Sir, with great interest we read the paper by Bianco et al, 1 presenting the association between genetic modifiers of liver injury and outcomes in patients with COVID‐19. Whilst Bianco et al 1 showed that the PRS‐HFC genetic score does not affect studied phenotypes, they also demonstrated that C3 and AB0 genetic variants are associated with the severity of COVID‐19. In this study, we would like to add to these interesting observations and present first data concerning the MBOAT7 polymorphism rs641738 (which is included among other variants in the PRS‐HFC score), a known risk factor for liver steatosis and fibrosis. 2
Prospectively, we analysed a cohort of 44 patients with COVID‐19 (64% men; median age 62 years, range 21‐91 years) hospitalized between March and October 2020 at our centre in Saarland, Germany. The MBOAT7 variant was genotyped using TaqMan® assays. The median length of stay in hospital was 14 days (range 1‐227 days) and abnormal liver parameters (ie at least one of ALT, AST, ALP, GGT, bilirubin, albumin, or INR > ULN) were present in 66% of patients at admission and in 82% during the hospital stay. Among recruited patients, only eight carried the wild‐type (WT) MBOAT7 allele in comparison to 36 patients carrying the variant allele (19 heterozygous and 17 homozygous carriers). We detected a significant (P = .013) correlation between abnormal values of liver parameters at admission and the MBOAT7 polymorphism.
The presence of the MBOAT7 polymorphism was associated with more severe liver injury during hospitalization for COVID‐19. Indeed, as compared to individuals with the WT MBOAT7 genotype, carriers of the risk allele showed significantly increased ALP (P = .002, Figure 1A), GGT (P = .009, Figure 1B), ALT (P = .003) and bilirubin (P < .001) and decreased albumin levels (P < .001) during hospitalization as compared to admission. Panels A and B of Figure 1 illustrate the exemplary time courses of the ALP and GGT fluctuations in all hospitalized COVID‐19 patients, respectively. A total of 739 values (ALP) and 758 values (GGT) from 44 patients during their stay were analysed, and the groups (WT vs variant) were graphically compared using local regression (Loess). Notably, 16/36 (44%) risk allele carriers and only 2/8 (25%) WT allele carriers presented ALP activities exceeding ULN (♂ >129 U/l; ♀ >104 U/l). In the exploratory analysis, we observed that 80% of the MBOAT7 risk allele carriers developed pneumonia in comparison to 50% of the non‐risk allele carriers. Of note, 94% of patients hospitalized in the intensive care unit, 93% of patients requiring artificial ventilation and 100% of patients in need of extracorporeal membrane oxygenation (ECMO) carried at least one copy of the MBOAT7 risk allele. Twenty‐two percent and 25% of the patients with or without the MBOAT7 polymorphism died, respectively.
Figure 1.
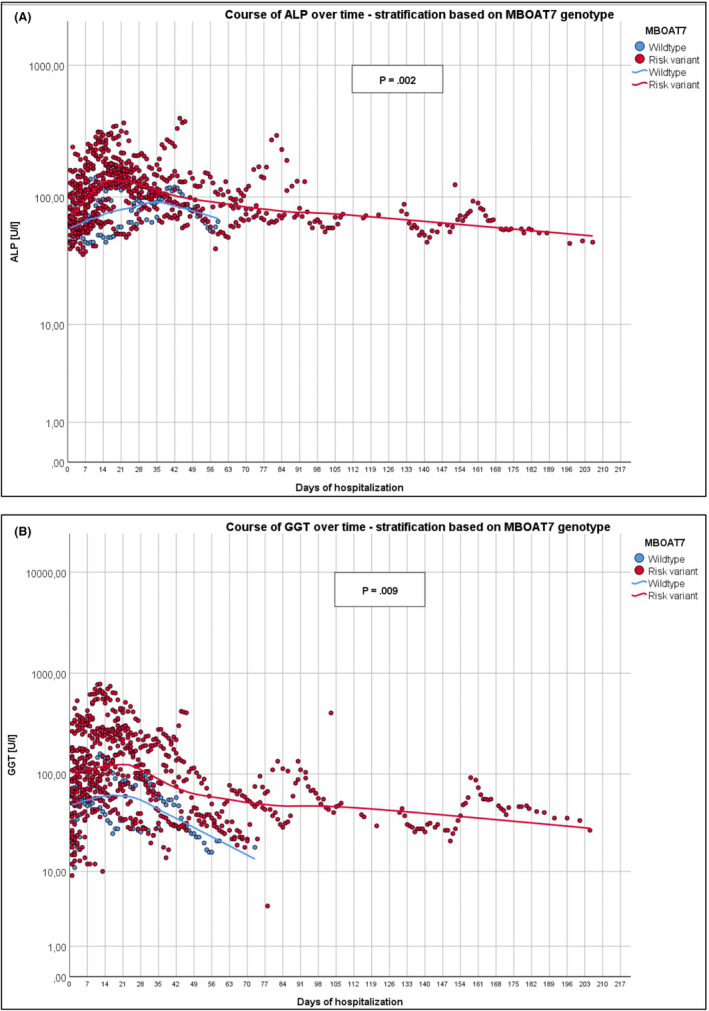
Course of ALP (A) and GGT activities (B) during patients’ entire stay in the hospital, stratified based on MBOAT7 genotypes. Data are presented on a logarithmic scale
Individuals with metabolic‐associated fatty liver disease (MAFLD) are at‐risk of more severe COVID‐19. 3 Here, by analysing trajectories of liver tests in hospitalized patients with COVID‐19, we show that the MAFLD‐predisposing MBOAT7 variant might also be linked to worsening of liver function during SARS‐CoV2 infection. COVID‐19 is a progressive disease characterized by dynamically changing clinical status and lab tests. Hence, it is obvious that analyses of liver tests coming from one single day strongly limit the chance to capture genetic modifiers of hepatic injury. For this reason, we analysed the cumulative laboratory data from the entire hospital stay and found that carriers of the MBOAT7 variant are at risk of severe liver injury. Still, we reckon that further studies are needed to confirm this association and its role in the course and treatment of COVID‐19.
CONFLICT OF INTEREST
None.
REFERENCES
- 1. Bianco C, Baselli G, Malvestiti F, et al.Genetic insight into Covid‐19 related liver injury. Liver Int. in press 10.1111/liv.14708 [DOI] [PubMed]
- 2. Krawczyk M, Rau M, Schattenberg JM, et al. Combined effects of the PNPLA3 rs738409, TM6SF2 rs58542926, and MBOAT7 rs641738 variants on NAFLD severity: A multicenter biopsy‐based study1. J Lipid Res. 2017;58:247‐255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhou Y‐J, Zheng KI, Wang X‐B, et al. Metabolic‐associated fatty liver disease is associated with severity of COVID‐19. Liver Int. 2020;40:2160‐2163. [DOI] [PMC free article] [PubMed] [Google Scholar]


