Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) can trigger a cytokine storm in the pulmonary tissue by releasing various types of mediators, leading to acute respiratory distress syndrome (ARDS). Increased neutrophil‐to‐lymphocyte ratio, as well as CD4+ T lymphopenia, is reported in cases with novel coronavirus disease (COVID‐19), meanwhile, lymphopenia is a significant finding in the majority of COVID‐19 cases with a severe phenotype. Moreover, excessive activation of monocyte/macrophage and cytokine storms are associated with the severity of the disease and the related complications in SARS‐CoV‐2 infection. Understanding the immune response dysregulation in COVID‐19 is essential to develop more effective diagnostic, therapeutic, and prophylactic strategies in this pandemic.
Keywords: acute respiratory distress syndrome, coronavirus disease, cytokine storm, immune dysregulation, lymphopenia, severe acute respiratory syndrome coronavirus
Abbreviation
- ACE2
angiotensin‐converting enzyme 2
- ARDS
acute respiratory distress syndrome
- β CoV
beta coronavirus
- COVID‐19
coronavirus disease
- FABP4
fatty acid‐binding protein‐4
- ICU
intensive care unit
- MASP‐2
mannose associated serine protease 2
- NLR
neutrophil‐to‐lymphocyte ratio
- NK
natural killer
- NKG2A
NK group 2 member A
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- WHO
World Health Organization
1. INTRODUCTION
The first patients with pneumonia of an unknown origin were reported in early December 2019 in Wuhan, Hubei province, China. The cause of pneumonia in these cases was a novel beta coronavirus (β CoV) with enveloped RNA that was previously called severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) due to the similar phylogenetic with SARS‐CoV (Golshani et al., 2020; Lotfi & Rezaei, 2020). The World Health Organization (WHO) has stated that coronavirus disease 2019 (COVID‐19) is a public health emergency of pandemic proportions (Guan et al., 2020). In addition to a huge burden on public health with a high rate of mortality, this pandemic has already distinctly affected the global economy and civil societies (Hanaei & Rezaei, 2020; Jabbari et al., 2020; Momtazmanesh et al., 2020). The risk of the severe form of the disease is more likely in patients with underlying conditions such as hypertension, cardiovascular disease, and diabetes mellitus (Huang et al., 2020; Qin et al., 2020). The virus can trigger a terrible cytokine storm in the pulmonary tissue by releasing various types of mediators causing edema, air exchange dysfunction and acute respiratory distress syndrome (ARDS), acute cardiac injury followed by a secondary infection which may lead to death (Huang et al., 2020; A. Saghazadeh & N. Rezaei, 2020; Xu et al., 2020). Several studies highlight relevant dysregulation both in innate and adaptive immune systems in COVID‐19 patients (Qin et al., 2020). Dysregulation of the immune response influences the outcome in critical COVID‐19 patients. For the development of drugs and vaccines during the epidemic and pandemic outbreak of a new virus, understanding the immune responses against the virus is essential (Lotfi, Hamblin, & Rezaei, 2020; Mansourabadi et al., 2020).
2. DYSREGULATED IMMUNITY IN COVID‐19
In a cohort study in Wuhan, China, the dysregulated immune system has been confirmed in 452 patients with laboratory‐confirmed COVID‐19. The increased neutrophil‐to‐lymphocyte ratio (NLR) as well as T lymphopenia, especially decreased number of CD4+ T cells, was typical in COVID‐19 patients, particularly among the severe cases. However, no significant alteration in the CD8+ cells and B cells was reported, suggesting the lymphocytes impairment, especially T cells immune system dysfunction during the period of illness in COVID‐19 (Qin et al., 2020; Wei et al., 2020). NLR, which is a well‐known factor to show systemic infection and inflammation, has been studied as a predictor of bacterial infection including pneumonia (Berhane et al., 2019; X. Liu et al., 2016). An increased number of neutrophil and a decreased number of lymphocyte were reported in several patients with COVID‐19 during the severe phase, indicating a serious disturbance in the internal environment and potential critical condition in those severe infected cases (Fathi & Rezaei, 2020; J. Liu, Wan, et al., 2020).
