Abstract
The COVID‐19 pandemic caused by SARS‐CoV‐2 is a deadly disease afflicting millions. The pandemic continues affecting population due to nonavailability of drugs and vaccines. The pathogenesis and complications of infection mainly involve hyperimmune‐inflammatory responses. Thus, therapeutic strategies rely on repurposing of drugs aimed at reducing infectivity and inflammation and modulate immunity favourably. Among, numerous therapeutic targets, the endocannabinoid system, particularly activation of cannabinoid type‐2 receptors (CB2R) emerged as an important one to suppress the hyperimmune‐inflammatory responses. Recently, potent antiinflammatory, antiviral and immunomodulatory properties of CB2R selective ligands of endogenous, plant, and synthetic origin were showed mediating CB2R selective functional agonism. CB2R activation appears to regulate numerous signaling pathways to control immune‐inflammatory mediators including cytokines, chemokines, adhesion molecules, prostanoids, and eicosanoids. Many CB2R ligands also exhibit off‐target effects mediating activation of PPARs, opioids, and TRPV, suggestive of adjuvant use with existing drugs that may maximize efficacy synergistically and minimize therapeutic doses to limit adverse/ side effects. We hypothesize that CB2R agonists, due to immunomodulatory, antiinflammatory, and antiviral properties may show activity against COVID‐19. Based on the organoprotective potential, relative safety, lack of psychotropic effects, and druggable properties, CB2R selective ligands might make available promising candidates for further investigation.
Keywords: cannabinoids, COVID‐19, immunomodulators, inflammation
1. INTRODUCTION
The pathogenesis and complications of COVID‐19 infection mainly involve immune‐inflammatory cascade. Therapeutic strategies currently rely on the repurposing of antivirals and immunomodulators to reduce infectivity and inflammation, and favorably modulate the immune system (Wu et al., 2020). In principle, immune responses and resultant inflammatory processes work simultaneously to abolition of viral infections, but this may significantly influence pathogenesis of viral infections, similar believed to take place in infections with SARS‐CoV‐2 and contribute to the clinical spectrum of COVID‐19 (Song et al., 2020).
Identifying candidate drugs to ameliorate infectivity, severity, mortality, and improve the prognosis, are needed, given the rapid emergence of COVID‐19 (Altay et al., 2020). Use of antiviral agents alone are insufficient for preventing the cytokine storm and related complications in critically ill patients. Immune dysregulation with hyperinflammatory conditions lead to complications, worsening, and poor prognosis rather than viremia (Channappanavar et al., 2016). In patients with COVID‐19, immunomodulators are regarded as “subetiological treatment” in the absence of effective antiviral drugs. Therefore, along with repurposing old drugs, novel candidate drugs that target hyperimmune‐inflammatory mechanisms are urgently needed to attenuate the cytokine storm, limit severity, and improve survival and prognosis (Wu et al., 2020).
The endocannabinoid system (ECS), a physiologically important, pharmacologically relevant, pharmaceutically feasible, and clinically successful target can regulate innate and adaptive immunity, inflammatory responses, pain, viral pathogenesis, and oxidative stress (Tay, Poh, Rénia, MacAry, & Ng, 2020). ECS typically consists of two receptors, cannabinoid type 1 and type 2 (CB1R and CB2R). Endogenous ligands (endocannabinoids) and metabolic enzymes mediating CB1R and CB2R participate in a variety of diseases by modulating immune‐inflammatory states (Oláh, Szekanecz, & Bíró, 2017). CB2R, a G protein‐coupled receptor receives attention for therapeutic targeting due to its major presence and role in organs including spleen, tonsils, thymus, and the lymphatic system and immune cells such as B lymphocytes, macrophages, mast cells, natural killer cells, microglia, dendritic cells. Ligands that activate CB2R, pharmacologically termed CB2 agonists have received enormous interest in recent years due to lack of psychotropic effects upon activation, along with its potent immunomodulatory, antiinflammatory, antioxidant activities, and potential benefits in targeting viral‐mediated immune‐inflammatory pathogenesis (Stasiulewicz, Znajdek, Grudzień, Pawiński, & Sulkowska, 2020; van Niekerk, Mabin, & Engelbrecht, 2019). Numerous cannabinoid ligands showing CB2 full functional agonism along with high affinity and selectivity toward CB2R, are classified into classical, nonclassical, aminoalkylindole, and eicosanoids and include GW842166X, CP‐55,940, S‐777469, JTE‐907, JBT‐101, WIN‐55,212‐2, JWH015, AM1241, HU308, HU‐910, AM1710, AZD1940, and MDA7 (Morales, Goya, & Jagerovic, 2018). L‐759633, L‐759656, and JWH‐133, are all structural analogs of Δ9‐THC. Other notable examples include the nonclassical cannabinoid, HU‐308, and the aminoalkylindole, AM1241 both of which have been well studied (Huffman et al., 2010). CB2R agonists also showed organoprotective effects including cardioprotective, hepatoprotective, neuroprotective, nephroprotective, anticonvulsive, and antipsychotic mediating selective activation of CB2R.
