Abstract
Aims
Interstitial pneumonia due to coronavirus disease 2019 (COVID‐19) is often complicated by severe respiratory failure. In addition to reduced lung compliance and ventilation/perfusion mismatch, a blunted hypoxic pulmonary vasoconstriction has been hypothesized, that could explain part of the peculiar pathophysiology of the COVID‐19 cardiorespiratory syndrome. However, no invasive haemodynamic characterization of COVID‐19 patients has been reported so far.
Methods and results
Twenty‐one mechanically‐ventilated COVID‐19 patients underwent right heart catheterization. Their data were compared both with those obtained from non‐mechanically ventilated paired control subjects matched for age, sex and body mass index, and with pooled data of 1937 patients with ‘typical’ acute respiratory distress syndrome (ARDS) from a systematic literature review. Cardiac index was higher in COVID‐19 patients than in controls [3.8 (2.7–4.5) vs. 2.4 (2.1–2.8) L/min/m2, P < 0.001], but slightly lower than in ARDS patients (P = 0.024). Intrapulmonary shunt and lung compliance were inversely related in COVID‐19 patients (r = −0.57, P = 0.011) and did not differ from ARDS patients. Despite this, pulmonary vascular resistance of COVID‐19 patients was normal, similar to that of control subjects [1.6 (1.1–2.5) vs. 1.6 (0.9–2.0) WU, P = 0.343], and lower than reported in ARDS patients (P < 0.01). Pulmonary hypertension was present in 76% of COVID‐19 patients and in 19% of control subjects (P < 0.001), and it was always post‐capillary. Pulmonary artery wedge pressure was higher in COVID‐19 than in ARDS patients, and inversely related to lung compliance (r = −0.46, P = 0.038).
Conclusions
The haemodynamic profile of COVID‐19 patients needing mechanical ventilation is characterized by combined cardiopulmonary alterations. Low pulmonary vascular resistance, coherent with a blunted hypoxic vasoconstriction, is associated with high cardiac output and post‐capillary pulmonary hypertension, that could eventually contribute to lung stiffness and promote a vicious circle between the lung and the heart.
Keywords: Haemodynamics, Pulmonary hypertension, Heart failure, COVID‐19, Acute respiratory distress syndrome
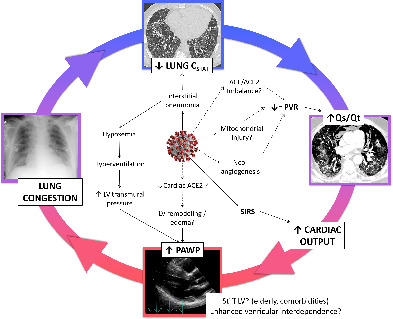
Vicious circle between the lung and the heart in COVID‐19. Coronavirus‐2 causes an interstitial pneumonia characterized by low lung compliance. The ventilation/perfusion mismatch of non‐ventilated but perfused lung zones is enhanced by specific virus‐related mechanisms, with blunted hypoxic pulmonary vasoconstriction and normal PVR, further promoting the intrapulmonary shunt. High cardiac output due to acute inflammation and hypoxaemia, with low PVR and unimpeded left ventricular preload, predisposes to high filling pressure, which might be favoured by patient characteristics (elderly with cardiovascular comorbidities) and further exacerbated by virus‐related cardiac remodelling. High left ventricular filling pressure promotes lung congestion with further reduction of lung compliance. ACE, angiotensin‐converting enzyme; CSTAT, static lung compliance; LV, left ventricle; PAWP, pulmonary artery wedge pressure; PVR, pulmonary vascular resistance; Qs/Qt, intrapulmonary shunt; SIRS, systemic inflammatory response syndrome.
Introduction
The current pandemic due to novel coronavirus disease 2019 (COVID‐19) represents an unprecedented and severe public health problem, burdened with high rates of hospitalization and mortality, as in the case of Northern Italy. 1 , 2 , 3 It may cause interstitial pneumonia and, in up to 15% of patients, it progresses towards severe acute respiratory syndrome, frequently complicated by acute respiratory distress syndrome (ARDS). 4 ARDS is typically characterized by inflammatory alveolar oedema associated with stiff lungs and severe gas exchange impairment, presenting as acute onset of non‐cardiogenic pulmonary oedema and severe hypoxaemia. 5 Non‐ventilated, poorly compliant lung zones generally represent the anatomical bases for intrapulmonary shunting, which further deteriorates arterial oxygenation. Pulmonary hypertension is a frequent finding, mainly attributed to hypoxic pulmonary vasoconstriction, thromboembolism, and, eventually, vascular remodelling. 6
It has been reported that the ARDS caused by COVID‐19 may present some ‘atypical’ features, including a relatively preserved lung compliance and a high intrapulmonary shunt fraction (increased Qs/Qt). 7 The latter could be a major contributor to the severity of the respiratory failure, and has been speculatively attributed to an abnormally blunted hypoxic pulmonary vasoconstriction. 7 , 8 , 9 Coherently, preliminary data collected with dual energy computed tomography of the chest have shown dilated subsegmental pulmonary arteries proximal to, and within the lung consolidation areas, with increased perfusion, that could represent the anatomical‐functional basis of intrapulmonary shunt. 8 However, a thorough haemodynamic characterization in COVID‐19 patients with ARDS needing mechanical ventilation has not been described so far. 9
During the emergency conditions imposed by the COVID‐19 outbreak in Northern Italy regions, allocation of resources dramatically changed. 2 Due to the limited amount of beds in general intensive care units, a number of COVID‐19 patients at our centres were randomly admitted to the cardiac surgery intensive care unit beds equipped for invasive haemodynamic monitoring, which is frequently a standard of care for attending physicians. In this context, some of the patients admitted to the intensive care unit underwent right heart catheterization to help guide management and gain a better understanding of the cardiovascular factors 10 , 11 , 12 , 13 that might be contributing to the high mortality rates observed with COVID‐19. The aim of this study was therefore to describe cardiopulmonary haemodynamics of mechanically ventilated patients with ARDS due to COVID‐19.
Methods
We retrospectively analysed data of consecutive patients with ARDS due to laboratory‐confirmed COVID‐19, admitted to the Intensive Care Unit of Ospedale San Luca (Istituto Auxologico Italiano, Milan), and Ospedale Papa Giovanni XXIII (Bergamo), Italy, between February 25th and April 15th, 2020. We included patients needing mechanical ventilation and who underwent right heart catheterization.
We excluded from this analysis COVID‐19 patients with: incomplete haemodynamic data, pre‐existing severe cardiac or respiratory disease, such as reduced left ventricular (LV) ejection fraction, more than mild chronic obstructive pulmonary disease, pulmonary vascular disease, cirrhosis, malignancy and those with acute extensive pulmonary thromboembolic manifestations (i.e. bilateral pulmonary artery peripheral involvement, or obstruction of the left or the right branches of the pulmonary artery), that could relevantly affect haemodynamics. Due to the high prevalence of pulmonary embolism reported in this cohort, 14 patients with previous demonstration of small, segmental or subsegmental pulmonary embolism were included, provided that they had been appropriately treated. Indeed, at least 30% of the pulmonary vascular bed should be involved before haemodynamic changes appear. 15 Additionally, it has been previously reported that pulmonary microthrombosis, which could be an issue in mechanically ventilated ARDS patients, should not relevantly affect haemodynamics. 16
We compared invasive haemodynamics of mechanically ventilated COVID‐19 ARDS patients with non‐ventilated controls, in analogy to pioneering studies that could demonstrate hypoxic pulmonary vasoconstriction as a peculiar characteristic of ARDS. 17 , 18 Furthermore, in order to strengthen our results, we also compared cardiorespiratory characteristics of our patients with pooled data obtained from a systematic literature review on haemodynamics in mechanically ventilated patients with ARDS.