The gravity of a viral disease can be determined by direct cytopathic effects exerted by the virus as well as the circumvention of host immune responses (Channappanavar & Perlman, 2017; Min et al., 2016). A rapid and well‐coordinated innate immune response is believed to play a critical role as the first line of defense against viral infections; however, dysregulation of the immune response leads to exacerbated inflammatory responses, even resulting in death (Shaw et al., 2013). Increased loss of dendritic cell (DC) function could result in delayed T cell responses in COVID‐19 patients. DCs play a substantial role in the interaction between innate and adaptive immunity. Plasmacytoid dendritic cell (pDC) is the main potent IFN‐I producer against viral infection. Accordingly, the substantial loss of pDC together with a decrease in NK cell could lead to instant destruction of innate immunity against SARS‐CoV‐2 infection (Chu et al., 2020; R. Zhou et al., 2020).
Massive upregulation of proinflammatory cytokines and chemokines and CD4+ and CD8+ T cells consumption, as well as reduced regulatory T cells in patients with COVID‐19, mainly in the severe illness, may contribute to exacerbated inflammatory responses, the cytokine storm induction, and ultimately aggravated lung injury (Grifoni et al., 2020). However, correlative evidence from those severe patients with a lower number of lymphocytes reveals the role of dysregulated immune responses in the pathogenesis of COVID‐19 (J. Chen et al., 2010; Qin et al., 2020; Rokni et al., 2020). So, SARS‐CoV‐2 infection might majorly affect the lymphocytes, especially T cells, and induce a cytokine storm in the body, resulting in several immune responses to impair the corresponding organs. NLR can be served as an independent prognostic marker of disease severity in COVID‐19 (Rokni et al., 2020). In addition, lymphopenia and enhanced NLR are highly correlated with COVID‐19 disease severity/outcome. Therefore, surveillance of NLR and lymphocyte subsets is a useful marker in the early screening of patients and is a specific strategy to diagnose and treat COVID‐19 patients (J. Liu, Li, et al., 2020; Qin et al., 2020; A. Saghazadeh & N. Rezaei, 2020; Figure 1).
Figure 1.
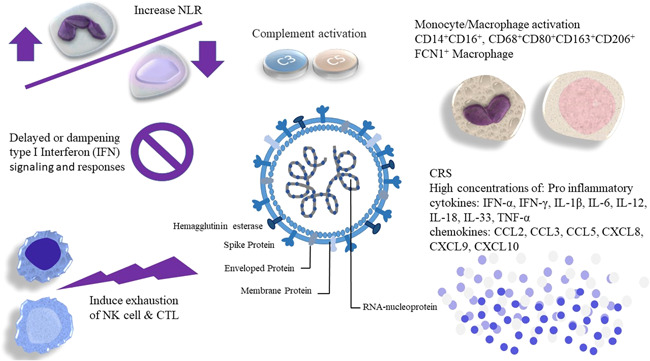
Dysfunctional and dysregulation of innate and adaptive immune responses. Uncontrolled monocyte‐macrophage activation, complement hyper‐activation, and inflammatory responses resulting in tissue damage and systemic inflammation; both contribute to morbidity and mortality in patients with coronavirus disease 2019
3. DYSREGULATION OF MONOCYTE/MACROPHAGE IN SARS‐COV‐2
Lymphopenia is as commonplace as the hematologic abnormality in up to 85% of patients with severe COVID‐19 (Qin et al., 2020). Regulatory T cells have been shown to play an essential role in weakening the immune response. Thus, a massive reduction of these types of T cells may lead to uncontrolled innate immune responses and unchecked inflammatory responses (Qin et al., 2020). Indeed, excessive activation of monocyte/macrophage and cytokine storms are linked with the gravity of the disease and the related complications in SARS‐CoV‐2 infection (Fung et al., 2020; Giamarellos‐Bourboulis et al., 2020).
The study on 28 patients with SARS‐CoV‐2 infection showed an alteration in the morphology and action of monocytes/macrophages as a predictive value for the severity of the disease, potential risk of intensive care unit (ICU) admission, length of stay in the hospital, and full recovery (Zhang et al., 2020).
