Abstract
Although people living with human immunodeficiency virus and other comorbidities are expected to experience more grievous consequences with corona virus disease 2019 (COVID‐19), recent cohort studies did not indicate this. Antiretrovirals (ARVs) might have a prophylactic role in these patients. The purpose of this study was to review the most recently published articles on the possible role of ARVs for pre‐ or postexposure prophylaxis against COVID‐19. From June to October 2020, we searched scientific databases using specific key words to identify ongoing trials or articles published before October 2020 investigating any subgroups of ARVs for prophylaxis against COVID‐19. Apart from molecular docking studies, in vitro, animal, and human studies are very limited for evaluating the prophylactic role of ARVs against severe acute respiratory syndrome‐corona virus 2 (SARS‐CoV‐2) infection. According to our findings, there is no definite evidence to support use of protease inhibitors for this purpose, despite the promising results of molecular studies and limited clinical evidence for ritonavir‐boosted lopinavir, darunavir, and nelfinavir when used early in the course of the disease. Nucleotide/nucleoside reverse‐transcriptase inhibitors (NRTI) also have shown binding affinity to main enzymes of SARS‐CoV‐2 in molecular, in vitro, and animal studies. NRTIs like tenofovir and emtricitabine might exhibit a prophylactic role against SARS‐CoV‐2 infection. In conclusion, currently there is no evidence to justify the use of ARVs for prophylaxis against COVID‐19.
Keywords: antiretroviral drugs, COVID‐19, HIV, prophylaxis, SARS‐CoV‐2
Currently the coronavirus disease 2019 (COVID‐19) pandemic is spreading very rapidly and has become a health crisis. The number of confirmed cases at the time of writing this article has reached more than 42 million on October 27, 2020. 1
According to a recently published prospective cohort study, frontline health care workers (HCWs) are 3.4 times more likely to be infected with severe acute respiratory syndrome‐corona virus 2 (SARS‐CoV‐2) than the general population. Epidemiologic findings also show that health care workers, especially of black and Asian descent as well as other ethnic minorities are at least 5 times more likely to be infected with SARS‐CoV‐2 than the non‐Hispanic white ethnicity. 2
Current public health strategies are rapid identification of cases, isolation, and self‐quarantine to mitigate transmission. In the absence of an effective vaccine or treatment, additional strategies are required, especially for high‐risk groups (eg, health care workers) in addition to adequate and efficient use of personal protective equipment (PPE). Pharmacological pre‐ or postexposure prophylaxis against COVID‐19 could be a cornerstone in controlling this disease.
Although immune deficiency and comorbidities make patients prone to more severe cases of COVID‐19, people living with human immunodeficiency virus (PLWH), especially those with undetectable viral loads and acceptable immunologic status, do not have a higher incidence of COVID‐19 compared with the general population. 2 , 3 , 4
A Spanish cohort study of 77 590 human immunodeficiency virus (HIV)‐positive persons who were mostly under treatment with antiretrovirals (ARVs) and in a well‐controlled state reported that the rate of COVID‐19 diagnosis and hospitalization decreased to 30.0 cases per 10 000 versus 41.7 cases per 10 000 in the general population. 6 Moreover, another study from Italy reported a 4% mortality rate among 47 HIV‐positive patients infected with SARS‐CoV‐2 compared with a 17% crude mortality rate in HIV‐negative patients with COVID‐19 treated in the same hospital. 7
The observed lower incidence and mortality of COVID‐19 in PLWH might be the result of antiretroviral therapy (ART) regimens acting as a simultaneous means of chemoprophylaxis for COVID‐19. This prophylactic effect might be because of inhibition of essential SARS‐CoV‐2 functional proteins. Also, ARVs have been shown to improve levels of C‐reactive protein, interleukin (IL)‐6, IL‐12, tumor necrosis factor (TNF)‐α, and d‐dimer. 7 , 8 Considering the role of cytokines in the pathogenesis of COVID‐19, these drugs may have potential efficacy against the ensuing cytokine storm. Therefore, ARVs might be demonstrating both antiviral and anti‐inflammatory effects against SARS‐CoV‐2.
We should also bear in mind that the excessive use of antiretroviral drugs for COVID‐19 has caused a concerning dip in drug deposits of health care systems throughout the world, especially in low‐income countries that also house the majority of the PLWH. Unless we succeed in making a balance between production of these expensive drugs and the excessive use of them, PLWH will be deprived of their vital therapeutics, and irreversible health consequences will ensue.
In this article we highlight the currently existing evidence regarding the potency of ARVs as preventive therapy against COVID‐19.
Methods
The current study is a comprehensive review of the existing literature since the SARS‐CoV‐2 outbreak before October 2020 on the suggested prophylactic indications of ARVs for COVID‐19. From June to October 23, 2020, the PubMed, Google Scholar, and Medline databases were searched using MeSH‐compliant key words. The key words included “prophylaxis,” “prevention,” “SARS‐CoV‐2,” “COVID‐19,” “coronavirus,” “antiretroviral,” “protease inhibitor,” “ARV,” “ART,” and names of antiretroviral drugs including “lopinavir,” “ritonavir,” “darunavir,” “atazanavir,” “tenofovir,” “emtricitabine,” “zidovudine,” “raltegravir,” and “dolutegravir.” The initial search identified 176 studies including clinical trials, observational studies, animal, and in vitro studies. Case reports and review articles were excluded. Title and abstract screening was conducted by 2 reviewers, and relevant articles were included. Search terms and selection were restricted to articles available in English. Referenced articles were hand‐searched to identify further relevant studies. Finally, data from included articles were summarized and reported.
Also, ongoing randomized, controlled trials and cohort studies relevant to any subgroup of antiretrovirals investigating pre‐ or postexposure prophylaxis of COVID‐19 were searched in clinicaltrials.gov and clinicaltrialsregister.eu registries (the results are presented in Table 1).
Table 1.