Clinical characteristics of patients, including ventilatory parameters, blood tests and medical treatment at the time of haemodynamic assessment, were retrieved from medical records.
Controls were selected among outpatients who underwent an elective right heart catheterization for unexplained dyspnoea after a comprehensive non‐invasive evaluation at Ospedale San Luca, Istituto Auxologico Italiano, Milan, between June 2016 and December 2019. A 1:1 matching by age, sex and body mass index (BMI) with COVID‐19 patients was performed after having excluded patients with reduced LV ejection fraction, more than mild chronic pulmonary obstructive disease, pulmonary vascular disease.
Furthermore, we conducted a systematic Medline literature review, updated to August 26th, 2020, in order to compare haemodynamic characteristics of our COVID‐19 patients with available published data. We used the following combinations of search terms: (‘pulmonary vascular resistance’ OR ‘pulmonary haemodynamics’ OR ‘pulmonary haemodynamics’ OR ‘pulmonary circulation’ OR ‘pulmonary vessels’ OR ‘pulmonary vasoconstriction’ OR ‘pulmonary hypertension’ OR ‘intrapulmonary shunt’) AND (‘ARDS’ OR ‘acute respiratory distress syndrome’ OR ‘acute lung injury’ OR ‘acute respiratory failure’). We only considered English‐written studies reporting pulmonary vascular resistance (PVR) or PVR index in mechanically ventilated patients with ARDS. We excluded studies on paediatric patients only, studies on animals, reviews, case reports, studies not reporting haemodynamic measurements. When it was evident that the same patients were included in more than one study, we considered only the larger one.
The study was approved by the Ethics Committees of the Istituto Auxologico Italiano, Milan, and Ospedale Papa Giovanni XXIII, Bergamo, Italy (HEMO‐COVID protocol). Informed consent for the anonymized use of clinical data for research purposes was waived because the patients were unconscious and in critical conditions, and due to the impossibility of having a physical contact with their relatives. Researchers analysed only deidentified (anonymized) data. Conversely, all controls signed a written informed consent for their clinical data to be used for research purposes.
Echocardiography
An experienced echocardiographer performed two‐dimensional and Doppler echocardiography studies following current recommendations. 19 Images were stored in digital format for quantitative analysis blinded to haemodynamic data. LV geometry was assessed using two‐dimensional echocardiography in parasternal long‐axis view. 19 Representative echocardiographic images are reported in the online supplementary material. Only the echocardiographic studies performed within 24 h after the haemodynamic assessment were considered.
Right heart catheterization
A 7 F fluid‐filled Swan–Ganz catheter was placed in the pulmonary artery through the right internal jugular vein by two skilled operators at each centre, who then performed haemodynamic readings (L.G. and F.R. at Ospedale Papa Giovanni XXIII, and S.C. and C.B. at Istituto Auxologico Italiano). The transducer was zeroed at the midthoracic line, halfway between the anterior sternum and the bed surface. 20 Proper pulmonary artery wedge positioning was confirmed by the appearance of a typical pulmonary artery wedge pressure (PAWP) trace and by an oxygen saturation sampled at the tip of the wedged catheter ≤5% than arterial oxygen saturation. Pulmonary haemodynamic measures were averaged throughout several heartbeats and respiratory cycles. The Shrout–Fleiss intraclass correlation was calculated in a sub‐sample of our dataset to assess the interrater reliability of mean PAWP.
Cardiac output was measured either by direct Fick method (controls) or by using Vigilance Monitor II, Edwards Lifescience, Irvine, CA (ventilated COVID‐19 patients). Two millilitres of blood were simultaneously sampled from the tip of the Swan–Ganz catheter and from the radial artery for blood gas analyses. Detailed methods on the measurements and calculation of Qs/Qt and static lung compliance are reported in the online supplementary material. Complete haemodynamic measurements were taken in triplicate and then averaged.
Statistics
The data are expressed as median (interquartile range) or as absolute numbers and percentage, where appropriate. Distribution of variables in terms of proximity to the normal curve and the homogeneity of variances were detected by Shapiro–Wilk test and Bartlett test, respectively. Numerical variables were analysed with t‐test or Wilcoxon rank sum, according to their distributions. Categorical variables were analysed with Chi‐squared test or Fisher exact test in case of small cell sizes. Correlation analysis was performed with the Pearson product–moment correlation. The data of the main haemodynamic characteristics found in the literature were pooled by random effect models. We then used a t‐test to compare the mean of our data with pooled mean. An α level of 0.05 was used for all hypothesis tests. All data analyses were performed using R Core Team (2019), Vienna, Austria.
Results
Clinical characteristics and echocardiographic parameters
COVID‐19 patients
Between February 25th and April 15th, 2020, 255 consecutive patients with radiologically and laboratory confirmed COVID‐19 pneumonia were admitted to the intensive care units of the two institutions. Fifty‐five (21.6%) of them underwent right heart catheterization. Out of these 55 patients, 22 (40%) had complete clinical, respiratory and haemodynamic data. After having excluded one patient with bilateral pulmonary embolism, our final cohort included 21 patients (online supplementary Figure S1 ). Demographics and clinical characteristics of these 21 patients are reported in Table 1 . The mean age was 65 years, the majority of patients were men (86%), overweight, 38% were obese. Most of patients were hypertensive (62%), 43% had diabetes mellitus, and only 5% had renal dysfunction. They had no previous history of cardiac disease or heart failure.
Table 1.
Demographics, anthropometrics and clinical characteristics of COVID‐19 patients with acute respiratory distress syndrome requiring mechanical ventilation, and controls
| COVID‐19 (n = 21) | Controls (n = 21) | P‐value | |
|---|---|---|---|
| Demographics and anthropometrics | |||
| Age, years | 67 [60–70] | 71 [66–75] | 0.144 |
| Male sex, n (%) | 18 (86) | 18 (86) | 1.00 |
| BMI, kg/m2 | 29 [25–31] | 27 [26–33] | 0.783 |
| BMI 25–30 kg/m2, n (%) | 9 (43) | 10 (48) | 0.757 |
| BMI ≥30 kg/m2, n (%) | 8 (38) | 8 (38) | 1.000 |
| Previous medical history, n (%) | |||
| Arterial hypertension | 13 (62) | 17 (81) | 0.171 |
| Diabetes mellitus | 9 (43) | 2 (10) | 0.014 |
| Dyslipidaemia | 3 (14) | 9 (43) | 0.040 |
| Chronic kidney disease | 1 (5) | 0 | 0.312 |
| COPD | 1 (5) | 8 (38) | 0.008 |
| Cerebrovascular disease | 1 (5) | 2 (10) | 0.549 |
| Coronary artery disease | 3 (14) | 6 (29) | 0.259 |
| Heart failure | 0 | 2 (10) | 0.147 |
| Atrial fibrillation | 0 | 4 (19) | 0.036 |
| Immunological disorder | 1 (5) | 0 | 0.312 |
| Smoking habitus | 1 (5) | 1 (5) | 1.000 |
Continuous variables are shown as median [interquartile range].
