Abstract
Background
Autoimmune hemolytic anemia (AIHA) has many known disease associations, including autoimmune, lymphoproliferative, and certain infectious diseases, as well as various medications. Studies have found that severe cases of coronavirus disease 2019 (COVID‐19) may be associated with coagulopathies; however, the potential association with AIHA is not clear.
Case Report
A patient with no known risk factors or underlying predisposition for developing AIHA presented to a hospital with vague symptoms and profound anemia with a complicated blood bank evaluation. She was found to have COVID‐19 and AIHA, for which extensive laboratory testing was performed, including direct antiglobulin tests, elution studies, and cold agglutinin titers, to identify the causative autoantibody. She required multiple blood transfusions and therapeutic interventions before clinical stabilization.
Discussion
AIHA is a complex disease with a spectrum of presentations and clinical severity. Many diseases have been associated with a propensity for developing AIHA; however, there are few cases in the literature of patients with COVID‐19 and AIHA. Most of the reports involve patients with other underlying conditions that are known to be associated with the development of AIHA. The presentation, clinical findings, and therapeutic interventions in a patient with severe AIHA, without other underlying conditions, in the setting of COVID‐19 are discussed.
Conclusions
There are few reports of patients with concurrent COVID‐19 and AIHA, and the association is not clear. Although COVID‐19 has been shown to be associated with coagulopathies, more research is required to determine whether AIHA may also be a potential complication.
Keywords: anemia, autoimmune hemolytic anemia, COVID‐19, infectious disease, SARS‐coronavirus‐2
Abbreviations
- AIHA
autoimmune hemolytic anemia
- CBC
complete blood count
- COVID‐19
coronavirus disease 2019
- DAT
direct antiglobulin testing
- HAV
hepatitis A virus
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- HIV
human immunodeficiency virus
- IAT
indirect antiglobulin testing
- IS
immediate spin
- LDH
lactate dehydrogenase
- PBS
phosphate buffered saline
- RT
room temperature
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
1. BACKGROUND
The novel coronavirus severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), the agent responsible for coronavirus disease 2019 (COVID‐19), was first reported in late 2019 and declared a pandemic on 11 March 2020 by the World Health Organization. 1 Among patients with COVID‐19, there is increasing evidence that a subset, particularly those with severe disease, may have a higher likelihood of developing certain hematologic abnormalities such as coagulopathies. 2 , 3 There are only two separate reports consisting of a total of eight patients with COVID‐19 and autoimmune hemolytic anemia (AIHA) in the literature. 4 , 5 Because of the paucity of data, the association between AIHA and COVID‐19 remains unclear. Of the eight documented cases, five patients reportedly had underlying lymphoid neoplasms, including chronic lymphocytic leukemia (two), marginal zone lymphoma (two), and monoclonal gammopathy of undetermined significance (one); one patient had prostate cancer, and one patient had congenital thrombocytopenia. 4 , 5
AIHA has a known association with lymphoproliferative neoplasms, autoimmune diseases, immunodeficiencies, medications, and certain infectious diseases. 6 We report a case of a patient with COVID‐19 and profound anemia secondary to hemolysis with no known underlying cause typically associated with AIHA.
2. CASE PRESENTATION
A 33‐year‐old woman with a reported history of hypothyroidism presented to an outside hospital complaining of headache for 2 days and family concern for altered mental status. A head computed tomography scan was performed and demonstrated no acute intracranial abnormalities. A complete blood count (CBC) was notable for a hemoglobin of 1.3 g/dL (reference, 12.0‐15.0 g/dL) and hematocrit of 6% (reference, 36%‐45%), while a complete metabolic panel showed a total bilirubin of 2.4 mg/dL (reference, 0.2‐1.3 mg/dL). Per the outside hospital report, the patient's blood type was unable to be determined due to an ABO discrepancy, and the patient's plasma reacted against both screening cells. Given these findings, she was transfused 1 unit of type O D‐negative red blood cells (RBCs) for symptomatic anemia and transferred to our facility for further care.
Upon presentation at our facility, the patient was confused, and the physical exam was significant for scleral icterus; tachycardia, with heart rate 120 beats per minute; tachypnea, with respiratory rate 30 breaths per minute; and an abnormal neurological exam, including delayed responses upon questioning and sluggish reactions to verbal commands.
