levels of pandemic influenza virus RNA in cells at 72 h after infection (Figure 1E) (data collected on Days 1, 5, and 7 are not shown).
3.3. Concentration‐dependent effects of nafamostat
Pretreatment with nafamostat decreased the titers of the pandemic influenza virus in supernatants in a concentration‐dependent manner, and a significant reduction was observed at 100 ng/ml and higher concentrations in HTE cells and at 10 ng/ml and higher concentrations in HNE cells (Figure 1F,G).
3.4. Effects of camostat
The effects of the serine protease inhibitor camostat (10 μg/ml) 16 were also examined and compared with those of nafamostat (10 μg/ml). Pretreatment of HTE or HNE cells with camostat reduced the titers of the pandemic [A/Sendai‐H/N0633/2009 (H1N1) pdm09] influenza virus (Figure 1H,I). The potency of the inhibitory effect of camostat on the viral titer was significantly lower than that of nafamostat (Figure 1H,I).
3.5. Effects of nafamostat on TMPRSS expression
Significant amounts of TMPRSS2, TMPRSS4, and TMPRSS11D mRNA were detected in HTE and HNE cells (Figure 2A,B). An indirect immunofluorescence assay confirmed the expression of the TMPRSS2 proteins in HTE and HNE cells (Figure 2C,D). In addition, significant amounts of the TMPRSS2 protein were detected in the supernatants of HTE and HNE cells (Figure 2E,F).
Figure 2.
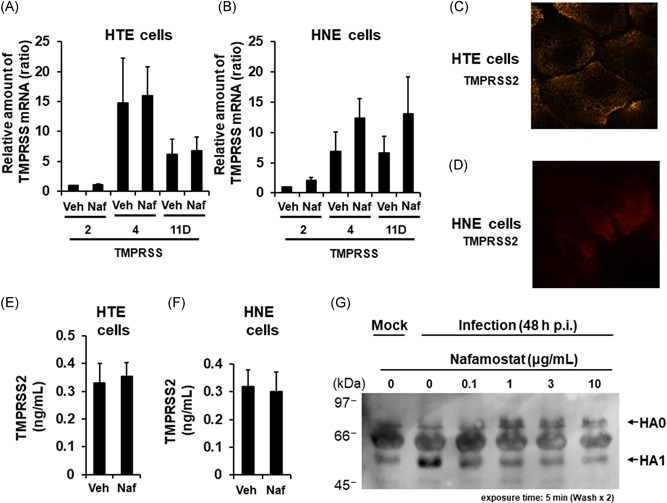
A and B, The mRNA expression levels of TMPRSS2, TMPRSS4, or TMPRSS11D in uninfected HTE or HNE cells treated with nafamostat (Naf, 10 μg/ml) or vehicle (Veh) for 72 h. The results for TMPRSS mRNA levels are expressed as the relative amount of mRNA (ratio) compared to the TMPRSS2 mRNA in the vehicle‐pretreated cells. The results are expressed as the mean ± SEM of five different tracheal or nasal samples. C and D, Indirect immunofluorescence staining of TMPRSS2 in HTE (C) and HNE (D) cells. TMPRSS2 was stained orange. Magnification; 630× (C) or 1000× (D). E and F, The TMPRSS2 protein concentration in the supernatants of HTE or HNE cells treated with nafamostat (Naf, 10 μg/ml) or vehicle (Veh) for 72 h. The results are expressed as the mean ± SEM of five different tracheal or nasal samples. G, Western blot analysis of proteins in the supernatants of primary cultures of HTE cells collected at 48 h post‐infection with the pandemic influenza virus in the presence of nafamostat (0.1, 1, 3, or 10 μg/ml) or vehicle (0) showing inhibition of HA0 cleavage. HA0, hemagglutinin precursor protein; HA1, hemagglutinin subunit; Mock, without infection
The mRNA expression levels of TMPRSS2, TMPRSS4, and TMPRSS11D in HTE and HNE cells and the TMPRSS2 protein concentration in supernatants did not differ between nafamostat‐ and vehicle‐treated cells (Figure 2A,B,E,F).
3.6. Effects of nafamostat on HA cleavage
In the absence of nafamostat, the cleaved HA1 subunit predominated in the supernatants of HTE cells (Figure 2G). In contrast, the amount of cleaved HA1 subunit decreased as the nafamostat concentration increased, while the amount of uncleaved HA0 correspondingly increased (Figure 2G).