Given the pharmacological effects, molecular mechanisms and therapeutic potential of CB2R agonists in past few years, we hypothesize that CB2R agonists due to its notable immunomodulatory, antiinflammatory, and antiviral properties could be possibly novel candidates for further investigation against COVID‐19. Our proposition is to scientifically envisage the perspective and prospect of CB2R agonists for therapeutic evaluation to curb the severity, progression, complications, and prognosis targeting infection, immunity, and inflammation in COVID‐19. CB2R, mainly expressed in immune cells, participate in the inflammatory process by regulating proinflammatory mediators, including cytokines, chemokines, adhesion molecules, and the polarization of macrophages. The latter is a key regulator of proinflammatory (M1)/antiinflammatory (M2) pathways.
CB2R primarily couples to Gi upon activation resulting in inhibition of an adenylyl cyclase agonist and further activates 5′‐AMP‐activated protein kinase (AMPK) pathways. These actions result in reduced anabolic reactions, and in turn, promote oxidative phosphorylation and exert antiinflammatory effects (Stasiulewicz et al., 2020; van Niekerk et al., 2019). Macrophages present in human lung expresses CB2R, and upon activation, significantly inhibits the onset of cytokine storm by suppressing proinflammatory cytokines, chemokines, growth factors, and adhesion molecules (Staiano et al., 2016). Activation of CB2R produces antiinflammation by inhibiting recruitment of leukocytes, reducing synthesis and release of chemokines, adhesion molecules, prostanoids, eicosanoids, reactive oxygen species, and proinflammatory cytokines (Stasiulewicz et al., 2020). Proinflammatory cytokine expression, including IL‐1β, TNF‐α, and particularly a massive rise in IL‐6, reflect the severity of pathology, prognosis, and mortality in acute lung injury associated with COVID‐19 infection. Inhibition of IL‐6 production mitigates acute lung injuries (Bohn et al., 2020). Further, activation of inflammasomes are also triggers of the cytokine storm and participate in clinical and pathological manifestations of COVID‐19 (Tay et al., 2020).
Additionally, CB2 gene (CNR2) polymorphism shown to play a role in the immunopathogenesis associated with severe necroinflammation in patients with respiratory syncytial virus (Tahamtan et al., 2018), chronic hepatitis C (Coppola et al., 2014), childhood immune thrombocytopenic purpura (Rossi et al., 2011), and neuroinflammation in HIV/HCV co‐infected patients (Sagnelli et al., 2017). CB2R knockout mice showed increased susceptibility and vulnerability to influenza infection demonstrated the importance of CB2R in immunoregulation in respiratory viral infections (Kapellos et al., 2019). The activation of CB2R is reported to suppress lung pathology in infants infected with acute respiratory syncytial virus by reducing cytokines and chemokines (Tahamtan et al., 2018). In HIV patients, CB2R activation suppresses infectivity, transmission and replication of the virus in monocytes and macrophages (Ramirez et al., 2013).
CB2R activation shows protection against acute lung injury, drug‐induced lung injuries, airway hyperresponsiveness, cough centers, pulmonary inflammation, and fibrosis, by correcting lung permeability, leukocyte trafficking, and preserving tight junctions (Pacher & Mechoulam, 2011). Further, at the doses at which cannabinoids produce bronchodilation, CB2R activation did not elicit central respiratory depression. Some patients that recover from COVID‐19 are reported to develop persistent lung dysfunction and fibrosis, initiated by microinjury, inflammation, and fibroblast activation (Bohn et al., 2020). CB2R activation may prevent lung fibrosis via antiinflammatory and antifibrogenic activity.