The patient was found to be positive for SARS‐CoV‐2, and a chest x‐ray was interpreted by a radiologist to indicate patchy opacities, consistent with infection. Given the report from the outside hospital of significant anemia and an undetermined blood type and screen due to spontaneous agglutination, extensive laboratory and serologic testing was performed. Over the course of her admission, the patient received a total of 10 units of RBCs through a blood warmer. As shown in Figure 1, despite receiving multiple units of RBCs with an appropriate increase in hemoglobin immediately following transfusion, the hemoglobin values did not remain stable, and several therapeutic interventions were performed over the course of her admission. These therapies included prednisone 1 mg/kg daily (total daily dose of 60 mg), which was initiated on Day 1 of hospitalization and continued throughout her stay, one dose of tocilizumab 4 mg/kg (total dose of 220 mg) on Day 6 of hospitalization, and one dose of rituximab 600 mg on Day 10 of hospitalization. During the first week of her admission, her respiratory and clinical status deteriorated, with an increasing oxygen demand and worsening opacities on repeat chest x‐ray. Following rituximab therapy, her hemoglobin began to stabilize, she began to clinically improve, and she was discharged on Day 13 of hospitalization.
FIGURE 1.
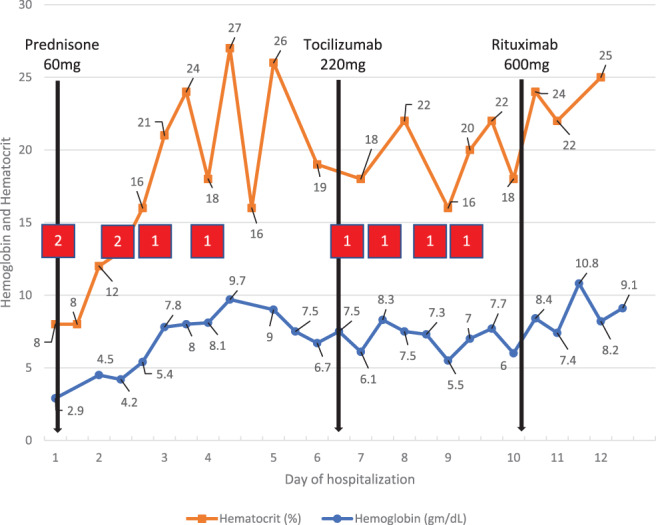
Hemoglobin and hematocrit values throughout hospitalization in relation to medication administration and RBC transfusion. (red boxes indicate approximately when RBCs were administered and the number of units transfused; black lines indicate when a specific medication was administered [prednisone was continued daily for the entire hospitalization]) [Color figure can be viewed at wileyonlinelibrary.com]
3. MATERIALS AND METHODS
Laboratory evaluation included peripheral smear; CBC, with differential, hemolysis biomarkers including lactate dehydrogenase (LDH), haptoglobin, and total bilirubin; and coagulation function tests including prothrombin time, partial thromboplastin time, and fibrinogen.
Infectious disease testing included evaluation for SARS‐CoV‐2 using a direct real‐time reverse transcriptase polymerase chain reaction assay, human immunodeficiency virus (HIV), hepatitis A virus (HAV), hepatitis B virus (HBV), hepatitis C virus (HCV), influenza A and B viruses, and Mycoplasma pneumoniae nucleic acid and immunoglobulin (IgG and IgM) testing.
Laboratory testing for immunologic, autoimmune, and lymphoproliferative disorders was performed, including antinuclear antibodies; rheumatoid factor; antimyeloperoxidase antibodies and antiprotease 3 antibodies; total IgG, IgM, and IgA immunoglobulins; monoclonal proteins by kappa and lambda immunofixation electrophoresis; and cryoglobulins via immunofixation electrophoresis. Complement levels were not performed.
3.1. RBC testing
ABO typing, direct antiglobulin testing (DAT), and antigen typing were performed by standard tube methods. ABO typing was performed with anti‐A and anti‐B reagents (BioClone, Ortho‐Clinical Diagnostics, Inc.) for forward grouping, and reagent RBCs (AFFIRMAGEN, Ortho‐Clinical Diagnostics, Inc.) for reverse grouping by indirect antiglobulin testing (IAT). To disperse spontaneous agglutination, RBCs were washed repeatedly with phosphate buffered saline (PBS) warmed to 37°C.
