Abstract
Currently, four non-vitamin K antagonists oral anticoagulants (NOACs) are available for stroke prevention in atrial fibrillation (AF). These have been in clinical use for up to 10 years now. Besides data of the initial phase III clinical trials, now clinical data, several sub-studies, meta-analyses, and studies in special clinical settings and specific patient populations are available. This review shall give an overview on the history of NOAC development, sum up study data and ‘real-world’ clinical data as well as discuss several special clinical settings like NOAC treatment in patients that require coronary artery stenting or cardioversion (CV). Furthermore, treatment considerations in special patient populations like patients with renal impairment, obesity, or patients requiring NOACs for secondary prevention are discussed. The significance of NOAC treatment will be discussed under consideration of the recently published 2020 ESC/EACTS Guidelines for the diagnosis and management of AF.
Keywords: Fibrillation, NOAC, Review, Stroke, Warfarin
History of non-vitamin K antagonists oral anticoagulants—for how long is ‘new’ still actually ‘new’?
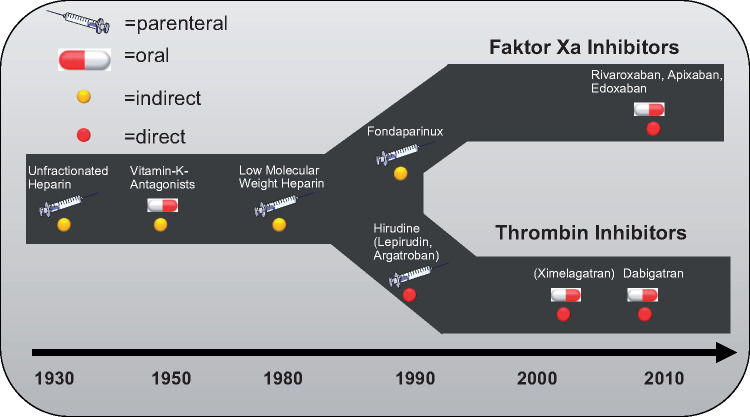
‘NOAC’ is an abbreviation used to group together ‘new oral anticoagulants’—thrombin inhibitors and factor Xa inhibitors. There is currently one direct thrombin inhibitor (dabigatran) and three different factor Xa inhibitors (in order of market release: rivaroxaban, apixaban, and edoxaban) available on the market. All of them have undergone large-scale phase III clinical trials for deep vein thrombosis (DVT) and pulmonary embolism (PE) prevention and treatment, as well as for the prophylaxis of atrial fibrillation (AF) associated thromboembolic events. The history and development of parenteral, orally available direct and indirect anticoagulants are depicted in Figure 1.
Figure 1.
Showing the development timeline of several parenteral and enteral anticoagulants with either direct or indirect mode of action.
Initially, all substances where tested a setting of a relatively short treatment period like in perioperative prophylaxis of DVT or PE. These studies were followed by phase three trials with a longer treatment period such as in the treatment and subsequent secondary prophylaxis after DVT and PE. Finally, all NOACs have subsequently been studied in a trial on the prophylaxis of thromboembolism in non-valvular AF vs. the vitamin K antagonist (VKA) warfarin.
The term NOAC is rather unfortunate: none of the NOACs were new at the time they received marketing authorization or during the registration studies, since all substances were synthesized ∼20 years before first clinical use. Today, all four substances have been on the market for several years. It has therefore been proposed to exchange the ‘N’ as new in the acronym for ‘D’ as direct, depicting their mode of action. Although the acronym DOAC seems sensible and distinguishes these direct inhibitors from other substances with indirect mode of action, there is a major drawback to the new acronym: several older and current guidelines still use the term NOAC. It is therefore necessary to use this term, in order to capture all results in a database search for these substances. Overall, the acronym NOAC should be retained, but the definition of NOAC was changed to non-Vitamin K oral anticoagulants.
Very recently, the 2020 ESC guideline on diagnosis and management of AF has upgraded treatment recommendation for switching from VKA- to NOAC therapy from Class IIb (may be considered) to a Class I recommendation (recommended or indicated) for patients on VKA that have a time in therapeutic range below 70%.1
Prospective clinical phase III trials and meta-analyses
All NOACs were tested in large-scale phase III clinical trials vs. warfarin. The RE-LY trial evaluated the efficacy of dabigatran for the prevention of thromboembolic events in AF patients in an Intention-to-treat (ITT) analysis for non-inferiority and superiority using a PROBE (Prospective Randomized Open, Blinded End-point) design.2
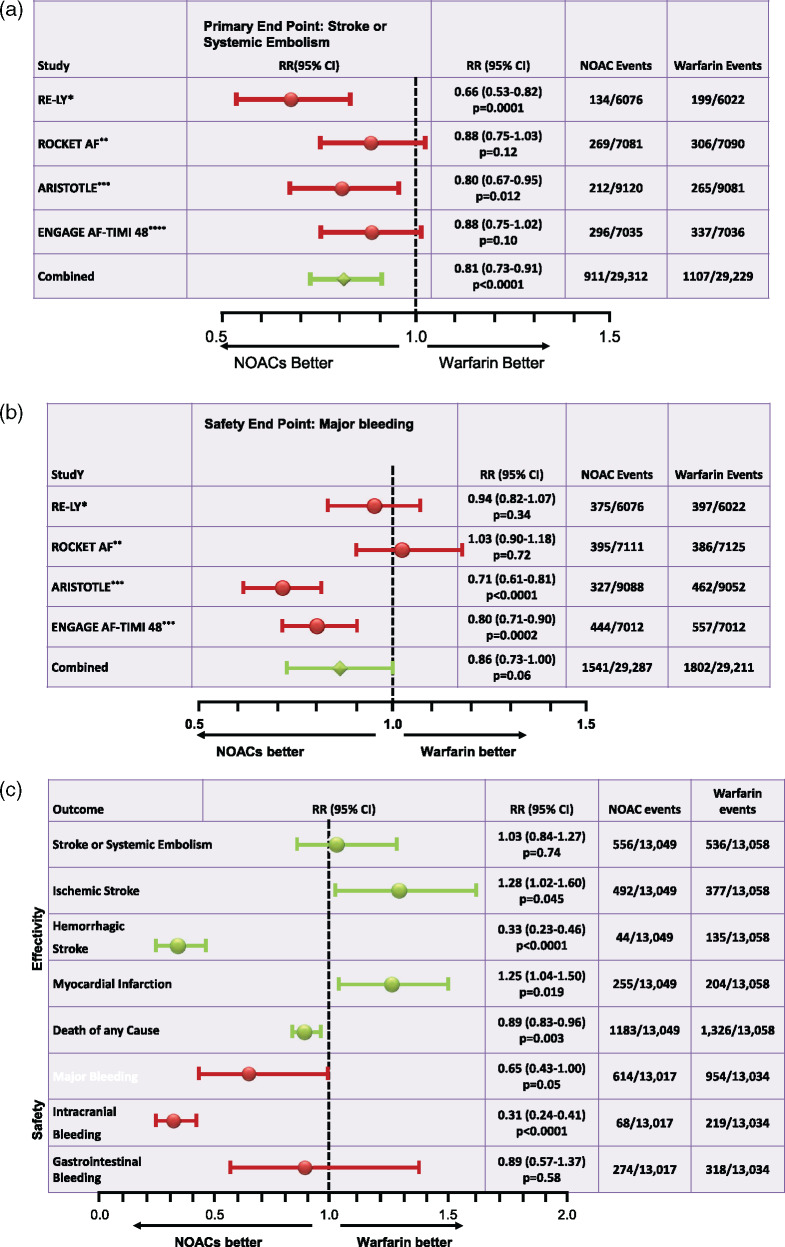
ROCKET-AF, the shortly later published study tested Rivaroxaban in a randomized, double-blind, double-dummy design that was powered for non-inferiority using a per-protocol/on treatment (OT) analysis.3 Therefore, not all enrolled patients were included into the analyses but only those, who had taken the study drug at least once. In the event of non-inferiority, an evaluation for superiority of the safety population on treatment was then done. Secondary endpoints were also evaluated in a superiority analysis. The next direct factor Xa inhibitor available on the market, apixaban, was tested in the ARISTOTLE trial primarily for non-inferiority and secondarily for superiority.4 Finally, trial results for the fourth NOAC, edoxaban, were published in 2013.5 In this study, named ENGAGE AF-TIMI 48, edoxaban was evaluated in an ITT analysis for superiority and also an OT analysis for non-inferiority. While ROCKET-AF had major and non-major clinically significant bleeding events as a safety endpoint, all other studies defined only major bleeding as such. All studies used the CHADS2 score as a risk score for increased thromboembolic risk in their inclusion criteria. ENGAGE AF-TIMI 48 and ROCKET-AF, the CHADS2 score had to be ≥2, in ROCKET-AF proportionally even ≥3 during the course of the trial. Therefore, in contrast to RE-LY and ARISTOTLE, ROCKET-AF and ENGAGE AF-TIMI 48 did not enrol patients with a CHADS2 score of 0–1. The mean CHADS2 score in ARISTOTLE was 2.1 and in RE-LY it was 2.1 to 2.2. According to the inclusion criteria, it was higher in both other studies: In ENGAGE AF-TIMI 48, it was 2.8 and in ROCKET-AF it was 3.5. Table 1 summarizes the studies. The 2014 published meta-analysis by Ruff et al.6 pooled data from all four NOAC studies. Compared with warfarin it showed a better effect of NOACs concerning the primary endpoint [stroke or systemic embolism (SE)]. Dabigatran with a dose of 2 × 150 mg per day and apixaban at a dose of 2 × 5 mg per day showed a significant benefit in the superiority analysis. Primary endpoint data of all studies and pooled data are shown in Figure 2A. The risk of the primary safety endpoint of each study including pooled data is depicted in Figure 2B, while Figure 2C shows efficacy and safety data for the low-dose regimens of dabigatran (2 × 110 mg per day) and apixaban (2 × 2.5 mg per day). It is important to say that no direct comparison of NOACs can be derived from these data as neither their study designs nor their study populations were comparable as mentioned above.
