Abstract
Background/Objectives
Several scoring systems have been specifically developed for risk stratification in COVID‐19 patients.
Design
We compared, in a cohort of confirmed COVID‐19 older patients, three specifically developed scores with a previously established early warning score. Main endpoint was all causes in‐hospital death.
Setting
This is a single‐center, retrospective observational study, conducted in the Emergency Department (ED) of an urban teaching hospital, referral center for COVID‐19.
Participants
We reviewed the clinical records of the confirmed COVID‐19 patients aged 60 years or more consecutively admitted to our ED over a 6‐week period (March 1st to April 15th, 2020). A total of 210 patients, aged between 60 and 98 years were included in the study cohort.
Measurements
International Severe Acute Respiratory Infection Consortium Clinical Characterization Protocol‐Coronavirus Clinical Characterization Consortium (ISARIC‐4C) score, COVID‐GRAM Critical Illness Risk Score (COVID‐GRAM), quick COVID‐19 Severity Index (qCSI), National Early Warning Score (NEWS).
Results
Median age was 74 (67–82) and 133 (63.3%) were males. Globally, 42 patients (20.0%) deceased. All the score evaluated showed a fairly good predictive value with respect to in‐hospital death. The ISARIC‐4C score had the highest area under ROC curve (AUROC) 0.799 (0.738–0.851), followed by the COVID‐GRAM 0.785 (0.723–0.838), NEWS 0.764 (0.700–0.819), and qCSI 0.749 (0.685–0.806). However, these differences were not statistical significant.
Conclusion
Among the evaluated scores, the ISARIC‐4C and the COVID‐GRAM, calculated at ED admission, had the best performance, although the qCSI had similar efficacy by evaluating only three items. However, the NEWS, already widely validated in clinical practice, had a similar performance and could be appropriate for older patients with COVID‐19.
Keywords: COVID‐19, NEWS, COVID‐GRAM, ISARIC‐4C, qCSI
1. INTRODUCTION
The novel coronavirus designated SARS‐CoV‐2, has determined an international outbreak of respiratory illness named COVID‐19. 1 , 2 Older adults and patients with previous comorbid conditions are at higher risk of developing severe disease, and death. 3 , 4 , 5
The prevalence of hypoxic respiratory failure in patients hospitalized with COVID‐19 was estimated to be about 19%, with up to 12% of patients requiring mechanical ventilation. 1 , 2 , 4 Indeed, based on available data, from 5% to 10% among hospitalized patients will require ICU admission, with rates even higher in older patients. 5 , 6 , 7 , 8
In this context of critically ill patients' overflow, it is mandatory to establish clear and objective criteria to stratify COVID‐19 risk for death. To date, already available national early warning scores (NEWS), and specifically developed clinical rules and scores, have been proposed for risk stratification in COVID‐19 patients. 9 , 10 , 11 , 12 , 13 Currently, even though most of developed scores include age among the factors evaluated for risk prediction, none of these tools was validated in a geriatric population, which indeed carries the highest risk of worse outcome in COVID‐19.
The aim of this study is to evaluate, in older patients with COVID‐19, the performance for death risk stratification of specifically developed scoring systems, including the International Severe Acute Respiratory Infection Consortium Clinical Characterization Protocol‐Coronavirus Clinical Characterization Consortium (ISARIC‐4C) score, the COVID‐GRAM Critical Illness Risk Score (COVID‐GRAM), the quick COVID‐19 Severity Index (qCSI). 11 , 12 , 13 These specifically developed scores were compared with the widely validated NEWS risk score. 14
2. METHODS
2.1. Study Design
This is a single‐center, retrospective observational study, conducted in the ED of an urban teaching hospital, which is a referral center for COVID‐19, in central Italy.
We reviewed the clinical records of all the patients 60 years or more old consecutively admitted to our ED over a six‐week period (from March 1st to April 15th, 2020). COVID‐19 was diagnosed on the basis of the WHO interim guidance. We included in the analysis only patients with positive result on real‐time reverse‐transcriptase–polymerase‐chain‐reaction assay of nasal and pharyngeal swab specimens. 15
We excluded patients already on orotracheal intubation at ED arrival, and patients for whom a do not resuscitate order was in place.