DAT was performed on a room temperature (RT) pretransfusion patient blood sample using anti–human globulin anti‐IgG and anti–human globulin anti‐C3b, ‐C3d reagents (Ortho‐Clinical Diagnostics, Inc.). A second DAT was performed with warm‐washed RBCs with 42°C saline and anti–human globulin anti‐IgG and anti–human globulin anti‐C3b, ‐ C3d reagents.
3.2. Plasma testing
Serologic detection of antibodies to RBC antigens was performed by standard tube methods with RT patient plasma and reagent RBCs (ORTHO RESOLVE Panel A, Ortho‐Clinical Diagnostics, Inc.), as well as patient plasma prewarmed to 37°C and reagent RBCs (SELECTOGEN I and II, Ortho‐Clinical Diagnostics, Inc.). Plasma samples were tested at immediate spin (IS), 37°C when appropriate, and by IAT without the use of potentiators.
Eluates were prepared from pretransfusion patient RBCs with a glycine acid RBC elution kit (ELUclear, Hemo bioscience). Eluates and last washes were tested by standard tube methods with type O (SELECTOGEN I and II) and type A1 and B (AFFIRMAGEN) reagent RBCs in the IAT, and against reagent RBCs (ORTHO RESOLVE Panel A) with the ORTHO ID‐MTS Gel Card (Ortho‐Clinical Diagnostics, Inc.).
Cold agglutinin testing was performed using three type O, DAT‐negative, pooled cord blood samples and reagent RBCs (SELECTOGEN I). For titration studies, serial dilutions of patient plasma were made in PBS at pH 7.0‐7.2 and tested by standard tube methods. The plasma dilutions were tested in saline at IS and in the IAT. Results were read at 4°C and at 37°C. Testing at 30°C was not performed.
No studies were performed using dithiothreitol, 2‐mercaptoethanol, or ficin‐treated cells. No potentiating agents were used in any of the serological tests.
4. RESULTS
The CBC was significant for an abnormal hemoglobin value of 2.9 g/dL (reference, 11.8‐16.0 g/dL), hematocrit of 8% (reference, 36%‐43%), and reticulocyte count of 14.4% (reference 0.5%‐1.8%). Lactate dehydrogenase (LDH) of 731 U/L (reference, 125‐220 U/L), total bilirubin of 2.0 mg/dL (reference, 0.2‐1.2 mg/dL), and haptoglobin of <8 mg/dL (reference 14‐258 mg/dL) were abnormal. Coagulation function was evaluated and generally unremarkable, with a normal prothrombin time of 14.4 seconds (reference, 11.9‐14.5 seconds), mildly prolonged partial thromboplastin time of 38.2 seconds (reference, 23.5‐33.5 seconds), and normal fibrinogen of 418 mg/dL (reference, 188‐450 mg/dL). Significant RBC agglutination was noted upon review of a peripheral blood smear prepared from an RT pretransfusion blood sample.
The patient tested positive for SARS‐CoV‐2. All other tested infectious diseases, including HIV, HAV, HBV, HCV, influenza A and B viruses, and M pneumoniae nucleic acid and IgM antibody were negative, while M pneumoniae IgG antibody was mildly elevated at 0.11 U/L (reference, ≤0.09 U/L), consistent with historical infection and recovery.
Testing for autoimmune and lymphoproliferative disorders was significant for mildly elevated total IgG at 1780 mg/dL (reference, 552‐1631 mg/dL). Total IgA and IgM were within normal limits. Evaluation for cryoglobulins revealed polyclonal IgG and kappa light chain (1% of cryocrit). No monoclonal protein was identified by kappa and lambda serum and urine protein electrophoresis. Testing for antinuclear antibodies, rheumatoid factor, antimyeloperoxidase antibodies, and antiprotease 3 antibodies was negative.
4.1. RBC testing
ABO typing with use of a RT blood sample was attempted; however, upon forward grouping, the patient RBCs exhibited spontaneous agglutination, precluding accurate ABO typing. When tested with a prewarmed sample and warm‐washed RBCs, the patient's predicted ABO type was O.
The first DAT, performed on a RT sample, was significant for both IgG (4+ reactivity) and C3 (3+ reactivity) bound to the RBCs, with agglutination of the RBCs in the saline auto‐control. The second DAT, using warm‐washed RBCs, was significant for both IgG (2+ reactivity) and C3 (2+ reactivity) bound to the RBCs, with agglutination of the warm‐washed RBCs in the saline auto‐control.