Extrapulmonary manifestations of COVID‐19 also include cardiac injury in patients with critical illness, and patients with preexisting cardiovascular disease involving systemic infection and inflammation leads to acute thrombosis (Capone et al., 2020). CB2R activation shows vasodilatory, positive inotropic and cardioprotective effects in acute myocardial injury, drug‐induced cardiotoxicity, and cardiac fibrosis. CB2R activation showed cardioprotective effects by inhibiting inflammasomes, downregulating RIP1/RIP3/MLKL‐mediated necroptosis, inhibition of hypertrophy through AMPK‐eNOS signaling, increasing ERK1/2 phosphorylation and inhibiting MPTP opening, suppressing the Na+/Ca2+ exchanger and upregulating integrin CD18/CD11b (Mac‐1) on human neutrophils in TNF‐α‐induced chemotaxis (Pacher & Mechoulam, 2011).
Cardiovascular complications in patients with COVID‐19 are aggravated further by the high incidence of venous and arterial thrombosis and coagulopathy involving platelet activation. Further issues include the formation of platelet‐monocyte aggregates, complement activation, increase in lipoproteins, endothelial dysfunction, stasis, hypoxia, and overexpression of tissue factors following the cytokine storm, also referred to as capillary leak syndrome in reference to thrombosis (Bohn et al., 2020; Tay et al., 2020). CB2R expressed in hematopoietic and endothelial cells upon activation attenuates inflammatory responses including endothelial activation, immune cell adhesion, and migration and protected against inflammation by increasing transendothelial resistance, tight junction, and inhibiting inflammatory mediators including adhesion molecules (Pacher & Mechoulam, 2011).
Liver injury/dysfunction in COVID‐19 patients is a common complication due to the virus itself or other concurrent complications, such as hepatotoxicity of drugs, mainly antipyretics or immunomodulators, currently used in COVID‐19 management. Preexisting chronic liver disease is an indicator of poorer prognosis (Feng et al., 2020). CB2 gene polymorphism with indices of liver damage in obese children suggests a hepatoprotective role of CB2R (Rossi et al., 2011). CB2R activation ameliorated portal hypertension, the severity of portosystemic collaterals and mesenteric angiogenesis, intrahepatic angiogenesis, and fibrosis in cirrhotic rats. CB2R activation was showed hepatoprotective against acute liver injury or failure, septic liver, liver cirrhosis, and hepatic ischemia–reperfusion injury, fibrosis, steatosis as well as ascites and peritonitis.
Acute kidney injury includes direct viral‐induced tubular and glomerular injury, sepsis‐associated injury, and thrombotic disease (Farouk, Fiaccadori, Cravedi, & Campbell, 2020). CB2R activation salvages kidneys in acute renal injury by inhibiting proinflammatory cytokines, chemokines, and apoptosis. Intestinal inflammation and diarrhea also, occur as complications in COVID‐19 patients. A reduction of mucosal ACE2 following virus entry, resulting in altered elevated angiotensin levels, increased TNF‐α and tryptophan deficiency (Taxonera et al., 2020). CB2R activation showed to correct motility impairment, intestinal secretion and integrity, neurogenic intestinal inflammation, intestinal I/R injury and attenuation of intestinal inflammation by enhancing apoptosis in activated T cells, decreasing activated T cells and inhibiting induction of mast cells, NK cells, and neutrophils at sites of inflammation. CB2R activation is suggested for diarrhea‐predominant inflammatory bowel (Pacher & Mechoulam, 2011).
Additionally, COVID‐19 has a significant impact on mental health and may adversely impact immune functioning (Rajkumar, 2020). Psychosocial issues such as stress, anxiety, and depression are believed to increase susceptibility to viral upper respiratory infections (Pedersen, Zachariae, & Bovbjerg, 2010). Psychological distress is also linked with immune‐inflammatory responses and suggests that psycho‐neuroimmunity axis can be therapeutically important in COVID‐19 infection. Further, stress exposure causes excitotoxicity and neuroinflammation that contribute to stress‐related neuropathology, such as depression (Cristino, Bisogno, & Di Marzo, 2020). CB2R activation has been shown beneficial in relieving stress, anxiety, and depression and post‐stroke depression (Cristino et al., 2020).