Table 1.
List of differences in the atrial fibrillation studies on the non-vitamin K antagonists oral anticoagulants dabigatran, rivaroxaban, apixaban, and edoxaban, modified from Ref.6
| RE-LY (dabigatran) | ROCKET-AF (rivaroxaban) | ARISTOTLE (apixaban) | ENGAGE AF (edoxaban) | |
|---|---|---|---|---|
| Number of included pts. | 18 113 | 14 264 | 18 201 | 21 105 |
| Mean age (a) | 72 ± 9 | 73 [65–78] | 70 [63–76] | 72 [64–78] |
| Female pts. (%) | 37 | 40 | 35 | 38 |
| CHADS2 score ≥3 (%) | 32 | 87 | 30 | 53 |
| Paroxysmal AF (%) | 32 | 18 | 15 | 25 |
| Status post-TIA or stroke (%) | 20 | 55 | 19 | 28 |
| VKA naïve (%) | 50 | 38 | 43 | 41 |
| ASA use (%) | 40 | 36 | 31 | 29 |
| Median follow-up(a) | 2.0 | 1.9 | 1.8 | 2.8 |
| Median time in therapeutic range (%) | 66 | 58 | 66 | 68 |
| CHADS2 score % | ||||
| 0–I | 32 | 0 | 34 | 0 |
| 1–2 | 35 | 13 | 36 | 47 |
| 3–6 | 33 | 87 | 30 | 53 |
ASA, acetylsalicylic acid; NOAC, non-vitamin K oral anticoagulant; TIA, transient ischaemic attack;, VKA, vitamin K antagonist.
Figure 2.
(A) Presentation of the primary study endpoints in a comparison of studies on atrial fibrillation for *dabigatran 2 × 150 mg, **rivaroxaban 1 × 20 mg, ***apixaban 2 × 5 mg, and ****edoxaban 1 × 60 mg compared with warfarin with a target INR of 2–3, modified from Ref.6; RR, relative risk. (B) Presentation of the safety endpoint ‘major bleeding’ in a comparison of studies on atrial fibrillation for *dabigatran 2 × 150 mg, **rivaroxaban 1 × 20 mg, ***apixaban 2 × 5 mg, and ****edoxaban 1 × 60 mg vs. warfarin with a target INR of 2–3, modified from Ref.6; RR, relative risk. (C) Presentation of the results of low-dose non-vitamin K oral anticoagulant treatment in atrial fibrillation. The results for dabigatran 2 × 110 mg and edoxaban 1 × 30 mg daily were pooled; modified from Ref.6; RR, relative risk reduction.
In 2014, Skjøth et al. did an indirect comparison analysis of the relative safety and efficacy of edoxaban (both high- and low-dose strategy) against the three other NOACs (dabigatran with two doses, rivaroxaban and apixaban with one dose).7 As a limitation of the analysis it has to be mentioned that their indirect comparison analysis based on the assumption of comparability of all study populations. In their analysis, there was no significant difference between high-dose edoxaban and apixaban for efficacy endpoints, mortality, myocardial infarction (MI), and major bleeding. However, apixaban was associated with fewer major or clinically relevant non-major bleeding (CRNMB) [hazard ratio (HR) 0.79; 95% confidence interval (CI) 0.70–0.90] and gastrointestinal bleeding (HR 0.72; 95% CI 0.53–0.99). Low-dose dabigatran (2 × 110 mg per day) showed no significant difference for efficacy or safety endpoint, but high-dose dabigatran (2 × 150 mg per day) was associated with lower rates of stroke or SE (HR 0.75; 95% CI 0.56–0.99), stroke (HR 0.73; 95% CI 0.55–0.96), and haemorrhagic stroke (HR 0.48; 95% CI 0.23–0.99). There were no significant differences between high-dose edoxaban vs. rivaroxaban for efficacy endpoints or mortality. However, rivaroxaban had more major and/or CRNMB events. In their analysis, apixaban was associated with lower stroke/SE (HR 0.70; 95% CI 0.55–0.89), stroke (HR 0.70; 95% CI 0.55–0.92), and ischaemic stroke (HR 0.65; 95% CI 0.50–0.89), but more major bleeding (HR 1.47; 95% CI 1.20–1.80) when compared with low-dose edoxaban (30 mg once daily). For dabigatran 110 mg bid, there were no significant differences in the efficacy endpoints, but dabigatran 110 mg bid had higher major (and gastrointestinal) bleeding. Dabigatran 150 mg bid and rivaroxaban were associated with lower stroke/SE and ischaemic stroke, but higher bleeding rates.
Another more recent analysis by Fernandez et al.8 compared relative efficacy and safety of edoxaban vs. other NOACs in a systemic review and network meta-analysis of all four large phase III trials in patients with non-valvular AF published in 2015. They adjusted for between-trial differences in CHADS2 score and length of follow-up by analysing annualized event rates among patients with CHADS2 score ≥2 using a mixed Poisson’s regression model. Their analysis demonstrated a significantly lower major bleeding risk for once-daily high-dose edoxaban compared with once-daily rivaroxaban (HR 0.76; 95% CI 0.66–0.89), twice-daily dabigatran 150 mg (HR 0.78; 95% CI 0.61–0.84), and twice-daily dabigatran 110 mg (HR, 0.83; 95% CI 0.71–0.98). Bleeding risk was similar for to twice-daily apixaban (HR 1.08; 95% CI 0.91–1.28). Risk of stroke and SE was similar for the high-dose edoxaban and other non-VKA oral anticoagulant regimens. The low-dose edoxaban regimen was associated with a significant lower risk of major bleeding than other non-VKA oral anticoagulants and a significant higher risk of stroke and SE compared with apixaban and dabigatran 150 mg. The low-dose edoxaban regimen was again associated with a significantly lower risk of major bleeding when compared with other NOACs. However, there was a significantly higher risk of stroke and SE compared with apixaban and dabigatran 150 mg twice daily.
A large comparison of NOACs was done by López-López et al.9 in 2017.
