We present the case of a 61‐year‐old woman who attended the Emergency Department with fever, tachypnea, abdominal discomfort, pain, and coldness in the right lower leg suggestive of ischemia. Her medical history included hypertension treated with losartan and depression, with a recent history of cognitive impairment.
Physical examination showed punctiform purpura on the arms and lower legs (Fig. 1a). Peripheral pulses in the right leg were absent. Oxygen saturation (SaO2) was 98% in air, and the blood pressure was 70/56 mmHg.
Figure 1.
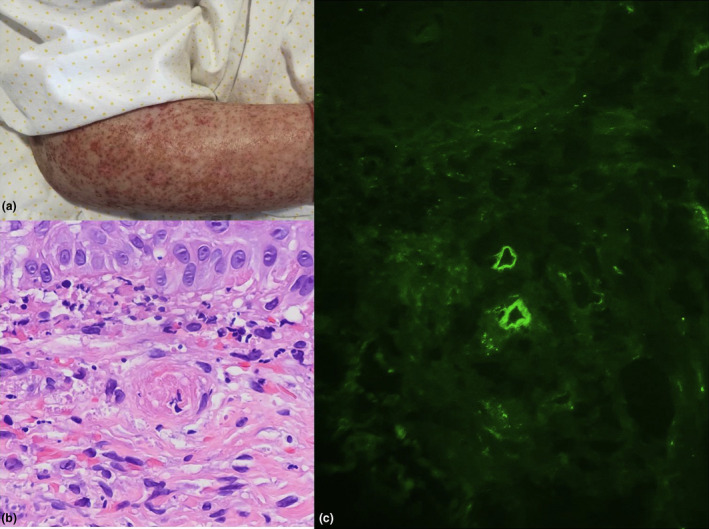
(a) Punctiform purpura on the arms. (b) A capillary with signs of thrombotic microangiopathy (small intraluminal fibrin‐hematic thrombus) is seen in the center of the image. (H&E, ×400). (c) C3 deposits in the superficial dermal capillary plexus. (immunofluorescence ×200)
Laboratory values included hemoglobin 8.1 g/dl (12–16.5);14,600 white blood cells/μl (4,000–11,500); creatinine 3 mg/dl (0.43–0.96); D‐dimer level 5,977 ng/ml (220–500); LDH 281 U per liter (120–246); elevated C reactive protein 279 mg/l; and troponin I 128 ng/l (3–58). The platelet count as well as prothrombin and activated partial thromboplastin times were all normal. Doppler ultrasound showed complete occlusion of the right common femoral artery, requiring supracondylar amputation.
A skin biopsy of purpuric lesion revealed small intraluminal fibrin‐hematic thrombi (Fig. 1b). Direct immunofluorescence showed C3 deposition in the wall of the superficial dermal capillary plexus (Fig. 1c). Autoantibody studies showed a positive direct Coombs test, and direct antibody testing was positive for C3. Anticardiolipin, anti–β2‐glycoprotein antibodies, and lupus anticoagulant were all negative.
Chest and abdominal computed tomography studies showed bilateral pulmonary thromboembolism (Fig. 2a), as well as bilateral peripheral ground‐glass opacities and focal consolidation (Fig. 2b) suggestive of COVID‐19 infection. There was also a peripheral hypodense lesion with triangular morphology in the spleen compatible with splenic infarction (Fig. 2c). Three blood cultures and oropharyngeal swab for COVID‐19 testing were negative. IgG SARS‐CoV‐2 was positive, and IgM was negative.
Figure 2.
