Abstract
Objectives
Information on the recently COVID‐19‐associated pulmonary aspergillosis (CAPA) entity is scarce. We describe eight CAPA patients, compare them to colonised ICU patients with coronavirus disease 2019 (COVID‐19), and review the published literature from Western countries.
Methods
Prospective study (March to May, 2020) that included all COVID‐19 patients admitted to a tertiary hospital. Modified AspICU and European Organization for Research and Treatment of Cancer/Mycoses Study Group (EORTC/MSG) criteria were used.
Results
COVID‐19‐associated pulmonary aspergillosis was diagnosed in eight patients (3.3% of 239 ICU patients), mostly affected non‐immunocompromised patients (75%) with severe acute respiratory distress syndrome (ARDS) receiving corticosteroids. Diagnosis was established after a median of 15 days under mechanical ventilation. Bronchoalveolar lavage was performed in two patients with positive Aspergillus fumigatus cultures and galactomannan (GM) index. Serum GM was positive in 4/8 (50%). Thoracic CT scan findings fulfilled EORTC/MSG criteria in one case. Isavuconazole was used in 4/8 cases. CAPA‐related mortality was 100% (8/8). Compared with colonised patients, CAPA subjects were administered tocilizumab more often (100% vs. 40%, p = .04), underwent longer courses of antibacterial therapy (13 vs. 5 days, p = .008), and had a higher all‐cause mortality (100% vs. 40%, p = .04). We reviewed 96 similar cases from recent publications: 59 probable CAPA (also putative according modified AspICU), 56 putative cases and 13 colonisations according AspICU algorithm; according EORTC/MSG six proven and two probable. Overall, mortality in the reviewed series was 56.3%.
Conclusions
COVID‐19‐associated pulmonary aspergillosis must be considered a serious and potentially life‐threatening complication in patients with severe COVID‐19 receiving immunosuppressive treatment.
Keywords: antifungal therapy, Aspergillus infection, COVID‐19, fungal diseases, fungal infections, intensive care, invasive pulmonary aspergillosis, SARS‐CoV‐2 infection
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19) patients with acute respiratory distress syndrome (ARDS), usually admitted to intensive care units (ICUs), frequently receive corticosteroids, broad‐spectrum antibiotics and immunomodulatory agents. Under these circumstances, it is not surprising that the patients develop secondary complications like invasive pulmonary aspergillosis (IPA). A new term has been coined for this condition: COVID‐19‐associated pulmonary aspergillosis (CAPA).
COVID‐19‐associated pulmonary aspergillosis has been described to occur in patients that do not fulfil typical risk factors for IPA (such as underlying haematological malignancy or neutropenia). 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 The criteria described by Blot et al and Schauwvlieghe et al may help differentiate between patients who are just colonised with Aspergillus spp. and real CAPA cases.
In this work, we aim to report a series of eight CAPA cases among our COVID‐19 patients, review other cases reported in literature, and compare invasive and colonisation episodes.
2. MATERIALS AND METHODS
2.1. Hospital setting
This study was conducted at a tertiary hospital in Madrid (Spain) during the COVID‐19 pandemic, between March 1 and May 31, 2020. The normal capacity of our hospital is 1200 beds, 67 in adults ICUs. With the pandemic, the number of beds was increased to 1572 beds in hospitalisation areas and 135 adult ICU beds.
The Department of Clinical Microbiology and Infectious Diseases has maintained a surveillance and registry of invasive fungal infections and an antifungal stewardship programme since 2010.
2.2. Study design and data collection
We carried out a prospective single‐centre, observational study. Every COVID‐19 adult patient with suspected IPA, based on the isolation of Aspergillus spp. from one or more respiratory samples, were followed‐up and classified as proven/probable/putative aspergillosis or colonisation.
Clinical records were reviewed, including demographic, clinical and radiological features, treatment and outcomes. The number of IPA cases during the COVID‐19 pandemic was compared to the previous incidence at our centre.
Finally, we reviewed the literature regarding CAPA cases in which enough individual data were reported.
2.3. Definitions
COVID‐19 confirmed cases were those with a positive result in the reverse transcription‐polymerase chain reaction (RT‐PCR) assay for severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) in respiratory samples (nasopharyngeal swab, tracheal aspirate, bronchial aspirate or bronchoalveolar lavage fluid).
COVID‐19‐associated CAPA was classified as possible/probable/proven aspergillosis according to the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) if the patient was immunosuppressed. 19 In non‐immunocompromised patients, the modified AspICU algorithm was used. 20 Accordingly, patients could be classified as:
Proven aspergillosis: idem to EORTC/MSG criteria. 19
Putative aspergillosis: all four criteria had to be met: (i) Aspergillus‐positive lower respiratory tract culture; (ii) compatible signs and symptoms—one or more of the following: fever refractory to at least three days of appropriate antibiotic therapy, recrudescent fever after a period of defervescence, pleuritic chest pain, pleuritic rub, dyspnoea, haemoptysis, worsening respiratory insufficiency despite appropriate antibiotic therapy and ventilatory support; (iii) abnormal chest X‐ray or computed tomography (CT) scan; (iv) either presence of host risk factors—one of the following (neutropenia, underlying haematological or oncological malignancy, glucocorticoid treatment with prednisone equivalent >20 mg/day, congenital or acquired immunodeficiency) or microbiological criterion with Aspergillus‐positive culture of bronchoalveolar lavage (BAL) fluid without bacterial growth and a positive cytological smear showing branching hyphae. Galactomannan (GM) detection values in BAL and serum were added to this algorithm as a microbiological criterion (modified AspICU). 20
Based on the literature, we accepted COVID‐19‐induced ARDS as a host risk factor. 7
Aspergillus colonisation: Isolation of Aspergillus spp. in one or more respiratory samples for which the former criteria was not fulfilled.
Acute respiratory distress syndrome diagnosis was based on the Berlin definition 21 which requires the occurrence of timing, radiological, and clinical criteria.
COVID‐19‐associated pulmonary aspergillosis‐related mortality was defined as death with current signs of IPA. 22
Obesity was defined as a body mass index >25 kg/m2.
2.4. Microbiology data
Presence of SARS‐CoV‐2 in respiratory samples was confirmed by RT‐PCR (TaqManTM 2019‐nCoV Assay; Applied Biosystems). Detection of 1,3‐β‐d‐glucan (BDG) in serum was performed with the Wako β‐glucan test (Fujifilm Wako Pure Chemical Corporation). A cut‐off value of 11 pg/mL was used. GM testing was performed using Platelia™ Aspergillus (Bio‐Rad Laboratories) with a cut‐off value of ≥0.5 in serum and ≥1.0 in BAL. Serum GM and BDG were performed in cases of suspected fungal infection. Culture of respiratory samples was performed upon clinical request on Sabouraud dextrose agar and BHI (Brain Heart Infusion) agar.