3.7. Effects on the acidification of endosomes
Treatment with a vehicle for 72 h did not alter the number of acidic endosomes in uninfected HTE or HNE cells, as determined by evaluation of the presence of green fluorescence (Figure 3A,C,D,F) and the fluorescence intensity of acidic endosomes (Figure 3G,H) compared with the fluorescence intensity of cells before any treatment.
Figure 3.
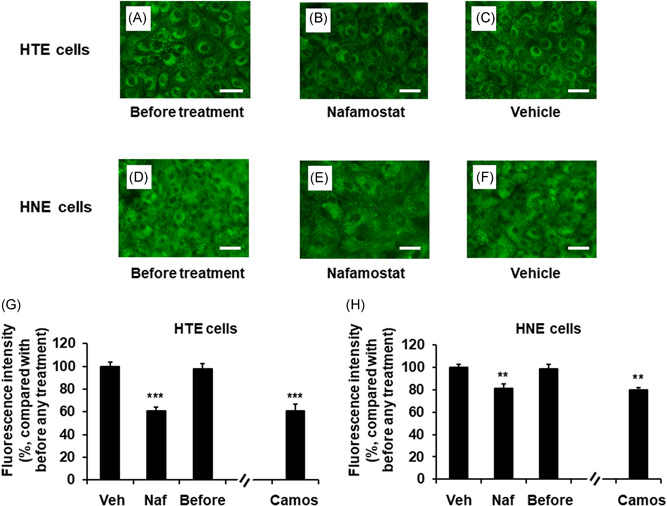
A–F, Changes in the distribution of acidic endosomes exhibiting green fluorescence in uninfected HTE (A–C) or HNE (D–F) cells at 72 h after treatment with Naf (10 μg/ml) (B and E) or Veh (C and F) or untreated cells cultured in medium alone (before treatment) (A and D) (scale bar = 100 μm). G and H, The effects of treatment with Naf (10 μg/ml), Veh, or Camos (10 μg/ml) on the fluorescence intensity of acidic endosomes in HTE cells (G) and HNE cells (H) at 72 h after treatment or in untreated cells (before treatment; before). The results are expressed as the relative intensity (%) compared to the mean intensity value of the vehicle‐treated cells. The results are expressed as the mean ± SEM for seven tracheal or nasal mucosa tissue samples. Camos, camostat; HNE, human nasal; HTE, human tracheal; Naf, nafamostat; Veh, vehicle. Significant differences versus values from the cells treated with Veh are indicated by **p < .01 and ***p < .001
In contrast, treatment with nafamostat (10 μ/ml) reduced the number of acidic endosomes in HTE and HNE cells (Figure 3B,E). Moreover, treatment with nafamostat reduced the fluorescence intensity compared to that in cells treated with vehicle and cells before treatment (Figure 3G,H). Treatment with camostat (10 μ/ml) also reduced the fluorescence intensity of the cells (Figure 3G,H). The potency of the inhibitory effects of nafamostat did not differ from that of camostat.
3.8. Effects of nafamostat on cytokine release
A significant amount of IL‐6 was detected in the supernatants of HTE and HNE cells before viral infection and at 72 h after sham infection (Figure 4A,C). The IL‐6 levels of sham‐infected cells at 72 h did not differ from those measured in cells before infection (Figure 4A,C). In contrast, IL‐6 levels increased after infection with the pandemic influenza virus (Figure 4A,C). The maximum IL‐6 levels were observed 72 h after infection in the supernatants of HTE and HNE cells (data collected at 24 and 120 h not shown). Treatment with nafamostat reduced the IL‐6 concentrations in supernatants after infection (Figure 4A,C).
Figure 4.