They included 23 randomized trials involving 94 656 patients. Thirteen compared an NOAC with warfarin dosed to achieve a target INR of 2.0–3.0. Apixaban 5 mg twice daily (HR 0.79, 95% CI 0.66–0.94), dabigatran 150 mg twice daily (HR 0.65, CI 0.52–0.81), edoxaban 60 mg once daily (HR 0.86, CI 0.74–1.01), and rivaroxaban 20 mg once daily (HR 0.88, CI 0.74–1.03) reduced the risk of stroke or SE in comparison to warfarin. The risk of stroke or SE was higher with edoxaban 60 mg once daily (HR 1.33, 95% CI 1.02–1.75) and rivaroxaban 20 mg once daily (HR 1.35, 95% CI 1.03–1.78) than with dabigatran 150 mg twice daily. The risk of all-cause mortality was lower with all NOACs than with warfarin. Apixaban 5 mg twice daily (HR 0.71, 95% CI 0.61–0.81), dabigatran 110 mg twice daily (HR 0.80, 95% CI 0.69–0.93), edoxaban 30 mg once daily (HR 0.46, 95% CI 0.40–0.54), and edoxaban 60 mg once daily (HR 0.78, 95% CI 0.69–0.90) reduced the risk of major bleeding compared with warfarin. The risk of major bleeding was higher with dabigatran 150 mg twice daily than apixaban 5 mg twice daily (HR 1.33, 95% CI 1.09–1.62), rivaroxaban 20 mg twice daily than apixaban 5 mg twice daily (HR 1.45, 95% CI 1.19–1.78), and rivaroxaban 20 mg twice daily than edoxaban 60 mg once daily (HR 1.31, 95% CI 1.07–1.59). The risk of intracranial bleeding was substantially lower for most NOACs compared with warfarin, whereas the risk of gastrointestinal bleeding was higher with some NOACs than warfarin. Apixaban 5 mg twice daily was ranked the highest for most outcomes, and was cost effective compared with warfarin.
Sub-studies of the phase III NOAC trials have shown that the beneficial effects of NOACs compared with warfarin are preserved in geriatric patients. A sub-study of the ROCKET-AF study found that more than 60% of AF patients were on five or more medications and that increasing medication use was associated with a higher risk of bleeding, but not stroke.10 Dose reductions for elderly patients have been studied for dabigatran and edoxaban.2,5 The risk of intracranial haemorrhage was lower in dabigatran-treated patients compared with warfarin-treated patients in those older than 75 years. The ENGAGE TIMI-AF 48 trial demonstrated that patients aged <75 or ≥75 years showed comparable rates of stroke/systemic embolic events (SEE) for both doses of edoxaban vs. warfarin and a lower rate of major bleeding compared with warfarin.5 For apixaban, dose reduction to 2.5 mg twice daily is recommended in patients ≥80 years old, but only in the presence of either a low body weight (≤60 kg) or serum creatinine ≥1.5 mg/dL.4 For Rivaroxaban, no dose reduction is necessary in elderly patients with normal kidney function. Therefore, the use of NOAC in the elderly might be a sufficient strategy if pharmacological aspects of the different NOACS are considered in individual patients. Impaired renal function appears to be one important factor.11–14
Non-vitamin K antagonists oral anticoagulant real-world data, non-vitamin K antagonists oral anticoagulant adherence and secondary prevention
Besides the results of phase II trials, the use of NOACs was assessed in several registries and smaller non-randomized trials representing a real-world scenario. Although the gold standard to assess the efficacy and safety of NOACs are randomized controlled trials (RCTs), real-world data (RWD) may help to gather information in subgroups of AF patients. In a 2017 published analysis, all available evidence from high-quality real-world observational studies about efficacy and safety of NOACs compared with vitamin K antagonists in AF patients were summarized.15
This large-scale meta-analysis included 28 studies of dabigatran, rivaroxaban, and apixaban compared with vitamin K antagonists. The analysed outcomes included ischaemic stroke, ischaemic stroke or SE, any stroke or SE, MI, intracranial haemorrhage, major haemorrhage, gastrointestinal haemorrhage, and death. All three NOACs studied were associated with a reduction of intracranial haemorrhage. In contrast, similar rates of ischaemic stroke and ischaemic stroke or SE were seen with NOACs compared with warfarin. Interestingly, apixaban and dabigatran were associated with lower mortality. In addition, apixaban therapy had fewer gastrointestinal bleedings and major haemorrhages. Dabigatran and rivaroxaban, however, were associated with more gastrointestinal haemorrhages. Thus, the meta-analysis confirms the main findings of the RCTs of dabigatran, rivaroxaban, and apixaban in the real-world setting.
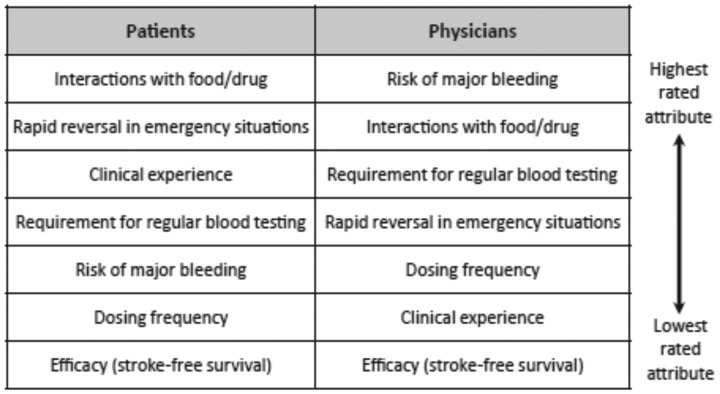
Real-world data have also shown that many physicians tend to underdose NOACs. This may be due to the fact that upon choosing NOAC substance and dosing, more emphasis is given to safety than to efficacy (Table 2).16 However, underdosing has been shown to be associated with increased stroke rates.1 Thus, RWD are helpful to learn about the current use of NOACs in routine practice.
Table 2.
Differences between patients and physicians in importance rating of factors for decision-making about non-vitamin K oral anticoagulant therapy from Ref.16
|
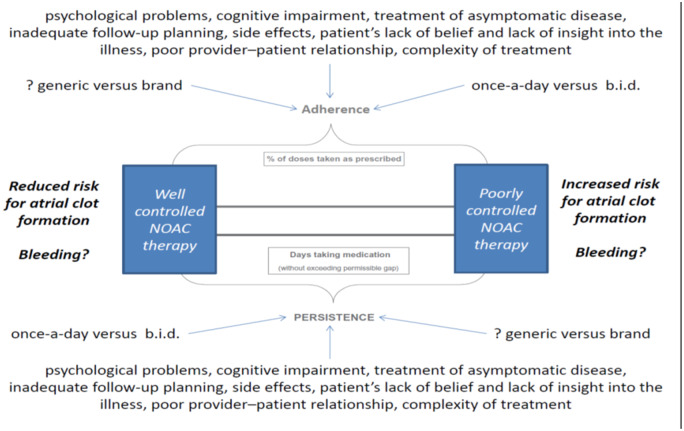
The average rates of adherence in clinical studies were reported to be 43–78% among patients receiving treatment for chronic diseases.16,17 Rates greater than 80% appear acceptable for adequate adherence. The ability of physicians to recognize non-adherence is poor if there is no specific test to measure to correct drug intake. The potential benefit of NOACs, which require no drug monitoring might therefore appear as a disadvantage with regard to the assessment of treatment adherence. Predictors of adherence and persistence to NOAC therapy as well as possible confounders are illustrated in Figure 3. The life-long use of a complex therapy like NOAC for primary prevention of stroke may have a particular trend to poor adherence if patients are not clearly educated about the benefits of therapy.17
Figure 3.
Illustration of adherence and persistence to non-vitamin K oral anticoagulants and potential confounders, which may affect the overall outcome of non-vitamin K oral anticoagulant therapy in patients with atrial fibrillation. B.i.d., twice daily.
