The prevalence of SARS‐coronavirus‐2 in asymptomatic elective surgical patients during a second wave was approximately 1 in 833. Given the very low likelihood of coronavirus transmission and with existing current hospital capacity, elective surgery can recommence at near‐normal levels.

Keywords: anaesthesia, coronavirus, COVID‐19, public health, surgery, surveillance
Abstract
Background
The COVID‐19 pandemic has greatly affected access to elective surgery, largely because of concerns for patients and healthcare workers. A return to normal surgery workflow depends on the prevalence and transmission of coronavirus in elective surgical patients. The aim of this study was to determine the prevalence of active SARS‐coronavirus‐2 infection during a second wave among patients admitted to hospital for elective surgery in Victoria.
Methods
Prospective cohort study across eight hospitals in Victoria during July–August 2020 was conducted enrolling adults and children admitted to hospital for elective surgery or interventional procedure requiring general anaesthesia. Study outcomes included a positive polymerase chain reaction (PCR) test for SARS‐CoV‐2 in the preoperative period (primary outcome), and for those with a negative test preoperatively, the incidence of a positive PCR test for SARS‐CoV‐2 in the post‐operative period.
Results
We enrolled 4965 elective adult and paediatric surgical patients from 15 July to 31 August 2020. Four patients screened negative on questionnaire but had a positive PCR test for coronavirus, resulting in a Bayesian estimated prevalence of 0.12% (95% probability interval 0–0.26%). There were no reports of healthcare worker infections linked to elective surgery during and up to 2 weeks after the study period.
Conclusion
The prevalence of SARS‐CoV‐2 in asymptomatic elective surgical patients during a second wave was approximately 1 in 833. Given the very low likelihood of coronavirus transmission, and with existing current hospital capacity, recommencement of elective surgery should be considered. A coronavirus screening checklist should be mandated for surgical patients.
Introduction
The COVID‐19 pandemic has led to anxiety among patients and healthcare workers (HCWs) alike. A second wave of SARS‐coronavirus‐2 (‘coronavirus’) transmissions hit Victoria in late June this year. The daily number of cases rose from approximately 30–50 cases per day in late June to 500–700 cases per day in late July. The chief health officer identified many northern and western suburbs in Melbourne as ‘hotspots’ at the beginning of the second wave, first defined by a daily incidence of at least 20 cases per 100 000 local population. As of 1 September, after the Victorian government initiated a stage 3 lockdown on 3 July, and a stage 4 lockdown on 2 August, this second wave has abated (Figs 1, 2,S1). Through this time, many municipalities in Melbourne had coronavirus prevalence rates of 100–1000 per 100 000 population.
Fig. 1.
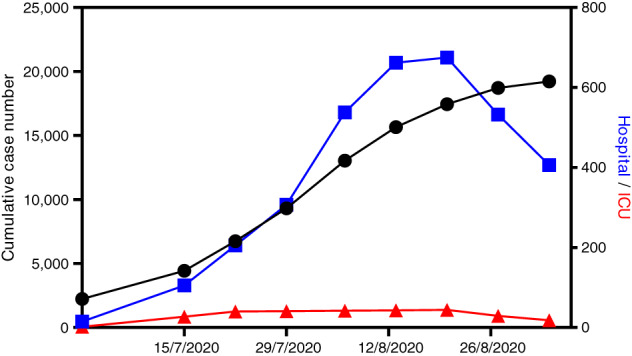
The number of cumulative cases and hospitalizations (including in the intensive care unit (ICU)) in Victoria over the study period from 13 July to 31 August 2020 ( , total cases;
, total cases;  , in hospital;
, in hospital;  , in ICU).
, in ICU).
Fig. 2.
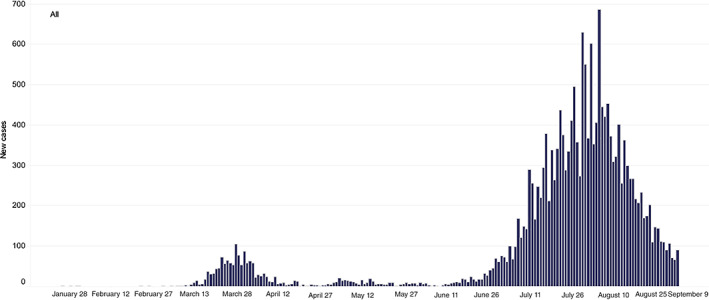
Daily new cases in Victoria.Source: https://www.dhhs.vic.gov.au/victorian‐coronavirus‐covid‐19‐data.