2.2. Study Variables
The following information were extracted from computerized clinical records: age, sex, clinical presentation symptoms, temperature, heart rate (HR), respiratory rate (RR), blood pressure (BP), Glasgow Coma Scale (GCS) score, oxygen supplementation, peripheral oxygen saturation (SpO2), laboratory values, radiographic imaging, and clinical history. Physiological parameters were assessed at ED admission. Comorbidities were evaluated according to Charlson comorbidity index. 16
2.3. Early Warning Scores for COVID‐19 Risk Stratification
Four early warning scores were evaluated: three were specifically developed for COVID‐19 (ISARIC‐4c, COVID‐GRAM, qCSI), while the NEWS score was recently validated in this setting. 11 , 12 , 13 , 14 The qCSI assesses the respiratory function; the COVID‐GRAM, the ISARIC 4C, and the NEWS also include the assessment of cardiovascular function, level of consciousness, age, number of comorbidities, and a selection of laboratory tests (Supplementary Table S1).
All the parameters evaluated for scores calculation were obtained from ED electronic records.
2.4. Study Endpoint
The primary study endpoint was all‐causes in hospital death.
2.5. Statistical Analysis
Continuous variables are reported as median (interquartile range), and are compared at univariate analysis by Mann–Whitney U test. Categorical variables are reported as absolute number (percentage), and are compared by chi‐square test (with Fisher's test if appropriate).
For patients with incomplete dataset of parameters to calculate the scores (either vital parameters or laboratory values), we utilized a data imputation by using a multiple imputation approach. 17 We excluded patients with three or more parameters missing, since the effect on final scores calculation would have been highly unpredictable. The missing parameters were imputed by using a multiple regression model including the available parameters in the dataset, the triage code at ED admission, and patient age. The limit of imputed parameters was set according to each parameter range in the study cohort.
Once the selected scores were calculated for each patient, receiver operating characteristic (ROC) curve analysis was used to evaluate the overall performance in predicting the defined adverse outcome. Youden's index was used to estimate optimal cutoff points and corresponding sensitivity and specificity at selected score threshold values. The comparison between the ROC AUCs was made according to DeLong method. 18
A two sided P value .05 or less was regarded as significant. Data were analyzed by SPSS v25® (IBM, IL).
2.6. Statement of Ethics
The study was conducted in accordance with the Declaration of Helsinki and its later amendments, and was approved by the local Institutional Review Board (IRB #001705520).
3. RESULTS
A total of 210 patients, aged between 60 and 98 years met the inclusion criteria and were included in the study cohort (Supplementary Figure S1). Median age was 74 (67–82) and 133 (63.3%) were males (Table 1).
Table 1.
Demographic and Clinical Characteristics of Enrolled Patients
| All Population | Survived | Deceased | ||
|---|---|---|---|---|
| Variable | n = 210 | n = 168 | n = 42 | P |
| Age | 74 (67–82) | 72 (66–80) | 81 (74–85) | <.001 |
| Sex (male) | 133 (63.3) | 106 (63.1) | 27 (64.3) | .886 |
| Physiological parameters at ED presentation | ||||
| Peripheral oxygen saturation (%) | 94 (90–96) | 94 (92–96) | 89 (80–92) | <.001 |
| Respiratory rate (breaths/min) | 18 (16–20) | 18 (15–20) | 19 (16–22) | .032 |
| Heart rate (beats/min) | 84 (72–99) | 82 (71–95) | 89 (76–110) | .011 |
| Systolic blood pressure (mmHg) | 130 (114–140) | 129 (113–141) | 128 (111–135) | .790 |
| Diastolic blood pressure (mmHg) | 78 (66–86) | 78 (70–87) | 73 (65–84) | .404 |
| Axillary temperature (°C) | 36.6 (36.0–37.5) | 36.5 (36.1–37.3) | 37.2 (36.2–38.2) | .711 |