Mature macrophages can alter their phenotypes and undergo functional polarization in response to signals from the local microenvironment that induce naïve or resting “M0 macrophages” to adopt classically activated “M1‐like” or anti‐inflammatory “M2‐like” phenotypes or promote M1‐like and M2‐like macrophage interconversion. M1 macrophages secrete elevated levels of proinflammatory cytokines, such as IL‐6, IL‐1β, and TNF‐α, to regulate local tissue and immune responses that promote pathogen clearance. Elevated serum levels of IL‐6, a hallmark of severe SARS‐CoV‐2 infections, correlate with ARDS, respiratory failure, and adverse clinical outcomes in COVID‐19 patients (Jingping Liu, Li, et al., 2020).
The genome of SARS‐CoV and MERS‐CoV translates multiple proteins, which contribute to antagonizing the IFN responses, with preliminary antagonism of IFN resulting in delayed or evaded innate immune responses (Channappanavar & Perlman, 2017; Channappanavar et al., 2016). Followed by postponed IFN cascade, the inflammatory monocyte/macrophage responses are further organized and T cells are sensitized to apoptosis; as a result, the dysregulated inflammatory response is more activated (Channappanavar et al., 2016). These inflammatory macrophages (M1) accumulate in the lungs; they are the potential sources of proinflammatory cytokines and chemokines associated with fatal diseases induced by human coronavirus infections such as SARS and COVID‐19. The autopsy findings of COVID‐19 are correlated with these consequences (Yao et al., 2020).
Remarkably, the monocytes isolated from infected patients are identified to be angiotensin‐converting enzyme 2 (ACE2)‐positive cells. The ACE2 receptors are utilized by SARS‐CoV‐2 to invade the host cells, suggesting the probability of direct infection of monocytes in COVID‐19 patients and leading to abortive replication of the virus and postponed type I IFN signaling in these types of cells (Acharya et al., 2020). As was shown earlier, monocyte infection by SARS‐CoV was responsible for the activation of inflammatory responses and altered gene expression related to the immune system signaling (Dosch et al., 2009; Hu et al., 2012).
The differentiation of monocytes to macrophages is induced by inflammatory mediators, e.g. GM‐CSF, IFN‐α, IL6, or TNF‐α. Recently, Y. Zhou et al. (2020) demonstrated that severe pulmonary syndrome patients admitted in ICU had a higher percentage of CD14+CD16+ monocytes. In addition, the ability of these monocytes in the production of mediators, such as GM‐CSF and IL6‐associated with cytokine storm, has been proven. A model also was defined by this study group, whereby an abnormal Th1 response activates GM‐CSF induced monocyte/macrophage, correlated by enhanced IL6 CD14+ CD16+ producing monocytes. Then, these monocytes migrate into the lung tissue and along with an inflammatory cytokine storm induce the subsequent lung injury. This condition was additionally supported by the recent data from a study performed on single‐cell RNA sequencing on the immune cells isolated from bronchoalveolar lavage samples of COVID‐19 patients that provide critical insights into the immune microenvironment of the infected pulmonary tissue (Liao et al., 2020; Schulte‐Schrepping et al., 2020). There was an increase in HLA‐DRhiCD11chi inflammatory monocytes with an interferon‐stimulated gene effect in mild COVID‐19. Severe COVID‐19 was signaled by the incidence of neutrophil precursors, as evidence of serious myelopoiesis, dysfunctional mature neutrophils, and HLA‐DRlo monocytes (Schulte‐Schrepping et al., 2020).
The main macrophage subset in the lungs of patients with ARDS was proven to be alveolar macrophages expressing supplant fatty acid binding protein‐4 (FABP4) and the Ficolin 1 expressing monocyte‐derived macrophages. These cells are highly implicated in the inflammatory responses and produce a wide range of chemokines involved in a cytokine storm (Qin et al., 2020). This evidence confirms the movement of inflammatory monocyte/macrophages into the lung tissue and other infected organs. On the other hand, there is a nonspecific finding demonstrating that somewhat larger, vacuolated, and atypical monocytes were observed in the morphological assessment of peripheral blood films of infected patients (Zhang et al., 2020).