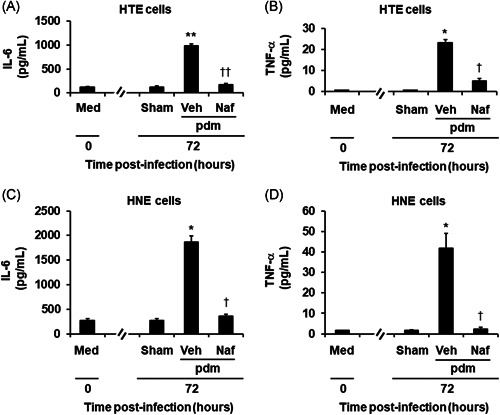
The release of IL‐6 (A, C) and TNF‐α (B, D) into the supernatants of HTE (A, B) or HNE (C, D) cells treated with Naf (10 μg/ml) or Veh collected before (time 0) and between 24 and 72 h after infection with the pdm or after sham infection (Sham). The results are reported as the mean ± SEM for cells from five different subjects. IL‐6, interleukin‐6; pdm, pandemic influenza virus; TNF‐α, tumor necrosis factor‐α; Veh, vehicle. Significant differences versus values from the cells before infection (Med) are indicated by *p < .05 and **p < .01. Significant differences versus values from the cells infected with the pandemic influenza virus alone in the presence of vehicle (Veh) are indicated by † p < .05 and †† p < .01
The concentration of TNF‐α in the supernatants of HTE cells was undetectable (<0.55 pg/ml) before infection and at 72 h after sham infection (Figure 4B). In contrast, a small but significant amount of TNF‐α was detected in the supernatants of HNE cells before viral infection and at 72 h after sham infection (Figure 4D). The TNF‐α levels of sham‐infected HNE cells at 72 h did not differ from those measured in cells before infection (Figure 4D). TNF‐α levels in the supernatants of HTE cells and HNE cells increased after infection with the pandemic influenza virus (Figure 4B,D). Pretreatment with nafamostat reduced the concentrations of TNF‐α in the supernatants at 72 h after infection (Figure 4B,D).
3.9. Effects of nafamostat and camostat on mice
Significant viral titers were detected in lung samples at 72 h after infection with the pandemic [A/Hyogo/YS/2011 (H1N1) pdm09] influenza virus (Figure 5A). Treatment of mice with 30 mg/kg/day nafamostat, camostat or oseltamivir reduced the titers measured by lung sampling (Figure 5A), and the titers in the mice treated with nafamostat were lower than those in the mice treated with oseltamivir (Figure 5A). Treatment with oseltamivir improved the survival rate and bodyweight reduction after infection (Figure 5B,C); however, treatment with nafamostat or camostat did not improve the survival rate or body weight reduction (Figure 5B,C).
Figure 5.
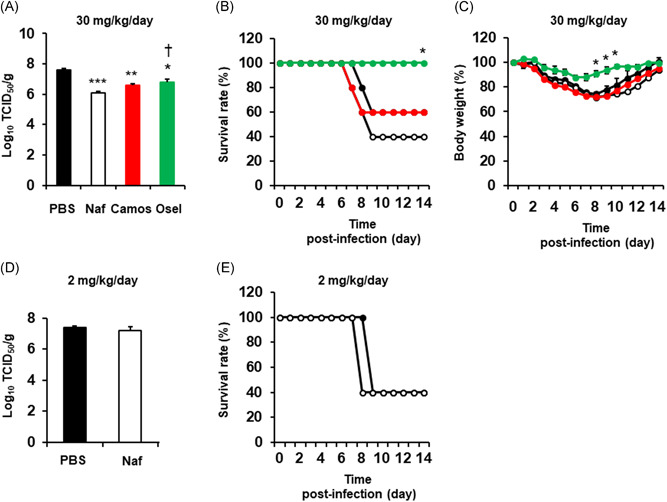
A, The titers of a pandemic [A/Hyogo/YS/2011 (H1N1) pdm09] influenza virus in lung samples collected from mice treated with 30 mg/kg/day nafamostat (Naf), camostat (Camos), oseltamivir (Osel), or vehicle (PBS) at 72 h after infection. The viral titers are expressed as TCID50/g of mouse lung. The results are expressed as the mean ± SEM of five mice. Significant differences versus the vehicle alone group are indicated by *p < .05, **p < .01 and ***p < .001. Significant differences versus the nafamostat group are indicated by † p < .05. B and C, Time courses of the survival rate (B) or body weight (C) of mice treated with 30 mg/kg/day nafamostat (open circles with black line), camostat (closed red circles with red line), oseltamivir (closed green circles with green line), or PBS (closed circles with black line). Five mice were used in each group in the study. The survival rate and body weight at the time of infection were set to 100%. The results are expressed as the relative survival rate or body weight (%) compared to the values at the time of infection. The body weight results are expressed as the mean ± SEM. The body weight in the nafamostat group 9 days and later after infection was expressed as the mean value because the mouse number was reduced due to death. Significant differences versus the PBS alone group are indicated by *p < .05. D, The titers of a pandemic [A/Hyogo/YS/2011 (H1N1) pdm09] influenza virus in lung samples collected from mice treated with 2 mg/kg/day nafamostat (Naf) or PBS at 72 h after infection. The results are expressed as the mean ± SEM of five (Naf) or four (PBS) mice. E, Time course of the survival rate of mice treated with intraperitoneal injection of 2 mg/kg/day nafamostat (open circles with black line) or PBS (closed circles with black line). Five mice were used in the study. The survival rate at the time of infection was set to 100%
Treatment with a decreased amount of nafamostat (2 mg/kg/day) 27 did not reduce the viral titers in lung samples (Figure 5D) or improve the survival rate after infection (Figure 5E).