Ongoing Studies on Effectiveness of Antiretrovirals for Prophylaxis of COVID‐19
| Study | Study Type | Prophylaxis Mode | Country | (Expected) Publication Date | Intervention |
|---|---|---|---|---|---|
| COVID‐19 Ring‐Based Prevention Trial With Lopinavir/Ritonavir (CORIPREV‐LR) | RCT | PEP | Canada |
March 2022 Ongoing |
LPV/r (400/100 mg Q12h × 14 days) versus no treatment |
| Treatment of Non‐severe Confirmed Cases of COVID‐19 and Chemoprophylaxis of Their Contacts as Prevention Strategy: A Cluster Randomized Clinical Trial (PEP CoV‐2) | RCT | PEP | Spain | Unknown | DRV/c (800/150 mg QD) versus HCQ (200 mg QD) |
| Impact of Long‐Term Protease Inhibitors in Patients Living With HIV on the Incidence of COVID‐19 (COVIP) | Cohort | PrEP | France |
July 2021 Ongoing |
Protease inhibitors (dosage not specified) |
| Randomized Clinical Trial for the Prevention of SARS‐CoV‐2 Infection (COVID‐19) in Healthcare Personnel (EPICOS) | RCT | PrEP | Spain |
December 2020 Ongoing |
TDF/FTC (245/200 mg QD), HCQ (200 mg QD), TDF/FTC (245/200 mg QD) plus HCQ (200 mg QD), and placebo |
| TAF/FTC for Pre‐exposure Prophylaxis of COVID‐19 in Healthcare Workers (CoviPrep Study) | RCT | PrEP | Argentina |
November 2020 Ongoing |
FTC/TAF (200/25 mg QD) versus placebo |
| Chemoprophylaxis of SARS‐CoV‐2 Infection (COVID‐19) in Exposed Healthcare Workers (COVIDAXIS) | RCT | PrEP | France |
November 2020 Ongoing |
HCQ (400 mg Q12h for 2 doses, then 200 mg Q12h) or LPV/r (400/100 mg Q12h) versus placebo |
| Daily Regimen of Tenofovir/Emtricitabine as Prevention for COVID‐19 in Health Care Personnel in Colombia | RCT | PrEP | Colombia |
April 2021 Ongoing |
TDF/FTC (300/200 mg QD) pus PPE versus placebo plus PPE |
DRV/c, darunavir/cobicistat; FTC, emtricitabine; HCQ, hydroxychloroquine; LPV/r, lopinavir/ritonavir; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate; NNRTI, nonnucleoside reverse‐transcriptase inhibitor; NRTI, nucleoside reverse‐transcriptase inhibitor; PEP, postexposure prophylaxis; PPE, personal protective equipment, PrEP, preexposure prophylaxis; Q12h, every 12 hours; QD, every day; RCT, randomized, controlled trial.
Ongoing studies on the effectiveness of antiretrovirals for prophylaxis of COVID‐19.
Discussion
Based on the existing understanding of pathogenesis, 2 phases can be considered for the course of COVID‐19. The first one is the viral response phase with earlier and milder symptoms because of viral replication in targeted organs, and the second phase is exaggerated host reaction through immune system response and cytokines to protect against SARS‐CoV‐2. 10 Therefore, treatment strategy can include early administration of antivirals to prevent viral replication in host cells along with establishing a balance between tissue damage caused by hyperactivation of the immune system and effective immune host response against SARS‐CoV‐2.
From the beginning of this pandemic, there have been scattered reports that, contrary to expectations, the prevalence of COVID‐19 is low in PLWH. The use of antiretroviral drugs is hypothesized to be one of the possible explanations for this phenomenon. A systematic review and meta‐analysis concluded that the prevalence of HIV/SARS‐CoV‐2 coinfection was low and, in terms of clinical characteristics and outcomes, was comparable to other patients without HIV. 11
The suggestion of using ARVs as preventive strategies has been raised since the severe acute respiratory syndrome (SARS) epidemic in 2002‐2003. 12 Also, a study conducted in South Korea showed effectiveness of ribavirin plus lopinavir/ritonavir as postexposure prophylaxis (PEP) in health care workers against Middle East respiratory syndrome (MERS). 13
Here we report the existing evidence regarding potential effectiveness of the most common ARVs for prophylaxis against COVID‐19.
Protease Inhibitors
Protease inhibitors are competitive inhibitors of HIV protease that prevent cleavage of polypeptide precursors that form viral capsid subunits at the end of the viral life cycle. They exhibit activity against clinical isolates of both HIV‐1 and HIV‐2. 14 In this section we will discuss our findings on lopinavir/ritonavir, atazanavir, darunavir, and other protease inhibitors.
Lopinavir/Ritonavir
Ritonavir‐boosted lopinavir (LPV/r) is a protease inhibitor most known for its use in the treatment of HIV. In a recent study by Nutho et al 15 with computer‐based molecular dynamics simulation, it was demonstrated that ritonavir and lopinavir interacted well with their binding sites on SARS‐CoV‐2 3CLpro protein (or main protease), with the former having about twice the affinity of the latter.
Another computational study with the objective of identifying appropriate drug complexes for drug repurposing reported that a combination of ritonavir, lopinavir, and oseltamivir was more effective than each drug individually for interactions with the target SARS‐CoV‐2 main protease. 16
Using chemoinformatic analysis, Copertino et al 17 identified protease inhibitors including ritonavir and lopinavir as capable of binding catalytic sites of both main protease and RNA‐dependent RNA polymerase (RdRp) of SARS‐CoV‐2. They also argue that PLWH contracting COVID‐19 less frequently than the normal population, based on anecdotal reports, might be because of the preexposure prophylaxis that they acquire by taking antiretroviral drugs including protease inhibitors and nucleoside and nucleotide reverse‐transcriptase inhibitors.
The first randomized, controlled trial on the efficacy of LPV/r was conducted by Cao et al on 199 patients. 18 In that study, the authors reported that LPV/r was not associated with clinical improvement or reduced mortality in severely ill COVID‐19 patients; however, in a post hoc analysis of their study participants, those who received LPV/r for less than 12 days after symptom onset had accelerated clinical recovery and reduced mortality (19% vs 27.1%). However, this study was underpowered to rule out the possible beneficial effects of LPV/r. Recently, the results of the largest randomized clinical trial comparing LPV/r (400/100 mg twice daily for 10 days) plus standard care (1616 patients) versus standard care only (3424 patients) in COVID‐19 patients was reported. This large‐scale trial (RECOVERY Trial) 19 was conducted in 176 UK hospitals, and the study outcomes were 4‐week all‐cause mortality, duration of hospital stay, and progression to mechanical ventilation or death. There was no significant difference between study arms regarding any of the predefined outcomes. Moreover, a subgroup analysis for RECOVERY participants did not indicate any significant difference in subgroups of age, sex, ethnicity, days since symptom onset, and respiratory support at randomization. After the results of the RECOVERY trial were made public, WHO's SOLIDARITY trial 20 stopped further participant recruitment for its LPV/r arm. Later, their published results on 1411 patients who took LPV/r (400/100 mg twice daily for 14 days) indicated no significant difference compared with the control group on study outcomes including mortality, duration of hospital stay, and initiation of ventilation.