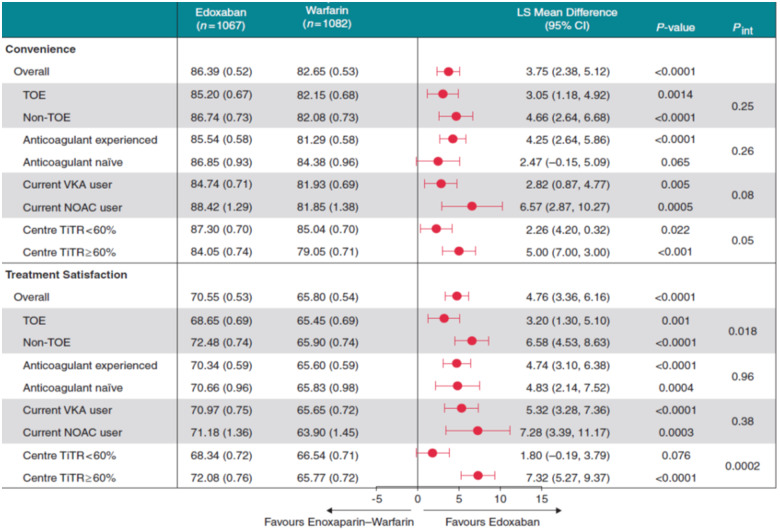
Of note, rates of non-adherence have been reported in the range of 22–58% for VKAs. Initially, there was a discussion about the impact of dosing frequency of NOAC. In several surveys, however, most AF patients consider safety (i.e. rapid reversal in emergency situations and risk of major bleeding) to be more important than dosing frequency or efficacy (Table 2). Stroke risk reduction and a slight increase in bleeding risk are important factors for an AF patient when deciding whether they are for or against NOAC therapy. Furthermore, a parameter that effect patient adherence to NOAC therapy is low consult frequency with medical specialists. The ENSURE-AF study was a multicentre prospective, randomized, open-label, blinded endpoint evaluation trial comparing edoxaban with enoxaparin/warfarin followed by warfarin alone in 2199 non-valvular AF patients undergoing electrical cardioversion (CV).18 Atrial fibrillation patients treated with edoxaban were more satisfied than enoxaparin/warfarin in both treatment satisfaction and convenience scores (Table 3). Edoxaban therapy also was associated with fewer hospital visits. Of note, NOAC therapy was estimated to reduce healthcare costs by €107.73, €437.92, €336.75, and $246.32 per patient in German, Spanish, Italian, and US settings, respectively.19
Table 3.
Treatment satisfaction of edoxaban vs. enoxaparin/warfarin in the setting of cardioversion for atrial fibrillation
|
The PACT-Q©2-Score (Perception of Anticoagulant Treatment Questionnaire 2) covers three domains (convenience, burden of disease and treatment, and anticoagulant treatment satisfaction) is used after having received the treatment.
CI, confidence interval; LS, least squares; NOAC, non-vitamin K oral anticoagulant; SE, standard error; TiTR, time in therapeutic range; TOE 0, treansoesophageal echocardiography; VKA, vitamin K antagonist.
Non-vitamin K antagonists oral anticoagulants in atrial fibrillation ablation
Catheter ablation of AF appears as a very promising technique to reduce arrhythmia episodes in patients with AF.1,20
The 2020 ESC guideline on diagnosis and management of AF has upgraded AF catheter ablation for pulmonary veins (PVI) for rhythm control after one failed or intolerant class I or III antiarrhythmic drug, to improve symptoms of AF recurrences in patients with paroxysmal and persistent AF with or with or without major risk factors for AF recurrence from Class IIa (should be considered) to Class I (recommended or indicated).1 For the decision on AF catheter ablation, the guideline gives a new and strong Class I recommendation to take into consideration the procedural risks and the major risk factors for AF recurrence following the procedure and discuss them with the patient.1 The major adverse events of such procedures are bleeding complications and stroke. Therefore, several studies were performed to assess efficacy and safety of different oral anticoagulants like warfarin (VKA) and non-VKA oral anticoagulants (NOAC). Non-vitamin K antagonists oral anticoagulant therapy has been explored in patients with chronic AF and in patients undergoing antiarrhythmic procedures.18,21,22 Overall, NOAC therapy appears non-inferior to VKA (although less intracranial bleeds) and cost effective.18 Nevertheless, due to the overall low rates of ischaemic events, no definite information is available about superiority of NOACs vs. VKA with regard to stroke and embolic events in patients undergoing electrophysiological interventions like CV and catheter ablation.18 So far, four randomized controlled clinical trials were published to assess the effect of rivaroxaban, dabigatran, and apixaban in comparison to VKA in patients undergoing catheter ablation of AF with isolation of the PVI. The VENTURE-AF study assessed the effect of an evening dose of 20 mg rivaroxaban once daily vs. VKA in patients undergoing pulmonary vein isolation.21 These studies showed that the overall safety profile of this approach is comparable to VKA. The study was not powered to assess efficacy. Of note, VENTRUE-AF showed that the periprocedural heparin dosing was significantly higher in comparison to VKA. Nevertheless, bleeding events were similar in the two groups. Importantly, all patients were pre-treated with anticoagulants for several weeks before PVI was performed. The RE-CIRCUIT studies showed that dabigatran 150 mg bid (last dabigatran dose given even hours before the procedure) was non-inferior to VKA in patients undergoing PVI.22 Of note, major bleeding events were less in comparison to VKA. Similar to the VENTURE-AF trial, the RE-CIRCUIT trial consisted of a substantial pre-treatment periods: a screening period of 0–2 weeks and a pre-ablation treatment period of 4–8 weeks, to achieve the desired stable anticoagulation range in patients receiving warfarin. The third NAOC trial was the AXAFA-AFNET 5 trial was published.23 In this trial, apixaban (last apixaban dose also given at the day of the procedure) was compared with VKA. Similar to the two other trials, a continuous sufficient anticoagulation in the VKA group was requested for 30 days prior to catheter ablation.
The most recent study, ELIMINATE AF, evaluated uninterrupted use of edoxaban 60 mg once daily in comparison to VKA.24 Six hundred and thirty-two patients were enrolled, 614 randomized, and 553 received study drug and underwent ablation. The primary endpoint (only major bleeds occurred) was observed in 0.3% (1 patient) on edoxaban and 2.0% (2 patients) on VKA (HR 0.16 95% CI 0.02–1.73). In the ablation population (modified intent-to-treat population including patients with ablation), the primary endpoint was observed in 2.7% of edoxaban (N = 10) and 1.7% of VKA patients (N = 3) between start of ablation and end of treatment.
Zhao et al.25 merged all trial data on NOACs in patients undergoing PVI in their meta-analysis. The authors identified six RCTs with a total of 1903 patients. There was no significant difference between NOACs group and VKAs group in incidence of stroke or TIA (HR 1.00; 95% CI 0.23–4.40, P = 1.00), silent cerebral thromboembolic events (HR 1.09; 95% CI 0.67–1.75, P = 0.74), or minor bleeding (HR 1.01; 95% CI 0.78–1.31, P = 0.93), which were consistent in subgroup analysis of individual NOACs vs. VKA. Non-vitamin K antagonists oral anticoagulant treatment was associated with reduced risk of major bleeding as compared with VKAs (HR 0.45; 95% CI 0.26–0.81, P < 0.01). In the subgroup analyses, only the dabigatran group showed significant lower incidence of major bleeding compared with VKA. In conclusion, uninterrupted NOAC therapy is as effective as uninterrupted VKA treatment. Uninterrupted dabigatran (150 mg twice daily) may be superior to other uninterrupted OACs strategies. Thus, the present analysis provides very reassuring data that NOAC therapy is non-inferior to VKA in patients undergoing PVI.