4. DISCUSSION
In the present study, the serine protease inhibitor nafamostat, which has been used to treat patients with acute pancreatitis and disseminated intravascular coagulation, 17 , 18 reduced the replication of pandemic and seasonal influenza viruses in HTE and HNE cells. Nafamostat reduced the cleavage of an influenza virus precursor protein, HA0, into subunit HA1. The mRNA expression of TMPRSS2, TMPRSS4, and TMPRSS11D in these cells and the TMPRSS2 protein in cell supernatants and cells were detected. These findings suggest that the serine protease inhibitor nafamostat may inhibit the replication of influenza virus in HTE and HNE cells through the inhibition of HA cleavage by host proteases.
Various types of cells, such as HTE, HNE, and Calu‐3 human lung cancer cells, express proteases, including trypsin, furins, TMPRSS2, TMPRSS4, and TMPRSS11D which promote influenza virus replication. 7 , 8 , 9 , 10 , 28 The TMPRSS expression observed in the present study is consistent with previous reports demonstrating that TMPRSS2, TMPRSS4, and/or TMPRSS11D are also expressed in various types of cells, such as those in the human nasal and tracheal mucosae, distal airways, lungs, 29 , 30 , 31 and swine airway epithelium 24 , and the Caco‐2 human colon cancer cell line. 25 Treatment with nafamostat reduced the cleavage of the pandemic influenza virus precursor protein HA0 into the HA1 subunit. In contrast, treatment with nafamostat did not reduce the mRNA levels of these proteases or the protein level of TMPRSS2. These findings suggest that nafamostat may inhibit the replication of influenza viruses by inhibiting protease activity rather than by reducing protease expression.
In the present study, treatment with nafamostat (10 μg/ml) did not reduce the cell viability of attached cells or increase the number of detached cells or LDH levels in supernatants. In addition, treatment of cells with nafamostat did not reduce the TMPRSS2, TMPRSS4, and TMPRSS11D mRNA levels in the cells or the TMPRSS2 protein levels in the cell supernatants. These findings are consistent with results showing that treatment of MDCK cells with nafamostat (10 μg/ml) inhibits influenza virus infection without cytotoxicity 12 and suggest that nafamostat is not cytotoxic toward HTE or HNE cells and that the observed reductions in viral titers result from an effect on protease activity rather than from cytotoxicity.
The maximal plasma concentration of nafamostat is 90 ng/ml after intravenous infusion of 40 mg of nafamostat into human subjects. 32 In the present study, the titers of the pandemic influenza virus were reduced by nafamostat at concentrations of 100 ng/ml and above in HTE cells and 10 ng/ml and above in HNE cells. Thus, nafamostat may reduce the release of the pandemic influenza virus at clinical concentrations.
Treatment of cells with nafamostat reduced the production of IL‐6 and TNF‐α, which are associated with disease symptoms and severity in influenza‐infected patients 21 , 33
and with cell damage. 34 The results of the present study showing that treatment of cells with nafamostat reduced cytokine production are consistent with those of previous studies demonstrating the inhibitory effects of protease inhibitors, including camostat, gabexate, and aprotinin, 16 , 35 , 36 on cytokine production induced by influenza virus infection. These findings suggest that serine protease inhibitors may have anti‐inflammatory effects on the lungs and airways in the context of influenza virus infection.