BMI, body mass index; COPD, chronic obstructive pulmonary disease.
The great majority of patients had a relevant respiratory compromise, as witnessed by a Berlin score of moderate or severe ARDS in 95% of them (moderate: 52%; severe 43%). Median APACHE IV score was 57 (47–67), with an estimated mortality rate of 40.7% (29.8–51.5).
Patients with COVID‐19 spent in median 6 (5–11) days with symptoms at home before hospital admission and 4 (1–7) days in hospital before endotracheal intubation. Sixty‐two percent of patients received non‐invasive ventilation for 4 (1–8) days before endotracheal intubation. Right heart catheterization was performed in median 3 (2–7) days after admission to the intensive care unit.
Medical treatment at the time of haemodynamic assessment included antiretroviral agents, steroids, antibiotics, hydroxychloroquine, and low‐molecular weight heparin (Table 2 ). Sixty‐two percent of patients were treated with intravenous furosemide (median daily dose 40 mg). Twenty‐eight percent of patients were treated with vasopressors.
Table 2.
Pharmacological treatment and blood tests at the time of right heart catheterization in COVID‐19 patients (n = 21)
| Medical treatment in the intensive care unit | |
| Antiretroviral agents | 6 (29) |
| Steroids | 15 (71) |
| Antibiotics | 15 (71) |
| Hydroxychloroquine | 6 (29) |
| Low molecular weight heparin | |
| Parenteral anticoagulation | 14 (67) |
| Thromboembolic prophylaxis | 7 (34) |
| Adrenergic agents | |
| Norepinephrine alone | 3 (14) |
| Norepinephrine + adrenaline | 3 (14) |
| Norepinephrine dose (µg/kg/min) | 0.07 [0.04–0.1] |
| Furosemide | 13 (62) |
| Furosemide dose, mg | 40 [30–120] |
| Blood tests | |
| High‐sensitivity troponin, ng/mL | 23 [6–94] |
| Haemoglobin, g/dL | 10.3 [8.6–11.5] |
| Creatinine, mg/dL | 0.8 [0.6–1.1] |
| Azotaemia, mg/dL | 56 [39–82] |
| White blood cells, 103/µL | 10.3 [7.0–15.4] |
| C‐reactive protein, mg/dL | 12.7 [2.7–19.0] |
| Procalcitonin, mg/mL | 0.6 [0.2–1.8] |
| D‐dimer, ng/mL | 1470 [1057–2384] |
| Fibrinogen, mg/dL | 564 [372–681] |
Values are given as n (%), or median [interquartile range].
Blood tests at the time of the haemodynamic assessment reflected the systemic inflammatory status, with mildly elevated C‐reactive protein and D‐dimer values, leucocytosis and mild anaemia. High‐sensitivity troponin was mildly increased (Table 2 ).
Clinically indicated computed tomography angiography (CTA) of the chest was performed in 11/21 patients, revealing pulmonary embolism in three of them. One patient presented with involvement of one segmental and one sub‐segmental pulmonary artery at the CTA 6 days before the haemodynamic study. Another had involvement of segmental pulmonary arteries of only one lobe at the CTA performed 3 days before the haemodynamic study. The last patient showed segmental and sub‐segmental pulmonary embolism 13 days before the haemodynamic study, with complete resolution of the obstruction at the CTA 2 days after the haemodynamic study.
Echocardiographic data were available in 13 COVID‐19 patients (Table 3 ). LV ejection fraction was >50% in all of them. LV wall thickness was increased, mainly due to concentric remodelling, which was present in 77% of patients. Left atrial dilatation was present in only one patient. The right ventricle was normally sized with normal systolic function.
Table 3.
Echocardiographic data
| COVID‐19 (n = 13) | Controls (n = 21) | P‐value | |
|---|---|---|---|
| LVEDV, mL | 109 [95–119] | 101 [89–110] | 0.395 |
| LVEF, % | 67 [61–72] | 64 [60–65] | <0.001 |
| IVS thickness, mm | 12 [11–13] | 11 [10–12] | 0.024 |
| PW thickness, mm | 11 [10–11] | 10 [9–11] | 0.045 |
| LV mass, g/m2 | 88 [75–108] | 89 [80–102] | 0.915 |
| LV RWT, cm | 0.46 [0.43–0.54] | 0.40 [0.38–0.44] | 0.008 |
| LV geometry | <0.001 | ||
| Normal geometry | 0 (0) | 14 (66) | |
| Concentric remodelling | 10 (77) | 5 (24) | |
| Concentric hypertrophy | 1 (8) | 2 (10) | |
| Eccentric hypertrophy | 2 (15) | 0 (0) | |
| LAVI, mL/m2 | 24 [22–28] | 30 [27–46] | 0.007 |
| RV basal diameter, mm | 36 [32–38] | 40 [36–43] | 0.151 |
| RV/LV ratio | 0.8 [0.7–0.9] | 0.8 [0.7–1.0] | 0.567 |
| RVEDA, cm2 | 21 [19–23] | 18 [16–22] | 0.249 |
| RV FAC, % | 42 [40–56] | 47 [43–49] | 0.669 |
| TAPSE, mm | 22 [17–26] | 25 [21–27] | 0.463 |
| S′ RV wave, cm/s | 14 [13–16] | 15 [13–15] | 0.481 |
| RAVI, mL/m2 | 21 [15–29] | 32 [18–38] | 0.220 |
Values are given as n (%), or median [interquartile range].
FAC, fractional area change; IVS, interventricular septum; LAVI, left atrial volume index; LV, left ventricle; LVEDV, left ventricular end‐diastolic volume; LVEF, left ventricular ejection fraction; PW, posterior wall; RAVI, right atrial volume index; RV, right ventricle; RVEDA, right ventricular end‐diastolic area; RWT, relative wall thickness; TAPSE, tricuspid annular plane systolic excursion.
Characteristics of mechanical ventilation and gas exchange data at the time of cardiac catheterization are reported in Table 4 . Static lung compliance was low [31 (24–42) mL/cmH2O] and inversely related (r = −0.54, P = 0.012) to the duration of the disease (i.e. the longer the duration of symptoms, the lower the lung compliance).
Table 4.