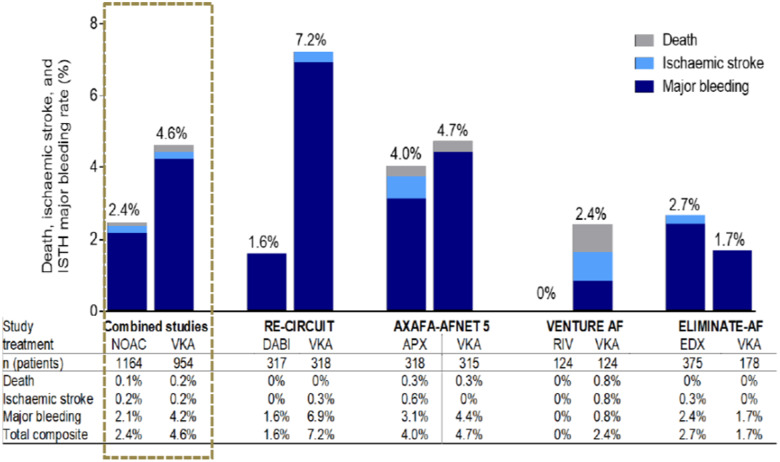
Nevertheless, some strategic questions remain. All NOAC studies focused on uninterrupted anticoagulation. Why is this strategy so important to the investigators? Stroke rates during PVI were very low in all presented trials at a rate of about 0.2%. Major bleeding rates, however, were 1.6% during NOAC therapy, whereas all bleeding rates were at 15%.26 In summary, major bleeding was eight-times more common than stroke, whereas the risk of any bleeding was 70 times higher than stroke risk during PVI. Single study and pooled study data on safety and efficacy of NOAC therapy in AF ablation are shown in Figure 4.
Figure 4.
Data on death, ischaemic stroke, and major bleeding under NOAC therapy during catheter ablation of atrial fibrillation. Pooled study data on the left. APX, apixaban; DABI, dabigatran; EDX, edoxaban; ISTH, International Society on Thrombosis and Hemostasis; NOAC, non-vitamin K oral anticoagulant; RIV, rivaroxaban; VKA, vitamin K antagonist.
Non-vitamin K antagonists oral anticoagulant therapy around cardioversion
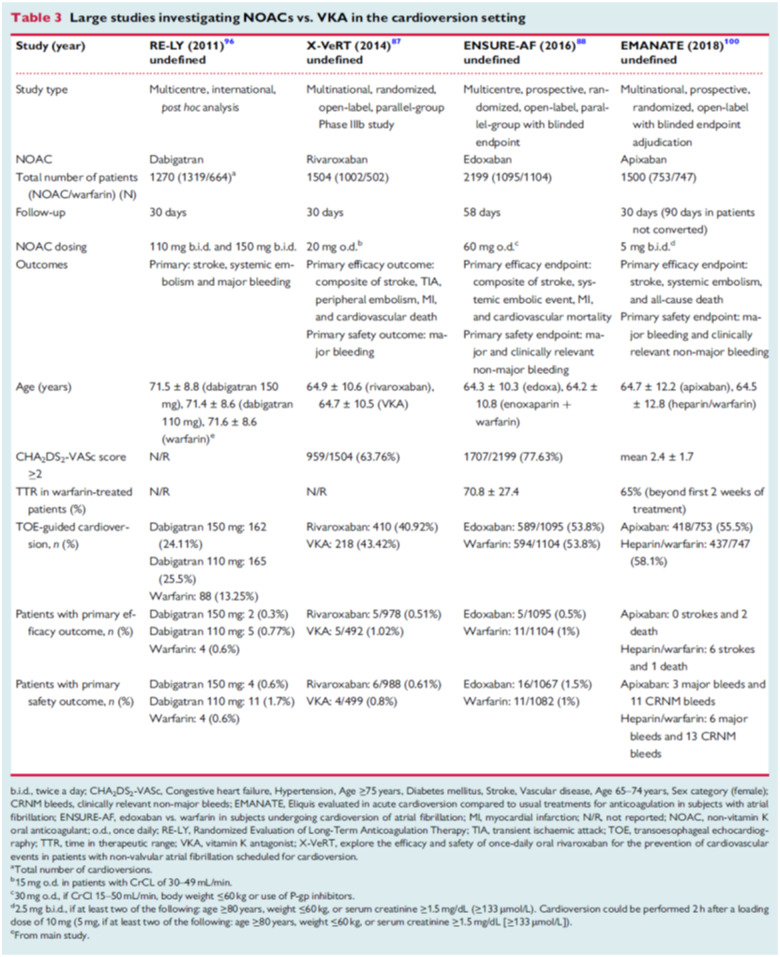
The RE-LY study was the only phase III trial that allowed inclusion of patients that were cardioverted. Therefore, only data on the direct thrombin inhibitor dabigatran were available in the setting of CV. Nagarakanti et al.27 have reported on the results of this subgroup of patients. Data from before, during, and 30 days after CV were analysed. A total of 1983 CVs were performed in 1270 patients: 647 in then dabigatran group that took 110 mg twice daily, 672 in the 150 mg twice daily group, and 664 in the warfarin group. Stroke and SE rates at 30 days were 0.8%, 0.3%, and 0.6%, respectively (no significant difference). They were similar in patients with and without transoesophageal echocardiography. Major bleeding rates were 1.7%, 0.6%, and 0.6% (dabigatran 110 mg twice daily vs. warfarin, P = 0.06).
While dabigatran was the first NOAC to provide data from the pivotal randomized controlled AF trial in the setting of CV, the direct factor Xa inhibitors were tested in specifically designed CV studies. Rivaroxaban 20 mg once daily was tested in the X-VeRT study that assigned 1504 patients for rivaroxaban (20 mg once daily, 15 mg if creatinine clearance was between 30 and 49 mL/min) or dose-adjusted VKA in a 2:1 ratio. The primary efficacy outcome was the composite of stroke, transient ischaemic attack, peripheral embolism, MI, and cardiovascular death. The primary safety outcome was major bleeding. The primary efficacy outcome occurred in 5 (two strokes) of 978 patients (0.51%) in the rivaroxaban group and in 5 (two strokes) of 492 patients (1.02%) in the VKA group. Major bleeding occurred in six patients (0.6%) in the rivaroxaban group and four patients (0.8%) in the VKA group. The largest NOAC CV study was the ENSURE-AF study for edoxaban with 2199 patients enrolled.18 It also had the longest follow-up of 58 days. Primary efficacy endpoint was a composite of stroke, SE, MI, and cardiovascular mortality (edoxaban group: 5/1095 = 0.5%; warfarin group 11/1104 = 1%). Primary safety endpoint were major and CRNMB (edoxaban group: 16/1067 = 1.5%; Warfarin group: 11/1082 = 1%).
The most recently published study was the EMANATE trial evaluating safety and efficacy of apixaban compared with VKA in the setting around CV.28 Like ENSURE-AF, it was a multicentre, prospective, randomized, parallel-group trial with blinded endpoint enrolling 1500 patients on apixaban or VKA. The apixaban dose of 5 mg b.i.d. was reduced to 2.5 mg b.i.d. in patients with two of the following: age ≥80 years, weight ≤60 kg or serum creatinine ≥133 mmol/L. To expedite CV, at the discretion of the investigator, imaging and/or a loading dose of 10 mg (down-titrated to 5 mg) was allowed. The endpoints for efficacy were stroke, SE, and death. The endpoints for safety were major bleeding and CRNMB. All three factor Xa inhibitor trials comparing NOAC with VKA therapy showed overall low rates of primary efficacy and safety endpoints for NOAC therapy around CV. Thus, NOAC use in this setting appears convenient and safe. However, the 2020 ESC AF guideline has included a new Class I recommendation that the importance of adherence and persistence to NOAC treatment both before and after CV is strongly emphasized to patients.1
Trials regarding NOAC use during CV and primary study results are summarized in Table 4.
Table 4.