Stroke is reported as a complication in COVID‐19 patients as a common accompaniment with atherosclerosis, hypertension, and atrial fibrillation (Bohn et al., 2020; Tay et al., 2020). CB2R activation showed to ameliorate neuroinflammation, brain edema, neuronal degeneration, microglial accumulation, and phosphorylated extracellular signal‐regulated kinase (p‐ERK) proteins. Protection of the blood–brain barrier by reducing extravasation, MMP‐9 and MMP‐12 activities and number of microglia is also shown with CB2R activation (Cristino et al., 2020). The antiinflammatory action of CB2R activation is involved in interactions with the cholinergic system and upregulation of serotonergic receptor, 5‐HT2A. Additionally, CB2R activation exhibits antihyperalgesic and antinociceptive effect in neuropathic pain induced by retrovirus infections by suppressing neuroinflammation, macrophage activation, and T‐cell infiltration via blocking JAK/STAT3 pathway (Cristino et al., 2020).
Uncontrolled infection and increased inflammatory mediators may cause systemic inflammatory responses and sepsis (Bohn et al., 2020). CB2R activation exerts organoprotective effects by suppressing inflammatory states and sepsis. Activation of CB2R also attenuates oxidative stress in liver, lungs, heart, kidney, intestine, and brain and by inhibiting inflammatory cell recruitment, proinflammatory cytokines, and increasing antiinflammatory cytokines (He et al., 2019). CB2R agonists show the ability to suppress redox‐inflammatory states, improve antioxidant power and favorably regulate immune system. Agonist activity on CB2R involves modulation of umerous redox immune‐inflammatory signaling pathways mainly, toll‐like receptors, opioid receptors, SIRT1/PGC‐1α, AMPK/CREB, MAPK/ERK, Nrf2/Keap1/HO‐1, and peroxisome proliferator‐activated receptors (PPARs). Many cannabinoid agonists also elicit off‐target effects by activating the PPARs family, including PPAR‐γ that inhibits inflammatory responses (O'Sullivan, 2016), enhance host responses against respiratory viral infections and inhibit replication of numerous viruses. Such pathogens include human immunodeficiency virus, respiratory syncytial virus, influenza A, hepatitis B, and hepatitis C (Bassaganya‐Riera, Song, Roberts, & Hontecillas, 2010; Du, Ma, Liu, Yan, & Tang, 2017; Skolnik, Rabbi, Mathys, & Greenberg, 2002). These off target effects of CB2R activation could be additionally effective in ameliorating pulmonary inflammation via inhibiting the generation of proinflammatory cytokines, collagen secretion, apoptosis of alveolar type II epithelial cells, and promoting surfactant‐associated protein A expression (Huang et al., 2019). The beneficial effects of CB2R activation and associated off‐target effects in models of endotoxemia, sepsis, allergic airway inflammation and acute lung injury, and acute respiratory distress syndrome suggest that cannabinoid ligands targeting CB2R may be novel candidates targeting the trinity of COVID‐19; infection, immunity, and inflammation (Figure 1).
FIGURE 1.