Selected isolates – one isolate per species and patient – were molecularly identified after amplification and further sequencing of the beta‐tubulin gene. 23 Antifungal susceptibility testing to amphotericin B, itraconazole, voriconazole, posaconazole (Sigma‐Aldrich), and isavuconazole (Basilea Pharmaceutica International Ltd.) was performed according to EUCAST 9.3.2 methodology. 24 Resistant isolates defined based on updated 2020 EUCAST breakpoints. 25
2.5. Statistical analysis
Clinical records were recorded onto a data collection form and transferred to an anonymised database for statistical analysis with the IBM SPSS Statistics package version 24.0 for Macintosh. The Shapiro‐Wilk normality test was applied; considering that some variables did not have a normal distribution, non‐parametric tests were performed. Categorical variables are presented as frequencies and percentages. Continuous variables results are expressed as medians and interquartile range (IQR).
To detect significant differences between groups, the Mann‐Whitney U test for continuous variables and χ2 or Fisher's exact test (when at least one expected frequency in a fourfold table is less than five) for categorical variables were used.
2.6. Ethical approval
The study was approved by the Research Ethics Committee with Medicines of Hospital General Universitario Gregorio Marañón (study MICRO.HGUGM.2020‐027) and granted a waiver of informed consent from study participants.
3. RESULTS
During the study period, 2723 patients were admitted to our hospitalisation wards and of those, 239 to ICUs (8.7%) due to COVID‐19.
3.1. Aspergillus spp. cultures and invasive infection during the COVID‐19 pandemic
Culture of respiratory samples yielded positive results for Aspergillus spp. in 17 COVID‐19 patients, corresponding to eight cases with probable CAPA and nine colonisations (52.9%). CAPA prevalence was 0.3% among all COVID‐19 hospitalised patients (8/2723) and 3.3% in COVID‐19 patients admitted to the ICU (8/239).
When a comparison of IPA cases was made considering monthly records, the mean number increased 4.4 fold in non‐immunocompromised patients (from 0.5 cases/month in 2019 to 2.2 cases/month in 2020) during the pandemic (Figure 1), and all were CAPA cases. The incidence of IPA in haematological patients doubled (0.3 cases/month in 2019 and 0.6 cases/month in 2020).
FIGURE 1.
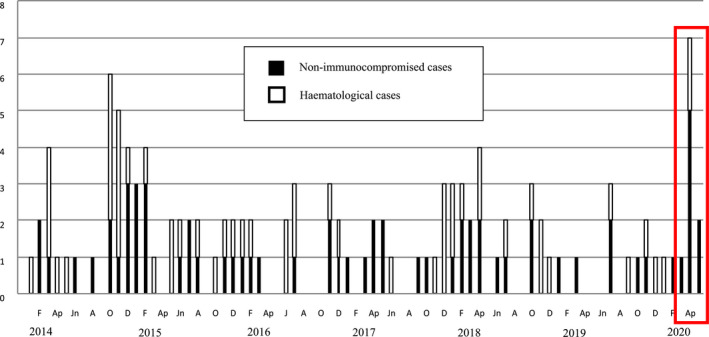
Monthly registry of invasive pulmonary aspergillosis in Hospital General Universitario Gregorio Marañón (COMIC study group)
3.2. Description of COVID‐19‐associated pulmonary aspergillosis patients
The characteristics of the eight CAPA patients are detailed in Table 1. Median age was 64.5 years (IQR 61.0–73.5) and 75% were male. Underlying conditions were cardiovascular disease (7/8), chronic kidney disease (3/8) and severe asthma (2/8). Obesity was present in 4/8 and it was the only pre‐existing comorbidity in one of the cases. Two patients were immunosuppressed (25%): one patient with past chronic lymphocytic leukaemia without active treatment and one liver transplant recipient.
TABLE 1.
Epidemiological and clinical characteristics of 8 patients with COVID‐19‐associated pulmonary aspergillosis
| Age (years)/Gender | Medical History | ARDS Severity (P/F ratio) | Cortico steroids a (days) | Prone position (number of times) | CRRT | Concomitant/previous superinfection (days to CAPA) | Antimicrobial treatment before IPA | Anti‐COVID‐19 treatment | Thoracic CT scan | EORTC/MSG criteria | AspICU | Modified AspICU | Microbiology results | Antifungal treatment | CAPA‐related mortality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
#1 74/M |
HTA, COPD | Severe (85mmHg) | Yes (8) | Yes (4) | Yes | CoNS CR‐BSI (−3) |
Ceftriaxone Piperacillin/tazobactam Ceftaroline fosamil |
LPV/r → Remdesivir Hydroxychloroquine IFN‐B1b |
Not performed | N/C | Putative | N/C |
TA: Aspergillus fumigatus Serum: GM: 0.10/BDG: 2.6 |
No | Yes |
|
#2 52/M |
Obesity | Severe (93 mmHg) | Yes (8) | Yes (7) | No | No |
Ceftriaxone Piperacillin/tazobactam Linezolid |
LPV/r Hydroxychloroquine IFN‐B1b |
Not performed | N/C | Putative | Putative |
BAS: A. citrinoterreus Serum: GM: 1.94/BDG: 2.6 |
VRC → ISV | Yes |
|
#3 66/M |
HTA, obesity | Moderate (102 mmHg) | Yes (11) | Yes (7) | No | No |
Ceftriaxone Meropenem Linezolid Trimethoprim/sulfamethoxazole |
LPV/r Hydroxychloroquine IFN‐B1b |
Not performed | N/C | Putative | Putative |
BAL: A. fumigatus (GM 2.80) Serum: GM: 0.21/BDG: 2.5 |
VRC → ISV | Yes |
|
#4 63/M |
HTA, CKD, asthma, CLL without active treatment | Severe (95 mmHg) | Yes (25) | Yes (3) | No | Pseudomonas aeruginosa VAP and BSI (−2) |
Ceftriaxone Linezolid Meropenem Ceftolozane/tazobactam |
LPV/r Hydroxychloroquine Azithromycin |
Bilateral patchy areas of GGO and pneumothorax | Probable | Putative | Putative |
BAS: A. fumigatus + A. awamori + A. terreus Serum: GM: 0.56/BDG: 5.4 |
ISV | Yes |
|
#5 73/M |
HTA | Severe (74 mmHg) | Yes (8) | Yes (2) | Yes | No |
Ceftriaxone Piperacillin/tazobactam Meropenem Linezolid |
LPV/r Hydroxychloroquine Azithromycin |
Bilateral patchy areas of GGO and pneumothorax | N/C | Putative | N/C |
BAS: A. fumigatus Serum: GM: 0.12/BDG: 2.6 |
L‐AMB | Yes |
|
#6 60/F |
HTA, CKD, asthma | Severe (98 mmHg) | Yes (18) | Yes (4) | Yes | Enterococcus faecium UTI (−5) |