However, a recent study suggested that even if LPV/r is not effective for treatment of severe COVID‐19, it could be used as treatment of early nonsevere patients to decrease the viral load and subsequent immune‐related aggravation of the disease. Also, as oral‐fecal transmission tends to extend late into the disease course, it was recommended that LPV/r be prescribed for at least 14 days. 21
Lopinavir is metabolized by the hepatic CYP3A enzyme, whereas ritonavir is both metabolized by and inhibits this same enzyme, increasing the plasma levels of lopinavir and itself. 22
Lopinavir exhibited antiviral activity against SARS‐CoV‐2 in Vero E6 cells, with a maximum effective concentration (EC50) of 26.63 μM. 23
From a kinetic point of view, suboptimal antiviral response of LPV may be because of factors such as inadequate concentration at the site of infection and/or insufficient plasma drug exposure, especially across the entire dosing interval. 24
Although in a pharmacokinetics study by Arshad et al, 25 LPV/r was predicted to achieve lung concentrations more than 10‐fold higher than the reported EC50, another recent study stated that LPV/r with poor lung distributions subsequently failed to inhibit viral replication, at least in the lungs. 26
According to pharmacokinetic studies, ritonavir and lopinavir reach much higher trough concentrations in patients with COVID‐19 compared with HIV patients. 26 , 27 But despite this high plasma concentration observed in COVID‐19 patients, the unbound fraction is not affected. 24 This higher plasma concentration has been attributed to the hepatic impairment caused by SARS‐CoV‐2 and the enzymatic inhibitory effects of these drugs. In addition, concurrent use with hydroxychloroquine and the subsequent drug‐drug reaction have been hypothesized for this observed effect.
In the study by Cao et al, 18 14% of their intervention group failed to complete the 14‐day course of treatment because of numerous side effects. Whatever the mechanism for the higher toxicity in COVID‐19 patients is, this higher concentration predisposes the patients to experience more frequent adverse effects related to these drugs.
Although protease inhibitors can cause serious side effects when used over long periods in HIV patients, they could still be used for prophylaxis against COVID‐19 in the short‐term with acceptable side effect profiles. However, even if it is determined that these drugs have prophylactic capabilities against COVID‐19, the exact dosing and frequency of use would be quite challenging.
LPV/r plus ribavirin was used in a study 13 as postexposure prophylaxis (PEP) in MERS in health care workers who had unprotected contact with infected patients. In that study, the LPV/r plus ribavirin resulted in a 40% reduction of MERS‐CoV infection (0% [0 of 22] in HCWs receiving PEP versus 28.6% [6 of 21] in HCWs not receiving it; OR, 0.405; 95%CI, 0.274‐0.599; P = .009). However, almost all patients receiving PEP medication reported side effects of mostly nausea and diarrhea, and all of them experienced hyperbilirubinemia without need for discontinuation of treatment.
Currently, the CORIPREV‐LR 29 trial from Canada is using lopinavir‐ritonavir versus control group against SARS‐CoV‐2 for postexposure prophylaxis on a target sample size of 1220 participants. The intervention group will receive oral lopinavir/ritonavir 400/100 mg twice daily for 14 days initiated within the first week of exposure. The control group will receive no treatment. The primary outcome is microbiological confirmation of SARS‐CoV‐2 infection by day 14 of the study. Data collection is expected to end in late March 2021.
Another placebo‐controlled, randomized trial (COVIDAXIS 2), in France, is also investigating lopinavir‐ritonavir 400/100 mg twice daily for 2 months in 600 health care workers for preexposure prophylaxis against COVID‐19. 30
To clarify the possibility of LPV/r use for preventing COVID‐19, we have to wait for the results of the ongoing clinical trials.
Atazanavir
Atazanavir is another protease inhibitor used for prophylaxis and treatment of HIV.
In a study by Beck et al 31 using MT‐DTI (a deep learning‐based drug‐target interaction prediction model), they found that atazanavir had the highest affinity for 3CLpro of SARS‐CoV‐2 among a group of drugs including remdesivir, efavirenz, ritonavir, and dolutegravir. Moreover, in this model atazanavir showed a potential binding affinity to almost all replication subunits of SARS‐CoV‐2 virus including RdRp, helicase, exonuclease, and endoRNAse.
However, a recent study has warned against use of atazanavir and LPV/r in COVID‐19 patients receiving antiplatelet and new oral anticoagulants. 32 Currently, a phase 2/3 randomized, controlled trial (NATADEX) 33 is recruiting patients with mild COVID‐19 symptoms to receive either atazanavir or NA‐831 (a neuroprotective drug) with or without dexamethasone combination.
To date, there is no evidence justifying the use of atazanavir as a means for COVID‐19 prophylaxis.
Darunavir
To our knowledge, the only published study to assess darunavir (another protease inhibitor used for treatment of HIV) efficacy for treatment of COVID‐19 patients was released in June 2020. 34 This study was a relatively small randomized, controlled trial (n = 30) and showed that the addition of darunavir/cobicistat (DRV/c) for 5 days to the standard treatment including inhalation interferon α 2b did not increase the negative conversion of polymerase chain reaction results on day 7. Also, a recent study demonstrated that DRV/c showed no in vitro antiviral activity at clinically relevant concentrations against SARS‐CoV‐2 (EC50 > 100 μM). 35
Four other trials are currently ongoing in China, Thailand, and Qatar to assess DRV/c for treatment of COVID‐19, in line with other agents like thymosin a‐1, oseltamivir, hydroxychloroquine, and favipiravir. 35 , 36 , 37 , 38 , 39
Another case series of 3 patients with HIV who were under treatment with DRV/c reported that these patients improved after contracting COVID‐19 disease; however, one of them required mechanical ventilation. 41 This study argues that darunavir might not be effective in preventing SARS‐CoV‐2 infection.
Currently, the only known trial to evaluate darunavir as a means for postexposure prophylaxis of SARS‐CoV‐2, is the Spanish PEP CoV‐2 study, 42 which will administer DRV/c or hydroxychloroquine to 3040 adult contacts of laboratory‐confirmed SARS‐CoV‐2‐infected patients to measure prophylaxis effectiveness in reducing secondary cases within 14 days after start of treatment.
At the present time, the evidence to support use of darunavir for prophylaxis or treatment of COVID‐19 is very limited, and there is a high risk of bias for published studies, so we have to wait for the results of the ongoing clinical trials.