Acidic endosomes are the organelles through which viruses release their RNPs containing viral RNA into the cytoplasm 37 and H+‐ATPase or Na+/H+exchangers act in the acidification of endosomes. 38 , 39 In the present study, nafamostat reduced the number of acidic endosomes in the cells. Although there have not been any studies demonstrating nafamostat‐induced inhibitory effects on H+‐ATPase or Na+/H+ exchangers, nafamostat acts on acid‐sensing ion channels. 40 Therefore, nafamostat may modulate the function of ion channels that act on the acidification of endosomes and may reduce the number of acidic endosomes. In contrast, in the present study, the potency of the inhibitory effects of nafamostat did not differ from that of camostat. Further studies are required to define the cause of the additive inhibitory effects of nafamostat on viral replication.
We detected TMPRSS2 protein in the supernatants of HTE and HNE cells. The precise mechanisms of the shedding of the membrane protein TMPRSS2 are uncertain; however, Wang et al. reported that the transmembrane serine protease matriptase 41 is stored in secretory granules in the Caco‐2 cells. 42 They also suggested that the secretory granules move to the cell surface, fuse with the plasma membrane and secrete matriptase. Therefore, this pathway may be associated with the shedding of TMPRSS2 from HTE and HNE cells.
In an in vivo study using mice, treatment with intraperitoneal injection of 30 mg/kg/day nafamostat or camostat reduced lung viral titers. These findings are consistent with those of previous reports using mice treated with camostat. 13 Furthermore, the viral titers in mice treated with nafamostat were lower than those in mice treated with oseltamivir.
Thus, treatment with nafamostat reduced mouse lung viral titers but did not improve the survival rate or bodyweight reduction after infection. The reasons for the discrepancy are uncertain; however, metabolic and hematological adverse effects reported in patients treated with nafamostat, including hyperkalemia and eosinophilia, 43 , 44 might be associated with the mechanisms of the discrepant effects on mice treated with 30 mg/kg/day nafamostat in the present study. Further studies are required to confirm the most efficient doses and administration routes of nafamostat, including the nasal and intravenous routes, as shown in studies using zanamivir, peramivir or laninamivir. 26 , 45 , 46 , 47
Nafamostat has been suggested to inhibit SARS‐CoV‐2 replication by inhibiting TMPRSS2‐mediated viral entry and to be a candidate drug to treat COVID‐19 patients. 11 , 48 Therefore, nafamostat may become a candidate drug to treat patients infected with SARS‐CoV‐2 and/or influenza viruses in the winter season.
In conclusion, nafamostat may inhibit influenza virus replication in human airway epithelial cells and mouse lungs and reduce infection‐induced airway inflammation by modulating cytokine production.
CONFLICT OF INTERESTS
This study was partially supported by a Research Grant from the Sendai Medical Center.
AUTHOR CONTRIBUTIONS
Mutsuo Yamaya: the design of the work and the acquisition, analysis and interpretation of data, manuscript writing and revision. Yoshitaka Shimotai: the acquisition and interpretation of data. Ayako Ohkawara, Enkhbold Bazarragchaa: the acquisition of data. Masatoshi Okamatsu, Yoshihiro Sakoda, Hiroshi Kida: design of the work, the analysis and interpretation of data. Hidekazu Nishimura: the design of the work and the analysis and interpretation of data.
ACKNOWLEDGMENTS
We thank Dr. Mitsuru Sugawara for providing human nasal polyps and Dr. Xue Deng and the staff at the Biomedical Research Unit and the Department of Pathology at Tohoku University Hospital for providing technical support.