Ceftriaxone Meropenem Linezolid Vancomycin |
LPV/r Hydroxychloroquine IFN‐B1b |
Bilateral patchy areas of GGO and lung fibrosis | N/C | Putative | Putative |
BAS: A. fumigatus Serum: GM: 0.56/BDG: 17.8 |
L‐AMB → ISV | Yes |
|
#7 74/M |
HTA, obesity | Severe (73 mmHg) | Yes (37) | No | Yes |
Enterococcus faecium BSI (−33) CMV reactivation (−24) |
Ceftriaxone Piperacillin/tazobactam Meropenem Vancomycin Ganciclovir |
LPV/r Hydroxychloroquine IFN‐B1b |
Bilateral patchy areas of GGO and cavitated nodule | Probable | Putative | Putative |
BAS: A. lentulus Serum: GM: 1.94/BDG: 15.7 |
No | Yes |
|
#8 62/F |
HTA, DM, obesity, CKD, CNS disease, NAFLD with liver SOT recipient‐ (08/02/20) | Severe (74 mmHg) | Yes (30) | No | No | CDI (−14) |
Ceftriaxone Meropenem Oral vancomycin |
Hydroxychloroquine Azithromycin |
Bilateral patchy areas of GGO | Probable | Putative | Putative |
BAL: A. fumigatus (GM > 7) Serum: GM: 0.05/BDG: 3.7 |
No | Yes |
Abbreviations: ARDS, Acute Respiratory Distress Syndrome; BAL, Bronchoalveolar Lavage fluid; BAS, Bronchial Aspirate; BDG, 1,3 β‐D‐glucan; BSI, Bloodstream Infection; CAPA, COVID‐19‐associated pulmonary aspergillosis; CDI, Clostridioides difficile infection; CKD, Chronic Kidney Disease; CLL, Chronic Lymphocytic Leukaemia; CoNS, Coagulase‐Negative Staphylococci; CR‐BSI, Catheter‐related Bloodstream Infection; CRRT, Continuous Renal Replacement Therapy; CSP, caspofungin; DM, Diabetes Mellitus; GGO, Ground‐Glass Opacities; GM, Galactomannan; HTA, Hypertension; ISV, isavuconazole; L‐AMB, liposomal Amphotericin B; LPV/r, Lopinavir/ritonavir; N/C, Not Classifiable; NAFLD, Non‐Alcoholic Fatty Liver Disease; P/F, PaO2/FiO2; TA, Tracheal Aspirate; UTI, Urinary Tract Infection; VAP, ventilator‐associated pneumonia; VRC, voriconazole.
Values marked in bold means that are positive results.
Equivalent >20 mg/day of prednisone.
All CAPA patients were admitted to the ICU and received mechanical ventilation (MV) after a median of two days (IQR 2.0–14.3) since hospital admission due to ARDS [Median 89 mmHg (IQR 74.0–97.3) P/F ratio]. Median duration of ICU stay plus MV was eight days until the first Aspergillus spp. isolation and 15 days until the definite CAPA diagnosis.
Previous or concomitant bacterial and/or viral infections were found in 5/8 patients (62.5%), as described in Table 1. All patients received antimicrobials and corticosteroids (≥20 mg/day of prednisone equivalent) during a median of 13 and 10 days before Aspergillus spp. isolation, respectively. Four patients required continuous renal replacement therapy before CAPA diagnosis.
Regarding COVID‐19 treatment, all patients received hydroxychloroquine [median 12 days (IQR 11.0–12.5)] plus lopinavir/ritonavir [median 12 days (IQR 11.0–13.0)]. Additionally, five patients received interferon beta‐1b (IFN‐β‐1b) every 48 h [median 7 days (IQR 5.0–7.5)] as established by the local protocol for severe COVID‐19 pneumonia. Due to IFN‐β‐1b stock depletion and updated scientific evidence, 26 IFN‐β‐1b was replaced by azithromycin in the remaining three patients. All CAPA patients received tocilizumab.
Thoracic CT scans were performed in five patients showing bilateral ground‐glass opacities (five patients), pneumothorax (two patients), lung fibrosis and a cavitary nodule (one patient in each case).
Microbiological results are detailed in Table 1. All patients had positive Aspergillus fumigatus (6/8) or other species (A. lentulus, A. citrinoterreus) respiratory tract cultures. Direct examination of respiratory samples with calcofluor white staining was not performed to avoid contamination of laboratory personnel/technician. BAL was performed in two patients with positive A. fumigatus culture and positive GM index (2.8 and >7). Serum GM was positive in 4/8 patients (50%) and serum BDG in 2/8 (25%). All studied isolates were fully susceptible to the azoles tested, with the exception of the A. lentulus isolate that showed resistance to voriconazole (Table S1).
Antifungal (AF) treatment was administered to 5/8 patients (the three non‐treated patients were in ominous clinical state and died within the first 48 hours following CAPA diagnosis). Isavuconazole was given to four patients due to difficulties with voriconazole therapeutic drug monitoring (TDM) during the COVID‐19 pandemic. Median duration of AF treatment was 11 days (IQR 8–17).
All patients died supposedly due to CAPA, a median of 4 days (IQR 2–15) since diagnosis.
3.3. Differences between COVID‐19‐associated pulmonary aspergillosis and Aspergillus colonisation
All CAPA patients were admitted to the ICU. Thus, we compared the characteristics of the eight CAPA patients with the five COVID‐19 patients also in the ICU but who were only colonised by Aspergillus spp. (AC) (Table 2). Patients with CAPA stayed significantly longer on MV before obtaining a positive culture for Aspergillus spp. (15 days vs. 3 days, p = .02); had received tocilizumab more frequently (100% vs. 40%, p = .04), and longer courses of antibacterial therapy before the isolation of Aspergillus spp. (13 days vs. 5 days, p = .008). There were no differences on serum GM and BDG positivity. All‐cause mortality was higher in CAPA patients (100% vs. 40%, p = .04).
TABLE 2.