| Radiological findings | ||||
| Negative | 27 (12.9) | 26 (15.5) | 1 (2.4) | |
| Interstitial/monolateral | 110 (52.3) | 101 (60.1) | 9 (21.4) | <.001 |
| Bilateral pneumonia | 73 (34.8) | 41 (24.4) | 32 (76.2) | |
| Comorbidities | ||||
| Charlson comorbidity index | 4 (3–5) | 4 (3–5) | 5 (4–6) | <.001 |
| Hypertension | 120 (57.1) | 92 (54.8) | 28 (66.7) | .163 |
| Obesity | 4 (1.9) | 3 (1.8) | 1 (2.4) | 1.000 |
| Coronary artery disease | 45 (21.4) | 38 (22.6) | 7 (16.7) | .400 |
| Congestive heart failure | 39 (18.6) | 30 (17.9) | 9 (21.4) | .594 |
| Diabetes mellitus | 27 (12.9) | 23 (13.7) | 4 (9.5) | .471 |
| Dementia | 10 (5.7) | 3 (2.2) | 7 (17.5) | <.001 |
| COPD | 19 (9.0) | 13 (7.7) | 6 (14.3) | .186 |
| Renal disease | 27 (12.9) | 13 (7.7) | 14 (33.3) | <.001 |
| Malignancy | 15 (7.1) | 12 (7.1) | 3 (7.1) | 1.000 |
| Laboratory values | ||||
| Hemoglobin (g/dl) | 12.7 (9.0–14.3) | 12.9 (9.0–14.2) | 12.4 (9.1–14.5) | .953 |
| Neutrophil (cells/mm3) | 4,890 (3,570–6,930) | 4,690 (3,540–6,610) | 5,930 (3,715–8,935) | .019 |
| Lymphocyte (cells/mm3) | 940 (670–1,290) | 950 (695–1,280) | 825 (570–1,600) | .591 |
| Neutrophil/lymphocyte ratio | 5.3 (3.3–8.1) | 5.1 (3.2–7.6) | 6.5 (3.9–12.2) | .026 |
| Creatinine (mg/dl) | 0.98 (0.78–1.42) | 0.96 (0.75–1.27) | 1.45 (0.88–2.05) | .003 |
| Blood urea nitrogen (mg/dl) | 20 (16–33) | 19 (15–25) | 37 (20–58) | <.001 |
| Sodium (mEq/L) | 138 (135–140) | 138 (135–140) | 138 (134–141) | .692 |
| Lactate dehydrogenase (UI/L) | 324 (241–440) | 306 (233–412) | 511 (314–801) | <.001 |
| Alanina transpherase (UI/L) | 19 (13.5–32.5) | 18.5 (13–30.5) | 21 (15–46) | .248 |
| Direct bilirubin (mg/dl) | 0.6 (0.4–0.9) | 0.6 (0.4–0.9) | 0.6 (0.4–1.0) | .725 |
| C‐reactive protein (mg/L) | 66.8 (28.1–141.0) | 53.1 (25.7–105.6) | 145 (77.9–210.5) | <.001 |
| Prothrombin time (s) | 11.2 (10.7–11.9) | 11.2 (10.6–11.8) | 11.4 (10.8–12.4) | .220 |
| Fibrinogen (mg/dl) | 478 (392–580) | 465 (390–550) | 512 (405–703) | .088 |
| D‐dimer (ng/ml) | 1,230 (709–3,359) | 1,228 (619–2,771) | 2,071 (900–5,412) | .194 |
| Risk scores | ||||
| NEWS | 3 (2–6) | 3 (1–5) | 6 (3–9) | <.001 |
| ISARIC 4C | 9 (7–10) | 8 (6–10) | 11 (9–12) | <.001 |
| COVID‐GRAM | 17.3 (8.5–34.4) | 13.9 (7.1–26.9) | 38.1 (23.8–56.8) | <.001 |
| qCSI | 4 (0–6) | 2 (0–5) | 7 (4–10) | <.001 |
Abbreviations: COPD, chronic obstructive pulmonary disease; COVID‐GRAM, COVID‐Gram Critical Illness Risk Score; ISARIC‐4C, International Severe Acute Respiratory Infection Consortium Clinical Characterization Protocol‐Coronavirus Clinical Characterization Consortium; NEWS, national early warning score; qCSI, quick COVID severity index.
Globally, 42 patients (20.0%) deceased (Table 1). When compared with survived patients, we found that deceased patients were significantly older (81 (74–85) vs 72 (66–80); P < .001), had worse radiological findings, and had a higher number of comorbidities (Charlson comorbidity index 5 (4–6) vs 4 (3–5); P < .001) (Table 1). In particular, deceased patients had a higher rate of dementia (17.5% vs 2.2%, P < .001), and a higher rate of renal disease (33.3% vs 7.7%, P < .001).
Among vital parameters at admission SpO2, respiratory rate and heart rate were significantly worse in deceased patients, whereas the two groups had similar admission values in term of temperature and blood pressure (Table 1).
Laboratory values at admission associated to death were higher C‐reactive protein (CRP), blood urea nitrogen, LDH, absolute neutrophil count, and neutrophil/lymphocyte ratio (Table 1).
All the four scores evaluated showed a fairly good predictive value with respect to in‐hospital death. The ISARIC‐4C score had the highest area under ROC curve (AUROC) 0.799 (0.738–0.851), followed by the COVID‐GRAM 0.785 (0.723–0.838), NEWS 0.764 (0.700–0.819), and qCSI 0.749 (0.685–0.806) (Figure 1). However, these differences were not statistically significant.