Other Protease Inhibitors
Saquinavir and indinavir‐2 protease inhibitors used for treatment of HIV were identified as hit compounds for the SARS‐CoV‐2 3CLpro main protease in a study that incorporated in silico molecular docking models. 43
Another study indicated that indinavir has the highest docking score with 3CLpro, followed closely by atazanavir, remdesivir, and amprenavir (another protease inhibitor used for treatment of HIV). 44
Musarrat et al 45 reported in May 2020 that nelfinavir drastically inhibits virus‐induced cell‐to‐cell fusion of Vero cells infected with SARS‐CoV‐2 at a dose of 10 μM without changing cell surface expression of N‐MYC or FLAG glycoproteins. The authors concluded that nelfinavir should be used early in the course of COVID‐19 disease to minimize virus spread and gain time for the immune system to respond (Figure 1).
Figure 1.
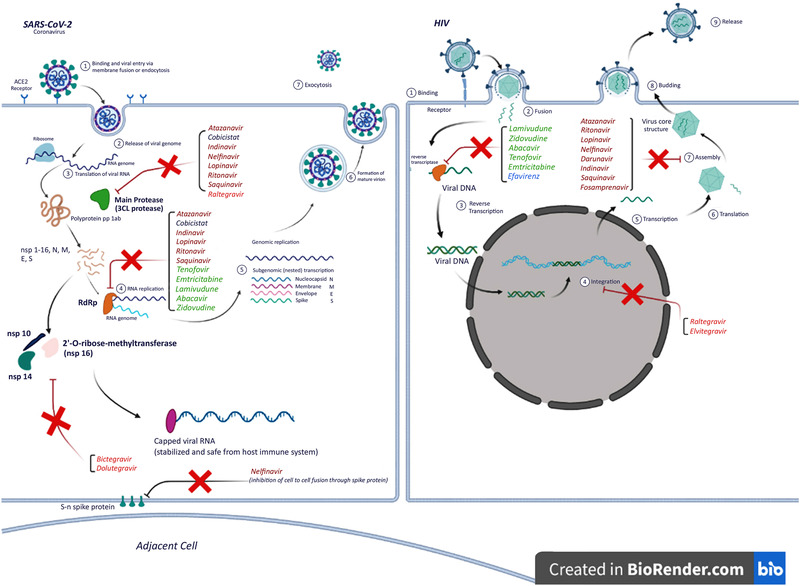
Mechanism of action of antiretroviral drugs through the life cycle of HIV and SARS‐CoV‐2 viruses. This figure was created using BioRender.com.
To identify a possible preventive role of protease inhibitors on the incidence of COVID‐19 among PLWH, a prospective multicenter cohort study is underway in France. 46 In this study patients in the long‐term protease inhibitors group will be compared with patients in the long‐term ARV regimen without protease inhibitors in terms of incidence rate of COVID‐19.
Drug to Increase HIV Drug Exposure
Cobicistat
Cobicistat is used as a pharmacokinetic boosting agent and a part of ART regimens to decrease protease inhibitors’ liver metabolism via CYP3A inhibition. Cobicistat is suggested to have potential activity against SARS‐CoV‐2 through inhibition of the main protease and RdRp to some extent in molecular docking studies. 17 A molecular study has explained that cobicistat has a high binding affinity to main protease via several hydrogen bonds with amino acids inside the active site of the main protease. 47 Apart from the trials investigating coadministration of cobicistat with darunavir, we did not find any relevant trials to assess the efficacy of cobicistat for prophylaxis of COVID‐19.
Nucleotide/Nucleoside Reverse‐Transcriptase Inhibitors
Tenofovir Disoproxil Fumarate and Tenofovir Alafenamide
Tenofovir disoproxil fumarate (TDF) is a prodrug for tenofovir, a nucleotide reverse‐transcriptase inhibitor. Tenofovir is an analogue of adenosine 5ʹ‐monophosphate, which is a competitive inhibitor of HIV reverse transcriptase, resulting in blockage of viral replication. 48
Characteristics such as high barrier resistance and long plasma and intracellular half‐life have caused tenofovir to become the most widely used antiretroviral agent. Tenofovir alafenamide (TAF) is a next‐generation tenofovir prodrug that has demonstrated equal efficacy with less adverse effects, especially regarding nephrotoxicity and drug‐induced osteoporosis. 49
Currently, a large number of patients with HIV are receiving some forms of tenofovir (TDF or TAF) as a part of backbone antiretroviral treatment. 50 Tenofovir, in combination with emtricitabine (FTC) or lamivudine is used for postexposure prophylaxis to reduce the risk of HIV infection in adults and adolescents. According to recent studies, tenofovir may also have a place in treatment or prophylaxis against COVID‐19. In a cohort study conducted by Del Amo et al, the authors concluded that risk of COVID‐19 and related hospitalization is lower among HIV‐positive patients receiving TDF/FTC. 6 Favorable pharmacological characteristics of tenofovir such as few adverse effects or drug‐drug interactions make it a good choice for preexposure prophylaxis. 51
A few studies using in silico molecular docking analysis have identified tenofovir and emtricitabine as potential candidates for inhibition of RdRp, which could play a role as chain terminators in the replication of SARS‐CoV‐2 RNA. 16 , 51 , 52 , 53
Moreover, TDF significantly reduced SARS CoV‐2 particle production in a cell culture study by concentrations between 3 and 90 μM. 55 Considering these findings and the similarities between RdRp from previous coronaviruses and HIV reverse transcriptase, 56 tenofovir remains an encouraging agent against SARS‐CoV‐2.
Park et al 57 tested lopinavir‐ritonavir, hydroxychloroquine sulfate, and emtricitabine‐tenofovir against SARS‐CoV‐2 infection in a ferret infection model to identify potential antiviral candidates for future human efficacy trials. The results obtained showed that all 3 drug treatments exhibited lower clinical symptoms compared with the control group, but they did not reduce virus titers with the exception of the emtricitabine‐tenofovir‐treated group, which led to diminished virus titers in nasal washes 8 days postinfection compared with the control group.