Comparison between COVID‐19 patients colonised by Aspergillus spp. (AC) and with COVID‐19‐associated pulmonary aspergillosis (CAPA)
|
CAPA patients n = 8 |
AC n = 5 |
p | |
|---|---|---|---|
| Age years – mean (SD) | 64.5 (60.5–73.8) | 64 (42.5–74.0) | .77 |
| Gender (male %) | 6 (75.0) | 5 (100) | .49 |
| Comorbidity | |||
| Hypertension | 7 (87.5) | 4 (80.0) | 1.00 |
| Diabetes mellitus | 1 (12.5) | 1 (20.0) | 1.00 |
| COPD | 1 (12.5) | 0 | 1.00 |
| Asthma | 2 (25.0) | 0 | .49 |
| Obesity | 4 (50.0) | 4 (80.0) | .56 |
| Lymphocytes at admission (cell/mm3) | 700 (700–1200) | 500 (400–1000) | .22 |
| Immunomodulation | |||
| Tocilizumab | 8 (100) | 2 (40.0) | .04 |
| Corticosteroids (>=20 mg/day Prednisone) | 8 (100) | 4 (80.0) | .38 |
| Days of corticosteroids till diagnosis | 10 (7.3–26.3) | 5.5 (0.5–11.3) | .20 |
| Time since COVID‐19 detection a (median days, IQR) | 21 (15.8–37.5) | 6 (3.0–10.5) | .003 |
| ICU stay before diagnosis a (median days, IQR) | 15 (9.75–19.0) | 3 (2.0–8.0) | .02 |
| MV before diagnosis a (median days, IQR) | 15 (9.75–19.0) | 3 (2.0–8.0) | .02 |
| Antimicrobial therapy before diagnosis a (median days, IQR) | 13 (10.5–18.8) | 5 (2.0–5.5) | .008 |
| Microbiological findings | |||
| Aspergillus fumigatus | 6 (75.0) | 3 (60.0) | .51 |
| Positive serum BDG | 2/6 (33.3) | 0/2 | 1.00 |
| Positive serum GM | 3/7 (42.9) | 0/3 | .47 |
| Outcome | |||
| Overall mortality | 8 (100) | 2 (40.0) | .04 |
| Days from diagnosis a till death | 4 (2.0–15.0) | 21 (16.0–26.0) | .08 |
p values comparing Aspergillus colonisation and invasive aspergillosis are tested by Kruskal–Wallis (continuous variables) or Chi‐square test (categorical variables).
p values numbers marked in bold indicate numbers that are significant (p<.05).
Abbreviations: AC, Aspergillus colonisation; BDG, 1,3 β‐D‐glucan; CAPA, COVID‐19‐associated pulmonary aspergillosis; COPD, chronic obstructive pulmonary disease; GM, galactomannan; ICU, intensive care unit; IQR, interquartile range; MV, mechanical ventilation; SD, standard deviation.
Until diagnosis of CAPA or until Aspergillus culture (AC).
3.4. Review of the literature
Table 3 summarises the cases reported in Western literature, containing the ones described here. Overall, 96 potentially eligible for CAPA cases have been reported, from which 93 had been admitted in ICUs. The disease was detected after a mean of 7 days of stay in the ICU. Seven patients (7.3%) had EORTC/MSG host risk factors and eight fulfilled criteria for IPA (8.3%), six proven and two probable. We analysed each case individually and classified them according to the recent definition of influenza‐associated pulmonary aspergillosis 27 and also according to previous definition of putative IPA (AspICU and modified AspICU): 59 cases fulfilled probable CAPA definition, same as modified AspICU algorithm, and according to AspICU algorithm, 56 cases are putative and 13 would be colonisations. Six patients are considered proven in all classifications. The remaining patients who do not meet the definitions in the different categories could be also considered as colonisations.
TABLE 3.
Reports of invasive pulmonary aspergillosis in patients with COVID‐19 from Western countries
| Reference | Number of patients included | ICU stay before CAPA‐days (IQR) | CAPA definition (Verweij) | AspICU (Blot) | Modified AspICU(Schauwvlieghe) | EORTC/MSG | Aspergillus LRT culture |
BAL GM (+) |
Serum GM (+) | Serum BDG (+) | CT scan performed and results | AF treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bartoletti M. (Italy) 1 | 30/108 ICU patients (27.7%) | 4 (2–8) | 30 Probable | 19 Putative | 30 Putative | 0 |
15 A. fumigatus 3 A. niger 1 A. flavus |
30/30 | 1/30 | NP | NR | 13 VRC |
44% 30‐day mortality rate |
| Alanio A. (France) 2 | 9/27 ICU patients (33.3%) | NR | 6 Probable |
5 Putative 2 Colonised |
6 Putative | 1 Probable | 7/9 A. fumigatus in (5 BAL, 2 TA) | 1/7 | 1/9 | 4/7 |
3/9. Bilateral COVID‐19 pneumonia One with peripheral nodule |
1 VRC 1 CSP |
3/9 Deaths (33.3%) |
| Falces‐Romero I. (Madrid, Spain) 3 | 10 COVID‐19 patients with positive A. fumigatus culture (7 under MV) | NR | 0 | 7 Putative | 0 | 1 Probable |
9 A. fumigatus (BAS) 1 A. nidulans (BAS) |
2/2 | 1/2 | NP | 1/10 Interstitial infiltrates and GGO |
2 VRC 1 VRC + CSP 1 L‐AMB 1 L‐AMB → ISV 1 L‐AMB → VRC 1 ANI → L‐AMB 1 MICA → VRC→ISV → L‐AMB |
7/10 Deaths (70%) |
|
Machado M. (Spain) Present report |
8/239 ICU patient (3.3%) | 15 (9.7–19.0) | 6 Probable | 8 Putative | 6 Putative | 3 Probable |
5/8 A. fumigatus (2 BAS, 2 BAL, 1 TA); 1 A. citrinoterreus (BAS); 1 A. lentulus (BAS) 1 A. fumigatus + A. awamori + A. terreus (BAS) |
2/8 | 4/8 | 2/8 |
5/8. Typical bilateral COVID‐19 pneumonia One pneumothorax and other with cavitated nodule |
3 ISV 2 L‐AMB (one switched to ISV) |
8/8 Deaths (100%) |
| Gangneux JP. (France) 4 | 7/45 MV patients (15.5%) | NR | 3 Probable | 9 Putative | 3 Putative | 0 | 7 patients with positive Aspergillus spp cultures and/or PCR | NP | 2/7 | NP | Diffuse reticular or alveolar opacities, nodules in half of putative cases, and non‐specific signs in colonised patients. | VRC or ISV |
2/7 Detahs (28.5%) |
| Rutsaert L. (Belgium) 5 | 7/34 ICU patients (20.6%) | 8 (5–13) |
4 Proven 2 Probable |
4 Proven 1 Putative 1 Colonised |
4 Proven 2 Putative |
4 proven |
5 A. fumigatus (5 BAL); 1 A. flavus (TA) 1 (‐) BAL |
5/6 | 1/6 | NP | 1/7; Results NR | 4 VRC (two switched to ISV) | 4/7 Deaths (57.1%) |
| Van Arkel A. (The Netherlands) 6 | 6/31 ICU patients (19.3%) | 5 (3–14) | 3 Probable |
2 Putative 3 Colonised 1 NC |
3 Putative | 0 |
5/6 A. fumigatus in (2 TA, 2 BAL, 1 sputum) 1 (‐) BAL |
2/3 NP in 3 patients |
0/3 | NP | 1/6; No signs of IFI |
5 VRC + Anidulaf. 1 L‐AMB |
3/6 Deaths (50%) |
| Koehler P. (Germany) 7 | 5/19 ICU patients (26.3%) | NR | 4 Probable |
1 Putative 2 Colonised |
4 Putative | 0 | 3/5 A. fumigatus (1 BAL, 2 TA) |
3/3 NP in 2 patients |