4. FUNCTIONAL EXHAUSTION OF ANTIVIRAL LYMPHOCYTES IN SARS‐COV‐2
Several studies have proven a remarkable decrease in the total number of natural killer (NK) and CD8+ T cells in COVID‐19 patients. Also, the exhausted activation of NK and CD8+ T cells due to enhanced expression of CD94/NK group 2 member A (NKG2A) was observed in these cases. Notably, the enhanced number of NK and CD8+ T cells was correlated with decreased NKG2A expression after therapy and gradual recovery in patients, suggesting that the functional exhaustion of CTLs is related to SARS‐CoV‐2 infection (Diao et al., 2020). Moreover, CD8+ T cells and CD4+ T cells have higher levels of PD‐1 and Tim‐3, exhaustion markers, in patients (Diao et al., 2020; H.‐Y. Zheng et al., 2020). In the early stages, SARS‐CoV‐2 infection may break down antiviral immunity (N. Chen et al., 2020; R. Zhou et al., 2020). Importantly, the effects of NKG2A expression on NK and CD8+ T cells activation lead to functional depletion of NK and CTLs. According to results, NKG2A expression was upregulated on NK cells and CD8+ T cells in COVID‐19 patients with a reduced ability to produce CD107a, granzyme B, IFN‐γ, and TNF‐α (Haanen & Cerundolo, 2018).
The number of NKG2A+ cytotoxic lymphocytes was also reduced in recovered COVID‐19 patients, emphasizing the important role of NKG2A expression in the pathogenesis of COVID‐19 in the early stage, correlated with the functional exhaustion of cytotoxic lymphocytes and progression of the disease (Haanen & Cerundolo, 2018).
Exhausted NK cells and CD8+ T cells arise from during chronic conditions such as chronic infection and tumorigenesis. Also, T cell apoptosis with identical underlying mechanisms was observed both in chronic infection and cancer. This condition was also proven in patients with SARS‐CoV infection (Barathan et al., 2018). Therefore, exhaustion of NKG2A+ cytotoxic lymphocytes may be predicted in patients with SARS‐CoV infection. In addition, effective control of SARS‐CoV‐2 infection is dependent on the decreased expression of NKG2A in cytotoxic lymphocytes, as the percentage of NKG2A+ cytotoxic lymphocytes was reduced following the antiviral therapy (94% of patients were administered antiviral therapy [Kaletra]) in the patients with COVID‐19. Consequently, SARS‐CoV‐2 infection‐induced NKG2A expression may be correlated with the functional exhaustion of cytotoxic lymphocytes at the early stage in COVID‐19 patients with severe pulmonary inflammation, leading to the progression of the illness (M. Zheng et al., 2020).
5. DYSREGULATION OF COMPLEMENT SYSTEM IN SARS‐COV‐2
The complement system plays a pivotal role in the host immune response to COVID‐19. Complement activation was also observed in COVID‐19 patients, which might be involved in the pathogenesis of severe lung injury and ARDS. Furthermore, complement activation may contribute to the maladaptive inflammatory and immune response rather than increased viral loads seen in some patients with acute COVID‐19 (Guan et al., 2020; Li et al., 2020).
The virus uses virus‐encoded proteins to avoid being detected by the complement system, emphasizing the critical role of complements in antiviral responses. C3a and C5a with potential characteristics of proinflammatory factors are able to initiate the recruitment of inflammatory cells and activate neutrophils (Gralinski et al., 2018).
As a curative approach to acute lung injury, the obstruction of C3a and C5a has been proved to be fruitful. Anti‐C5a antibody was reported to mitigate the gravity of the adverse impacts arising from MERS‐CoV in mice (Gralinski et al., 2018; Sun et al., 2013). In a study by Gao et al. (2020) N proteins were shown to empower SARS‐CoV‐2 to attach to mannose‐associated serine protease 2 (MASP‐2). MASP‐2 is a key serine protease in the lectin pathway of complement activation, resulting in aberrant C5 activation and aggravated inflammatory lung injury. The complement cascade is activated during SARS‐CoV‐2 and participates in the pathogenesis of the disease (Gao et al., 2020; Sun et al., 2013).
6. CONCLUSION
SARS‐CoV‐2 can evade the immune responses, which permits the virus to produce enormous copy numbers in infected tissues. Through the dysregulation and infection of the immune cells as well as the recruitment of other immune cells from the circulation to the site of infection, acute immune reactions induce inflammation resulting in a cytokine storm and life‐ menacing problems (Schurink et al., 2020; Yazdanpanah et al., 2020). Dysregulation of host immunity, including hyper‐activation of macrophage and complement system, NLR induction, as well as NK cells and CTLs exhaustion, could be involved in the disease severity and outcomes. Understanding of the immune system dysregulation seems to be critical for disease control in patients with COVID‐19.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHORS CONTRIBUTIONS
N. R. proposed the general concept and supervised the project. L. M. K. contributed to the data gathering, writing the manuscript, and preparing the figure, while N. R. contributed to study design, scientific, and structural editing. N. R. and L. M. K. critically revised the manuscript. Both authors approved the final draft of the manuscript before submission.