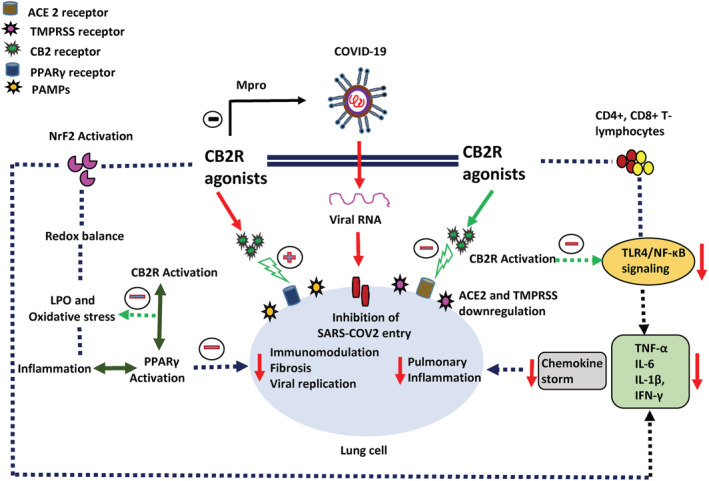
The proposed possible mechanisms andpotential of CBR2 agonists in SARS‐CoV2 infection
Much information presented is solely based on reports from previously published studies on immunomodulatory, anti‐inflammatory, and antimicrobial potential of CB2R agonists. Synthesis of CB2R ligands and preclinical and clinical studies for their efficacy are promising in numerous related pathogenesis, thus the CB2R selective ligands can be evaluated in experimental models of COVID‐19. However, a reliable preclinical SARS‐CoV‐2‐infected animal model is not available for preclinical evaluations. The potent pleiotropic properties of CB2R ligands could be leveraged as effective compounds in supportive treatment of COVID‐19 infection.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
ACKNOWLEDGMENTS
The authors are grateful for providing research facilities and research grant supports from the United Arab Emirates University, Al Ain, UAE.
DATA AVAILABILITY STATEMENT
The present manuscript is a perspective/commentary based on the published reports. The information and data presented in this manuscript are appropriately cited in the manuscript.
REFERENCES
- Altay, O. , Mohammadi, E. , Lam, S. , Turkez, H. , Boren, J. , Nielsen, J. , … Mardinoglu, A. (2020). Current Status of COVID‐19 Therapies and Drug Repositioning Applications. iScience, 23(7), 101303. 10.1016/j.isci.2020.101303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassaganya‐Riera, J. , Song, R. , Roberts, P. C. , & Hontecillas, R. (2010). PPAR‐gamma activation as an anti‐inflammatory therapy for respiratory virus infections. Viral Immunology, 23(4), 343–352. 10.1089/vim.2010.0016 [DOI] [PubMed] [Google Scholar]
- Bohn, M. K. , Hall, A. , Sepiashvili, L. , Jung, B. , Steele, S. , & Adeli, K. (2020). Pathophysiology of COVID‐19: Mechanisms underlying disease severity and progression. Physiology (Bethesda, MD.), 35(5), 288–301. 10.1152/physiol.00019.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capone, V. , Cuomo, V. , Esposito, R. , Canonico, M. E. , Ilardi, F. , Prastaro, M. , … Santoro, C. (2020). Epidemiology, prognosis and clinical manifestation of cardiovascular disease in COVID‐19. Expert Review of Cardiovascular Therapy, 8, 531–539. 10.1080/14779072.2020.1797491 [DOI] [PubMed] [Google Scholar]
- Channappanavar, R. , Fehr, A. R. , Vijay, R. , Mack, M. , Zhao, J. , Meyerholz, D. K. , & Perlman, S. (2016). Dysregulated type I interferon and inflammatory monocyte‐macrophage responses cause lethal pneumonia in SARS‐CoV‐infected mice. Cell Host & Microbe, 19(2), 181–193. 10.1016/j.chom.2016.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppola, N. , Zampino, R. , Bellini, G. , Macera, M. , Marrone, A. , Pisaturo, M. , … Rossi, F. (2014). Association between a polymorphism in cannabinoid receptor 2 and severe necroinflammation in patients with chronic hepatitis C. Clinical Gastroenterology and Hepatology, 12(2), 334–340. 10.1016/j.cgh.2013.05.008 [DOI] [PubMed] [Google Scholar]
- Cristino, L. , Bisogno, T. , & Di Marzo, V. (2020). Cannabinoids and the expanded endocannabinoid system in neurological disorders. Nature reviews. Neurology, 16(1), 9–29. 10.1038/s41582-019-0284-z [DOI] [PubMed] [Google Scholar]