Figure 1.
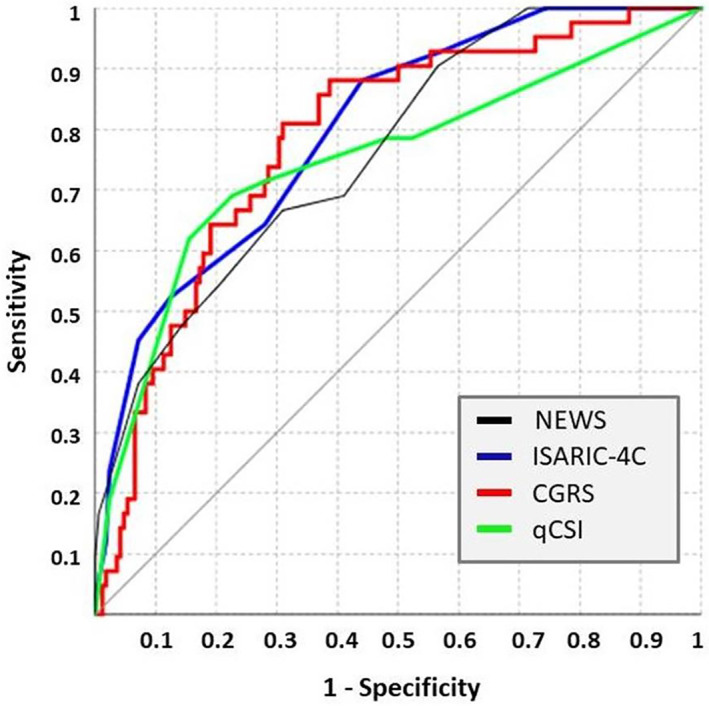
Graphical representation of the receiver operating characteristic (ROC) curve of the evaluated score.
When comparing score sensitivity, COVID‐GRAM and ISARIC‐4C had the best performance, both reaching 88.1% sensitivity for COVID‐GRAM greater than 17.7 and ISARIC‐4C greater than 8 (Table 2). However, COVID‐GRAM had a slightly higher negative predictive value (Table 2). The qCSI had the best specificity, thus having a qCSI greater than 5 the highest positive predictive value for death (43.3 (35.1–51.9)) (Table 2). The worst performer in this group was the NEWS, still keeping a fair negative predictive value of 89.2 (84.2–92.8) at selected cutoff.
Table 2.
Sensitivities, Specificities, Negative and Positive Predictive Values, Positive and Negative Likelihood Ratios for NEWS, COVID‐GRAM, ISARIC‐4C, and qCSI Scoring Systems for Predicting Death of COVID‐19 Older Patients
| Score | ROC AUC | Cut off value | Sensitivity (%) | Specificity (%) | +LR | −LR | PPV | NPV |
|---|---|---|---|---|---|---|---|---|
| NEWS | 0.764 (0.700–0.819) | >4 | 66.7 (50.5–80.4) | 69.0 (61.5–75.9) | 2.1 (1.6–2.9) | 0.5 (0.3–0.7) | 35.0 (28.3–42.4) | 89.2 (84.2–92.8) |
| COVID‐GRAM | 0.785 (0.723–0.838) | >17.7 | 88.1 (74.4–96.0) | 61.3 (53.5–68.7) | 2.3 (1.8–2.8) | 0.2 (0.1–0.4) | 36.3 (31.3–41.5) | 95.4 (90.0–97.9) |
| ISARIC‐4C | 0.799 (0.738–0.851) | >8 | 88.1 (74.4–96.0) | 55.9 (48.1–63.6) | 2.0 (1.6–2.5) | 0.2 (0.1–0.5) | 33.3 (29.0–38.0) | 94.9 (89.1–97.7) |
| qCSI | 0.749 (0.685–0.806) | >5 | 69.0 (52.9–82.4) | 77.4 (70.3–83.5) | 3.0 (2.2–4.3) | 0.4 (0.3–0.6) | 43.3 (35.1–51.9) | 90.9 (86.3–94.1) |
Note: Optimal cutoff was chosen according to Youden index J. Differences among area under ROC curves did not reach statistical significance.
Abbreviations: NEWS, National early warning score; COVID‐GRAM, COVID‐Gram Critical Illness Risk Score; ISARIC‐4C, International Severe Acute Respiratory Infection Consortium Clinical Characterization Protocol‐Coronavirus Clinical Characterization Consortium; qCSI, quick COVID severity index; +LR, positive likelihood ratio; −LR, negative likelihood ratio; NPV, negative predictive value; PPV, Positive predictive value.