Furthermore, tenofovir regulates the cytokine networks and shifts the IL‐12/IL‐10 balance toward decreasing IL‐10 and increasing IL‐12 levels. 9 Activated antigen‐presenting cells (APCs) including dendritic cells, macrophages, and astrocytes secrete the key cytokine IL‐12, which promotes differentiation of naive T cells into T‐helper 1 and activates natural killer (NK) cells. These activated cells play a major role in inhibition of viral replication and clearance of virus from host cells. IL‐12 also primes interferon gamma (IFN‐γ) production by T cells and NK cells, which can in turn upregulate IL‐12 synthesis by the APCs, and this positive feedback loop is essential for adaptive immune response against viral pathogens. CD4+ T cells also help B cells through IL‐12 and IFN‐γ to produce antibodies. This antibody‐mediated immunity, which is activated indirectly by IL‐12, plays a crucial role in host defense against extracellular pathogens. 58
We hypothesize that by increasing IL‐12 levels directly or indirectly through increasing IFN‐γ and TNF‐α levels in serum and stimulation of antibody production, tenofovir could prepare better antiviral response against SARS‐CoV‐2 for the host. Considering all the above, it can be concluded that tenofovir could potentially play a role as an antiviral agent along with prophylactic immune modulation against COVID‐19; however, no definite conclusions could be made without evidence from further clinical studies.
Emtricitabine
Emtricitabine (FTC) is a cytosine nucleoside analogue that inhibits HIV reverse transcription. As previously mentioned, like tenofovir, emtricitabine can be used as a permanent terminator for the SARS‐CoV‐2 RdRp enzymatic function. 54
Because emtricitabine can be coadministered with tenofovir in HIV postexposure prophylaxis or as a backbone of antiretroviral therapies, these 2‐drug combinations can be used as lead compounds in upcoming studies to test the inhibitory effects on SARS‐CoV‐2.
The first study on prophylactic efficacy of tenofovir/emtricitabine against COVID‐19 indicated a higher seroprevalence of anti SARS‐CoV‐2 immunoglobulin G among preexposure prophylaxis (PrEP) users than the control group with no statistically significant differences in clinical manifestations. 59 PrEP users had fewer symptoms and for a shorter duration than the control group, as did those receiving TDF/FTC when compared with TAF/FTC, although no statistically significant differences could be found.
To investigate prevention of SARS‐CoV‐2 infection through preexposure prophylaxis in health care personnel aged 18 to 70 years in Spain, the EPICOS randomized, double‐blinded clinical trial is in process. 60 This study has 4 arms comparing TDF/FTC, hydroxychloroquine, TDF/FTC plus hydroxychloroquine, and placebo. Four thousand participants will be assigned to 1 of the 4 groups. The primary outcome is the number of confirmed symptomatic infections of SARS‐CoV‐2 at 12 weeks. Also, a similar controlled trial will be conducted in Colombia to assess TDF/FTC plus PPE versus PPE alone in 950 health care workers. 61
Another ongoing randomized, double‐blinded, placebo‐controlled clinical trial on this subject is the CoviPrep study from Argentina. 62 The purpose of this study is to investigate daily doses of FTC/TAF 200‐25 mg versus placebo for preexposure prophylaxis in health care workers at a duration of 12 weeks for each group. Its main objective is to evaluate the risk of developing COVID‐19 in health care workers in addition to the currently recommended control measures.
Other Nucleoside/Nucleotide Reverse‐Transcriptase Inhibitors
Molecular basic studies have demonstrated positive response with different effectiveness for other nucleotide analogues such as the active triphosphate form of zidovudine, abacavir, and lamivudine in inhibition of SARS‐CoV‐2 RdRp. 16 , 51 However, zidovudine and lamivudine did not show inhibitory effects against SARS‐CoV‐2 in an in vitro study. 63
In a cohort study from South Africa, the mortality rate of PLWH with COVID‐19 was lower on TDF compared with zidovudine‐ or abacavir‐based regimens (HR, 0.41; 95%CI, 0.21‐0.78; P = .007) and other antiretrovirals. 64
There are no published or ongoing trials to effectively assess the prophylactic role of any of these agents against COVID‐19.
Integrase Strand Transfer Inhibitors
A newer class of ARVs is integrase strand transfer inhibitors (INSTIs) that inhibit HIV integrase enzyme and thus prevent HIV from incorporating its genome into the host DNA. Raltegravir was the first INSTI approved by the US Food and Drug Administration in 2007. Two newer INSTIs, dolutegravir and elvitegravir, were subsequently approved, in 2013 and 2014, respectively, and are used in many ART regimens along with the backbone drugs. 65
An important viral enzyme that could be pharmacologically targeted, is the 2’‐O‐ribose‐methyltransferase, which is the non‐structural‐protein 16 in SARS‐CoV‐2 (Figure 1). This enzyme ensures the integrity of viral RNA and its safety from the innate host immune system. It functions by putting a “cap” (specific arrangement at the 5’ end of the RNA on the ribose 2’‐O position) on one end of the viral RNA, which resembles the native mRNA of the host cell. This process guarantees survival and replication of the viral RNA. 65 , 66
A recent study of molecular drug simulation for the 2’‐O‐ribose‐methyltransferase enzyme identified raltegravir, dolutegravir, and bictegravir as potential therapeutic choices for drug repurposing. 66 Another molecular docking study revealed that raltegravir, alongside protease inhibitors like lopinavir‐ritonavir and tipranavir, shows the best molecular interaction with the main protease of SARS‐CoV‐2. 68 However, there is a need for further in vitro studies and clinical trials to find out the effectiveness of these drugs on COVID‐19.
Recently, 2‐drug ART regimens including dolutegravir and lamivudine were approved for PLWH. A study is being conducted in Iran (IRCT20191005044984N1) to evaluate and compare the effectiveness of a 2‐drug regimen of dolutegravir and tenofovir alafenamide in PLWH that coincides with the outbreak of COVID‐19. In addition to the main objectives of this study, it may be possible to compare the incidence and severity of COVID‐19 in patients receiving either 2‐drug or standard triple‐drug ART regimens and also the general population to reveal any further clues toward repurposing of ARVs for COVID‐19.
Summary
To date there have been few clinical trials designed to evaluate ARVs as prophylactic therapies against SARS‐CoV‐2 infection, and some of them are currently underway.
Recent cohorts and observational studies have reported that HIV‐infected patients and PrEP users are not at increased risk of contracting COVID‐19 compared with other people if a similar age. 4 , 5 , 6 , 68 If the role of ARVs in prevention of COVID‐19 in PLWH is proven, then we can extend their use to the population at higher risk of infection with SARS‐CoV‐2.
Currently, there is no evidence to justify the use of protease inhibitors for early prevention or postexposure prophylaxis against SARS‐CoV‐2. However, the CORIPREV‐LR, 29 COVIP, 46 and COVIDAXIS 2 30 trials and the Spanish PEP CoV‐2 study 42 will shed light on the use of ritonavir‐boosted lopinavir, protease inhibitors, and darunavir, respectively, for this purpose. Other protease inhibitors such as atazanavir, indinavir, and nelfinavir have gained notice because of their promising interactions with specific SARS‐CoV‐2 viral proteins, revealed through molecular docking analyses and in vitro studies. Therefore, it seems that these drugs merit more precise evaluation by further clinical studies.