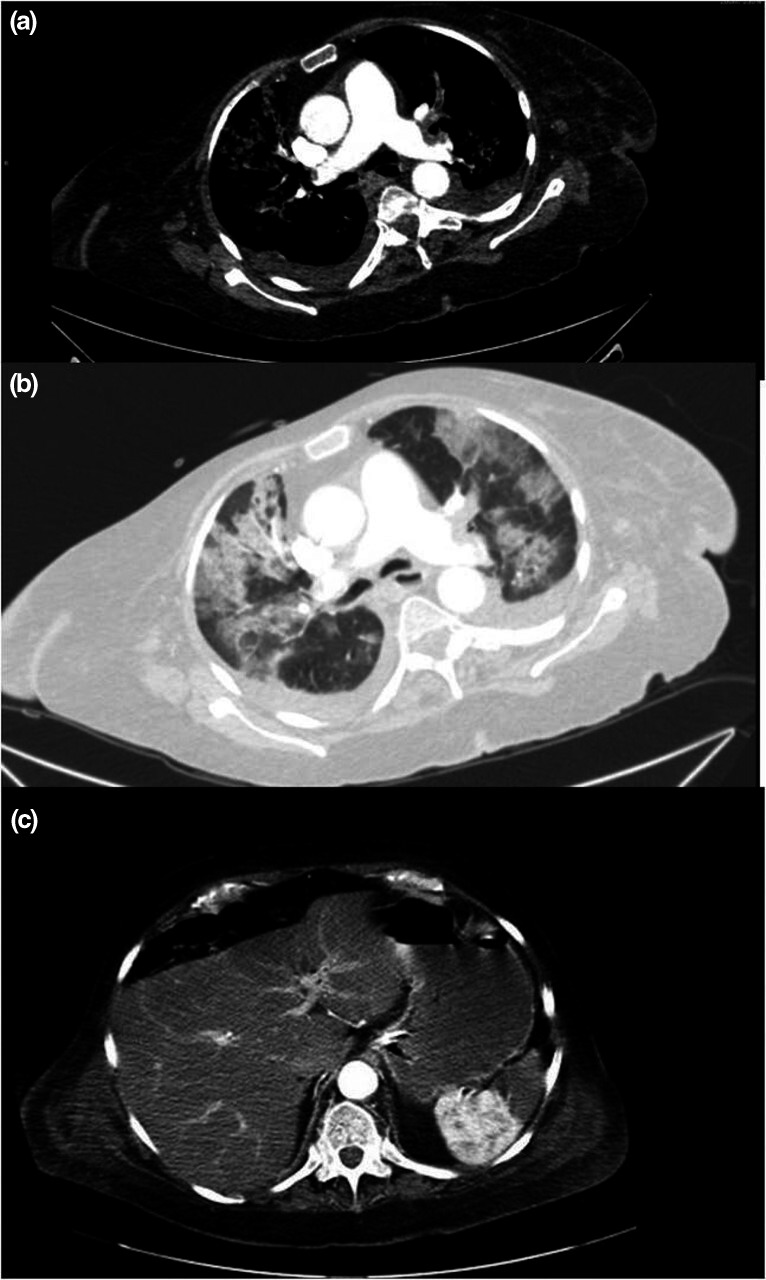
(a) Computed tomography angiography showed massive bilateral pulmonary thromboembolism. (b) Computed tomography showed bilateral and peripheral ground‐glass opacities with consolidations. (c) Computed tomography angiography showed a hypodense lesion with triangular morphology in the spleen, compatible with splenic infarction
After 5 days taking enoxaparin 1 mg/kg daily (dosing adjustment due to renal insufficiency and monitoring with anti‐Xa measurement) and oxygen therapy, the patient suffered from upper gastrointestinal bleeding and died.