4. DISCUSSION
The main result of present study is that among COVID‐19 older patients the specifically developed scores ISARIC‐4C, COVID‐GRAM, and qCSI, although slightly superior in terms of overall AUROC and sensitivity, do not perform significantly better than the standard NEWS. However, the qCSI gave the best results in terms of specificity by evaluating only three parameters.
The SARS‐CoV‐2 primarily infects the upper respiratory and gastrointestinal tracts, 19 binding to human angiotensin‐converting enzyme 2 for cell entry. 1 , 2 , 4 , 5 , 20 Severe hypoxia and respiratory distress are common features of COVID‐19, and septic shock occurs mainly as a result of end‐stage organ failure. 1 , 2 , 5 Radiological findings confirm the extensive lung involvement, and up to 98% of symptomatic patients show bilateral ground glass opacity, and multiple lobular and subsegmental consolidation areas at chest imaging. 20
The results of the present study are largely explained by both the underlying pathophysiological mechanisms and the clinical presentation of COVID‐19. Indeed, since the acute hypoxia is the main determinant of disease progression and severity, the evaluation of respiratory function is crucial for score prediction ability.
All the evaluated scores include an assessment of respiratory function, even if obtained in different ways. The NEWS includes both the SpO2 and the respiratory rate in the calculation, as well as the ISARC‐4C and the qCSI. For the COVID‐GRAM calculation, the respiratory function is indirectly derivated by the assessment of X‐ray abnormalities, and directly evaluated as the presence of dyspnea, as reported by the patient. The qCSI and the NEWS both evaluate the supplemental oxygen flow given to patients, although this latter measure has a high variability, being not always directly linked to effective patient's respiratory distress.
Apart from qCSI that evaluate only respiratory distress, all the scores evaluate neurological status, by using a simplified version of GCS (normal or <15 for ISARIC‐4C), the Alert, Verbal, Pain, Unresponsive (AVPU) scale (for NEWS), and simply conscious/unconscious for COVID‐GRAM. However, although neurologic involvement is common in COVID‐19, a severe depression of consciousness is rare. 21 Indeed, in our cohort only seven (3.3%) patients presented with GCS less than 15 at admission, and three (1.4%) were unconscious. Hence, the contribution of this item to the score prediction was low.
The relatively low incidence of shock in COVID‐19 1 , 2 could explain why the blood pressure in ED did not seem to be associated to worse outcome (Table 1) in our population. Indeed, none of the specifically designed scores for COVID‐19 evaluates blood pressure.
Both COVID‐GRAM and ISARIC 4C evaluate patients comorbidities. While the latter utilizes the Charlson Index adding obesity, 22 the COVID‐GRAM evaluates a selected number of conditions, including hypertension and hepatitis B. As our study was conducted in a population of COVID‐19 older patients, most of them presenting comorbidities, the influence of this item was reduced for the overall prediction. Nevertheless, our data confirmed that deceased patients showed an overall higher Charlson comorbidity index, but dementia and renal disease were significantly higher. Indeed, COVID‐19 patients with cognitive impairment are at high risk of worse outcome, and this is a major challenge in geriatric populations. 6 , 23 , 24
Among the scores we assessed, ISARIC‐4C and COVID‐GRAM include laboratory tests in their model. ISARIC‐4C includes blood urea nitrogen and CRP, whereas COVID‐GRAM include lactate dehydrogenase (LDH) and direct bilirubin. CRP and LDH were already described to be associated to advanced pulmonary disease in COVID‐19, 25 , 26 as well as kidney damage and increased blood urea nitrogen, 27 as confirmed in our study. Conversely, we cannot confirm the usefulness of bilirubin evaluation since the hepatic involvement in our cohort was limited.
Both the COVID‐GRAM and the ISARIC‐4C assign an increased risk value for older age. However, in our selected population of patients above 60 years the weight of age was probably reduced because of the limited age range. This may partly explain why the PPV for hospital mortality of both COVID‐GRAM and ISARIC‐4C was lower than in the original reports. 11 , 13 Among the scores we tested, qCSI had the highest PPV for predicting hospital mortality (43.3% for qCSI > 5). The most likely reason is that qCSI is focused on respiratory failure, which is the major cause of death in COVID‐19 patients. Despite the AUROC of qCSI was the lowest among the scores we tested in our study, this score may be preferred for a quick bedside detection of patients at higher risk of adverse events. In fact, qCSI requires only three clinical parameters (respiratory rate, pulse oximetry, and oxygen flow rate). Conversely, despite the higher complexity and the need of laboratory tests, ISARIC‐4C and COVID‐GRAM showed a high NPV (95%) and, as such, they can be used to exclude the risk of subsequent deterioration in patients destined to a non‐critical area.