2/5 | NP | 5/5. Typical COVID‐19. pneumonia One patient with cavitated nodules and air crescent |
2 VRC 1 ISV 2 CSP switched to VRC |
3/5 Deaths (60%) |
| Lamoth F. (Switzerland) 8 | 3/80 MV patients (3.8%) | 7 (3–8) | 1 Probable | 3 colonised | 1 Putative | 0 | 3/3 A. fumigatus (BAS) | NP | 1/3 | 1/3 |
2/3 Multiple consolidations 1/3 Interstitial infiltrates and GGO |
3/3 VRC | 1/3 Deaths (33%) |
| Lahmer T. (Germany) 9 | 2 ICU patients | 5 and 6 | 2 Probable | 2 Putative | 2 Putative | 0 | A. fumigatus in 2 BAL | 2/2 | 1/2 | NP | 2/2; Typical signs of COVID‐19 pneumonia. No signs of IFI | 2 L‐AMB | 2/2 Deaths (100%) |
| Blaize M. (France) 10 | 1 ICU patient | 4 | 0 | Colonised | 0 | 0 | A. fumigatus (TA) | NP | Neg | Neg | NP | None | Death |
| Prattes J. (Austria) 11 | 1 ICU patient | 3 | 0 | Colonised | 0 | 0 | A. fumigatus (TA) | NP | Neg | Neg | Reversed halo sign | VRC | Death |
| Lescure F. (France) 12 | 1 ICU patient | NR | 0 | Colonised | 0 | 0 | A. flavus (TA) | NP | NP | NP | Pleural effusion, alveolar condensations, ground‐glass opacities, and pulmonary cysts | VRC switched to ISV | Death |
| Meijer (The Netherlands) 13 | 1 ICU patient | 1 | 0 | Putative | 0 | 0 | A. fumigatus (TA) azole resistant | Pos | Neg | Pos |
Bilateral GGO No specific suggestions of aspergillosis |
VRC + CSP→Oral VRC → L‐AMB | Death |
| Antinori S. (Italy) 14 | 1 ICU patient | 6 | Proven | Proven | Proven | Proven | A. fumigatus (BAL) | NP | Pos | NP | NP | L‐AMB → ISV | Death |
| Sharma A. (Australia) 15 | 1 ICU patient | 10 | 0 | Colonised | 0 | 0 | A. fumigatus (TA) | NP | NP | NP | NP | VRC | Alive |
| Mohamed A. (Ireland) 16 | 1 ICU patient | 3 | Probable | Colonised | Putative | 0 | A. fumigatus tri‐azole resistant (TA) | Pos (TA) | Pos | Pos | NP | L‐AMB | Death |
| Fernandez NB. (Argentina) 17 | 1 ICU patient | 25 | Probable | Putative | Putative | 0 | A. flavus (TA) | NP | Pos | NP | NP | ANI → VRC | Death |
| Santana MF. (Brasil) 18 | 1 ICU patient | 3 | Proven | Proven | Proven | Proven | Histopathological findings of Aspergillus spp in lungs (autopsy) confirmed by PCR. | NP | NP | NP | NP | None | Death |
| Overall, including present report | 96 patients (93 ICU) | Mean of 7 days |
6 CAPA proven 59 CAPA probable 31 NC |
6 proven 56 putative 13 colonised 21 NC |
6 proven 59 putative 31 NC |
6 proven 2 probable 88 NC |
61 A. fumigatus 7 Aspergillus spp 4 A. flavus 3 A. niger 1 A. citrinoterreus 1 A. terreus 1 A. awamori 1 A. lentulus 1 A. nidulans |
49/63 (77.8%) | 17/81 (21%) | 9/22 (40.9%) |
31/96 had CT scan Four with data suggestive of angioinvasive IFI (EORTC/MSG) |
62/96 treated (39 azoles, 10 L‐AMB, 5 VRC + ANI, 2 VRC + CSP, 6 candins) b | 54/96 deaths (56.3%) |
Abbreviations: AF, antifungal; ANI, Anidulafungin; BAL, bronchoalveolar lavage; BAS, bronchial aspirate; BDG, 1,3‐β‐D‐glucan; CAPA, COVID‐19‐associated pulmonary aspergillosis; CSP, caspofungin; GGO, ground‐glass opacities; GM, galactomannan; ICU, intensive care unit; ISV, isavuconazole; L‐AMB, liposomal Amphotericin B; MICA, micafungin; MV, mechanical ventilation; NC, Not classifiable; NP, not performed; NR, not reported; PIPA, putative invasive pulmonary aspergillosis; TA, tracheal aspirate; VRC, voriconazole.
Use of corticosteroids >0.3 mg/kg of prednisone during 3 weeks.
Antifungal used as first line.
The following species were isolated: 61 A. fumigatus, seven Aspergillus spp, four A. flavus, three A. niger, one A. citrinoterreus, one A. terreus, one A. lentulus, one A. nidulans and one A. awamori.
As for biomarker performance, BAL‐GM was positive in 49/63 (77.8%) and 17/81 (21%) in serum samples; BDG was positive in 9/22 (40.9%). Thoracic CT scan was performed in 31/96 cases and showed lesions compatible with angioinvasive IPA in four cases. Antifungal therapy was given to 62/96 patients, and overall mortality rate was 56.3% (54/96).
4. DISCUSSION
This is the fourth largest study in number of CAPA cases published in the literature and the second in Spain. CAPA was diagnosed in 0.3% of the 2723 patients with COVID‐19 hospitalised at our centre, accounting for 3.3% of the 239 patients in the ICU. All patients were under MV, had received tocilizumab and corticosteroids, and eventually died.
There are no strong diagnostic criteria for this type of cases as tissue‐proven diagnosis is rare in this scenario and CT scans are not sensitive enough. Accordingly, different algorithms and non‐uniform criteria have been used to classify these patients. 20 , 27 , 28 Analysing the reports case‐by‐case in the literature review, 21 cases (21.8%) with AspICU algorithm and 31 cases (32.3%) with modified AspICU and CAPA definition were not classifiable.
In our institution, we observed an important increase in the number of IPA cases in non‐immunosuppressed patients during the COVID‐19 pandemic. However, the incidence rate in ICU patients with COVID‐19 (3.3%) is lower than the reported in other series from Western countries (3.8%–33.3%) (Table 3). 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 In a case series from China, the rate of CAPA was 7.7% among 104 COVID‐19 patients and 30.7% among 26 ICU patients. 29 The main difference may rely on the fact that there was no patient selection in our study and we used as the denominator all patients admitted to an ICU during the pandemic months. Moreover, in some series, Aspergillus colonisation may have been misclassified as CAPA, since 5/9 patients survived despite not receiving AF treatment. 2 We believe that our figure of 3.3% reflects more accurately current CAPA incidence.
Our study indicates that CAPA develops in ICU patients with COVID‐19‐related ARDS Aspergillus colonisation occurs soon after the patient did placed under MV in COVID‐19 patients, while CAPA usually appears later (3 days vs. 15 days under MV). As usually seen in ICU aspergillosis, 20 , 30 only a small proportion of patients with CAPA fulfilled EORTC/MSG's criteria (eight out of 96 cases).