The Victorian Department of Health and Human Services (DHHS) instituted mandatory reverse transcription polymerase chain reaction (PCR) testing for coronavirus in both elective and non‐elective surgical patients in stage 3 lockdown areas on 15 July, and later, use of level 3 (N95‐type) respirators for all aerosol‐generating procedures in all patients irrespective of PCR test result, among other interventions. These interventions were thought to be necessary in view of the known risks of COVID‐19 in surgical patients and potential risks to HCWs. 1 , 2 , 3 The Victorian DHHS also restricted elective surgery to more urgent (category 1 and urgent category 2) patients.
If and when surgery workflow can return to normal, and what level of personal protective equipment is required to keep HCWs safe will depend on the rates of prevalence and transmission in the community. However, a more useful metric is the prevalence rate in elective surgical patients. Local, up‐to‐date data will better inform planning for further increases in elective surgery. The aim of this study was to determine the prevalence of active coronavirus infection among patients admitted to hospital for elective surgery in Victorian hospitals.
Methods
We performed a multicentre, prospective, observational study across selected Melbourne hospitals, and later included one regional hospital that had comparable procedures and collected equivalent data. Our study hypothesis was that elective surgery patients would have a low prevalence (less than 1 in 100) of active (PCR positive) coronavirus infection.
The study population consisted of adults and children booked for elective surgery, endoscopy or interventional procedure requiring general anaesthesia at each of the participating hospitals, having no risk factors (or recent contact with a known case) identified by a screening questionnaire (Appendix S1), and having a pre‐admission PCR coronavirus test. During the study period, elective patients completed a screening questionnaire at the time of booking (typically 5 days prior to surgery), had a swab for coronavirus 2–5 days prior to surgery and then the screening questionnaire was repeated on the day of surgery. We excluded patients having non‐elective (urgent or emergency inpatient) surgery.
Patients enrolled at the Alfred Hospital had an additional nasopharyngeal swab done by the attending anaesthesiologist post‐anaesthetic induction. This was done to improve the detection of coronavirus infection and to account for the interim period between their pre‐admission PCR test and the day of surgery (patients were informed of this additional testing at the time of their consent for surgery).
We collected relevant patient demographic, recent travel and coronavirus contacts, and perioperative characteristics (Appendix S2). The hotspot suburbs were identified as those postcodes with a daily incidence >20 per 100 000 at the beginning of the second wave. Demographic and clinical data were abstracted from the medical record by local investigators and then entered into a password‐protected REDCap web database, 4 on a secure server managed by the Monash University.
Our primary outcome was a positive PCR test for coronavirus in patients who had screened negative on the questionnaire (asymptomatic prevalence). Our secondary outcomes were: (i) the number of patients who had risk factors identified by the screening questionnaire that led to their surgery being deferred but also had a positive PCR test for coronavirus, (ii) the number of patients who converted from a negative pre‐admission PCR test to a positive PCR test for coronavirus in the post‐operative period, and (iii) the incidence of coronavirus transmission (attack rate) in HCWs caring for elective surgical patients during and for 2 weeks after the study period.
The Alfred Hospital Ethics Committee approved this study with a waiver of consent because testing was occurring as part of clinical care, and data collection was conducted locally at each site and only de‐identified data were transcribed onto the central database (Appendix S3, Table S1). 5 All participating hospitals obtained ethics committee approval or otherwise had approval from their chief executive or chief medical officer as a quality assurance project.
Sample size and statistical analysis
We anticipated a prevalence of asymptomatic coronavirus infection in elective surgical patients to be no more than that seen in metropolitan Melbourne in July, being approximately 50–200 per 100 000. Calculating exact binomial confidence intervals, assuming no diagnostic test inaccuracy and using the upper 95% confidence limit, a sample size of at least 2000 patients with no observed positive test results could rule out a prevalence of less than 2 per 1000.
We estimated the prevalence of coronavirus infection using a Bayesian approach which incorporates uncertainty about the values of sensitivity and specificity of the PCR testing in the form of prior distributions for these parameters. We assumed a beta (15.5) prior to distribution for sensitivity, which has mean at 75% and 2.5–97.5 percentiles of 54–91%, and a beta (19.9, 0.01) prior to distribution for specificity, which has mean at 99.5% and 2.5–97.5 percentiles of 95–100%. Statistical analysis was conducted using RStan version 2.21.1 (Stan Governing Body, IO, USA), implementing 20 000 draws across four chains. Convergence and appropriate mixing of chains were confirmed with diagnostic plots and all R‐hat values being <1.01. Prevalence estimates are reported as the median of the posterior distribution, and 95% probability intervals use the highest posterior density. Data for summary statistics are presented as number (%), and mean (SD) or median (interquartile range) for numerical data.