LPV/r was the only protease inhibitor drug combination that entered national therapeutic protocols for treatment of COVID‐19 in many countries; however, large randomized clinical trials ruled out any benefits of this protease inhibitor on outcomes like mortality, duration of hospital stay, or mechanical ventilation.
Tenofovir and emtricitabine could potentially play a role as antiviral agents along with prophylactic immune modulation in COVID‐19. Three randomized, controlled trials from Spain (EPICOS), 60 Argentina (CoviPrep), 62 and Colombia 61 are investigating the role of a tenofovir and emtricitabine combination for preexposure prophylaxis in health care workers within 8 to 12 weeks.
Limitations
This comprehensive review article includes any molecular, in vitro, in vivo, animal, and human studies investigating the possible role of antiretrovirals in prophylaxis against COVID‐19. We must be aware that many in vitro studies use drug concentrations far exceeding the in vivo concentrations, and their results may not necessarily be relevant to clinical practice. Therefore, we should be careful when interpreting these data. Also, because of the scarcity of current clinical data and low certainty of evidence and high risk of bias in the published articles on this issue, we should be very careful in drawing any firm conclusions. Hopefully, publication of the currently ongoing randomized clinical trials and prospective cohort studies will bring more definitive evidence on the efficacy of antiretrovirals for pre‐ or postexposure prophylaxis against COVID‐19.
Conclusions
Many biomolecular studies strongly identify multiple antiretroviral drugs as potential inhibitors of SARS‐CoV‐2 main protease and RNA‐dependent RNA polymerase, and some limited evidence considers protease inhibitor subgroup of ARVs, especially ritonavir‐boosted lopinavir for decreasing mortality and accelerating recovery. However, currently there is no evidence to support the use of these ARVs for prophylaxis against COVID‐19, and we must await the results of ongoing trials that include them in their intervention groups. Ritonavir‐boosted lopinavir, nelfinavir, tenofovir, and emtricitabine are favorable pharmacologic candidates against SARS‐CoV‐2 and must be further investigated by adequately powered randomized clinical trials to determine their effectiveness for prophylaxis and treatment. There is insufficient evidence for darunavir, and more study is needed to evaluate atazanavir and integrase inhibitors for prophylaxis and treatment. Considering the cost and limited availability of these drugs and the risk of PLWH being deprived of these essential therapeutics, we must search for definite evidence that would justify using these ARVs.
Conflicts of Interest
All the authors state that they have no conflicts of interest to declare.
Author Contributions
G.A. and K.K. contributed to data collection, writing, and critical appraisal of the article. M.M. contributed to writing and final revision and drafting of the article. Z.T. contributed to study design, data gathering, writing, and final revision of the article.
Acknowledgments
We thank Rose Naderi of Duke University Trinity College of Arts and Sciences for English proofreading of the article. We also acknowledge the generous contribution of Shokoufeh Alavian of the University of Tehran College of Fine Arts for graphic enhancement of the disclosed figure.
References
- 1. World Health Organization. Weekly epidemiological update, coronavirus disease 2019 (COVID‐19). https://www.who.int/publications/m/item/weekly-epidemiological-update---27-october-2020. Published October 27, 2020. Accessed October 27, 2020.
- 2. Nguyen LH, Drew DA, Joshi AD, et al. Risk of COVID‐19 among frontline healthcare workers and the general community: a prospective cohort study. medRxiv. 2020. 10.1101/2020.04.29.20084111. Accessed October 20, 2020. [DOI] [Google Scholar]
- 3. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the New York City area. JAMA. 2020;323(20):2052‐2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shalev N, Scherer M, LaSota ED, et al. Clinical characteristics and outcomes in people living with HIV hospitalized for COVID‐19. Clin Infect Dis. 2020; 10.1093/infdis/jiaa380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Guo W, Ming F, Dong Y, et al. A survey for COVID‐19 among HIV/AIDS patients in two Districts of Wuhan, China. AIDS Patients in Two Districts of Wuhan, China; March 4, 2020, 2020. [Google Scholar]
- 6. Del Amo J, Polo R, Moreno S, et al. Incidence and severity of COVID‐19 in HIV‐positive persons receiving antiretroviral therapy: a cohort study. Ann Intern Med. 2020;173(7):536‐541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gervasoni C, Meraviglia P, Riva A, et al. Clinical features and outcomes of HIV patients with coronavirus disease 2019. Clin Infect Dis. 2020;71(16):2276‐2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hileman CO, Funderburg NT. Inflammation, immune activation, and antiretroviral therapy in HIV. Curr HIV/AIDS Rep. 2017;14(3):93‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Melchjorsen J, Risor MW, Sogaard OS, et al. Tenofovir selectively regulates production of inflammatory cytokines and shifts the IL‐12/IL‐10 balance in human primary cells. J Acquir Immune Defic Syndr. 2011;57(4):265‐275. [DOI] [PubMed] [Google Scholar]
- 10. Shetty R, Ghosh A, Honavar SG, Khamar P, Sethu S. Therapeutic opportunities to manage COVID‐19/SARS‐CoV‐2 infection: Present and future. Indian J Ophthalmol. 2020;68(5):693‐702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Baluku JB, Olum R, Agolor C, et al. Prevalence, clinical characteristics and treatment outcomes of HIV and SARS‐CoV‐2 co‐infection: a systematic review and meta‐analysis. medRxiv. https://www.medrxiv.org/content/10.1101/2020.05.31.20118497v1.full.pdf. Published 2020. Accessed October 22, 2020. [Google Scholar]
- 12. Chen XP, Cao Y. Consideration of highly active antiretroviral therapy in the prevention and treatment of severe acute respiratory syndrome. Clin Infect Dis. 2004;38(7):1030‐32. [DOI] [PubMed] [Google Scholar]