The physiopathology of CAPA infection remains undetermined. Immune dysregulation associated with ARDS 31 or its treatment (tocilizumab, corticosteroids) may predispose to infections with opportunists. 32 A recent study 33 analyses two possible explanations for this: first, the release of danger‐associated molecular patterns (DAMPs) in ARDS, which promote and exacerbate the immune and inflammatory response leading to lung injury. DAMPs have also been shown to regulate inflammation in fungal diseases; second, the involvement of IL‐1 and IL‐6 in immune dysregulation. Early hyperactivation of IL‐1 induced by SARS‐CoV‐2 infection may promote permissive inflammatory environment for developing fungal infection. Moreover, IL‐6 is also observed in epithelial cells following infection with A. fumigatus, suggesting that co‐infection may contribute to the increased levels of this cytokine in severe COVID‐19 patients. 34 The use of tocilizumab, a monoclonal antibody against IL‐6 receptor used as a therapeutic strategy in the immunomodulation of COVID‐19 patients, was detected in high concentrations in patients with CAPA. 1 Beside fungal infections, IL‐6 inhibition may increase the risk of tuberculosis or other viral aetiologies. 35 , 36 In our study, tocilizumab use was more common in infected than in colonised patients.
Risk factors described in CAPA patients include older age, lymphopenia, chronic respiratory diseases, corticosteroid therapy, antimicrobial therapy, MV or cytokine storm. 29 , 37 , 38
Due to the severity of the clinical situation, thoracic CT scans were performed only in 5/8 (62.5%) patients of our series, and globally in 31/96 patients (32.3%). Thoracic CT scans may be difficult to assess in patients with ARDS‐associated COVID‐19, 39 usually showing ground‐glass opacities, a crazy‐paving pattern and patchy consolidations. 40 As described in Table 3, typical COVID‐19 radiological findings are usually present in CAPA cases, sometimes with consolidating peribronchial patterns. However, classic findings of angioinvasive fungal infection (infarct shaped consolidation, cavity, halo signs, mass or nodules) 41 are anecdotic (four cases). The possibility of airway invasive aspergillosis has to be taken into account in order to improve diagnostic accuracy, but more data are needed.
Most cases of CAPA from our series and the literature with positive culture are caused by A. fumigatus (61/80, 76.3%). The usefulness of biomarkers in the diagnosis of CAPA is limited. In our cases, serum GM and the BDG were positive in 42% and 33%, respectively. Similar results are observed in the literature, 2 , 5 , 6 , 7 , 9 , 10 , 11 , 12 (serum GM 21% and BDG 40.9%). However, positive GM results in BAL samples are higher (77.8%), suggesting the possibility that CAPA patients are more prone to have an airway invasive infection than an angioinvasive one. 2 , 42 , 43 In our experience, fungal biomarkers are undoubtedly useful, but CAPA diagnosis in critical patients should not be exclusively based on them due to potential multiple causes that may lead to false positives. Thus, we do not currently recommend systematic screening of fungal biomarkers in COVID‐19 ICU patients until further scientific evidence is available. However, the use of GM in BAL, when feasible, may help physicians to properly identify aspergillosis cases.
Due to high CAPA‐related mortality, we support prompt initiation of AF treatment in ICU patients for whom respiratory Aspergillus spp. has been isolated and fulfil CAPA criteria. This becomes particularly relevant in patients with prolonged MV, high‐dose corticosteroids, long courses of antibiotic therapy and/or previous tocilizumab use. In our centre, isavuconazole proved to be very convenient due to the difficulties with voriconazole TDM. Its attractive pharmacokinetic profile is especially relevant in patients with variable volume of distribution, such as critical or obese subjects. Unfortunately, all our patients died soon after the diagnosis. The reported mortality in the literature is 65%, although several cases of patients who even survive without treatment may represent colonisations rather than real CAPA cases.
The limitations of this study are that it is based on data from a single centre and there is absence of tissue‐proven diagnosis.
In summary, severe COVID‐19 pneumonia requiring MV may be complicated due to the occurrence of a form of invasive aspergillosis that is difficult to diagnose because of its non‐specific clinical and radiological presentation. The current definitions of CAPA and modified algorithms derived from severe influenza infection allow to get closer to the proper diagnosis of this disease. Given the very high mortality and the difficult treatment due to coexisting comorbidity, it is necessary to promote the creation of internationally accepted diagnostic and therapeutic criteria.
CONFLICT OF INTEREST
Dr Machado reports personal speaker fees from Pfizer, outside the submitted work. Dr Valerio reports personal speaker fees from GSK, speaker fees from Pfizer, and speaker fees from MSD, outside the submitted work. Dr Muñoz reports personal fees from PFIZER, personal fees from GILEAD, outside the submitted work.
Dr Álvarez‐Uría, Dr Olmedo, Dr Veintimilla, Dr Padilla, Dr De la Villa, Dr Guinea, Dr Escribano, Dr Ruiz‐Serrano, Dr Reigadas, Dr Alonso, Dr Guerrero, Dr Hortal and Dr Bouza declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
Marina Machado: Conceptualization (equal); Data curation (lead); Formal analysis (lead); Investigation (equal); Methodology (equal); Writing‐original draft (lead); Writing‐review & editing (equal). Maricela Valerio: Conceptualization (equal); Methodology (equal); Writing‐review & editing (equal). Ana Álvarez‐Uría: Investigation (equal); Methodology (equal). María Olmedo: Investigation (equal). Cristina Veintimilla: Investigation (equal). Belén Padilla: Investigation (equal). Sofía de la Villa: Investigation (equal). Jesus Vicente Guinea Ortega: Investigation (equal); Methodology (equal); Writing‐review & editing (equal). Pilar Escribano: Investigation (equal); Methodology (equal). Maria Jesús Ruiz‐Serrano: Methodology (equal); Resources (equal). Elena Reigadas: Investigation (supporting); Resources (supporting). Roberto Alonso: Investigation (equal); Resources (equal). José Eugenio Guerrero: Resources (equal). Javier Hortal: Resources (equal). Emilio Bouza: Supervision (supporting); Writing‐review & editing (supporting). Patricia Muñoz: Conceptualization (equal); Supervision (lead); Writing‐review & editing (supporting).
Supporting information
Table S1
ACKNOWLEDGMENTS
The authors are grateful to Dainora Jaloveckas for editing assistance.