Results
We enrolled 4965 patients from 15 July to 31 August, 2020 (Fig. S2), during which the cumulative number of cases of coronavirus in Victoria went from 4428 to 19 224 (Fig. 1). A total of 497 (10.0%) patients were resident in Melbourne's designated hotspots at the beginning of the second wave. Through this time, the daily number of hospitalizations peaked at over 700 cases. Patient and surgical characteristics are outlined in Table 1; the STROBE checklist is available in Table S2.
Table 1.
Patient demographics and perioperative characteristics (n = 4965)
| Factor | Missing n (%) | |
|---|---|---|
| Mean (SD) age, years | 47.0 (25.1) | 0 |
| Range | 0–99 | |
| Sex | ||
| Female | 2349 (47.3) | 0 |
| Male | 2616 (52.7) | |
| ASA physical status | ||
| 1 | 986 (19.9) | 0 |
| 2 | 2217 (44.7) | |
| 3 | 1579 (31.8) | |
| 4 | 183 (3.7) | |
| Ethnicity | 283 (6.0) | |
| White | 3263 (69.7) | |
| Asian | 441 (9.4) | |
| ATSI | 25 (0.5) | |
| Black/African | 50 (1.1) | |
| Other | 903 (19.3) | |
| Socio‐economic classification (IRSAD) | 23 (0.5) | |
| 1–20% | 604 (12.2) | |
| 21–40% | 763 (15.4) | |
| 41–60% | 915 (18.5) | |
| 61–80% | 1072 (21.7) | |
| 81–100% | 1588 (32.1) | |
| Comorbidities | ||
| CVD (including HT, HF, CAD) | 1766 (35.6) | 2 (0.0) |
| Treated diabetes | 642 (12.9) | 2 (0.0) |
| COPD and/or asthma | 771 (15.5) | 2 (0.0) |
| Month of surgery | 5 (0.1) | |
| July | 1302 (26.3) | |
| August | 3658 (73.8) | |
| Preoperative screening on admission | 4618 (93.3) | 14 (0.3) |
| Temperature check on admission | 4861 (98.0) | 4 (0.1) |
| Overseas travel in 2020 | 27 (0.6) | 623 (14.3) |
| When was travel | 5 (19) | 0 |
| ≤2 weeks | 22 (81) | |
| >2 weeks | ||
| COVID‐19 contact | 13 (0.3) | 16 (0.3) |
| When was contact | 0 | |
| ≤2 weeks | 9 (69) | |
| >2 weeks | 4 (31) | |
| Health worker | 136 (2.7) | 3 (0.1) |
| Surgery type | 3 (0.1) | |
| Orthopaedic | 381 (7.7) | |
| Urology | 697 (14.0) | |
| Gynaecological | 361 (7.3) | |
| Gastrointestinal | 536 (10.8) | |
| Plastics | 415 (8.4) | |
| Neurological | 155 (3.1) | |
| Cardiac | 201 (4.1) | |
| Vascular | 127 (2.6) | |
| Ear, nose, throat | 286 (5.8) | |
| Endoscopy | 952 (19.2) | |
| Ophthalmology | 59 (1.2) | |
| Oral/faciomaxillary | 61 (1.2) | |
| Other | 731 (14.7) | |
| Unplanned ICU/HDU admission | 36(0.07) | 32 (0.6) † |
| Hospital stay | 7 (0.1) † | |
| ≥1 night stay | 2112 (42.6) | |
| Day case | 2846 (57.4) | |
| Number of nights in hospital, median (IQR) | 2.0 (1.0. 5.0) | 34 (1.6) † |
| In‐hospital mortality | 10 (0.2) | 37 (0.8) † |
Data are represented as number (%), unless otherwise indicated.
Patients still as inpatients on 22 September 2020.
ASA, American Society of Anesthesiologists; ATSI, Aboriginal or Torres Strait Islander; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; HDU, high‐dependency unit; HF, heart failure; HT, hypertension; ICU, intensive care unit; IQR, interquartile range; IRSAD, Index of Relative Socio‐economic Advantage and Disadvantage.