Mechanical ventilation characteristics and gas exchange data
| COVID‐19 patients | Control group | P‐value | |
|---|---|---|---|
| Mechanical ventilation | |||
| FiO2, % | 70 [60–80] | – | – |
| PEEP, cmH2O | 11 [8–14] | – | – |
| Peak inspiratory pressure, cmH2O | 28 [25–32] | – | – |
| Plateau pressure, cmH2O | 27 [23–29] | – | – |
| Tidal volume, mL | 470 [360–520] | – | – |
| Respiratory rate, /min | 24 [20–28] | – | – |
| Static lung compliance, mL/cmH2O | 31 [24–42] | – | – |
| Gas exchange | |||
| Arterial pH | 7.40 [7.33–7.44] | – | – |
| PaCO2, mmHg | 57 [43–66] | 39 [36–43] | <0.001 |
| PaO2, mmHg | 74 [69–93] | 89 [76–95] | 0.106 |
| SaO2, % | 95 [93–96] | 96 [94–97] | 0.174 |
| Lactate, mmoL/L | 1.1 [1.0–1.6] | 0.7 [0.6–0.8] | <0.001 |
| PvO2, mmHg | 43 [39–48] | 38 [37–40] | 0.091 |
| SvO2, % | 73 [68–77] | 71 [67–73] | 0.371 |
| PaO2/FiO2, mmHg | 103 [83–153] | 424 [361–451] | <0.001 |
| CaO2, mL/dL | 14.4 [12.8–16.7] | 18.4 [17.4–19.9] | <0.001 |
| CvO2, mL/dL | 11.2 [10.0–12.3] | 13.7 [12.3–14.8] | <0.001 |
| C(a‐v)O2, mL/dL | 3.3 [2.9–3.9] | 4.8 [4.5–5.3] | <0.001 |
Values are given as median [interquartile range].
CaO2, arterial oxygen content; C(a‐v)O2, artero‐venous oxygen difference; CvO2, venous oxygen content; FiO2, inspired oxygen fraction; PaCO2, arterial partial pressure of carbon dioxide; PaO2, arterial partial pressure of oxygen; PEEP, positive end‐expiratory pressure; PvO2, venous partial pressure of oxygen; SaO2, arterial oxygen saturation.
Control group, non‐ventilated subjects
Out of 69 patients who underwent right heart catheterization for unexplained dyspnoea, we could find 21 age‐, sex‐ and BMI‐matched controls for COVID‐19 patients. Demographic and clinical characteristics of control patients are summarized in Table 1 . Three of them had a previous hospitalization for heart failure, four had persistent or permanent atrial fibrillation, and eight had a history of mild chronic obstructive pulmonary disease (Global Initiative for Chronic Obstructive Lung Disease class 1). Median N‐terminal pro B‐type natriuretic peptide of the control cohort was 117 (47–408) ng/L.
Echocardiography parameters of control patients are summarized in Table 3 . Cardiac chambers showed normal size and function. Five patients (24%) showed concentric remodelling of the left ventricle, and two (10%) showed concentric LV hypertrophy, whereas 66% of controls showed normal LV geometry.
Eighty‐one percent of controls had haemodynamic parameters at rest within normal limits (Table 5 ).
Table 5.
Invasive haemodynamic data
| COVID‐19 patients | Control group | P‐value | |
|---|---|---|---|
| Cardiac output and shunt fraction | |||
| Heart rate, bpm | 89 [72–94] | 65 [58–78] | <0.001 |
| Cardiac output, L/min | 7.3 [5.3–8.8] | 4.5 [3.9–5.5] | <0.001 |
| Stroke volume, mL | 83 [68–105] | 69 [59–90] | 0.088 |
| Cardiac index, L/min/m2 | 3.8 [2.7–4.5] | 2.4 [2.1–2.8] | <0.001 |
| Qs/Qt | 0.35 [0.28–0.45] | 0.13 [0.06–0.17] | <0.001 |
| Systemic haemodynamics | |||
| Systolic BP, mmHg | 124 [110–143] | 140 [134–148] | 0.208 |
| Diastolic BP, mmHg | 62 [48–71] | 78 [66–80] | 0.007 |
| Mean BP, mmHg | 82 [70–100] | 101 [90–103] | 0.017 |
| Systemic vascular resistance, WU | 9.5 [8.1–13.0] | 18.4 [14.1–23.2] | 0.014 |
| Pulmonary and right heart haemodynamics | |||
| Systolic PAP, mmHg | 41 [34–48] | 25 [22–34] | <0.001 |
| Diastolic PAP, mmHg | 20 [15–26] | 14 [10–16] | <0.001 |
| Mean PAP, mmHg | 27 [25–33] | 17 [14–21] | <0.001 |
| PAWP, mmHg | 15 [11–18] | 9 [8–13] | 0.012 |
| RAP, mmHg | 11 [9–15] | 5 [4–7] | <0.001 |
| RAP/PAWP | 0.8 [0.7–0.9] | 0.5 [0.4–0.7] | 0.004 |
| PAWP – RAP, mmHg | 3 [2–6] | 4 [3–7] | 0.370 |
| Pulmonary vascular resistance, WU | 1.6 [1.1–2.5] | 1.6 [0.9–2.0] | 0.343 |
| Total pulmonary resistance, WU | 4.0 [3.1–4.7] | 3.9 [2.5–5.3] | 0.537 |
| Diastolic pressure gradient, mmHg | 5 [0–8] | 2 [1–3] | 0.047 |
| Transpulmonary pressure gradient, mmHg | 13 [8–14] | 6 [5–8] | 0.002 |
| Mean PAP ≥25 mmHg | 16 (76) | 4 (19) | <0.001 |
| PVR >3 WU | 0 | 0 | 1.000 |
| Post‐capillary PH | 12 (57) | 4 (19) | 0.011 |
| Isolated post‐capillary PH | 12 | 4 | |
| Combined post‐ and pre‐capillary PH | 0 | 0 |
Values are given as n (%), or median [interquartile range].
BP, blood pressure; PAP, pulmonary artery pressure; PAWP, pulmonary artery wedge pressure; PH, pulmonary hypertension; PVR, pulmonary vascular resistance; Qs/Qt, intrapulmonary shunt; RAP, right atrial pressure.
Comparison between COVID‐19 group and control group
Patients with COVID‐19 patients were more diabetic, had less atrial fibrillation and had lower levels of haemoglobin (10.3 [8.6–11.5] vs. 13.5 [12.7–15.1] g/dL, P < 0.001) than controls (Table 1 ). Moreover, COVID‐19 patients had higher LV ejection fraction, higher incidence of concentric LV remodelling, and smaller left atrial volumes than controls (Table 3 ) despite a non‐significantly higher prevalence of arterial hypertension in controls.
Complete haemodynamic data are displayed in Table 5 . Intra‐class correlation coefficient in PAWP readings was 0.98.
The Qs/Qt of COVID‐19 patients was 0.35 (0.28–0.45), which was significantly higher than that of controls. Qs/Qt was inversely related to static lung compliance (r = −0.57, P = 0.011; online supplementary Figure S2 ) but not to the ratio between arterial partial pressure and inspired oxygen fraction (r = 0.36, P = 0.120).
Pulmonary artery pressure (PAP) was higher in COVID‐19 patients than in controls (Table 5 ), with mean PAP ≥25 mmHg in 76% of COVID‐19 patients vs. 19% of controls (P < 0.001). PVR was similar in COVID‐19 patients and in controls (P = 0.343). No patients either in the COVID‐19 or in the control group had PVR >3 WU. PVR was not related to Qs/Qt (r = 0.21, P = 0.388).