Discussion
Acute respiratory failure and systemic coagulopathy are critical aspects of the morbidity of individuals with SARS‐CoV‐2. Recent data show that nearly 20% of COVID‐19 patients present with severe coagulation abnormalities. 1 A prothrombotic state in COVID‐19 infection is known to be associated with platelet hyper‐reactivity, hypofibrinolysis, hypercoagulability, complement overactivation, and renin–angiotensin–aldosterone systems derangement due to endothelial dysfunction. 2 Autopsy samples with skin and lung damage revealed that microvascular injury was mediated by complement, with generalized activation of both the alternative and lectin pathways. 3 The complement system is part of the immune system providing innate defense against microbes and mediating inflammatory responses. A link also exists among the complement system and platelet activation, leukocyte recruitment, endothelial cell activation, and coagulation. 4
C3 plays a role in detecting and disabling intracellular pathogens. C3 attaches to the surface of viruses and may be carried intracellularly. Recent studies suggest the concept of immunothrombosis and that C3 fragment deposition on target cells are the common denominator among endothelial dysfunction and microvascular thrombosis. 2 This generalized microvascular thrombotic disorder can manifest in the skin as retiform purpura or livedo racemosa and/or purpuric lesions. Severe COVID‐19 may define a type of catastrophic microvascular injury syndrome complement‐mediated, as in the presented case, showing C3 deposition on endothelial cells. Other authors have studied the deposition of C3d, C4d, and C5b‐9 in skin and lung biopsy samples. 3 Histopathologic features in COVID‐19 suggest a complement‐mediated endotheliopathy. 4 Evidence suggests that the signs of severe COVID‐19 infection resemble more the phenotype of complement‐mediated thrombotic microangiopathies rather than disseminated intravascular coagulation. 5
The use of complement inhibitors could be a possible new target in treating COVID‐19‐related systemic thrombosis, such as eculizumab or ravulizumab, 5 , 6 by reducing the innate immune‐mediated consequences of severe coronavirus infection. 7 , 8
Pulmonary thrombosis, immunothrombosis, and other types of thrombotic microangiopathies may share certain pathogenic mechanisms with the COVID‐19 microangiopathy, 5 and high‐dose heparin may be dangerous, possibly contributing to the hemorrhagic component of microangiopathy, 9 as happened in our patient.
With the present case, we suggest performing skin biopsy including immunohistologic studies in COVID‐19 patients presenting with cutaneous lesions. Immunofluorescence findings may help to understand pathogenesis of the hypercoagulopathy and microvascular immunothrombosis in COVID‐19. The immunofluorescence features could suggest possible targets for specific intervention.
Acknowledgments
To all the health care professionals and patients who are battling on the front line of the COVID‐19 epidemic.
Conflict of interest: None.
Funding source: None.
References
- 1. Zhai Z, Li C, Chen Y, et al. Prevention Treatment of VTE Associated with COVID‐19 Infection Consensus Statement Group. Prevention and Treatment of Venous Thromboembolism Associated with Coronavirus Disease 2019 Infection: A Consensus Statement before Guidelines. Thromb Haemost 2020; 120: 937–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Henry BM, Vikse J, Benoit S, et al. Hyperinflammation and derangement of renin‐angiotensin‐aldosterone system in COVID‐19: A novel hypothesis for clinically suspected hypercoagulopathy and microvascular immunothrombosis. Clin Chim Acta 2020; 507: 167–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID‐19 infection: a report of five cases. Transl Res 2020; 220: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Varga Z, Flammer A, Steiger O, et al. Endothelial cell infection and endotheliitis in COVID‐19. Lancet 2020; 395: 1417–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gavriilaki E, Brodsky RA. Severe COVID‐19 infection and thrombotic microangiopathy: success doesn't come easily. Br J Haematol 2020; 189: e227–e230. [DOI] [PubMed] [Google Scholar]
- 6. Mastaglio S, Ruggeri A, Risitano AM, et al. The first case of COVID‐19 treated with the complement C3 inhibitor AMY‐101. Clin Immunol 2020; 215: 108450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Campbell C, Kahwash R. Will complement inhibition be the new target in treating COVID‐19 related systemic thrombosis? Circulation 2020; 141: 1739–1741. [DOI] [PubMed] [Google Scholar]
- 8. Song W‐C, FitzGerald GA. COVID‐19, microangiopathy, hemostatic activation, and complement. J Clin Invest 2020; 130: 3950–3953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cattaneo M, Bertinato EM, Birocchi S, et al. Pulmonary embolism or pulmonary thrombosis in COVID‐19? Is the recommendation to use high‐dose heparin for thromboprophylaxis justified? Thromb Haemost 2020; 120: 1230–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]