4.2. Plasma testing
A RT sample of patient plasma reacted with all 11 of the reagent panel RBCs at the IAT phase (IS and 37°C phase results were not reported). The prewarmed patient plasma did not react with either reagent screening RBC in the IAT.
4.3. Eluate testing
The acid glycine eluate reacted with both O reagent screening RBCs, both A1 and B reagent screening RBCs, and with all 11 of the reagent panel RBCs. This reactivity was observed in the IAT phase (IS and 37°C phase results were not reported). The last wash control did not react against either of the O reagent screening RBCs, nor the A1 and B reagent screening RBCs.
4.4. Titration studies
In the cold agglutinin screen at 4°C, the patient's plasma reacted with a 1:4 dilution of reagent screening RBCs (I antigen), and with a 1:8 dilution of pooled cord cells (i antigen), indicating a cold agglutinin titer of 8. Testing at 37°C against both saline‐suspended reagent screening RBCs (I antigen) and pooled cord cells (i antigen) was significant for a titer of 1.
Since reactivity occurred at 37°C, testing at 30°C was not performed. Had reactivity not occurred at 37°C, testing at 30°C would have been performed.
5. DISCUSSION
The case reported here of a patient with COVID‐19 who presented with significant anemia secondary to hemolysis is one of only a few of documented reports of COVID‐19 and AIHA. 4 , 5 This case is unique in that the patient had no known underlying neoplastic, lymphoproliferative, autoimmune, or infectious disease typically associated with autoimmune hemolytic anemia. She also had no known exposure to medications known to cause drug‐induced AIHA.
The diagnostic evaluation for the underlying cause of the hemolysis in this patient was complicated, as there was evidence of a cold agglutinin, with RBC agglutination on peripheral smear, a positive room temperature antibody screen, and a negative antibody screen when a prewarmed sample at 37°C was used. However, the cold agglutinin titers were at a lower level than those typically associated with clinically significant hemolysis. 7 A DAT was performed, with evidence of both IgG and C3 bound to the patient's RBCs. When an acid glycine eluate was prepared from the patient's RBCs and tested against a panel of reagent cells, the eluate reacted with all reagent cells on the panel, suggesting the presence of a panagglutinin. This finding is more commonly seen in warm AIHA. 8 At the time of this report, the patient had definitive evidence of AIHA given her laboratory evaluation, although whether the AIHA was due to a cold agglutinin or warm autoantibody or was possibly a mixed AIHA is not clear.
There is scant evidence in the literature of patients presenting with COVID‐19 and AIHA; thus, the association between these diseases is unknown. Lazarian et al 4 identified seven patients with COVID‐19 and AIHA, with a DAT positive for IgG in two patients, C3d in two patients, and both IgG and C3d in three patients. They reported that four of the cases were warm antibodies and three were cold agglutinins. Of those seven patients, five had underlying lymphoid neoplasms, and one had prostate cancer. 4 Lopez et al 5 reported one patient with a history of congenital thrombocytopenia who presented with COVID‐19 and concurrent warm AIHA, with a DAT positive for IgG and C3.
AIHA is a heterogenous disease entity associated with destruction or increased clearance of RBCs due to antierythrocyte antibodies. 6 This destruction may occur in the intravascular space via complement‐mediated hemolysis, or in the extravascular space predominantly via antibody‐ and complement‐mediated phagocytosis or removal of RBCs. 9 Warm AIHA is typically associated with IgG antibodies, with the IgG binding to the RBC and mediating phagocytosis or destruction in the reticuloendothelial system. 9 , 10 In severe cases, intravascular hemolysis can occur secondary to complement activation and formation of the membrane attack complex. 10 Approximately one‐half of warm AIHA cases are idiopathic, while the rest are secondary to various disease conditions or drugs. 10 Conditions associated with warm AIHA include lymphoproliferative diseases such as chronic lymphocytic leukemia and non‐Hodgkin lymphoma, as well as autoimmune diseases such as rheumatoid arthritis and systemic lupus erythematosus. 10 Warm AIHA has also been associated with certain infectious diseases, including HIV, and medications, such as penicillin and cephalosporins. 10
AIHA due to cold agglutinins is typically caused by IgM immunoglobulins. 10 Reports have found that a low‐level of polyclonal cold agglutinins exist in a small percentage of the general population at low titer (typically <64) and are not associated with clinical manifestations. 11 Pathogenic cold agglutinins commonly have a much higher titer, although their thermal amplitude is generally a better predictor of their clinical significance, with reactivity at or above 30° C considered significant. 11 Cold agglutinins lead to destruction of RBCs by binding to their membrane and activating complement, which may lead to direct intravascular hemolysis or phagocytosis in the extravascular space. 12 Cold agglutinins are associated with infections, most frequently M pneumoniae, as well as hepatitis C virus, Epstein‐Barr virus, and cytomegalovirus. 11 They can also be seen in autoimmune conditions, while primary cold agglutinin disease is commonly associated with B‐cell lymphoproliferative disorders. 6
The literature demonstrates that cold agglutinins, as well as warm autoantibodies, can be associated with certain bacterial and viral infections resulting in clinically significant hemolytic anemia; however, the association between COVID‐19 and cold or warm autoantibodies leading to AIHA is not known.