- Du, L. , Ma, Y. , Liu, M. , Yan, L. , & Tang, H. (2017). Peroxisome proliferators activated receptor (PPAR) agonists activate hepatitis B virus replication in vivo. Virology Journal, 14(1), 96. 10.1186/s12985-017-0765-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farouk, S. S. , Fiaccadori, E. , Cravedi, P. , & Campbell, K. N. (2020). COVID‐19 and the kidney: What we think we know so far and what we don't. Journal of Nephrology, 1–6. 10.1007/s40620-020-00789-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng, G. , Zheng, K. I. , Yan, Q.‐Q. , Rios, R. S. , Targher, G. , Byrne, C. D. , … Zheng, M.‐H. (2020). COVID‐19 and liver dysfunction: Current insights and emergent therapeutic strategies. Journal of Clinical and Translational Hepatology, 8(1), 1–7. 10.14218/JCTH.2020.00018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, Q. , Xiao, F. , Yuan, Q. , Zhang, J. , Zhan, J. , & Zhang, Z. (2019). Cannabinoid receptor 2: A potential novel therapeutic target for sepsis? Acta Clinica Belgica, 74(2), 70–74. 10.1080/17843286.2018.1461754 [DOI] [PubMed] [Google Scholar]
- Huang, S. , Zhu, B. , Cheon, I. S. , Goplen, N. P. , Jiang, L. , Zhang, R. , … Sun, J. (2019). PPAR‐γ in macrophages limits pulmonary inflammation and promotes host recovery following respiratory viral infection. Journal of Virology, 93(9), e00030–19. 10.1128/JVI.00030-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffman, J. W. , Hepburn, S. A. , Lyutenko, N. , Thompson, A. L. S. , Wiley, J. L. , Selley, D. E. , & Martin, B. R. (2010). 1‐Bromo‐3‐(1′,1′‐dimethylalkyl)‐1‐deoxy‐Δ(8)‐tetrahydrocannabinols: New selective ligands for the cannabinoid CB(2) receptor. Bioorganic & Medicinal Chemistry, 18(22), 7809–7815. 10.1016/j.bmc.2010.09.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapellos, T. S. , Taylor, L. , Feuerborn, A. , Valaris, S. , Hussain, M. T. , Rainger, G. E. , … Iqbal, A. J. (2019). Cannabinoid receptor 2 deficiency exacerbates inflammation and neutrophil recruitment. FASEB Journal, 33(5), 6154–6167. 10.1096/fj.201802524R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales, P. , Goya, P. , & Jagerovic, N. (2018). Emerging strategies targeting CB 2 cannabinoid receptor: Biased agonism and allosterism. Biochemical Pharmacology, 157, 8–17. 10.1016/j.bcp.2018.07.031 [DOI] [PubMed] [Google Scholar]
- Oláh, A. , Szekanecz, Z. , & Bíró, T. (2017). Targeting cannabinoid signaling in the immune system: “High”‐ly exciting questions, possibilities, and challenges. Frontiers in Immunology, 8, 1487. 10.3389/fimmu.2017.01487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan, S. E. (2016). An update on PPAR activation by cannabinoids. British Journal of Pharmacology, 173(12), 1899–1910. 10.1111/bph.13497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacher, P. , & Mechoulam, R. (2011). Is lipid signaling through cannabinoid 2 receptors part of a protective system? Progress in Lipid Research, 50(2), 193–211. 10.1016/j.plipres.2011.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen, A. , Zachariae, R. , & Bovbjerg, D. H. (2010). Influence of psychological stress on upper respiratory infection—a meta‐analysis of prospective studies. Psychosomatic Medicine, 72(8), 823–832. 10.1097/PSY.0b013e3181f1d003 [DOI] [PubMed] [Google Scholar]
- Rajkumar, R. P. (2020). COVID‐19 and mental health: A review of the existing literature. Asian Journal of Psychiatry, 52, 102066. 10.1016/j.ajp.2020.102066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez, S. H. , Reichenbach, N. L. , Fan, S. , Rom, S. , Merkel, S. F. , Wang, X. , … Persidsky, Y. (2013). Attenuation of HIV‐1 replication in macrophages by cannabinoid receptor 2 agonists. Journal of Leukocyte Biology, 93(5), 801–810. 10.1189/jlb.1012523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi, F. , Bellini, G. , Nobili, B. , Maione, S. , Perrone, L. , & del Giudice, E. M. (2011). Association of the cannabinoid receptor 2 (CB2) Gln63Arg polymorphism with indices of liver damage in obese children: An alternative way to the CB2 hepatoprotective properties. Hepatology (Baltimore, MD.), 54(3), 1102. 10.1002/hep.24440 [DOI] [PubMed] [Google Scholar]