Finally, only ISARIC‐4C considers the gender in risk prediction. Nonetheless, although male sex was associated to worse outcome in several reports, 3 , 4 , 5 patient gender was not significantly associated to a different outcome in our population (Table 1).
4.1. Study Limitations
As for any retrospective study some limitations are worth considering. First, our sample size is limited and therefore, the global accuracy of our ROC curve estimation could be reduced, still keeping a good reliability in ROC curve comparison. Moreover, we did not collect data about total time of eventual O2 supplementation before ED admission, and this could affect the SpO2 measurement at ED arrival.
4.2. Conclusions
Among the evaluated scores, the ISARIC‐4C and the COVID‐GRAM, calculated at ED admission, had the best performance in predicting death in COVID‐19 older patients. Moreover, the qCSI, although not specifically designed for death risk prediction had similar efficacy by evaluating only three items, being the best choice for a quick assessment. However, the longtime validated NEWS had a similar performance and, since it represents the standard early warning score in many institutions, could be appropriate also for older patients with COVID‐19.
Supporting information
Supplementary Figure S1: Flow‐chart of the cohort selection for the study.
Supplementary Table S1: Early warning scores for COVID‐19 risk stratification.
ACKNOWLEDGMENTS
Members of the GEMELLI AGAINST COVID‐19 group include: Valeria Abbate, Nicola Acampora, Giovanni Addolorato, Fabiana Agostini, Maria E. Ainora, Elena Amato, Gloria Andriollo, Brigida E. Annicchiarico, Mariangela Antonelli, Gabriele Antonucci, Alessandro Armuzzi, Christian Barillaro, Fabiana Barone, Rocco D.A. Bellantone, Andrea Bellieni, Andrea Benicchi, Francesca Benvenuto, Filippo Berloco, Roberto Bernabei, Antonio Bianchi, Luigi M. Biasucci, Stefano Bibbò, Federico Biscetti, Nicola Bonadia, Alberto Borghetti, Giulia Bosco, Silvia Bosello, Vincenzo Bove, Giulia Bramato, Vincenzo Brandi, Dario Bruno, Maria C. Bungaro, Alessandro Buonomo, Maria Livia Burzo, Angelo Calabrese, Andrea Cambieri, Giulia Cammà, Marcello Candelli, Gennaro Capalbo, Lorenzo Capaldi, Esmeralda Capristo, Luigi Carbone, Silvia Cardone, Angelo Carfì, Annamaria Carnicelli, Cristiano Caruso, Francesco Antonio Casciaro, Lucio Catalano, Roberto Cauda, Andrea L. Cecchini, Lucia Cerrito, Michele Ciaburri, Rossella Cianci, Sara Cicchinelli, Arturo Ciccullo Francesca Ciciarello, Antonella Cingolani, Maria C. Cipriani, Gaetano Coppola, Andrea Corsello, Federico Costante, Marcello Covino, Stefano D'Addio, Alessia D'Alessandro, Maria E. D'Alfonso, Emanuela D'Angelo, Francesca D'Aversa, Fernando Damiano, Tommaso De Cunzo, Giuseppe De Matteis, Martina De Siena, Francesco De Vito, Valeria Del Gatto, Paola Del Giacomo, Fabio Del Zompo, Davide Antonio Della Polla, Luca Di Gialleonardo, Simona Di Giambenedetto, Roberta Di Luca, Luca Di Maurizio, Alex Dusina, Alessandra Esperide, Domenico Faliero, Cinzia Falsiroli, Massimo Fantoni, Annalaura Fedele, Daniela Feliciani, Andrea Flex, Evelina Forte, Francesco Franceschi, Laura Franza, Barbara Funaro, Mariella Fuorlo, Domenico Fusco, Maurizio Gabrielli, Eleonora Gaetani, Antonella Gallo, Giovanni Gambassi, Matteo Garcovich, Antonio Gasbarrini, Irene Gasparrini, Silvia Gelli, Antonella Giampietro, Laura Gigante, Gabriele Giuliano, Giorgia Giuliano, Bianca Giupponi, Elisa Gremese, Caterina Guidone, Amerigo Iaconelli, Angela Iaquinta, Michele Impagnatiello, Riccardo Inchingolo, Raffaele