The Gregorio Marañón Microbiology‐ID COVID 19 Study Group: Luis Alcalá, Teresa Aldámiz, Roberto Alonso, Beatriz Álvarez, Ana Álvarez‐Uría, Alexi Arias, Luis Antonio Arroyo, Elena Bermúdez, Emilio Bouza, Almudena Burillo, Ana Candela, Raquel Carrillo, Pilar Catalán, Emilia Cercenado, Alejandro Cobos, Cristina Díez, Pilar Escribano, Agustín Estévez, Chiara Fanciulli, Alicia Galar, Mª Dolores García, Darío García de Viedma, Paloma Gijón, Adolfo González, Helmuth Guillén, Jesús Guinea, Laura Vanessa Haces, Martha Kestler, Juan Carlos López, Carmen Narcisa Losada, Marina Machado, Mercedes Marín, Pablo Martín, Pedro Montilla, Zaira Moure, Patricia Muñoz, María Olmedo, Belén Padilla, María Palomo, Francisco Parras, María Jesús Pérez‐Granda, Laura Pérez, Leire Pérez, Paula Pescador, Elena Reigadas, Cristina Rincón, Belén Rodríguez, Sara Rodríguez, Adriana Rojas, María Jesús Ruiz‐Serrano, Carlos Sánchez, Mar Sánchez, Julia Serrano, Francisco Tejerina, Maricela Valerio, Mª Cristina Veintimilla, Lara Vesperinas, Teresa Vicente, Sofía de la Villa.
Funding informationJesús Guinea is a stabilised researcher contracted by Fundación para Investigación Sanitaria del Hospital Gregorio Marañón. PE (CPI15/00115) is a recipient of a Miguel Servet contract supported by the FIS. AAU (CM18/0089) is supported by a Río Hortega contract supported by the FIS. This study did not receive any funding
Contributor Information
Marina Machado, Email: marina.machado@salud.madrid.org.
the COVID‐19 Study Group:
Luis Alcalá, Teresa Aldámiz, Beatriz Álvarez, Alexi Arias, Luis Antonio Arroyo, Elena Bermúdez, Almudena Burillo, Ana Candela, Raquel Carrillo, Pilar Catalán, Emilia Cercenado, Alejandro Cobos, Cristina Díez, Agustín Estévez, Chiara Fanciulli, Alicia Galar, Mª Dolores García, Darío García de Viedma, Paloma Gijón, Adolfo González, Helmuth Guillén, Laura Vanessa Haces, Martha Kestler, Juan Carlos López, Carmen Narcisa Losada, Mercedes Marín, Pablo Martín, Pedro Montilla, Zaira Moure, María Palomo, Francisco Parras, María Jesús Pérez‐Granda, Laura Pérez, Leire Pérez, Paula Pescador, Cristina Rincón, Belén Rodríguez, Sara Rodríguez, Adriana Rojas, Carlos Sánchez, Mar Sánchez, Julia Serrano, Francisco Tejerina, Lara Vesperinas, and Teresa Vicente
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Bartoletti M, Pascale R, Cricca M, et al. Epidemiology of invasive pulmonary aspergillosis among COVID‐19 intubated patients: a prospective study. Clin Infect Dis. 2020. 10.1093/cid/ciaa1065 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alanio A, Delliere S, Fodil S, Bretagne S, Megarbane B. Prevalence of putative invasive pulmonary aspergillosis in critically ill patients with COVID‐19. Lancet Respir Med. 2020;8(6):e48‐e49. 10.1016/S2213-2600(20)30237-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Falces‐Romero I, Ruiz‐Bastian M, Diaz‐Pollan B, Maseda E, Garcia‐Rodriguez J, Group SA‐C‐W . Isolation of Aspergillus spp. in respiratory samples of patients with COVID‐19 in a Spanish Tertiary Care Hospital. Mycoses. 2020. 10.1111/myc.13155 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gangneux JP, Reizine F, Guegan H, et al. Is the COVID‐19 pandemic a good time to include Aspergillus molecular detection to categorize aspergillosis in ICU patients? A monocentric experience. J Fungi. 2020;6(3):1‐11. 10.3390/jof6030105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rutsaert L, Steinfort N, Van Hunsel T, et al. COVID‐19‐associated invasive pulmonary aspergillosis. Ann Intensive Care. 2020;10(1):71. 10.1186/s13613-020-00686-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van Arkel ALE, Rijpstra TA, Belderbos HNA, van Wijngaarden P, Verweij PE, Bentvelsen RG. COVID‐19–associated pulmonary aspergillosis. Am J Respir Crit Care Med. 2020;202(1):132‐135. 10.1164/rccm.202004-1038LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Koehler P, Cornely OA, Bottiger BW, et al. COVID‐19 associated pulmonary aspergillosis. Mycoses. 2020;63:528‐534. 10.1111/myc.13096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lamoth F, Glampedakis E, Boillat‐Blanco N, Oddo M, Pagani JL. Incidence of invasive pulmonary aspergillosis among critically ill COVID‐19 patients. Clin Microbiol Infect. 2020. 10.1016/j.cmi.2020.07.010 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lahmer T, Rasch S, Spinner C, Geisler F, Schmid RM, Huber W. Invasive pulmonary aspergillosis in severe coronavirus disease 2019 pneumonia. Clin Microbiol Infect. 2020;26:1428‐1429. 10.1016/j.cmi.2020.05.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Blaize M, Mayaux J, Nabet C, et al. Fatal invasive aspergillosis and coronavirus disease in an immunocompetent patient. Emerg Infect Dis. 2020;26(7):1636‐1637. 10.3201/eid2607.201603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Prattes J, Valentin T, Hoenigl M, Talakic E, Reisinger AC, Eller P. Invasive pulmonary aspergillosis complicating COVID‐19 in the ICU – a case report. Med Mycol Case Rep. 2020. 10.1016/j.mmcr.2020.05.001 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lescure FX, Bouadma L, Nguyen D, et al. Clinical and virological data of the first cases of COVID‐19 in Europe: a case series. Lancet Infect Dis. 2020;20(6):697‐706. 10.1016/S1473-3099(20)30200-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Meijer EFJ, Dofferhoff ASM, Hoiting O, Buil JB, Meis JF. Azole‐resistant COVID‐19‐associated pulmonary aspergillosis in an immunocompetent host: a case report. J Fungi. 2020;6(2):79. 10.3390/jof6020079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Antinori S, Rech R, Galimberti L, et al. Invasive pulmonary aspergillosis complicating SARS‐CoV‐2 pneumonia: a diagnostic challenge. Travel Med Infect Dis. 2020:101752. 10.1016/j.tmaid.2020.101752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sharma A, Hofmeyr A, Bansal A, et al. COVID‐19 associated pulmonary aspergillosis (CAPA): an Australian case report. Med Mycol Case Rep. 2020. 10.1016/j.mmcr.2020.06.002 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mohamed A, Hassan T, Trzos‐Grzybowska M, et al. Multi‐triazole‐resistant Aspergillus fumigatus and SARS‐CoV‐2 co‐infection: a lethal combination. Med Mycol Case Rep. 2020. 10.1016/j.mmcr.2020.06.005 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fernandez NB, Caceres DH, Beer KD, et al. Ventilator‐associated pneumonia involving Aspergillus flavus in a patient with coronavirus disease 2019 (COVID‐19) from Argentina. Med Mycol Case Rep. 2020. 10.1016/j.mmcr.2020.07.001 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Santana MF, Pivoto G, Alexandre MAA, et al. Confirmed invasive pulmonary aspergillosis and COVID‐19: the value of postmortem findings to support antemortem management. Rev Soc Bras Med Trop. 2020;53:e20200401. 10.1590/0037-8682-0401-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Donnelly JP, Chen SC, Kauffman CA, et al. Revision and update of the consensus definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin Infect Dis. 2019;71:1367‐1376. 10.1093/cid/ciz1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schauwvlieghe A, Rijnders BJA, Philips N, et al. Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: a retrospective cohort study. Lancet Respir Med. 2018;6(10):782‐792. 10.1016/S2213-2600(18)30274-1 [DOI] [PubMed] [Google Scholar]