- 13. Park SY, Lee JS, Son JS, et al. Post‐exposure prophylaxis for Middle East respiratory syndrome in healthcare workers. J Hosp Infect. 2019;101(1):42‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lv Z, Chu Y, Wang Y. HIV protease inhibitors: a review of molecular selectivity and toxicity. HIV AIDS (Auckl). 2015;7:95‐104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nutho B, Mahalapbutr P, Hengphasatporn K, et al. Why are lopinavir and ritonavir effective against the newly emerged coronavirus 2019? Atomistic insights into the inhibitory mechanisms. Biochemistry. 2020;59(18):1769‐1779. [DOI] [PubMed] [Google Scholar]
- 16. Muralidharan N, Sakthivel R, Velmurugan D, Gromiha MM. Computational studies of drug repurposing and synergism of lopinavir, oseltamivir and ritonavir binding with SARS‐CoV‐2 protease against COVID‐19. J Biomol Struct Dyn. 2020:1‐6. 10.1080/07391102.2020.1752802. [DOI] [PubMed] [Google Scholar]
- 17. Copertino DC Jr, Lima B, Duarte R, et al. Antiretroviral drug activity and potential for pre‐exposure prophylaxis against COVID‐19 and HIV infection. https://chemrxiv.org/ndownloader/articles/12250199/versions/1/export_pdf. Published 2020. Accessed October 22, 2020. [DOI] [PMC free article] [PubMed]
- 18. Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, et al. A trial of lopinavir‐ritonavir in adults hospitalized with severe Covid‐19. N Engl J Med. 2020;382(19):1787‐1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Horby PW, Mafham M, Bell JL, et al. Lopinavir–ritonavir in patients admitted to hospital with COVID‐19 (RECOVERY): a randomised, controlled, open‐label, platform trial. Lancet. 2020;396(10259):1345‐1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pan H, Peto R, Karim QA, et al. Repurposed antiviral drugs for COVID‐19; interim WHO SOLIDARITY trial results. medRxiv. https://www.medrxiv.org/content/10.1101/2020.10.15.20209817v1.full.pdf. Published 2020. Accessed October 22, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Klement‐Frutos E, Burrel S, Peytavin G, et al. Early administration of ritonavir‐boosted lopinavir could prevent severe COVID‐19. J Infect. 2020; 10.1016/j.jinf.2020.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bishara D, Kalafatis C, Taylor D. Emerging and experimental treatments for COVID‐19 and drug interactions with psychotropic agents. Ther Adv Psychopharmacol. 2020;10:2045125320935306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Choy K‐T, Wong AY‐L, Kaewpreedee P, et al. Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS‐CoV‐2 replication in vitro. Antiviral Res. 2020;178:104786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Venisse N, Peytavin G, Bouchet S, et al. Concerns about pharmacokinetic (PK) and pharmacokinetic‐pharmacodynamic (PK‐PD) studies in the new therapeutic area of COVID‐19 infection. Antiviral Res. 2020;181:104866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Arshad U, Pertinez H, Box H, et al. Prioritisation of anti‐SARS‐Cov‐2 drug repurposing opportunities based on plasma and target site concentrations derived from their established human pharmacokinetics. Clin Pharmacol Ther. 2020;108(4):775‐790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang Y, Chen L. Tissue distributions of antiviral drugs affect their capabilities of reducing viral loads in COVID‐19 treatment. Eur J Pharmacol. 2020;889:173634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Baldelli S, Corbellino M, Clementi E, Cattaneo D, Gervasoni C. Lopinavir/ritonavir in COVID‐19 patients: maybe yes, but at what dose? J Antimicrob Chemother. 2020;75(9):2704‐2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gregoire M, Le Turnier P, Gaborit BJ, et al. Lopinavir pharmacokinetics in COVID‐19 patients. J Antimicrob Chemother. 2020;75(9):2702‐2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. COVID‐19 Ring‐based Prevention Trial With Lopinavir/Ritonavir. https://ClinicalTrials.gov/show/NCT04321174. Accessed October 15, 2020.
- 30. Chemoprophylaxis of SARS‐CoV‐2 Infection (COVID‐19) in Exposed Healthcare Workers. https://ClinicalTrials.gov/show/NCT04328285. Accessed October 15, 2020.
- 31. Beck BR, Shin B, Choi Y, Park S, Kang K. Predicting commercially available antiviral drugs that may act on the novel coronavirus (SARS‐CoV‐2) through a drug‐target interaction deep learning model. Comput Struct Biotechnol J. 2020;18:784‐790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ghasemiyeh P, Borhani‐Haghighi A, Karimzadeh I, et al. Major neurologic adverse drug reactions, potential drug‐drug interactions and pharmacokinetic aspects of drugs used in COVID‐19 patients with stroke: a narrative review. Ther Clin Risk Manag. 2020;16:595‐605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. NA‐831, Atazanavir and Dexamethasone Combination Therapy for the Treatment of COVID‐19 Infection. https://ClinicalTrials.gov/show/NCT04452565. Accessed October 15, 2020.
- 34. Chen J, Xia L, Liu L, et al. Antiviral activity and safety of darunavir/cobicistat for the treatment of COVID‐19. Open Forum Infect Dis. 2020;7(7):ofaa241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. De Meyer S, Bojkova D, Cinatl J, et al. Lack of antiviral activity of darunavir against SARS‐CoV‐2. Int J Infect Dis. 2020;97:7‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Darunavir/Cobicistat vs. Lopinavir/Ritonavir in COVID‐19 Pneumonia in Qatar. https://ClinicalTrials.gov/show/NCT04425382.
- 37. Ivermectin vs Combined Hydroxychloroquine and Antiretroviral Drugs (ART) Among Asymptomatic COVID‐19 Infection. https://ClinicalTrials.gov/show/NCT04435587.
- 38. Efficacy and Safety of Darunavir and Cobicistat for Treatment of COVID‐19. https://ClinicalTrials.gov/show/NCT04252274. Accessed October 15, 2020.
- 39. Various Combination of Protease Inhibitors, Oseltamivir, Favipiravir, and Hydroxychloroquine for Treatment of COVID‐19 : A Randomized Control Trial. https://ClinicalTrials.gov/show/NCT04303299. Accessed October 12, 2020.
- 40. Fragkou PC, Belhadi D, Peiffer‐Smadja N, et al. Review of trials currently testing treatment and prevention of COVID‐19. Clin Microbiol Infect. 2020;26(8):988‐998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Riva A, Conti F, Bernacchia D, et al. Darunavir does not prevent SARS‐CoV‐2 infection in HIV patients. Pharmacol Res. 2020;157:104826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Treatment of non‐severe confirmed cases of COVID‐19 and chemoprophylaxis of their contacts as prevention strategy: a Cluster Randomized Clinical Trial (PEP CoV‐2 Study). https://www.clinicaltrialsregister.eu/ctr-search/search?query=2020-001031-27. Accessed October 25, 2020.