Iorio, Immacolata M. Izzi, Cristina Kadhim, Daniele I. La Milia, Francesco Landi, Giovanni Landi, Rosario Landi, Massimo Leo, Antonio Liguori, Rosa Liperoti, Marco M. Lizzio, Maria R. Lo Monaco, Pietro Locantore, Francesco Lombardi, Loris Lopetuso, Valentina Loria, Angela R. Losito, Noemi Macerola, Giuseppe Maiuro, Francesco Mancarella, Francesca Mangiola, Alberto Manno, Debora Marchesini, Giuseppe Marrone, Ilaria Martis, Anna M. Martone, Emanuele Marzetti, Maria V. Matteo, Luca Miele, Alessio Migneco, Irene Mignini, Alessandro Milani, Domenico Milardi, Massimo Montalto, Flavia Monti, Davide Moschese, Barbara P. L. Mothaenje, Celeste A. Murace, Rita Murri, Marco Napoli, Elisabetta Nardella, Gerlando Natalello, Simone M. Navarra, Antonio Nesci, Maria Anna Nicolazzi, Alberto Nicoletti, Tommaso Nicoletti, Rebecca Nicolò, Nicola Nicolotti, Enrico C. Nista, Eugenia Nuzzo, Veronica Ojetti, Francesco C. Pagano, Cristina Pais, Alfredo Papa, Luigi G. Papparella, Mattia Paratore, Giovanni Pecorini, Simone Perniola, Erika Pero, Giuseppe Parrinello, Luca Petricca, Martina Petrucci, Chiara Picarelli, Andrea Piccioni, Giulia Pignataro, Raffaele Pignataro, Marco Pizzoferrato, Fabrizio Pizzolante, Roberto Pola, Caterina Policola, Maurizio Pompili, Valerio Pontecorvi, Francesca Ponziani, Valentina Popolla, Enrica Porceddu, Angelo Porfidia, Giuseppe Privitera, Daniela Pugliese, Gabriele Pulcini, Simona Racco, Francesca Raffaelli, Gian L. Rapaccini, Luca Richeldi, Emanuele Rinninella, Sara Rocchi, Stefano Romano, Federico Rosa, Laura Rossi, Raimondo Rossi, Enrica Rossini, Elisabetta Rota, Fabiana Rovedi, Gabriele Rumi, Andrea Russo, Luca Sabia, Andrea Salerno, Sara Salini, Lucia Salvatore, Dehara Samori, Maurizio Sanguinetti, Luca Santarelli, Paolo Santini, Angelo Santoliquido, Francesco Santopaolo, Michele C. Santoro, Francesco Sardeo, Caterina Sarnari, Luisa Saviano, Tommaso Schepis, Francesca Schiavello, Giancarlo Scoppettuolo, Luisa Sestito, Carlo Settanni, Valentina Siciliano, Benedetta Simeoni, Andrea Smargiassi, Domenico Staiti, Leonardo Stella, Eleonora Taddei, Rossella Talerico, Enrica Tamburrini, Claudia Tarli, Pietro Tilli, Enrico Torelli, Matteo Tosato, Alberto Tosoni, Luca Tricoli, Marcello Tritto, Mario Tumbarello, Anita M. Tummolo, Federico Valletta, Giulio Ventura, Lucrezia Verardi, Lorenzo Vetrone, Giuseppe Vetrugno, Elena Visconti, Raffaella Zaccaria, Lorenzo Zelano, Lorenzo Zileri Dal Verme, Giuseppe Zuccalà.
Financial Disclosure
This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
Conflict of Interest
All authors declared no conflict of interests for this paper.
Author Contributions
Marcello Covino: conceptualization, methodology, software, data curation, formal analysis, writing—review & editing. Giuseppe De Matteis: conceptualization, validation, visualization, writing—original draft, writing—review & editing. Maria Livia Burzo: validation, visualization, writing—review & editing. Andrea Russo, Andrea Piccioni: software, formal analysis. Annamaria Carnicelli, Evelina Forte, Benedetta Simeoni: writing—review & editing. Antonio Gasbarrini, Francesco Franceschi: conceptualization, methodology, writing—review & editing. Claudio Sandroni: conceptualization, methodology, data curation, supervision.
Sponsor's Role
No sponsor.
Members of the GEMELLI AGAINST COVID‐19 Group are listed in the Acknowledgements.
REFERENCES
- 1. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. COVID‐19 situation reports; 2020. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports. Accessed September 25, 2020.