Non-vitamin K oral anticoagulant during cardioversion of atrial fibrilation
|
Non-vitamin K antagonists oral anticoagulant therapy in atrial fibrillation patients after stenting
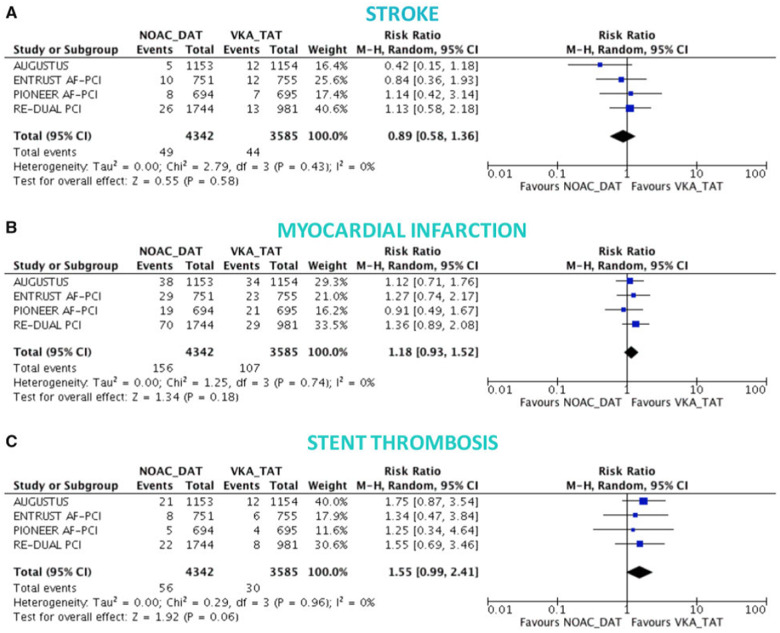
All published NOAC AF percutaneous coronary intervention (PCI) studies used safety parameters as primary endpoints (Table 2). Bleeding endpoints were typically defined as major bleeding or CRNMB.29 Secondary efficacy endpoints included all-cause death; cardiovascular death; trial-defined major adverse cardiovascular event; MI; stroke; and stent thrombosis (ST). The four NOAC AF PCI trials showed that NOAC-based dual antiplatelet therapy (DAT), compared with VKA-triple antiplatelet therapy (TAT), reduces major and CRNMB. Non-vitamin K antagonists oral anticoagulant-based DAT is associated with reduced rates of intracranial haemorrhage. The risk of MI and ST is increased in AF patients if aspirin therapy is stopped early after stenting. Thus, TAT is of importance in all AF patients after coronary artery stenting for some weeks to prevent ST.30–32 The mechanism through which early aspirin discontinuation expose AF patients to more ischaemic events remains unknown. Whether ticagrelor or prasugrel reduce the ischaemic risks in DAT warrants further investigations. Nevertheless, the results of the four AF PCI trials will influence clinical routine (Figure 5).31
Figure 5.
Ischaemic endpoints in non-vitamin K oral anticoagulant-based double antithrombotic therapy vs. vitamin k-based triple therapy from Ref.31 Random-effects risk ratios and 95% confidence intervals for stroke (A), myocardial infarction (B), and stent thrombosis. Number of events of stent thrombosis stratified by NOAC-DAT vs. VKA-TAT for AUGSTUS trial come from recent meta-analysis and likely correspond to definite or probable or possible stent thrombosis. NOAC_DAT, non-vitamin K oral anticoagulant dual antiplatelet therapy; VKA_TAT, vitamin K antagonist triple antiplatelet therapy.
The 2020 ESC guideline on diagnosis and management of AF early recommends cessation (≤1 week) of aspirin and continuation of dual therapy with an OAC and a P2Y12 inhibitor (preferably clopidogrel) for up to 12 months in AF patients with acute coronary syndrome (ACS) or chronic coronary syndrome (CCS) undergoing an uncomplicated PCI if the risk of ST is low or if concerns about bleeding risk prevail over concerns about risk of ST, irrespective of the type of stent used.1
However, a post-hoc study from AUGUSTUS suggests that aspirin should be provided up to 30 days in AF patients at high risk for ST.32 Compared with TAT, DAT has been shown to be associated with reduced major bleeding as well as intracranial haemorrhages. The benefit is somewhat counterbalanced by a higher risk of stent-related ischaemia during the early phase of DAT. Thus, TAT after stenting may be appropriate for at least 14 days with a maximum of 30 days. Thereafter, DAT including an NOAC is the therapy of choice in AF PCI patients (Figure 6).
Figure 6.
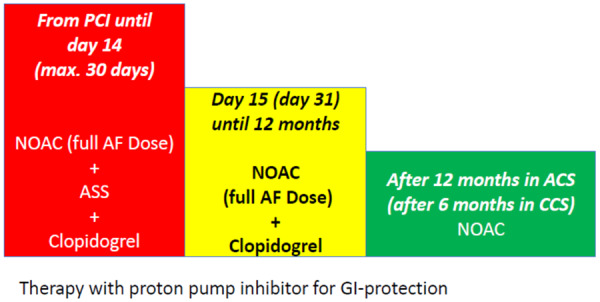
NOAC therapy after coronary artery stenting. ACS, acute coronary syndrome; ASS, acetylsalicylic acid; CCS, chronic coronary syndrome; GI, gastrointestinal tract; NOAC, non-vitamin K oral anticoagulant; PCI, percutaneous coronary intervention.
Further sub-studies are warranted to assess differences between various subgroups of AF patients like ACS and CCS.
Renal function and non-vitamin K antagonists oral anticoagulant therapy
All NOACs are to some extent excreted via the kidneys (varying from about 80% in Dabigatran to about 25% in apixaban). To date, any NOAC use is contraindicated in patients with end-stage kidney disease. It is also known that patients with end-stage renal disease do not benefit from oral anticoagulation with warfarin. Their bleeding risk is higher and stroke risk reduction is lower when compared with non-dialysis patients.33 In patients with mild to moderate CKD, dose adjustments were implemented for most NOACs in the above-mentioned phase III NOAC clinical trials. However, direct head-to-head comparisons between safety and efficacy of all currently available NOACs are missing. Andò and Capranzano34 have conducted a meticulous and important meta-analysis of five randomized clinical trials including 13 878 patients in order to evaluate and rank efficacy and safety of all available NOACs in comparison to Warfarin in CKD patient. In that study, data were analysed comparing three treatments: warfarin, low-dose NOAC (either dabigatran 110 mg or edoxaban low dose with dose reduction from 30 to 15 mg), and full/single dose NOACs (dabigatran 150 mg, rivaroxaban 20 mg, apixaban 5 mg twice daily, and edoxaban 60 mg, each with protocol defined dose reduction to 15 mg, 2.5 mg twice daily, or 30 mg, respectively). Of note, compared with warfarin, full-dose NOAC regiments were associated with significant relative reductions in both the ischaemic endpoint (21%) and major bleeding (26%). Warfarin, however, was associated with a trend towards better efficacy as compared with low-dose NOACs. A recent study assessed the use of NOAC by searching the Cochrane Kidney and Transplant Specialised Register.35 The authors included all RCTs, which directly compared the efficacy and safety of direct oral anticoagulants (direct thrombin inhibitors or factor Xa inhibitors) with dose-adjusted warfarin for preventing stroke and systemic embolic events in non-valvular AF patients with CKD [defined as creatinine clearance (CrCl) or eGFR between 15 and 60 mL/min]. The review included 12 545 AF participants with CKD from five studies. All participants were randomized to either NOAC (apixaban, dabigatran, edoxaban, and rivaroxaban) or dose-adjusted warfarin. Four studies used a central, interactive, automated response system for allocation concealment while the other did not specify concealment methods. Four studies were blinded while the other was partially open-label. However, given that all studies involved blinded evaluation of outcome events, we considered the risk of bias to be low. Funnel plots could not be generated due to the small number of studies, thwarting assessment of publication bias. Study duration ranged from 1.8 to 2.8 years. The large majority of participants included in this study were CKD stage G3 (12 155), and a small number were stage G4 (390). Of 12 545 participants from five studies, a total of 321 cases (2.56%) of the primary efficacy outcome occurred per year. Furthermore, of 12 521 participants from five studies, a total of 617 cases (4.93%) of the primary safety outcome occurred per year. Non-vitamin K antagonists oral anticoagulants appeared to probably reduce the incidence of stroke and SE events (5 studies, 12 545 participants: RR 0.81, 95% CI 0.65–1.00; moderate certainty evidence) and to slightly reduce the incidence of major bleeding events (5 studies, 12 521 participants: RR 0.79, 95% CI 0.59–1.04; low certainty evidence) in comparison with warfarin. The results show that NOAC are as likely as warfarin to prevent all strokes and systemic embolic events without increasing risk of major bleeding events among AF patients with kidney impairment. The major limitation of that study is that the results reflect CKD stage G3, only.