Cardiac output and heart rate were higher and systemic vascular resistance was lower in COVID‐19 patients than in controls. In COVID‐19 patients, total cardiac output was directly related to Qs, i.e. the amount of shunted blood (r = 0.71, P < 0.001), but not to haemoglobin levels (r = 0.33, P = 0.141).
Pulmonary hypertension was post‐capillary (PAWP ≥15 mmHg) in 57% of COVID‐19 patients and 19% of controls (P = 0.011). In COVID‐19 patients, PAWP was inversely related to lung compliance (r = −0.46, P = 0.038; Figure 1 ). Right atrial pressure (RAP) and the ratio between RAP and PAWP were higher in COVID‐19 patients than in controls (Table 5 ). Results did not change when we excluded the three COVID‐19 patients with documented segmental or sub‐segmental pulmonary embolism.
Figure 1.
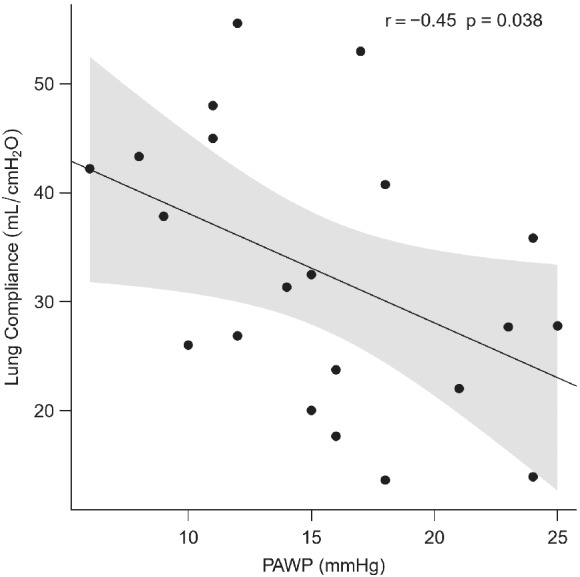
Inverse relationship between lung compliance and pulmonary artery wedge pressure (PAWP).
Control group, pooled analysis of haemodynamics in acute respiratory distress syndrome patients from the literature
Out of 1759 literature results, 58 studies reporting either PVR or PVR index were considered in this analysis (online supplementary Table S1 ; complete article list of this systematic review is available in the online supplementary material). They included 1937 patients with ARDS, whose mean age was 48.3 years (based on data available from 52 studies on 1578 patients). Sixty‐three percent patients were males (based on data available from 36 studies on 1292 patients). ARDS aetiology was infectious (bacterial, viral, or fungal) pneumonia in 31.5% of cases (based on data available from 51 studies on 1441 patients). In all studies but one patients received standard intensive care unit treatment for ARDS according to clinical needs, including vasoactive and cardioactive drugs, fluids, sedatives. Only 10 patients out of 1937 were not mechanically ventilated, while only 15 patients were assessed during extracorporeal membrane oxygenation. The pooled mean for relevant ventilatory and haemodynamic variables is reported in Table 6 .
Table 6.
Comparison of the mean value of cardiorespiratory variables in our sample of COVID‐19 patients with the pooled value calculated in acute respiratory distress syndrome patients from the literature
| Studies on ARDS from the literature, n | ARDS subjects, n | Pooled mean of ARDS patients from the literature | Confidence interval | COVID‐19 patients (n = 21) | P‐value | |
|---|---|---|---|---|---|---|
| PaO2/FiO2, mmHg | 43 | 1568 | 127 | 117–138 | 121 ± 47 | 0.560 |
| Qs/Qt | 35 | 1147 | 0.36 | 0.33–0.39 | 0.37 ± 0.11 | 0.815 |
| Static lung compliance, mL/cmH2O | 21 | 528 | 34 | 31–37 | 32 ± 12 | 0.691 |
| PEEP, cmH2O | 33 | 1389 | 10.2 | 9.3–11.2 | 11.1 ± 4.0 | 0.330 |
| Mean PAP, mmHg | 66 | 1832 | 31 | 30–32 | 27 ± 8 | 0.071 |
| PAWP, mmHg | 46 | 1580 | 13 | 12–13 | 16 ± 6 | 0.024 |
| CO, L/min | 16 | 261 | 6.9 | 6.3–7.5 | 7.3 ± 2.3 | 0.411 |
| CI, L/min/m2 | 43 | 1362 | 4.3 | 4.1–4.4 | 3.7 ± 1.1 | 0.024 |
| PVR, WU | 38 | 586 | 2.9 | 2.6–3.2 | 1.7 ± 0.8 | <0.001 |
| PVRI, mmHg/L/min/m2 | 34 | 1351 | 4.6 | 4.1–5.0 | 3.4 ± 1.6 | 0.002 |
| SVR, WU | 18 | 231 | 9.9 | 8.8–11.0 | 11.2 ± 4.3 | 0.182 |
| SVRI, mmHg/L/min/m2 | 17 | 1043 | 17.1 | 15.6–18.6 | 21.9 ± 8.2 | 0.013 |
ARDS, acute respiratory distress syndrome; CI, cardiac index; CO, cardiac output; FiO2, inspired oxygen fraction; PaO2, arterial partial pressure of oxygen; PAP, pulmonary artery pressure; PAWP, pulmonary artery wedge pressure; PEEP, positive end‐expiratory pressure; PVR, pulmonary vascular resistance; PVRI, pulmonary vascular resistance index; Qs/Qt, intrapulmonary shunt; SVR, systemic vascular resistance; SVRI, systemic vascular resistance index.
Comparison between COVID‐19 group and pooled data of acute respiratory distress syndrome patients from the literature
As compared with pooled data from the literature, our COVID‐19 patients had a similar ARDS severity as reflected by non‐different positive end‐expiratory pressure support (P = 0.330), lung compliance (P = 0.691), as well as the ratio between arterial oxygen partial pressure and inspired oxygen fraction (P = 0.560). In spite of this, PVR and PVR index were lower (P < 0.001 and P = 0.002, respectively) while PAWP was higher (P = 0.024) in our COVID‐19 patients than in pooled ARDS patients from the literature. Cardiac output and systemic vascular resistance did not diff between groups (P = 0.411 and P = 0.182, respectively), while cardiac index and systemic vascular resistance index were higher in COVID‐19 patients (P = 0.024 and P = 0.013, respectively).
Survivors vs. non‐survivors
Eleven COVID‐19 patients (52%) died in the intensive care unit and 10 survived. The patients who died, as compared to survivors, had similar age (P = 0.223), sex distribution (P = 0.476) and BMI (P = 0.672). As shown in online supplementary Table S2 , they were ventilated with higher inspired oxygen fraction [0.80 (0.75–0.88) vs. 0.68 (0.46–0.70), P = 0.025] and higher plateau pressure [28 (27–30) vs. 23 (21–25) cmH2O, P = 0.005], whereas lung compliance was similar [28 (21–39) vs. 34 (27–43) mL/cmH2O, P = 0.387]. COVID‐19 patients who died had a trend towards higher Qs/Qt [0.43 (0.34–0.49) vs. 0.30 (0.28–0.35), P = 0.079], had higher Qs [3.5 (2.6–4.0) vs. 1.9 (1.6–2.3) L/min, P = 0.016] and cardiac output [4.1 (3.4–4.6) vs. 2.7 (2.4–3.7) L/min/m2, P = 0.051], as shown in online supplementary Table S3 . Consequently, mean PAP was higher in non‐survivors as compared with survivors [31 (27–37) vs. 25 (18–27) mmHg, P = 0.032] despite non‐significantly higher PAWP [16 (13–18) vs. 13 (11–20) mmHg, P = 0.397] and PVR [2.1 (1.3–2.6) vs. 1.4 (0.8–2.2) WU, P = 0.387].