A total of eight patients had positive PCR tests either before or after surgery. Of these, four adult patients were asymptomatic and did not report any epidemiological risk factors but had a positive test, resulting in a Bayesian estimated prevalence of 0.12% (95% probability interval 0–0.26%). No patients enrolled at the Alfred Hospital (n = 713) had a positive PCR test from the additional nasopharyngeal swab done by the attending anaesthesiologist post‐anaesthetic induction. One patient had reported symptoms on the screening questionnaire and had their surgery cancelled; their PCR test for coronavirus was positive. Three (two adults and one child) patients were asymptomatic and had a negative PCR test for coronavirus preoperatively, underwent surgery and developed symptoms and had a positive test in the post‐operative period (at 2, 7 and 8 days, respectively); all three had undergone minor surgery and none required additional hospitalization. One of the eight patients who had tested positive before or after surgery resided in a hotspot suburb of Melbourne.
There were no reports of HCWs caring for the elective surgical patients testing positive to coronavirus during or up to 2 weeks after the study period that could be attributed to elective surgery. Thus, the attack rate was zero.
Discussion
Despite a second and much larger wave of coronavirus cases in Victoria during July–August 2020, particularly across the northern and western suburbs of Melbourne, we found a very low prevalence of coronavirus in elective surgical patients who had a negative screening questionnaire at the time of booking. Those (n = 3) who first developed symptoms of COVID‐19 post‐operatively had an otherwise uneventful recovery. In addition, there were no reports of associated HCW transmission linked to elective surgery in the participating hospitals during the data collection period. Our study included a variety of hospitals, both adult and paediatric, adjacent to coronavirus hotspots, and in metropolitan and regional areas.
Only one of the eight positives cases had come from one of the Melbourne's hotspot suburbs, weakening any ability to selectively identify at‐risk patients on this basis. We identified three cases in which the preoperative PCR test was negative but the patient was subsequently found to have coronavirus infection in the post‐operatively period – none of these had any complications. These cases might reflect a false‐negative preoperative test, or the patient was early in the incubation period at the time of preoperative testing. Both false‐negative and false‐positive coronavirus PCR tests results can occur. 6 We accounted for this with prior distributions for sensitivity and specificity in our Bayesian analysis. Such occurrences will be unavoidable, but there were no apparent adverse effects on their post‐operative recovery. The combination of screening questionnaire, preoperative swab testing and appropriate personal protective equipment has led to no HCW infections in our series. Data from high‐prevalence cities and countries around the world have found that anaesthesiologists and other HCWs have a worryingly high prevalence (3.1–7.1%) of probable COVID‐19. 7 , 8 , 9 , 10 These transmission rates mostly reflect community prevalence. 9
During the period of study, elective surgery was effectively restricted to 50% so that priority was being given to the more urgent category 1 and 2A cases (e.g. cancer surgery). Less urgent cases and emergency cases may have a different risk exposure to community transmission of coronavirus.
Some of us participated in a similar study conducted across Australia during the nadir period of coronavirus transmission through June and early July 2020. When combined, these pooled results offer great reassurance for those caring for elective surgical patients in Victoria and other states. Elective surgery should be able to return to near‐normal levels in Victoria. The global backlog of elective surgery has been predicted to take close to a year to resolve. 10 Given the moderate sensitivity and therefore worrying false‐negative rate of PCR testing, there is insufficient reassurance with a negative test. Also, even with high specificity (99.5%), there is very weak predictive utility with routine testing in the low‐risk setting of elective surgery: for a prevalence of 0.1%, the positive predictive value is only 13% (selective surveillance testing may however be done to reaffirm very low prevalence). Nor is there a strong need to require the use of COVID‐19 precautions if the patient has screened negative for coronavirus on preoperative questionnaire. These circumstances differ markedly from other parts of the world. For example, the preoperative coronavirus positive testing rate in North Carolina was 0.86% (61 of 7100). 11 This is eight‐fold higher than what has been experienced in Victoria.
In conclusion, the prevalence of coronavirus infection in a screened population of elective surgical patients in Victoria during a second a wave was very low, approximately 1 in 833. With existing current hospital capacity and decreasing case numbers, a staged recommencement of elective surgery should be considered. Patient and staff safety will be enhanced by preoperative screening to detect symptoms of coronavirus infection or recent contact with anyone known to have been infected; this should be mandated.
Author Contributions
Paul Myles: Conceptualization; data curation; funding acquisition; investigation; methodology; project administration; writing‐original draft; writing‐review and editing. Sophie Wallace: Data curation; investigation; methodology; project administration; writing‐review and editing. David Story: Investigation; methodology; writing‐review and editing. Allen Cheng: Methodology; validation; writing‐review and editing. Andrew Forbes: Data curation; formal analysis; writing‐original draft; writing‐review and editing. Sofia Sidiropoulos: Data curation; writing‐review and editing. Andrew Davidson: Data curation; writing‐review and editing. Niki Tan: Data curation; writing‐review and editing. Andrew Jeffries: Data curation; writing‐review and editing. David Scott: Data curation; writing‐review and editing. Jade Radnor: Conceptualization; data curation; investigation; writing‐review and editing.