Yamaya M, Shimotai Y, Ohkawara A, et al. The clinically used serine protease inhibitor nafamostat reduces influenza virus replication and cytokine production in human airway epithelial cells and viral replication in mice. J Med Virol. 2021;93:3484‐3495. 10.1002/jmv.26700
REFERENCES
- 1. Treanor JJ, Hayden FG, Vrooman PS, et al. Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza: a randomized controlled trial. US Oral Neuraminidase Study Group. JAMA. 2000;283:1016‐1024. 10.1001/jama.283.8.1016 [DOI] [PubMed] [Google Scholar]
- 2. Kumar A. Early versus late oseltamivir treatment in severely ill patients with 2009 pandemic influenza A (H1N1): speed is life. J Antimicrob Chemother. 2011;66:959‐963. 10.1093/jac/dkr090 [DOI] [PubMed] [Google Scholar]
- 3. Hayden FG, Sugaya N, Hirotsu N, et al. Baloxavir marboxil for uncomplicated influenza in adults and adolescents. N Engl J Med. 2018;379:913‐923. 10.1056/NEJMoa1716197 [DOI] [PubMed] [Google Scholar]
- 4. Perez‐Padilla R, de la Rosa‐Zamboni D, Ponce de Leon S, et al. INER Working Group on Influenza. Pneumonia and respiratory failure from swine‐origin influenza A (H1N1) in Mexico. N Engl J Med. 2009;361:680‐689. 10.1056/NEJMoa0904252 [DOI] [PubMed] [Google Scholar]
- 5. Gooskens J, Jonges M, Claas EC, Meijer A, van den Broek PJ, Kroes AM. Morbidity and mortality associated with nosocomial transmission of oseltamivir‐resistant influenza A (H1N1) virus. JAMA. 2009;301:1042‐1046. 10.1001/jama.2009.297 [DOI] [PubMed] [Google Scholar]
- 6. Takashita E, Ichikawa M, Morita H, et al. Human‐to human transmission of influenza A(H3N2) virus with reduced susceptibility to baloxavir, Japan, February 2019. Emerg Infect Dis. 2019;25:2108‐2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Klenk HD, Rott R, Orlich M, Blödorn J. Activation of influenza A viruses by trypsin treatment. Virology. 1975;68:426‐439. [DOI] [PubMed] [Google Scholar]
- 8. Böttcher E, Matrosovich T, BeyerleM, Klenk HD, Garten W, Matrosovich M. Proteolytic activation of influenza viruses by serine proteases TMPRSS2 and HAT from human airway epithelium. J Virol. 2006;80:9896‐9898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li F, Ma C, Wang J. Inhibitors targeting the influenza virus hemagglutinin. Curr Med Chem. 2015;22:1361‐1382. 10.2174/0929867322666150227153919 [DOI] [PubMed] [Google Scholar]
- 10. Laporte M, Naesens L. Airway proteases: an emerging drug target for influenza and other respiratory virus infections. Curr Opin Virol. 2017;24:16‐24. 10.1016/j.coviro.2017.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hoffmann M, Kleine‐Weber H, Schroeder S, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271‐280.e8. 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hosoya M, Matsuyama S, Baba M, Suzuki H, Shigeta S. Effects of protease inhibitors on replication of various myxoviruses. Antimicrob Agents Chemother. 1992;36:1432‐1436. 10.1128/aac.36.7.1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lee MG, Kim KH, Park KY, Kim JS. Evaluation of anti‐influenza effects of camostat in mice infected with non‐adapted human influenza viruses. Archiv Virol. 1996;141:1979‐1989. [DOI] [PubMed] [Google Scholar]
- 14. Zhirnov OP, Ikizler MR, Wright P. Cleavage of influenza A virus hemagglutinin in human respiratory epithelium is cell‐associated and sensitive to exogenous antiprotesases. J Virol. 2002;76:8682‐8689. 10.1128/jvi.76.17.8682-8689.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhirnov OP, Klenk HD, Wright PF. Aprotinin and similar protease inhibitors as drugs against influenza. Antiviral Res. 2011;92:27‐36. 10.1016/j.antiviral.2011.07.014 [DOI] [PubMed] [Google Scholar]