Discussion
To the best of our knowledge, our study is the first to report invasive haemodynamic characteristics of mechanically ventilated COVID‐19 patients with ARDS. In particular, our COVID‐19 patients seemed to present peculiar haemodynamic features, as outlined by (i) only mild increase of PAP with surprisingly low PVR despite respiratory failure, and (ii) high prevalence of increased LV filling pressures. Both these elements, either due to coronavirus itself or to the characteristics of infected patients (elderly subjects with cardiovascular comorbidities), in the context of an inflammation‐driven hyperdynamic circulation, might contribute to clinical manifestations, promoting a vicious circle between the heart and the lungs (Graphical Abstract). In particular, in this specific context, low PVR might facilitate both the development of high LV pressure as well as lung congestion and stiffening, since LV preload is not impeded and the capillary membrane is not protected by a pre‐capillary resistor.
Respiratory characteristics of our COVID‐19 patients would suggest, at a first glance, a ‘typical’ form of ARDS, the so‐called ‘type H’ COVID‐19 pneumonia. 21 Static lung compliance was roughly one third of normal values, which is coherent with previous data on ARDS in non‐COVID‐19 patients, reflecting extensive parenchymal disruption, changes in surfactant due to virus infection, as well as the severity of the respiratory failure. 22
Indeed, ARDS is generally believed to be characterized by high PVR due to hypoxic pulmonary vasoconstriction in pulmonary units with low alveolar oxygen pressure. 5 This reflex vascular modulation can reduce blood flow to atelectatic regions by 50%, with a significant improvement of ventilation/perfusion ratio 23 and may limit the intrapulmonary shunt, whereby increasing right ventricular afterload. Conversely, PVR was ‘atypically’ low in our COVID‐19 patients with ARDS, and not significantly different from measurements obtained in control patients without ARDS. In this perspective, our data seem to confirm the hypothesis that COVID‐19 might be associated with a blunted hypoxic pulmonary vasoconstriction, 7 , 8 , 9 even when pulmonary compliance is low and lung damage is relevant. This may occur through several purely speculative virus‐induced mechanisms, including: up‐ or down‐regulation of mitochondrial proteins involved in aerobic metabolism, 24 with a consequent interference with O2 sensing 8 ; COVID‐19‐related pulmonary neoangiogenesis 25 ; dysregulated angiotensin‐converting enzyme 2 metabolism 26 and/or inflammatory stimuli with a potential imbalance between vasodilatory and vasoconstrictor substances/substrates leading to a net effect of low PVR.
Clinical characteristics of coronavirus‐infected patients, including age and cardiovascular comorbidities, might also contribute to this ‘atypical’ presentation, characterized by more marked pulmonary than systemic vasoplegia and high LV filling pressure, as compared with ‘typical’ ARDS. Indeed, in elderly subjects, high cardiac output can be easily associated with high LV filling pressure, 27 especially if low pulmonary vascular tone does not impede LV preload. Obviously, not only local pulmonary but also systemic factors, such as cytokine storm, anaemia and hypercapnia, might contribute to this high‐output state, as it is generally the case in the context of ARDS, even though systemic vascular resistance was slightly higher in our COVID‐19 patients than in ARDS patients from the literature. This latter finding might once again point towards the peculiar characteristics of our COVID‐19 patients, who were older than ‘typical’ ARDS patients and with a high prevalence of cardiovascular risk factors, likely leading to higher degrees of arterial stiffening and lower systemic vasodilatation in response to cytokine storm. Additionally, the peculiar neurotropism of coronavirus might also play a role, accounting for alterations of normal cardiovascular reflexes. 28
Coherently with low PVR, also mean PAP was only mildly elevated in COVID‐19 patients, and such increase was totally explained by high cardiac output and high PAWP. 29 Interestingly, pulmonary hypertension was present in more than half of patients and was always post‐capillary, 30 and occurred in spite of a large use of intravenous diuretics at high dose. The non pre‐conditioned right ventricle of COVID‐19 patients behaved as expected, with a still preserved contractility without overt dilatation to face an acutely increased afterload (homeometric adaptation). 31 However, it has been repeatedly demonstrated that a higher than normal PAWP can increase the pulsatile afterload of the right ventricle in spite of normal PVR. 32 Thus, despite a still preserved RV morphology and function, which is at variance from previous reports including patients with acute pulmonary embolism during COVID‐19, 13 RAP was already increased in our patients, with a high ratio between RAP and PAWP, portending right heart failure, which is coherent with the evidence of liver congestion, associated with lung congestion, in a number of COVID‐19 patients who underwent autopsy at Ospedale Papa Giovanni XXIII (unpublished data). However, we cannot exclude technical limitations in measuring the right ventricle in mechanically ventilated patients, due to exquisite sensitivity of RV size with angular change. 19 Alternatively, we might hypothesize that the mild RAP increase we found in COVID‐19 patients, associated with high RAP/PAWP ratio, could reflect enhanced ventricular interdependence in mechanically ventilated patients, as a result of reduced lung compliance and high positive end‐expiratory pressure, 33 or COVID‐19 related ventricular concentric remodelling 10 , 26 , 34 associated with inflammation and increased myocardial oedema. 35 Indeed, also enhanced ventricular interdependence can be associated with high filling pressures and mildly elevated PAP, further exacerbated by increased metabolic demands. 36
The finding of mildly elevated PAWP in our population of COVID‐19 patients deserves particular attention. Firstly, pathological specimens from patients with ARDS frequently reveal diffuse alveolar damage, with both alveolar epithelial and lung endothelial injury, resulting in accumulation of protein‐rich inflammatory oedematous fluid in the alveolar space. 5 In this specific setting, a higher than normal PAWP may further promote interstitial and alveolar oedema. 37 This might help explaining the association between PAWP and lung compliance, suggesting that high PAWP may further contribute to worsen lung stiffness. Secondly, high PAWP is a hallmark of heart failure. All of our COVID‐19 patients, albeit burdened with a number of cardiovascular risk factors, did not have a previous history of heart failure. However, a number of otherwise healthy elderly people might present with ‘pathologically’ high PAWP during high‐output states, 27 basically unmasking age‐related LV stiffening, but potentially exposing the pulmonary capillary membrane to damage and interstitial oedema. 37 This behaviour might be further exacerbated by the presence of risk factors associated with cardiovascular ageing, even in the absence of LV hypertrophy or other overt cardiac abnormalities. 38 Indeed, our COVID‐19 patients were quite systematically found to present with LV concentric remodelling. We may hypothesize that this finding could simply reflect the limits of fine echocardiographic measurements in challenging conditions such as that encountered in mechanically ventilated patients with a hyperdynamic circulation. Additionally, LV concentric remodelling is not a necessary condition to develop high filling pressure during increased metabolic demands. 36 , 37 Moreover, arterial hypertension, which is a common comorbidity in COVID‐19 patients and could have been underdiagnosed, might have played a pre‐existing role in LV geometry changes. Finally, we cannot exclude a direct myocardial injury by coronavirus‐2, 11 with potential myocardial oedematous changes. 35
Study limitations
We enrolled a limited, but well phenotyped, cohort of COVID‐19 patients that, in the frame of the peculiar allocation of resources during the outbreak of COVID‐19 pandemic, 2 underwent invasive haemodynamic assessment in randomly assigned, dedicated beds of the cardiac surgery intensive care unit. Moreover, severe respiratory or cardiovascular comorbidities were not represented in our population. Thus, we cannot exclude a potential selection bias, even if the burden of mild comorbidities was quite consistent with recently published data. 4 Furthermore, our small sample size might have prevented us from detecting other significant differences in sub‐group analyses (e.g. survivors vs. non‐survivors).