- 43. Hall DC Jr, Ji HF. A search for medications to treat COVID‐19 via in silico molecular docking models of the SARS‐CoV‐2 spike glycoprotein and 3CL protease. Travel Med Infect Dis. 2020;35:101646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kumar S, Sharma PP, Shankar U, et al. Discovery of new hydroxyethylamine analogs against 3CL(pro) protein target of SARS‐CoV‐2: molecular docking, molecular dynamics simulation, and structure‐activity relationship studies. J Chem Inf Model. 2020; 10.1021/acs.jcim.0c00326. [DOI] [PubMed] [Google Scholar]
- 45. Musarrat F, Chouljenko V, Dahal A, et al. The anti‐HIV drug nelfinavir mesylate (Viracept) is a potent inhibitor of cell fusion caused by the SARSCoV‐2 spike (S) glycoprotein warranting further evaluation as an antiviral against COVID‐19 infections. J Med Virol. 2020; 10.1002/jmv.25985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Impact of long‐term protease inhibitors in patients living with HIV on the incidence of COVID‐19 (COVIP). https://ClinicalTrials.gov/show/NCT04357639. Accessed October 12, 2020.
- 47. Ibrahim MAA, Abdelrahman AHM, Hegazy MF. In‐silico drug repurposing and molecular dynamics puzzled out potential SARS‐CoV‐2 main protease inhibitors. J Biomol Struct Dyn. 2020:1‐12. 10.1080/07391102.2020.1791958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kearney BP, Flaherty JF, Shah J. Tenofovir disoproxil fumarate: clinical pharmacology and pharmacokinetics. Clin Pharmacokinet. 2004;43(9):595‐612. [DOI] [PubMed] [Google Scholar]
- 49. Atta MG, De Seigneux S, Lucas GM. Clinical Pharmacology in HIV Therapy. Clin J Am Soc Nephrol. 2019;14(3):435‐444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.WHO model list of essential medicines World Health Organization; 2013. http://apps.who.int/iris/bitstream/10665/93142/1/EML_18_eng.pdf. Accessed August 20, 2020. [Google Scholar]
- 51. Anderson PL, Kiser JJ, Gardner EM, Rower JE, Meditz A, Grant RM. Pharmacological considerations for tenofovir and emtricitabine to prevent HIV infection. J Antimicrob Chemother. 2011;66(2):240‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chien M, Anderson TK, Jockusch S, et al. Nucleotide analogues as inhibitors of SARS‐CoV‐2 polymerase, a key drug target for COVID‐19. J Proteome Res. 2020;19(11):4690‐4697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Elfiky AA. Ribavirin, remdesivir , sofosbuvir, galidesivir, and tenofovir against SARS‐CoV‐2 RNA dependent RNA polymerase (RdRp): a molecular docking study. Life Sci. 2020;253:117592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jockusch S, Tao C, Li X, Anderson TK, et al. Triphosphates of the two components in DESCOVY and TRUVADA are inhibitors of the SARS‐CoV‐2 polymerase. bioRxiv. 2020. https://www.biorxiv.org/content/10.1101/2020.04.03.022939v1. [Google Scholar]
- 55. Clososki GC, Soldi RA, RMd Silva, et al. Tenofovir disoproxil fumarate: new chemical developments and encouraging in vitro biological results for SARS‐CoV‐2. J Braz Chem Soc. 2020;31(8):1552‐1556. [Google Scholar]
- 56. Oberg B. Rational design of polymerase inhibitors as antiviral drugs. Antiviral Res. 2006;71(2‐3):90‐95. [DOI] [PubMed] [Google Scholar]
- 57. Park SJ, Yu KM, Kim YI, et al. Antiviral efficacies of FDA‐approved drugs against SARS‐CoV‐2 infection in ferrets. mBio. 2020;11(3):e01114‐e01120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Komastu T, Ireland DD, Reiss CS. IL‐12 and viral infections. Cytokine Growth Factor Rev. 1998;9(3‐4):277‐285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ayerdi O, Puerta T, Clavo P, et al. Preventive efficacy of tenofovir/emtricitabine against SARS‐CoV‐2 among PREP users. Open Forum Infect Dis. 2020;7(11):ofaa455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Randomized Clinical Trial for the Prevention of SARS‐CoV‐2 Infection (COVID‐19) in Healthcare Personnel. https://ClinicalTrials.gov/show/NCT04334928. Accessed October 14, 2020.
- 61. Daily Regimen of Tenofovir/Emtricitabine as Prevention for COVID‐19 in Health Care Personnel in Colombia. https://ClinicalTrials.gov/show/NCT04519125. Accessed October 15, 2020.
- 62. TAF/FTC for Pre‐exposure Prophylaxis of COVID‐19 in Healthcare Workers (CoviPrep Study). https://ClinicalTrials.gov/show/NCT04405271. Accessed October 12, 2020.
- 63. Tan EL, Ooi EE, Lin CY, et al. Inhibition of SARS coronavirus infection in vitro with clinically approved antiviral drugs. Emerg Infect Dis. 2004;10(4):581‐586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Davies MA. HIV and risk of COVID‐19 death: a population cohort study from the Western Cape Province, South Africa. medRxiv. 2020. 10.1101/2020.07.02.20145185. [DOI] [Google Scholar]
- 65. You J, Wang H, Huang X, et al. Therapy‐emergent drug resistance to integrase strand transfer inhibitors in HIV‐1 patients: a subgroup meta‐analysis of clinical trials. PLoS One. 2016;11(8):e0160087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Khan RJ, Jha RK, Amera GM, et al. Targeting SARS‐CoV‐2: a systematic drug repurposing approach to identify promising inhibitors against 3C‐like proteinase and 2'‐O‐ribose methyltransferase. J Biomol Struct Dyn. 2020:1‐14. 10.1080/07391102.2020.1753577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Krafcikova P, Silhan J, Nencka R, Boura E. Structural analysis of the SARS‐CoV‐2 methyltransferase complex involved in RNA cap creation bound to sinefungin. Nat Commun. 2020;11(1):3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kumar Y, Singh H, Patel CN. In silico prediction of potential inhibitors for the main protease of SARS‐CoV‐2 using molecular docking and dynamics simulation based drug‐repurposing. J Infect Public Health. 2020;13(9):1210‐1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Charre C, Icard V, Pradat P, et al. COVID‐19 attack rate in HIV‐infected patients and in PrEP users. AIDS. 2020. 10.1097/QAD.0000000000002639. [DOI] [PMC free article] [PubMed] [Google Scholar]