- 16. Yamaya M, Shimotai Y, Hatachi Y, et al. The serine protease inhibitor camostat inhibits influenza virus replication and cytokine production in primary cultures of human tracheal epithelial cells. Pulm Pharmacol Ther. 2015;33:66‐74. 10.1016/j.pupt.2015.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yamamoto T, Yamamura H, Yamamoto H, Mizobata Y. Comparison of the efficacy of continuous i.v. infusion versus continuous regional arterial infusion of nafamostat mesylate for severe acute pancreatitis. Acute Med Surg. 2016;3:237‐243. 10.1002/ams2.173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Minakata D, Fujiwara S, Ikeda T, et al. Comparison of gabexate mesilate and nafamostat mesilate for disseminated intravascular coagulation associated with hematologic malignancies. Int J Hematol. 2019;109:141‐146. 10.1007/s12185-018-02567-w [DOI] [PubMed] [Google Scholar]
- 19. Yamaya M, Finkbeiner WE, Chun SY, Widdicombe JH. Differentiated structure and function of cultures from human tracheal epithelium. Am J Physiol. 1992;262:L713‐L724. 10.1152/ajplung.1992.262.6.L713 [DOI] [PubMed] [Google Scholar]
- 20. Lusamba Kalonji N, Nomura K, Kawase T, et al. The non‐antibiotic macrolide EM900 inhibits rhinovirus infection and cytokine production in human airway epithelial cells. Physiol Rep. 2015;3. 10.14814/phy2.12557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Paquette SG, Banner D, Zhao Z, et al. Interleukin‐6 is a potential biomarker for severe pandemic H1N1 influenza A infection. PLOS One. 2012;7:e38214. 10.1371/journal.pone.0038214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Numazaki Y, Oshima T, Ohmi A, et al. A microplate method for isolation of viruses from infants and children with acute respiratory infections. Microbiol Immunol. 1987;31:1085‐1095. 10.1111/j.1348-0421.1987.tb01340.x [DOI] [PubMed] [Google Scholar]
- 23. Lorusso A, Faaberg KS, Killian ML, Koster L, Vincent AL. One‐step real‐time RT‐PCR for pandemic influenza A virus (H1N1) 2009 matrix gene detection in swine samples. J Virol Methods. 2010;164:83‐87. 10.1016/j.jviromet.2009.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Peitsch C, Klenk HD, Garten W, Böttcher‐Friebertshauser E. Activation of influenza A viruses by host proteases from swine airway epithelium. J Virol. 2014;88:282‐291. 10.1128/JVI.01635-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bertram S, Glowacka I, Blazejewska P, et al. Pöhlmann S. TMPRSS2 and TMPRSS4 facilitate trypsin‐independent spread of influenza virus in Caco‐2 cells. J Virol. 2010;84:10016‐10025. 10.1128/JVI.00239-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kobayashi M, Kodama M, Noshi T, et al. Therapeutic efficacy of peramivir against H5N1 highly pathogenic avian influenza viruses harboring the neuraminidase H275Y mutation. Antiviral Res. 2017;139:41‐48. 10.1016/j.antiviral.2016.12.011 [DOI] [PubMed] [Google Scholar]
- 27. Na KR, Choi H, Jeong JY, Lee KW, Chang YK, Choi DE. Nafamostat mesilate attenuates ischemia‐reperfusion‐induced renal injury. Transplant Proc. 2016;48:2192‐2199. 10.1016/j.transproceed.2016.03.050 [DOI] [PubMed] [Google Scholar]
- 28. Ohler A, Becker‐Pauly C. TMPRSS4 is a type II transmembrane serine protease involved in cancer and viral infections. Biol Chem. 2012;393:907‐914. 10.1515/hsz-2012-0155 [DOI] [PubMed] [Google Scholar]
- 29. Yamaoka K, Masuda K, Ogawa H, Takagi K, Umemoto N, Yasuoka S. Cloning and characterization of the cDNA for human airway trypsin‐like protease. J Biol Chem. 1998;273:11895‐11901. 10.1074/jbc.273.19.11895 [DOI] [PubMed] [Google Scholar]
- 30. Lin B, Ferguson C, White JT, et al. Prostate‐localized and androgen‐regulated expression of the membrane‐bound serine protease TMPRSS2. Cancer Res. 1999;59:4180‐4184. [PubMed] [Google Scholar]
- 31. Donaldson SH, Hirsh A, Li DC, et al. Regulation of the epithelial sodium channel by serine proteases in human airways. J Biol Chem. 2002;277:8338‐8345. 10.1074/jbc.M105044200 [DOI] [PubMed] [Google Scholar]
- 32. Abe T, Kinoshita T, Matsuda J, et al. Phase I study of FUT‐175—single and multiple dose study. Jpn Pharmacol Ther. 1984;12:4941‐4964. [Google Scholar]