- Rossi, F. , Mancusi, S. , Bellini, G. , Roberti, D. , Punzo, F. , Vetrella, S. , … Perrotta, S. (2011). CNR2 functional variant (Q63R) influences childhood immune thrombocytopenic purpura. Haematologica, 96(12), 1883–1885. 10.3324/haematol.2011.045732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagnelli, C. , Uberti‐Foppa, C. , Hasson, H. , Bellini, G. , Minichini, C. , Salpietro, S. , … Rossi, F. (2017). Cannabinoid receptor 2‐63 RR variant is independently associated with severe necroinflammation in HIV/HCV coinfected patients. PloS One, 12(7), e0181890. 10.1371/journal.pone.0181890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skolnik, P. R. , Rabbi, M. F. , Mathys, J.‐M. , & Greenberg, A. S. (2002). Stimulation of peroxisome proliferator‐activated receptors alpha and gamma blocks HIV‐1 replication and TNFalpha production in acutely infected primary blood cells, chronically infected U1 cells, and alveolar macrophages from HIV‐infected subjects. Journal of Acquired Immune Deficiency Syndromes (1999), 31(1), 1–10. 10.1097/00126334-200209010-00001 [DOI] [PubMed] [Google Scholar]
- Song, J.‐W. , Zhang, C. , Fan, X. , Meng, F.‐P. , Xu, Z. , Xia, P. , … Zhang, J.‐Y. (2020). Immunological and inflammatory profiles in mild and severe cases of COVID‐19. Nature Communications, 11(1), 3410. 10.1038/s41467-020-17240-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staiano, R. I. , Loffredo, S. , Borriello, F. , Iannotti, F. A. , Piscitelli, F. , Orlando, P. , … Marone, G. (2016). Human lung‐resident macrophages express CB1 and CB2 receptors whose activation inhibits the release of angiogenic and lymphangiogenic factors. Journal of Leukocyte Biology, 99(4), 531–540. 10.1189/jlb.3HI1214-584R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stasiulewicz, A. , Znajdek, K. , Grudzień, M. , Pawiński, T. , & Sulkowska, J. I. (2020). A guide to targeting the endocannabinoid system in drug design. International Journal of Molecular Sciences, 21(8), 2778. 10.3390/ijms21082778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahamtan, A. , Samieipoor, Y. , Nayeri, F. S. , Rahbarimanesh, A. A. , Izadi, A. , Rashidi‐Nezhad, A. , … Salimi, V. (2018). Effects of cannabinoid receptor type 2 in respiratory syncytial virus infection in human subjects and mice. Virulence, 9(1), 217–230. 10.1080/21505594.2017.1389369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taxonera, C. , Sagastagoitia, I. , Alba, C. , Mañas, N. , Olivares, D. , & Rey, E. (2020). Letter: Intestinal inflammation, COVID‐19 and gastrointestinal ACE2‐exploring RAS inhibitors. Alimentary Pharmacology & Therapeutics, 52(3), 569–570. 10.1111/apt.15814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay, M. Z. , Poh, C. M. , Rénia, L. , MacAry, P. A. , & Ng, L. F. P. (2020). The trinity of COVID‐19: Immunity, inflammation and intervention. Nature Reviews Immunology, 20(6), 363–374. 10.1038/s41577-020-0311-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Niekerk, G. , Mabin, T. , & Engelbrecht, A.‐M. (2019). Anti‐inflammatory mechanisms of cannabinoids: An immunometabolic perspective. Inflammopharmacology, 27(1), 39–46. 10.1007/s10787-018-00560-7 [DOI] [PubMed] [Google Scholar]
- Wu, R. , Wang, L. , Kuo, H.‐C. D. , Shannar, A. , Peter, R. , Chou, P. J. , … Kong, A.‐N. (2020). An update on current therapeutic drugs treating COVID‐19. Current Pharmacology Reports, 6(3), 56–70. 10.1007/s40495-020-00216-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The present manuscript is a perspective/commentary based on the published reports. The information and data presented in this manuscript are appropriately cited in the manuscript.