Conflicts of interest
None declared.
Supporting information
Appendix S1. Victorian elective surgery collaboration – contributors at each site.
Appendix S2. An example of the pre‐admission/admission screening questionnaire.
Appendix S3. Case report form.
Table S1. Ethics and quality assurance approvals.
Table S2. STROBE statement – checklist of items that should be included in reports of cohort studies.
Figure S1. SARS‐coronavirus‐2 cases as of 31 August 2020 reported by the Australian Government Department of Health.
Figure S2. Patient flow: details of elective surgical patients screened for eligibility and inclusion.
Acknowledgements
We thank the many nurses, anaesthesiologists and surgeons who cooperated with the study. We thank Dr Adam Jenney and colleagues of the Department of Microbiology at the Alfred Hospital who supported the additional intraoperative nasopharyngeal swab testing. The study was funded by Safer Care Victoria (DHHS) and the Medibank Better Health Foundation. The funders had no role in the design, conduct or interpretation of the study. PSM is funded by an NHMRC Practitioner Fellowship.
P. S. Myles MPH, DSc, FANZCA; S. Wallace BHlthSc, MPH; D. A. Story MD, FANZCA; W. Brown PhD, FRACS; A. C. Cheng PhD, FRACP; A. Forbes MSc, PhD; S. Sidiropoulos BN, MPH; A. Davidson MD, FANZCA; N. Tan MBBS, FANZCA; A. Jeffreys MBBS, FANZCA; R. Hodgson MBBS, FRACS; D. A. Scott PhD, FANZCA; J. Radnor MBBS, FANZCA.
References
- 1. COVIDSurg Collaborative . Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS‐CoV‐2 infection: an international cohort study. Lancet 2020; 396: 27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Myles PS, Maswime S. Mitigating the risks of surgery during the COVID‐19 pandemic. Lancet 2020; 396: 2–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Doglietto F, Vezzoli M, Gheza F et al. Factors associated with surgical mortality and complications among patients with and without coronavirus disease 2019 (COVID‐19) in Italy. JAMA Surg. 2020; 155: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Harris PA, Taylor R, Minor BL et al. The REDCap consortium: building an international community of software platform partners. J. Biomed. Inform. 2019; 95: 103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zeps N, Iacopetta BJ, Schofield L, George JM, Goldblatt J. Waiver of individual patient consent in research: when do potential benefits to the community outweigh private rights? Med. J. Aust. 2007; 186: 88–90. [DOI] [PubMed] [Google Scholar]
- 6. Watson J, Whiting PF, Brush JE. Interpreting a covid‐19 test result. BMJ 2020; 369: m1808. [DOI] [PubMed] [Google Scholar]
- 7. El‐Boghdadly K, Wong DJN, Owen R et al. Risks to healthcare workers following tracheal intubation of patients with COVID‐19: a prospective international multicentre cohort study. Anaesthesia 2020; 75: 1437–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bampoe S, Lucas DN, Neall G et al. A cross‐sectional study of immune seroconversion to SARS‐CoV‐2 in frontline maternity health professionals. Anaesthesia 2020; 75: 1614–9. [DOI] [PubMed] [Google Scholar]
- 9. Moscola J, Sembajwe G, Jarrett M et al. Prevalence of SARS‐CoV‐2 antibodies in health care personnel in the New York City area. JAMA 2020; 324: 893–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. COVIDSurg Collaborative . Elective surgery cancellations due to the COVID‐19 pandemic: global predictive modelling to inform surgical recovery plans. Br. J. Surg. 2020; 107: 1440–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kibbe MR. Surgery and COVID‐19. JAMA 2020; 324: 1151–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Victorian elective surgery collaboration – contributors at each site.
Appendix S2. An example of the pre‐admission/admission screening questionnaire.
Appendix S3. Case report form.
Table S1. Ethics and quality assurance approvals.
Table S2. STROBE statement – checklist of items that should be included in reports of cohort studies.
Figure S1. SARS‐coronavirus‐2 cases as of 31 August 2020 reported by the Australian Government Department of Health.
Figure S2. Patient flow: details of elective surgical patients screened for eligibility and inclusion.