Since pulmonary vessels and the heart are intrathoracic, mechanical ventilation can affect haemodynamic measurements. However, lung compliance in our cohort was markedly reduced, suggesting a negligible transmission of positive end‐expiratory pressure to intravascular and intracardiac compartments.
An ideal control group for our COVID‐19 patients would have been composed by non‐COVID‐19 ARDS patients. However, right heart catheterization is nowadays rarely performed during ARDS. We tried to overcome this limit performing a systematic review and pooled analysis of published data. These results from 1937 ARDS patients from the literature could corroborate and complement those obtained in the comparison of COVID‐19 patients with matched subjects without relevant comorbidities and without obvious causes for dyspnoea from our cardiac catheterization laboratory database. As such, these two groups represent the best control groups we could use: the former highly representative of the characteristics of mechanically ventilated but younger ARDS patients, and the latter characterized by haemodynamics closer to that of elderly, otherwise healthy subjects. Accordingly, the haemodynamic profile of the control group was roughly compatible with that of an aged, overweight and hypertensive population.
Conclusions
In our small but well phenotyped cohort of mechanically ventilated COVID‐19 patients, we found some ‘atypical’ ARDS features, either related to coronavirus itself or to the general characteristics of affected individuals (elderly patients with cardiovascular comorbidities), including a blunted hypoxic pulmonary vasoconstriction with high cardiac output and ‘unimpeded’ high LV filling pressure. These alterations may promote a vicious circle where the increase of PAWP might contribute to lung stiffening and to the severity of the respiratory insufficiency.
Supporting information
Table S1. Hemodynamic and ventilatory characteristics of patients with acute respiratory distress syndrome from the literature.
Table S2. Mechanical ventilation characteristics and gas exchange data of survivors and non‐survivors with COVID‐19.
Table S3. Invasive haemodynamic data of survivors and non‐survivors with COVID‐19.
Methods S1. Supplementary methods.
Figure S1. Patients' flow. COVID‐19, coronavirus disease 2019; ICU, intensive care unit; PE, pulmonary embolism.
Figure S2. Inverse relationship between intrapulmonary shunt (Qs/Qt) and lung compliance.
Video S1. Representative echocardiographic clips from two of our mechanically ventilated COVID‐19 patients.
Video S2. Representative echocardiographic clips from two of our mechanically ventilated COVID‐19 patients.
Acknowledgements
The authors would like to thank FROM (Fondazione per la Ricerca Ospedale Maggiore) for the technical support.
Funding
This study was funded by the Italian Ministry of Health (Progetti di Ricerca Corrente, IRCCS).
Conflict of interest: S.C. received the grant ‘Vera Srebot’ from the ‘Fondazione CNR/Regione Toscana per la Ricerca Medica e di Sanità Pubblica’. C.B. is the recipient of a research grant from the European Society of Cardiology. All other authors have nothing to disclose.
References
- 1. Senni M. COVID‐19 experience in Bergamo, Italy. Eur Heart J 2020;41:1783–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fagiuoli S, Lorini FL, Remuzzi G; Covid‐19 Bergamo Hospital Crisis Unit . Adaptations and lessons in the province of Bergamo. N Engl J Med 2020;382:e71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sanfilippo F, Bignami E, Lorini FL, Astuto M. The importance of a "socially responsible" approach during COVID‐19: the invisible heroes of science in Italy. Crit Care 2020;24:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, Cereda D, Coluccello A, Foti G, Fumagalli R, Iotti G, Latronico N, Lorini L, Merler S, Natalini G, Piatti A, Ranieri MV, Scandroglio AM, Storti E, Cecconi M, Pesenti A; COVID‐19 Lombardy ICU Network . Baseline characteristics and outcomes of 1591 patients infected with SARS‐CoV‐2 admitted to ICUs of the Lombardy region, Italy. JAMA 2020;323:1574–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Thompson BT, Chambers RC, Liu KD. Acute respiratory distress syndrome. N Engl J Med 2017;377:562–572. [DOI] [PubMed] [Google Scholar]
- 6. Ryan D, Frohlich S, McLoughlin P. Pulmonary vascular dysfunction in ARDS. Ann Intensive Care 2014;4:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gattinoni L, Coppola S, Cressoni M, Busana M, Rossi S, Chiumello D. Covid‐19 does not lead to a "typical" acute respiratory distress syndrome. Am J Respir Crit Care Med 2020;201:1299–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lang M, Som A, Mendoza DP, Flores EJ, Reid N, Carey D, Li MD, Witkin A, Rodriguez‐Lopez JM, Shepard JAO, Little BP. Hypoxaemia related to COVID‐19: vascular and perfusion abnormalities on dual‐energy CT. Lancet Infect Dis 2020;20:1365–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Archer SL, Sharp WW, Weir EK. Differentiating COVID‐19 pneumonia from acute respiratory distress syndrome and high altitude pulmonary edema: therapeutic implications. Circulation 2020;142:101–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Clerkin KJ, Fried JA, Raikhelkar J, Sayer G, Griffin JM, Masoumi A, Jain SS, Burkhoff D, Kumaraiah D, Rabbani L, Schwartz A, Uriel N. Coronavirus disease 2019 (COVID‐19) and cardiovascular disease. Circulation 2020;141:1648–1655. [DOI] [PubMed] [Google Scholar]
- 11. Bonow RO, Fonarow GC, O'Gara PT, Yancy CW. Association of coronavirus disease 2019 (COVID‐19) with myocardial injury and mortality. JAMA Cardiol 2020;5:751–753. [DOI] [PubMed] [Google Scholar]
- 12. Fried JA, Ramasubbu K, Bhatt R, Topkara VK, Clerkin KJ, Horn E, Rabbani LR, Brodie D, Jain SS, Kirtane AJ, Masoumi A, Takeda K, Kumaraiah D, Burkhoff D, Leon M, Schwartz A, Uriel N, Sayer G. The variety of cardiovascular presentations of COVID‐19. Circulation 2020;141:1930–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Szekely Y, Lichter Y, Taieb P, Banai A, Hochstadt A, Merdler I, Gal Oz A, Rothschild E, Baruch G, Peri Y, Arbel Y, Topilsky Y. The spectrum of cardiac manifestations in coronavirus disease 2019 (COVID‐19) – a systematic echocardiographic study. Circulation 2020;142:342–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Poissy J, Goutay J, Caplan M, Parmentier E, Duburcq T, Lassalle F, Jeanpierre E, Rauch A, Labreuche J, Susen S; Lille ICU Haemostasis COVID‐19 Group . Pulmonary embolism in COVID‐19 patients: awareness of an increased prevalence. Circulation 2020;142:184–186. [DOI] [PubMed] [Google Scholar]