Mohamed Khosroshahi L, Rezaei N. Dysregulation of the immune response in coronavirus disease 2019. Cell Biol Int. 2021;45:702–707. 10.1002/cbin.11517
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- Acharya, D. , Liu, G. , & Gack, M. U. (2020). Dysregulation of type I interferon responses in COVID‐19. Nature Reviews Immunology, 20, 1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barathan, M. , Mohamed, R. , Yong, Y. , Kannan, M. , Vadivelu, J. , Saeidi, A. , Larsson, M. , & Shankar, E. (2018). Viral persistence and chronicity in hepatitis C virus infection: Role of T‐cell apoptosis, senescence, and exhaustion. Cells, 7(10), 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berhane, M. , Melku, M. , Amsalu, A. , Enawgaw, B. , Getaneh, Z. , & Asrie, F. (2019). The role of neutrophil to lymphocyte count ratio in the differential diagnosis of pulmonary tuberculosis and bacterial community‐acquired pneumonia: A cross‐sectional study at Ayder and Mekelle hospitals, Ethiopia. Clinical Laboratory, 65(4), 527–533. [DOI] [PubMed] [Google Scholar]
- Channappanavar, R. , Fehr, A. R. , Vijay, R. , Mack, M. , Zhao, J. , Meyerholz, D. K. , & Perlman, S. (2016). Dysregulated type I interferon and inflammatory monocyte‐macrophage responses cause lethal pneumonia in SARS‐CoV‐infected mice. Cell host & microbe, 19(2), 181–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channappanavar, R. , & Perlman, S. (2017). Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Paper presented at the Seminars in immunopathology. [DOI] [PMC free article] [PubMed]
- Chen, J. , Lau, Y. F. , Lamirande, E. W. , Paddock, C. D. , Bartlett, J. H. , Zaki, S. R. , & Subbarao, K. (2010). Cellular immune responses to severe acute respiratory syndrome coronavirus (SARS‐CoV) infection in senescent BALB/c mice: CD4+ T cells are important in control of SARS‐CoV infection. Journal of Virology, 84(3), 1289–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, N. , Zhou, M. , Dong, X. , Qu, J. , Gong, F. , Han, Y. , Qiu, Y. , Wang, J. , Liu, Y. , Wei, Y. , Xia, J. , Yu, T. , Zhang, X. , & Zhang, L. (2020). Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. The Lancet, 395(10223), 507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu, H. , Chan, J. F.‐W. , Wang, Y. , Yuen, T. T.‐T. , Chai, Y. , Hou, Y. , Shuai, H. , Yang, D. , Hu, B. , Huang, X. , Zhang, X. , Cai, J. P. , Zhou, J. , Yuan, S. , Kok, K. H. , To, K. K. W. , Chan, I. H. Y. , Zhang, A. J. , Sit, K. Y. , Au, W. K. , & Yuen, K. Y. (2020). Comparative replication and immune activation profiles of SARS‐CoV‐2 and SARS‐CoV in human lungs: An ex vivo study with implications for the pathogenesis of COVID‐19. Clinical Infectious Diseases, 71, 1400–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao, B. , Wang, C. , Tan, Y. , Chen, X. , Liu, Y. , Ning, L. , Chen, L. , Li, M. , Liu, Y. , Wang, G. , Yuan, Z. , Feng, Z. , Zhang, Y. , Wu, Y. , & Chen, Y. (2020). Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID‐19). Frontiers in Immunology, 11(827). 10.3389/fimmu.2020.00827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosch, S. F. , Mahajan, S. D. , & Collins, A. R. (2009). SARS coronavirus spike protein‐induced innate immune response occurs via activation of the NF‐κB pathway in human monocyte macrophages in vitro. Virus Research, 142(1‐2), 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fathi, N. , & Rezaei, N. (2020). Lymphopenia in COVID‐19: Therapeutic opportunities. Cell Biology International, 44, 1792–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung, S.‐Y. , Yuen, K.‐S. , Ye, Z.‐W. , Chan, C.‐P. , & Jin, D.‐Y. (2020). A tug‐of‐war between severe acute respiratory syndrome coronavirus 2 and host antiviral defence: lessons from other pathogenic viruses. Emerging microbes & infections, 9(1), 558–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, T. , Hu, M. , Zhang, X. , Li, H. , Zhu, L. , Liu, H. , & Hu, Y. (2020). Highly pathogenic coronavirus N protein aggravates lung injury by MASP‐2‐mediated complement over‐activation. medRxiv. 10.1101/2020.03.29.20041962 [DOI] [Google Scholar]