6. CONCLUSIONS
AIHA is a rare but complex disorder with various presentations and a spectrum of clinical severity. Studies have shown that both cold and warm autoantibodies can develop secondary to a myriad of conditions, including certain infectious diseases. There have been few reports of patients with AIHA and COVID‐19; however, the majority of the patients in these reports also have other underlying diseases known to be associated with autoantibodies and the development of AIHA. Our case highlights the fact that SARS‐CoV‐2, the causative agent of COVID‐19, may itself be capable of inducing severe AIHA even in patients with no underlying predisposition.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
ACKNOWLEDGMENTS
The authors would like to express sincere gratitude to Mary Johnson, team leader at Vanderbilt University Medical Center Blood Bank, for her assistance in providing expert technical support, interpretation, and education.
Jacobs J, Eichbaum Q. COVID‐19 associated with severe autoimmune hemolytic anemia. Transfusion. 2021;61:635–640. 10.1111/trf.16226
REFERENCES
- 1. WHO Timeline ‐ COVID‐19 . World Health Organization. https://www.who.int/news-room/detail/27-04-2020-who-timeline-covid-19. Accessed May 28, 2020.
- 2. Thachil J, Tang N, Gando S, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID‐19. J Thromb Haemost. 2020;18(5):1023–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tang N, Li D, Wang X, et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lazarian G, Quinquenel A, Bellal M, et al. Autoimmune hemolytic anemia associated with Covid‐19 infection. Br J Haematol. 2020;190(1):29–31. 10.1111/bjh.16794. Published online ahead of print, May 6, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lopez C, Kim J, Pandey A, et al. Simultaneous onset of COVID‐19 and autoimmune hemolytic anemia. Br J Haematol. 2020;190(1):31–32. 10.1111/bjh.16786. Published online ahead of print, May 5, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Go RS, Winters JL, Kay NE. How I treat autoimmune hemolytic anemia. Blood. 2017;129(22):2971–2979. [DOI] [PubMed] [Google Scholar]
- 7. Swiecicki PL, Hegerova LT, Gertz MA. Cold agglutinin disease. Blood. 2013;122(7):1114–1121. [DOI] [PubMed] [Google Scholar]
- 8. Parker V, Tormey CA. The direct antiglobulin test: indications, interpretation, and pitfalls. Arch Pathol Lab Med. 2017;141(2):305–310. [DOI] [PubMed] [Google Scholar]
- 9. Liebman HA, Weitz I. Autoimmune hemolytic anemia. Med Clin Am Med. 2017;101(2):351–359. [DOI] [PubMed] [Google Scholar]
- 10. Brodsky RA. Warm autoimmune hemolytic anemia. N Engl J Med. 2019;381(7):647–654. [DOI] [PubMed] [Google Scholar]
- 11. Berentsen S, Randen U, Tjønnfjord GE. Cold agglutinin‐mediated autoimmune hemolytic anemia. Hematol Oncol Clin North Am. 2015;29(3):455–471. [DOI] [PubMed] [Google Scholar]
- 12. Bass GF, Tuscano ET, Tuscano JM. Diagnosis and classification of autoimmune hemolytic anemia. Autoimmun Rev. 2014;13(4–5):560–564. [DOI] [PubMed] [Google Scholar]