- 4. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934‐943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS‐CoV‐2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323(16):1574‐1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Covino M, De Matteis G, Santoro M, et al. Clinical characteristics and prognostic factors in COVID‐19 patients aged ≥80 years. Geriatr Gerontol Int. 2020;20(7):704‐708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shahid Z, Kalayanamitra R, McClafferty B, et al. COVID‐19 and older adults: what we know. J Am Geriatr Soc. 2020;68(5):926‐929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Niu S, Tian S, Lou J, et al. Clinical characteristics of older patients infected with COVID‐19: a descriptive study. Arch Gerontol Geriatr. 2020;89:104058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Covino M, Sandroni C, Santoro M, et al. Predicting intensive care unit admission and death for COVID‐19 patients in the emergency department using early warning scores. Resuscitation. 2020;156:84‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bernabeu‐Wittel M, Ternero‐Vega JE, Díaz‐Jiménez P, et al. Death risk stratification in elderly patients with COVID‐19. A comparative cohort study in nursing homes outbreaks. Arch Gerontol Geriatr. 2020;91:104240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liang W, Liang H, Ou L, et al. Development and validation of a clinical risk score to predict the occurrence of critical illness in hospitalized patients with COVID‐19. JAMA Intern Med. 2020;180(8):1081‐1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Haimovich A, Ravindra NG, Stoytchev S, et al. Development and validation of the quick COVID‐19 severity index (qCSI): a prognostic tool for early clinical decompensation. Ann Emerg Med. 2020;76(4):442‐453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Knight SR, Ho A, Pius R, et al. Risk stratification of patients admitted to hospital with COVID‐19 using the ISARIC WHO Clinical Characterisation Protocol: development and validation of the 4C Mortality Score. BMJ. 2020;370:m3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Smith GB, Prytherch DR, Meredith P, Schmidt PE, Featherstone PI. The ability of the National Early Warning Score (NEWS) to discriminate patients at risk of early cardiac arrest, unanticipated intensive care unit admission, and death. Resuscitation. 2013;84:465‐470. [DOI] [PubMed] [Google Scholar]
- 15. World Health Organization . Clinical management of severe acute respiratory infection when novel coronavirus (2019‐nCoV) infection is suspect: interim guidance. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance Accessed September 25, 2020
- 16. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373‐383. [DOI] [PubMed] [Google Scholar]
- 17. Sterne JA, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ (Clinical Research ed). 2009;338:b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. DeLong E, DeLong D, Clarke‐Pearson D. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837‐845. [PubMed] [Google Scholar]
- 19. Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cao Y, Liu X, Xiong L, Cai K. Imaging and clinical features of patients with 2019 novel coronavirus SARS‐CoV‐2: a systematic review and meta‐analysis. J Med Virol. 2020;92(9):1149–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Luigetti M, Iorio R, Bentivoglio AR, et al. Assessment of neurological manifestations in hospitalized patients with COVID‐19. Eur J Neurol. 2020;27(11):2322–2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sattar N, McInnes IB, McMurray JJV. Obesity is a risk factor for severe COVID‐19 infection: multiple potential mechanisms. Circulation. 2020;142(1):4‐6. [DOI] [PubMed] [Google Scholar]
- 23. Hägg S, Jylhävä J, Wang Y, et al. Age, frailty, and comorbidity as prognostic factors for short‐term outcomes in patients with coronavirus disease 2019 in geriatric care. J Am Med Dir Assoc. 2020;21(11):1555‐1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Garcez FB, Aliberti MJR, Poco PCE, et al. Delirium and adverse outcomes in hospitalized patients with COVID‐19. J Am Geriatr Soc. 2020;68(11):2440–2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhu J, Chen C, Shi R, Li B. Correlations of CT scan with high‐sensitivity C‐reactive protein and D‐dimer in patients with coronavirus disease 2019. Pak J Med Sci. 2020;36(6):1397‐1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Danwang C, Endomba FT, Nkeck JR, Wouna DLA, Robert A, Noubiap JJ. A meta‐analysis of potential biomarkers associated with severity of coronavirus disease 2019 (COVID‐19). Biomark Res. 2020;31(8):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nogueira SÁR, Oliveira SCS, Carvalho AFM, et al. Renal changes and acute kidney injury in COVID‐19: a systematic review. Rev Assoc Med Bras. 2020;66(Suppl. 2):112‐117. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1: Flow‐chart of the cohort selection for the study.
Supplementary Table S1: Early warning scores for COVID‐19 risk stratification.