- Giamarellos‐Bourboulis, E. J. , Netea, M. G. , Rovina, N. , Akinosoglou, K. , Antoniadou, A. , Antonakos, N. , & Katsaounou, P. (2020). Complex immune dysregulation in COVID‐19 patients with severe respiratory failure. Cell host & microbe, 27(6), 992–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golshani, M. , Saghazadeh, A. , & Rezaei, N. (2020). SARS‐CoV‐2–a tough opponent for the immune system. Archives of Medical Research, 51, 589–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gralinski, L. E. , Sheahan, T. P. , Morrison, T. E. , Menachery, V. D. , Jensen, K. , Leist, S. R. , Whitmore, A. , Heise, M. T. , & Baric, R. S. (2018). Complement activation contributes to severe acute respiratory syndrome coronavirus pathogenesis. mBio, 9(5), e01753–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grifoni, A. , Weiskopf, D. , Ramirez, S. I. , Mateus, J. , Dan, J. M. , Moderbacher, C. R. , Rawlings, S. A. , Sutherland, A. , Premkumar, L. , Jadi, R. S. , Marrama, D. , de Silva, A. M. , Frazier, A. , Carlin, A. F. , Greenbaum, J. A. , Peters, B. , Krammer, F. , Smith, D. M. , Crotty, S. , & Sette, A. (2020). Targets of T cell responses to SARS‐CoV‐2 coronavirus in humans with COVID‐19 disease and unexposed individuals. Cell, 181, 1489–1501.e15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan, W. , Ni, Z. , Hu, Y. , Liang, W. , Ou, C. , He, J. , Liu, L. , Shan, H. , Lei, C. , Hui, D. S. C. , Du, B. , Li, L. , Zeng, G. , Yuen, K. Y. , Chen, R. , Tang, C. , Wang, T. , Chen, P. … Zhong, N. (2020). Clinical characteristics of coronavirus disease 2019 in China. New England Journal of Medicine, 382, 1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haanen, J. B. , & Cerundolo, V. (2018). NKG2A, a new kid on the immune checkpoint block. Cell, 175(7), 1720–1722. [DOI] [PubMed] [Google Scholar]
- Hanaei, S. , & Rezaei, N. (2020). COVID‐19: Developing from an outbreak to a pandemic. Archives of Medical Research, 51, 582–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, W. , Yen, Y.‐T. , Singh, S. , Kao, C.‐L. , & Wu‐Hsieh, B. A. (2012). SARS‐CoV regulates immune function‐related gene expression in human monocytic cells. Viral Immunology, 25(4), 277–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, C. , Wang, Y. , Li, X. , Ren, L. , Zhao, J. , Hu, Y. , Zhang, L. , Fan, G. , Xu, J. , Gu, X. , Cheng, Z. , Yu, T. , Xia, J. , Wei, Y. , Wu, W. , Xie, X. , Yin, W. , Li, H. , Liu, M. … Cao, B. (2020). Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet, 395(10223), 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabbari, P. , Jabbari, F. , Ebrahimi, S. , & Rezaei, N. (2020). COVID‐19: A chimera of two pandemics. Disaster Medicine and Public Health Preparedness, 14, 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, G. , Fan, Y. , Lai, Y. , Han, T. , Li, Z. , Zhou, P. , Pan, P. , Wang, W. , Hu, D. , Liu, X. , Zhang, Q. , & Wu, J. (2020). Coronavirus infections and immune responses. Journal of Medical Virology, 92(4), 424–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao, M. , Liu, Y. , Yuan, J. , Wen, Y. , Xu, G. , Zhao, J. , & Wang, F. (2020). The landscape of lung bronchoalveolar immune cells in COVID‐19 revealed by single‐cell RNA sequencing. medRxiv. 10.1101/2020.02.23.20026690 [DOI] [Google Scholar]