Further trials are underway to assess the effect of NOAC in patients on haemodialysis. In the ENSURE-AF study of anticoagulation for electrical CV in non-valvular AF patients no effect of renal (dys)function was demonstrated in comparing edoxaban to enoxaparin/warfarin for electrical CV; efficacy and safety of edoxaban remained consistent even in patients with normal or supranormal renal function. Thus, during cardiac interventions in most AF patients with moderately impaired kidney function NOAC therapy can be considered as safe.
An observational study by Andreu-Cayuelas et al.36 found an interesting and clinically relevant interaction between recent acute heart failure and renal function influencing dosing recommendation of NOAC therapy for AF. Worsening renal function would have needed dabigatran dosage reduction in 44% of patients, for rivaroxaban in 35% of patients and in 29% of patients treated with apixaban. These mandatory dose adjustments were most frequent in patients older than 75 years and those with a baseline creatinine clearance below 60 mL/min.
Obesity
A meta-analysis analysed the effect of NOAC in obese AF patients compared with warfarin. Although the data are limited in class III obese patients (body mass index ≥40 kg/m2), the efficacy and safety of apixaban or edoxaban appear to be similar to warfarin in patients with BMI 40–50 kg/m2.37 An ENSURE-AF sub-study compared endpoints body mass index <30 vs. ≥30 kg/m2 in AF patients undergoing CV. Successful CV rate was higher in the BMI <30 vs. ≥30 kg/m2 subgroup. The BMI did not significantly impact the relative efficacy and safety of edoxaban vs. enoxaparin/warfarin.38
Warfarin
Non-vitamin K antagonists oral anticoagulant therapy has many advantages over VKA therapy. Equal or better efficacy and improved safety that has been demonstrated in various patient populations and clinical settings has led to upgraded recommendation levels for NOAC therapy in the current ESC/EACTS guideline for the management of AF.1 However, there are several settings, in which VKA are still indicated as the preferred treatment option. There is for example currently no indication for any NOAC in aortic or mitral valve replacement therapy.
A dose-finding study for the treatment of patients with aortic valve replacement was also initiated (RE-ALIGN) but was prematurely stopped because of negative interim results, and hence NOACs are not currently indicated for the treatment of patients with artificial valve replacement.39 Therefore, the 2020 ESC guideline for the management of AF gives a Class III level of evidence B recommendation (‘treatment not recommended’) for prosthetic mechanical valve patients.1
Furthermore, all phase III trials have evaluated NOACs in the setting of ‘non-valvular’ AF, a term that should not be used any more according to the new 2020 ESC guideline. None was tested in AF patients with moderate to severe mitral valve stenosis. This leads to another Class III level of evidence C recommendation for NOAC treatment in patients with moderate to severe mitral stenosis.1 Non-vitamin K antagonists oral anticoagulants also have not been specifically tested in the setting of atrial flutter. Therefore, in these cases, VKA are still the treatment of choice unless further studies demonstrate efficacy and safety of NOACs.
Currently, besides the fact that end-stage renal disease is a relative contraindication for VKA therapy, many AF patients on haemodialysis are treated with VKA, because all initial large-scale NOAC studies excluded these patients and are therefore strictly contraindicated. Original data and meta-analyses, however, indicate that in this patient population also VKA therapy shows reduced efficacy concerning stroke prevention. Moreover, VKA therapy is associated with excess bleeding risk.40,41 Therefore, optimal treatment of patients with ERD remains unsure and further studies for example with very low dose or increased interval NOAC therapy are warranted.
Funding
This paper was published as part of a supplement financially supported by Bayer AG and the scientific content has not been influenced in any way by the sponsor.
Conflict of interest: Dr M.H. has received honoraria and speaker fees from Astra Zeneca, Berlin Chemie Boehringer Ingelheim, Bayer, Bristol Myers Squibb, Pfizer, and Daiichi-Sankyo. Dr A.G. has received honoraria and speaker fees from Astra Zeneca, Berlin Chemie, Boehringer Ingelheim, Bayer, Bristol Myers Squibb, Pfizer, and Daiichi-Sankyo.
References
- 1. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom-Lundqvist C, Boriani G, Castella M, Dan GA, Dilaveris PE, Fauchier L, Filippatos G, Kalman JM, La Meir M, Lane DA, Lebeau JP, Lettino M, Lip GYH, Pinto FJ, Thomas GN, Valgimigli M, Van Gelder IC, Van Putte BP, Watkins CL; ESC Scientific Document Group. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS). Eur Heart J 2020;ehaa612. [DOI] [PubMed] [Google Scholar]
- 2. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139–1151. [DOI] [PubMed] [Google Scholar]
- 3. Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, Becker RC, Nessel CC, Paolini JF, Berkowitz SD, Fox KA, Califf RM; ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011;365:883–891. [DOI] [PubMed] [Google Scholar]
- 4. Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al-Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R, Easton JD, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S, Hermosillo AG, Hohnloser SH, Horowitz J, Mohan P, Jansky P, Lewis BS, Lopez-Sendon JL, Pais P, Parkhomenko A, Verheugt FW, Zhu J, Wallentin L; ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365:981–992. [DOI] [PubMed] [Google Scholar]
- 5. Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, Waldo AL, Ezekowitz MD, Weitz JI, Spinar J, Ruzyllo W, Ruda M, Koretsune Y, Betcher J, Shi M, Grip LT, Patel SP, Patel I, Hanyok JJ, Mercuri M, Antman EM; ENGAGE AF-TIMI 48 Investigators. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013;369:2093–2104. [DOI] [PubMed] [Google Scholar]
- 6. Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, Camm AJ, Weitz JI, Lewis BS, Parkhomenko A, Yamashita T, Antman EM. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014;383:955–962. [DOI] [PubMed] [Google Scholar]
- 7. Skjøth F, Larsen TB, Rasmussen LH, Lip GY. Efficacy and safety of edoxaban in comparison with dabigatran, rivaroxaban and apixaban for stroke prevention in atrial fibrillation. An indirect comparison analysis. Thromb Haemost 2014;111:981–988. [DOI] [PubMed] [Google Scholar]
- 8. Fernandez MM, Wang J, Ye X, Kwong WJ, Sherif B, Hogue S, Sherrill B. Systematic review and network meta-analysis of the relative efficacy and safety of edoxaban versus other nonvitamin K antagonist oral anticoagulants among patients with nonvalvular atrial fibrillation and CHADS2 score 2. SAGE Open Med 2015;3:205031211561335. 2050312115613350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. López-López JA, Sterne JAC, Thom HHZ, Higgins JPT, Hingorani AD, Okoli GN, Davies PA, Bodalia PN, Bryden PA, Welton NJ, Hollingworth W, Caldwell DM, Savovic J, Dias S, Salisbury C, Eaton D, Stephens-Boal A, Sofat R. Oral anticoagulants for prevention of stroke in atrial fibrillation: systematic review, network meta-analysis, and cost effectiveness analysis. BMJ 2017;359:j5058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Piccini JP, Hellkamp AS, Washam JB, Becker RC, Breithardt G, Berkowitz SD, Halperin JL, Hankey GJ, Hacke W, Mahaffey KW, Nessel CC, Singer DE, Fox KA, Patel MR. Polypharmacy and the efficacy and safety of rivaroxaban versus warfarin in the prevention of stroke in patients with nonvalvular atrial fibrillation. Circulation 2016;133:352–360. [DOI] [PubMed] [Google Scholar]