- 21. Force ADT, Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526‐2533. 10.1001/jama.2012.5669 [DOI] [PubMed] [Google Scholar]
- 22. Hahn‐Ast C, Glasmacher A, Muckter S, et al. Overall survival and fungal infection‐related mortality in patients with invasive fungal infection and neutropenia after myelosuppressive chemotherapy in a tertiary care centre from 1995 to 2006. J Antimicrob Chemother. 2010;65(4):761‐768. 10.1093/jac/dkp507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Escribano P, Pelaez T, Munoz P, Bouza E, Guinea J. Is azole resistance in Aspergillus fumigatus a problem in Spain? Antimicrob Agents Chemother. 2013;57(6):2815‐2820. 10.1128/AAC.02487-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Arendrup MC, Mouton JW, Lagrou K, Hamal P, Guinea J. EUCAST DEFINITIVE DOCUMENT E.DEF 9.3.2. Method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for conidia forming moulds. https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/AFST/Files/EUCAST_E_Def_9.3.2_Mould_testing_definitive_revised_2020.pdf [DOI] [PubMed]
- 25. Testing. TECoAS . Breakpoint tables for interpretation of MICs for antifungal agents, version 10.0. http://www.eucast.org/astoffungi/clinicalbreakpointsforantifungals/
- 26. Gautret P, Lagier JC, Parola P, et al. Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID‐19 patients with at least a six‐day follow up: a pilot observational study. Travel Med Infect Dis. 2020;34:101663. 10.1016/j.tmaid.2020.101663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Verweij PE, Rijnders BJA, Bruggemann RJM, et al. Review of influenza‐associated pulmonary aspergillosis in ICU patients and proposal for a case definition: an expert opinion. Intensive Care Med. 2020;46(8):1524‐1535. 10.1007/s00134-020-06091-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Blot SI, Taccone FS, Van den Abeele AM, et al. A clinical algorithm to diagnose invasive pulmonary aspergillosis in critically ill patients. Am J Respir Crit Care Med. 2012;186(1):56‐64. 10.1164/rccm.201111-1978OC [DOI] [PubMed] [Google Scholar]
- 29. Wang J, Yang Q, Zhang P, Sheng J, Zhou J, Qu T. Clinical characteristics of invasive pulmonary aspergillosis in patients with COVID‐19 in Zhejiang, China: a retrospective case series. Crit Care. 2020;24(1):299. 10.1186/s13054-020-03046-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Koehler P, Bassetti M, Kochanek M, Shimabukuro‐Vornhagen A, Cornely OA. Intensive care management of influenza‐associated pulmonary aspergillosis. Clin Microbiol Infect. 2019;25(12):1501‐1509. 10.1016/j.cmi.2019.04.031 [DOI] [PubMed] [Google Scholar]
- 31. Han S, Mallampalli RK. The acute respiratory distress syndrome: from mechanism to translation. J Immunol. 2015;194(3):855‐860. 10.4049/jimmunol.1402513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kolilekas L, Loverdos K, Giannakaki S, et al. Can steroids reverse the severe COVID‐19 induced "cytokine storm"? J Med Virol. 2020;92:2866‐2869. 10.1002/jmv.26165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Arastehfar A, Carvalho A, van de Veerdonk FL, et al. COVID‐19 associated pulmonary aspergillosis (CAPA)‐from immunology to treatment. J Fungi. 2020;6(2):91. 10.3390/jof6020091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Borger P, Koeter GH, Timmerman JA, Vellenga E, Tomee JF, Kauffman HF. Proteases from Aspergillus fumigatus induce interleukin (IL)‐6 and IL‐8 production in airway epithelial cell lines by transcriptional mechanisms. J Infect Dis. 1999;180(4):1267‐1274. 10.1086/315027 [DOI] [PubMed] [Google Scholar]
- 35. Rutherford AI, Subesinghe S, Hyrich KL, Galloway JB. Serious infection across biologic‐treated patients with rheumatoid arthritis: results from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis. Ann Rheum Dis. 2018;77(6):905‐910. 10.1136/annrheumdis-2017-212825 [DOI] [PubMed] [Google Scholar]
- 36. Zhao M. Cytokine storm and immunomodulatory therapy in COVID‐19: role of chloroquine and anti‐IL‐6 monoclonal antibodies. Int J Antimicrob Agents. 2020;55(6):105982. 10.1016/j.ijantimicag.2020.105982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gangneux JP, Bougnoux ME, Dannaoui E, Cornet M, Zahar JR. Invasive fungal diseases during COVID‐19: we should be prepared. J Mycol Med. 2020;30;100971. 10.1016/j.mycmed.2020.100971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Thompson GR III, Cornely OA, Pappas PG, et al. Invasive aspergillosis as an under recognized superinfection in COVID‐19. Open Forum Infect Dis. 2020;7(7). 10.1093/ofid/ofaa242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Li Y, Xia L. Coronavirus disease 2019 (COVID‐19): role of chest CT in diagnosis and management. AJR Am J Roentgenol. 2020;214(6):1280‐1286. 10.2214/AJR.20.22954 [DOI] [PubMed] [Google Scholar]
- 40. Guan CS, Lv ZB, Yan S, et al. Imaging features of coronavirus disease 2019 (COVID‐19): evaluation on thin‐section CT. Acad Radiol. 2020;27(5):609‐613. 10.1016/j.acra.2020.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Logan PM, Muller NL. High‐resolution computed tomography and pathologic findings in pulmonary aspergillosis: a pictorial essay. Can Assoc Radiol J. 1996;47(6):444‐452. [PubMed] [Google Scholar]
- 42. Henriet SS, Jans J, Simonetti E, et al. Chloroquine modulates the fungal immune response in phagocytic cells from patients with chronic granulomatous disease. J Infect Dis. 2013;207(12):1932‐1939. 10.1093/infdis/jit103 [DOI] [PubMed] [Google Scholar]
- 43. Verweij PE, Gangneux J‐P, Bassetti M, et al. Diagnosing COVID‐19‐associated pulmonary aspergillosis. Lancet Microbe. 2020;1:e53‐e55. 10.1016/S2666-5247(20)30027-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