- Liu, J. , Li, S. , Liu, J. , Liang, B. , Wang, X. , Wang, H. , Li, W. , Tong, Q. , Yi, J. , Zhao, L. , Xiong, L. , Guo, C. , Tian, J. , Luo, J. , Yao, J. , Pang, R. , Shen, H. , Peng, C. , Liu, T. … Zheng, X. (2020). Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS‐CoV‐2 infected patients. EBioMedicine, 55, 102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J. , Wan, M. , Lyon, C. J. , & Hu, T. Y. (2020). Nanomedicine therapies modulating macrophage dysfunction: A potential strategy to attenuate cytokine storms in severe infections. Theranostics, 10(21), 9591–9600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X. , Shen, Y. , Wang, H. , Ge, Q. , Fei, A. , & Pan, S. (2016). Prognostic significance of neutrophil‐to‐lymphocyte ratio in patients with sepsis: A prospective observational study. Mediators of Inflammation, 2016, 2016–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotfi, M. , Hamblin, M. R. , & Rezaei, N. (2020). COVID‐19: Transmission, prevention, and potential therapeutic opportunities. Clinica Chimica Acta, 508, 254–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotfi, M. , & Rezaei, N. (2020). SARS‐CoV‐2: A comprehensive review from pathogenicity of the virus to clinical consequences. Journal of Medical Virology, 92, 1864–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansourabadi, A. H. , Sadeghalvad, M. , Mohammadi‐Motlagh, H. R. , & Rezaei, N. (2020). The immune system as a target for therapy of SARS‐CoV‐2: A systematic review of the current immunotherapies for COVID‐19. Life Sciences, 258, 118185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min, C.‐K. , Cheon, S. , Ha, N.‐Y. , Sohn, K. M. , Kim, Y. , Aigerim, A. , Shin, H. M. , Choi, J. Y. , Inn, K. S. , Kim, J. H. , Moon, J. Y. , Choi, M. S. , Cho, N. H. , & Kim, Y. S. (2016). Comparative and kinetic analysis of viral shedding and immunological responses in MERS patients representing a broad spectrum of disease severity. Scientific Reports, 6(1), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momtazmanesh, S. , Ochs, H. D. , Uddin, L. Q. , Perc, M. , Routes, J. M. , Vieira, D. N. , Al‐Herz, W. , Baris, S. , Prando, C. , Rosivall, L. , Abdul Latiff, A. H. , Ulrichs, T. , Roudenok, V. , Aldave Becerra, J. C. , Salunke, D. B. , Goudouris, E. , Condino‐Neto, A. , Stashchak, A. , Kryvenko, O. … Rezaei, N. (2020). All together to fight novel coronavirus disease (COVID‐19). The American Journal of Tropical Medicine and Hygiene, 102, 1181–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin, C. , Zhou, L. , Hu, Z. , Zhang, S. , Yang, S. , Tao, Y. , & Wang, W. (2020). Dysregulation of immune response in patients with COVID‐19 in Wuhan, China. Clinical Infectious Diseases.71(15), 762–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rokni, M. , Ahmadikia, K. , Asghari, S. , Mashaei, S. , & Hassanali, F. (2020). Comparison of clinical, para‐clinical and laboratory findings in survived and deceased patients with COVID‐19: Diagnostic role of inflammatory indications in determining the severity of illness. BMC Infect Dis, 20, 869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saghazadeh, A. , & Rezaei, N. (2020). Immune‐epidemiological parameters of the novel coronavirus – a perspective. Expert Review of Clinical Immunology, 16, 465–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saghazadeh, A. , & Rezaei, N. (2020). Towards treatment planning of COVID‐19: Rationale and hypothesis for the use of multiple immunosuppressive agents: Anti‐antibodies, immunoglobulins, and corticosteroids. International Immunopharmacology, 84(106560), 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte‐Schrepping, J. , Reusch, N. , Paclik, D. , Baßler, K. , Schlickeiser, S. , Zhang, B. , Krämer, B. , Krammer, T. , Brumhard, S. , Bonaguro, L. , De