- 33. Hayden FG, Fritz R, Lobo MC, Alvord W, Strober W, Straus SE. Local and systemic cytokine responses during experimental human influenza A virus infection. Relation to symptom formation and host defense. J Clin Invest. 1998;101:643‐649. 10.1172/JCI1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yamaya M, Lusamba N, Ota C, et al. Magnitude of influenza virus replication and cell damage is associated with interleukin‐6 production in primary cultures of human tracheal epithelium. Respir Physiol Neurobiol. 2014;202:16‐23. 10.1016/j.resp.2014.07.010 [DOI] [PubMed] [Google Scholar]
- 35. Kosai K, Seki M, Yanagihara K, et al. Gabexate mesilate suppresses influenza pneumonia in mice through inhibition of cytokines. J Internat Med Res. 2008;36:322‐328. 10.1177/147323000803600215 [DOI] [PubMed] [Google Scholar]
- 36. Tumurkhuu G, Koide N, Hiwasa T, et al. ONO 3403, a synthetic serine protease inhibitor, inhibits lipopolysaccharide‐induced tumor necrosis factor‐α and nitric oxide production and protects mice from lethal endotoxic shock. Innate Immun. 2011;17:97‐105. 10.1177/1753425909353641 [DOI] [PubMed] [Google Scholar]
- 37. Sieczkarski SB, Brown HA, Whittaker GR. Role of protein kinase C βII in influenza virus entry via late endosomes. J Virol. 2003;77:460‐469. 10.1128/jvi.77.1.460-469.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mellman I, Fuchs R, Helenius A. Acidification of the endocytic and exocytic pathways. Ann Rev Biochem. 1986;55:663‐700. 10.1146/annurev.bi.55.070186.003311 [DOI] [PubMed] [Google Scholar]
- 39. Nass R, Rao R. Novel localization of a Na+/H+ exchanger in a late endosomal compartment of yeast. Implications for vacuole biogenesis. J Biol Chem. 1998;273:21054‐21060. 10.1074/jbc.273.33.21054 [DOI] [PubMed] [Google Scholar]
- 40. Liu S, Cheng XY, Wang F, Liu CF. Acid‐sensing ion channels: potential therapeutic targets for neurologic diseases. Transl Neurodegener. 2015;4:10. 10.1186/s40035-015-0031-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Beaulieu A, Gravel É, Cloutier A, et al. Matriptase proteolytically activates influenza virus and promotes multicycle replication in the human airway epithelium. J Virol. 2013;87:4237‐4251. 10.1128/JVI.03005-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang JK, Lee MS, Tseng IC, et al. Polarized epithelial cells secrete matriptase as a consequence of zymogen activation and HAI‐1‐mediated inhibition. Am J Physiol. 2009;297:C450‐C470. 10.1152/ajpcell.00201.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kitagawa H, Chang H, Fujita T. Hyperkalemia due to nafamostat mesylate. N Engl J Med. 1995;332:687. 10.1056/NEJM199503093321018 [DOI] [PubMed] [Google Scholar]
- 44. Nakanishi K, Kaneko T, Yano F, et al. Marked eosinophilia induced by nafamostat mesilate, an anticoagulant in a hemodialysis patient. Nephron. 1992;62:97‐99. 10.1159/000187004 [DOI] [PubMed] [Google Scholar]
- 45. Ryan DM, Ticehurst J, Dempsey MH, Penn CR. Inhibition of influenza replication by GG167 (4‐guanidino‐2,4‐dideoxy‐2,3‐dehydro‐N‐acetylneuraminic acid) is consistent with extracellular activity of viral neuraminidase. Antimicrob Agents Chemother. 1994;38:2270‐2275. 10.1128/aac.38.10.2270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kubo S, Tomozawa T, Kakuta M, Tokumitsu A, Yamashita M. Laninamivir prodrug CS‐8958, a long‐acting neuraminidase inhibitor, shows superior anti‐influenza virus activity after a single administration. Antimicrob Agents Chemother. 2010;54:1256‐1264. 10.1128/AAC.01311-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zablockinenė B, Kačergius T, Ambrozaitis A, et al. Zanamivir diminishes lung damage in influenza A virus‐infected mice by inhibiting nitric oxide production. In Vivo. 2018;32:473‐478. 10.21873/invivo.11263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yamaya M, Nishimura H, Deng X, Kikuchi A, Nagatomi R. Protease inhibitors: candidate drugs to inhibit severe acute respiratory syndrome coronavirus 2 replication. Tohoku J Exp Med. 2020;251:27‐30. 10.1620/tjem.251.27 [DOI] [PubMed] [Google Scholar]