- 15. Azarian R, Wartski M, Collignon MA, Parent F, Hervé P, Sors H, Simonneau G. Lung perfusion scans and hemodynamics in acute and chronic pulmonary embolism. J Nucl Med 1997;38:980–983. [PubMed] [Google Scholar]
- 16. Vesconi S, Rossi GP, Pesenti A, Fumagalli R, Gattinoni L. Pulmonary microthrombosis in severe adult respiratory distress syndrome. Crit Care Med 1988;16:111–113. [DOI] [PubMed] [Google Scholar]
- 17. Zapol WM, Snider MT. Pulmonary hypertension in severe acute respiratory failure. N Engl J Med 1977;296:476–480. [DOI] [PubMed] [Google Scholar]
- 18. Zimmerman GA, Morris AH, Cengiz M. Cardiovascular alterations in the adult respiratory distress syndrome. Am J Med 1982;73:25–34. [DOI] [PubMed] [Google Scholar]
- 19. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015;16:233–270. [DOI] [PubMed] [Google Scholar]
- 20. Caravita S, Faini A, Carolino D'Araujo S, Dewachter C, Chomette L, Bondue A, Naeije R, Parati G, Vachiéry JL. Clinical phenotypes and outcomes of pulmonary hypertension due to left heart disease: role of the pre‐capillary component. PLoS One 2018;13:e0199164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gattinoni L, Chiumello D, Caironi P, Busana M, Romitti F, Brazzi L, Camporota L. COVID‐19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med 2020;46:1099–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Henderson WR, Chen L, Amato MB, Brochard LJ. Fifty years of research in ARDS. Respiratory mechanics in acute respiratory distress syndrome. Am J Respir Crit Care Med 2017;196:822–833. [DOI] [PubMed] [Google Scholar]
- 23. Morrell NW, Nijran KS, Biggs T, Seed WA. Magnitude and time course of acute hypoxic pulmonary vasoconstriction in man. Respir Physiol 1995;100:271–281. [DOI] [PubMed] [Google Scholar]
- 24. Lai CC, Jou MJ, Huang SY, Li SW, Wan L, Tsai FJ, Lin CW. Proteomic analysis of up‐regulated proteins in human promonocyte cells expressing severe acute respiratory syndrome coronavirus 3C‐like protease. Proteomics 2007;7:1446–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, Vanstapel A, Werlein C, Stark H, Tzankov A, Li WW, Li VW, Mentzer SJ, Jonigk D. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid‐19. N Engl J Med 2020;383:120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Guo J, Huang Z, Lin L, Lv J. Coronavirus disease 2019 (COVID‐19) and cardiovascular disease: a viewpoint on the potential influence of angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers on onset and severity of severe acute respiratory syndrome coronavirus 2 infection. J Am Heart Assoc 2020;9:e016219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wolsk E, Bakkestrøm R, Thomsen JH, Balling L, Andersen MJ, Dahl JS, Hassager C, Møller JE, Gustafsson F. The influence of age on hemodynamic parameters during rest and exercise in healthy individuals. JACC Heart Fail 2017;5:337–346. [DOI] [PubMed] [Google Scholar]
- 28. Ellul MA, Benjamin L, Singh B, Lant S, Michael BD, Easton A, Kneen R, Defres S, Sejvar J, Solomon T. Neurological associations of COVID‐19. Lancet Neurol 2020;19:767–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Reddy YN, Melenovsky V, Redfield MM, Nishimura RA, Borlaug BA. High‐output heart failure: a 15‐year experience. J Am Coll Cardiol 2016;68:473–482. [DOI] [PubMed] [Google Scholar]
- 30. Vachiéry JL, Tedford RJ, Rosenkranz S, Palazzini M, Lang I, Guazzi M, Coghlan G, Chazova I, De Marco T. Pulmonary hypertension due to left heart disease. Eur Respir J 2019;53:1801897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vonk Noordegraaf A, Chin KM, Haddad F, Hassoun PM, Hemnes AR, Hopkins SR, Kawut SM, Langleben D, Lumens J, Naeije R. Pathophysiology of the right ventricle and of the pulmonary circulation in pulmonary hypertension: an update. Eur Respir J 2019;53:1801900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tedford RJ, Hassoun PM, Mathai SC, Girgis RE, Russell SD, Thiemann DR, Cingolani OH, Mudd JO, Borlaug BA, Redfield MM, Lederer DJ, Kass DA. Pulmonary capillary wedge pressure augments right ventricular pulsatile loading. Circulation 2012;125:289–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Verhoeff K, Mitchell JR. Cardiopulmonary physiology: why the heart and lungs are inextricably linked. Adv Physiol Educ 2017;41:348–353. [DOI] [PubMed] [Google Scholar]
- 34. Oudit GY, Pfeffer MA. Plasma angiotensin‐converting enzyme 2: novel biomarker in heart failure with implications for COVID‐19. Eur Heart J 2020;41:1818–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Huang L, Zhao P, Tang D, Zhu T, Han R, Zhan C, Liu W, Zeng H, Tao Q, Xia L. Cardiac involvement in recovered COVID‐19 patients identified by magnetic resonance imaging. J Am Coll Cardiol Img 2020;13:2330–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Obokata M, Reddy YN, Pislaru SV, Melenovsky V, Borlaug BA. Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation 2017;136:6–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Reddy YN, Obokata M, Wiley B, Koepp KE, Jorgenson CC, Egbe A, Melenovsky V, Carter RE, Borlaug BA. The haemodynamic basis of lung congestion during exercise in heart failure with preserved ejection fraction. Eur Heart J 2019;40:3721–3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Senni M, Caravita S, Paulus WJ. Do existing definitions identify subgroup phenotypes or reflect the natural history of heart failure with preserved ejection fraction? Circulation 2019;140:366–369. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Hemodynamic and ventilatory characteristics of patients with acute respiratory distress syndrome from the literature.
Table S2. Mechanical ventilation characteristics and gas exchange data of survivors and non‐survivors with COVID‐19.
Table S3. Invasive haemodynamic data of survivors and non‐survivors with COVID‐19.
Methods S1. Supplementary methods.
Figure S1. Patients' flow. COVID‐19, coronavirus disease 2019; ICU, intensive care unit; PE, pulmonary embolism.
Figure S2. Inverse relationship between intrapulmonary shunt (Qs/Qt) and lung compliance.
Video S1. Representative echocardiographic clips from two of our mechanically ventilated COVID‐19 patients.
Video S2. Representative echocardiographic clips from two of our mechanically ventilated COVID‐19 patients.