Domenico, E. , Wendisch, D. , Grasshoff, M. , Kapellos, T. S. , Beckstette, M. , Pecht, T. , Saglam, A. , Dietrich, O. , Mei, H. E. , … Ziebuhr, J. (2020). Severe COVID‐19 is marked by a dysregulated myeloid cell compartment. Cell, 182, 1419–1440.e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurink, B. , Roos, E. , Radonic, T. , Barbe, E. , Bouman, C. S. C. , de Boer, H. H. , de Bree, G. J. , Bulle, E. B. , Aronica, E. M. , Florquin, S. , Fronczek, J. , Heunks, L. M. A. , de Jong, M. D. , Guo, L. , du Long, R. , Lutter, R. , Molenaar, P. C. G. , Neefjes‐Borst, E. A. , Niessen, H. W. M. … Bugiani, M. (2020). Viral presence and immunopathology in patients with lethal COVID‐19: a prospective autopsy cohort study. The Lancet Microbe, 1, e290–e299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw, A. C. , Goldstein, D. R. , & Montgomery, R. R. (2013). Age‐dependent dysregulation of innate immunity. Nature Reviews Immunology, 13(12), 875–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, S. , Zhao, G. , Liu, C. , Wu, X. , Guo, Y. , Yu, H. , Song, H. , Du, L. , Jiang, S. , Guo, R. , Tomlinson, S. , & Zhou, Y. (2013). Inhibition of complement activation alleviates acute lung injury induced by highly pathogenic avian influenza H5N1 virus infection. American Journal of Respiratory Cell and Molecular Biology, 49(2), 221–230. [DOI] [PubMed] [Google Scholar]
- Wei, L.l , Wang, W.j , Chen, D.x , & Xu, B. (2020). Dysregulation of the immune response affects the outcome of critical COVID‐19 patients. Journal of Medical Virology, 92, 2768–2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, Z. , Shi, L. , Wang, Y. , Zhang, J. , Huang, L. , Zhang, C. , Liu, S. , Zhao, P. , Liu, H. , Zhu, L. , Tai, Y. , Bai, C. , Gao, T. , Song, J. , Xia, P. , Dong, J. , Zhao, J. , & Wang, F. S. (2020). Pathological findings of COVID‐19 associated with acute respiratory distress syndrome. The Lancet respiratory medicine, 8(4), 420–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao, X. , Li, T. , He, Z. , Ping, Y. , Liu, H. , Yu, S. , & Fu, W. (2020). A pathological report of three COVID‐19 cases by minimally invasive autopsies. Chinese Journal of Pathology, 49, E009. [DOI] [PubMed] [Google Scholar]
- Yazdanpanah, F. , Hamblin, M. R. , & Rezaei, N. (2020). The immune system and COVID‐19: Friend or foe? Life Sciences, 256, 117900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, D. , Guo, R. , Lei, L. , Liu, H. , Wang, Y. , Wang, Y. , & Wang, J. (2020). COVID‐19 infection induces readily detectable morphological and inflammation‐related phenotypic changes in peripheral blood monocytes, the severity of which correlate with patient outcome. medRxiv. [Google Scholar]
- Zheng, H.‐Y. , Zhang, M. , Yang, C.‐X. , Zhang, N. , Wang, X.‐C. , Yang, X.‐P. , Dong, X. Q. , & Zheng, Y. T. (2020). Elevated exhaustion levels and reduced functional diversity of T cells in peripheral blood may predict severe progression in COVID‐19 patients. Cellular & Molecular Immunology, 17(5), 541–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, M. , Gao, Y. , Wang, G. , Song, G. , Liu, S. , Sun, D. , & Tian, Z. (2020). Functional exhaustion of antiviral lymphocytes in COVID‐19 patients. Cellular & Molecular Immunology, 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, R. , Wong, K. K.‐W. , Liu, Y.‐C. , Zhou, L. , Li, B. , X., & Lau, T. T.‐K. (2020). Acute SARS‐CoV‐2 infection impairs dendritic cell and T cell responses. Immunity, 53, 864–877.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, Y. , Fu, B. , Zheng, X. , Wang, D. , & Zhao, C. (2020). Pathogenic T cells and inflammatory monocytes incite inflammatory storm in severe COVID‐19 patients. National Science Review, 7, 998–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