- 11. Goette A. Atrial fibrillation and stroke risk factors induce decline in creatinine clearance: is there a specific “fibrillatory kidney disease”? Int J Cardiol 2018;253:82–83. [DOI] [PubMed] [Google Scholar]
- 12. Go AS, Fang MC, Udaltsova N, Chang Y, Pomernacki NK, Borowsky L, Singer DE; ATRIA Study Investigators. Impact of proteinuria and glomerular filtration rate on risk of thromboembolism in atrial fibrillation: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. Circulation 2009;119:1363–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Beyer-Westendorf J, Kreutz R, Posch F, Ay C. The CHA2DS2-VASc score strongly correlates with glomerular filtration rate and predicts renal function decline over time in elderly patients with atrial fibrillation and chronic kidney disease. Int J Cardiol 2018;253:71–77. [DOI] [PubMed] [Google Scholar]
- 14. Bukowska A, Lendeckel U, Krohn A, Keilhoff G, Have S. T, Neumann KH, Goette A. Atrial fibrillation down-regulates renal neutral endopeptidase expression and induces profibrotic pathways in the kidney. Europace 2008;10:1212–1217. [DOI] [PubMed] [Google Scholar]
- 15. Ntaios G, Papavasileiou V, Makaritsis K, Vemmos K, Michel P, Lip GYH. Real-world setting comparison of nonvitamin-K antagonist oral anticoagulants versus vitamin-K antagonists for stroke prevention in atrial fibrillation: a systematic review and meta-analysis. Stroke 2017;48:2494–2503. [DOI] [PubMed] [Google Scholar]
- 16. Andrade JG, Krahn AD, Skanes AC, Purdham D, Ciaccia A, Connors S. Values and preferences of physicians and patients with nonvalvular atrial fibrillation who receive oral anticoagulation therapy for stroke prevention. Can J Cardiol 2016;32:747–753. [DOI] [PubMed] [Google Scholar]
- 17. Goette A, Hammwöhner M. How important it is for therapy adherence to be once a day? Eur Heart J Suppl 2016;18:I7–I12. [Google Scholar]
- 18. Goette A, Merino JL, Ezekowitz MD, Zamoryakhin D, Melino M, Jin J, Mercuri MF, Grosso MA, Fernandez V, Al-Saady N, Pelekh N, Merkely B, Zenin S, Kushnir M, Spinar J, Batushkin V, de Groot JR, Lip GY; ENSURE-AF Investigators. Edoxaban versus enoxaparin-warfarin in patients undergoing cardioversion of atrial fibrillation (ENSURE-AF): a randomised, open-label, phase 3b trial. Lancet 2016;388:1995–2003. [DOI] [PubMed] [Google Scholar]
- 19. Goette A, Kwong WJ, Ezekowitz MD, Banach M, Hjortshoj SP, Zamoryakhin D, Lip GYH. Edoxaban therapy increases treatment satisfaction and reduces utilization of healthcare resources: an analysis from the EdoxabaN vs. warfarin in subjectS UndeRgoing cardiovErsion of atrial fibrillation (ENSURE-AF) study. Europace 2018;20:1936–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Calkins H, Hindricks G, Cappato R, Kim Y-H, Saad EB, Aguinaga L, Akar JG, Badhwar V, Brugada J, Camm J, Chen P-S, Chen S-A, Chung MK, Nielsen JC, Curtis AB, Davies DW, Day JD, d’Avila A, de Groot NMS(N), Di Biase L, Duytschaever M, Edgerton JR, Ellenbogen KA, Ellinor PT, Ernst S, Fenelon G, Gerstenfeld EP, Haines DE, Haissaguerre M, Helm RH, Hylek E, Jackman WM, Jalife J, Kalman JM, Kautzner J, Kottkamp H, Kuck KH, Kumagai K, Lee R, Lewalter T, Lindsay BD, Macle L, Mansour M, Marchlinski FE, Michaud GF, Nakagawa H, Natale A, Nattel S, Okumura K, Packer D, Pokushalov E, Reynolds MR, Sanders P, Scanavacca M, Schilling R, Tondo C, Tsao H-M, Verma A, Wilber DJ, Yamane T. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: executive summary. Heart Rhythm 2017;14:e445–e494. [DOI] [PubMed] [Google Scholar]
- 21. Cappato R, Marchlinski FE, Hohnloser SH, Naccarelli GV, Xiang J, Wilber DJ, Ma CS, Hess S, Wells DS, Juang G, Vijgen J, Hugl BJ, Balasubramaniam R, De Chillou C, Davies DW, Fields LE, Natale A; VENTURE-AF Investigators. Uninterrupted rivaroxaban vs. uninterrupted vitamin K antagonists for catheter ablation in non-valvular atrial fibrillation. Eur Heart J 2015;36:1805–1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Calkins H, Willems S, Gerstenfeld EP, Verma A, Schilling R, Hohnloser SH, Okumura K, Serota H, Nordaby M, Guiver K, Biss B, Brouwer MA, Grimaldi M; RE-CIRCUIT Investigators. Uninterrupted dabigatran versus warfarin for ablation in atrial fibrillation. N Engl J Med 2017;376:1627–1636. [DOI] [PubMed] [Google Scholar]
- 23. Kirchhof P, Haeusler KG, Blank B, De Bono J, Callans D, Elvan A, Fetsch T, Van Gelder IC, Gentlesk P, Grimaldi M, Hansen J, Hindricks G, Al-Khalidi HR, Massaro T, Mont L, Nielsen JC, Nolker G, Piccini JP, De Potter T, Scherr D, Schotten U, Themistoclakis S, Todd D, Vijgen J, Di Biase L. Apixaban in patients at risk of stroke undergoing atrial fibrillation ablation. Eur Heart J 2018;39:2942–2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hohnloser SH, Camm J, Cappato R, Diener HC, Heidbuchel H, Mont L, Morillo CA, Abozguia K, Grimaldi M, Rauer H, Reimitz PE, Smolnik R, Monninghoff C, Kautzner J. Uninterrupted edoxaban vs. vitamin K antagonists for ablation of atrial fibrillation: the ELIMINATE-AF trial. Eur Heart J 2019;40:3013–3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhao Y, Lu Y, Qin Y. A meta-analysis of randomized controlled trials of uninterrupted periprocedural anticoagulation strategy in patients undergoing atrial fibrillation catheter ablation. Int J Cardiol 2018;270:167–171. [DOI] [PubMed] [Google Scholar]
- 26. Goette A. Uninterrupted NOAC therapy in patients undergoing catheter ablation of atrial fibrillation: “Dual anticoagulant therapy” ready for primetime or systematic overtreatment? Int J Cardiol 2018;270:185–186. [DOI] [PubMed] [Google Scholar]
- 27. Nagarakanti R, Ezekowitz MD, Oldgren J, Yang S, Chernick M, Aikens TH, Flaker G, Brugada J, Kamensky G, Parekh A, Reilly PA, Yusuf S, Connolly SJ. Dabigatran versus warfarin in patients with atrial fibrillation: an analysis of patients undergoing cardioversion. Circulation 2011;123:131–136. [DOI] [PubMed] [Google Scholar]
- 28. Ezekowitz MD, Pollack CV Jr, Halperin JL, England RD, VanPelt Nguyen S, Spahr J, Sudworth M, Cater NB, Breazna A, Oldgren J, Kirchhof P. Apixaban compared to heparin/vitamin K antagonist in patients with atrial fibrillation scheduled for cardioversion: the EMANATE trial. Eur Heart J 2018;39:2959–2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Goette A, Vranckx P. Atrial fibrillation patients undergoing percutaneous coronary intervention: dual or triple antithrombotic therapy with non-vitamin K antagonist oral anticoagulants. Eur Heart J Suppl 2020;22:I22–I31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vranckx P, Valgimigli M, Eckardt L, Lewalter T, Unikas R, Marin F, Schiele F, Laeis P, Reimitz PE, Smolnik R, Zierhut W, Tijssen J, Goette A. Edoxaban in atrial fibrillation patients with percutaneous coronary intervention by acute or chronic coronary syndrome presentation: a pre-specified analysis of the ENTRUST-AF PCI trial. Eur Heart J 2020;ehaa617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gargiulo G, Goette A, Tijssen J, Eckardt L, Lewalter T, Vranckx P, Valgimigli M. Safety and efficacy outcomes of double vs. triple antithrombotic therapy in patients with atrial fibrillation following percutaneous coronary intervention: a systematic review and meta-analysis of non-vitamin K antagonist oral anticoagulant-based randomized clinical trials. Eur Heart J 2019;40:3757–3767. [DOI] [PubMed] [Google Scholar]
- 32. Lopes RD, Leonardi S, Wojdyla DM, Vora AN, Thomas L, Storey RF, Vinereanu D, Granger CB, Goodman SG, Aronson R, Windecker S, Thiele H, Valgimigli M, Mehran R, Alexander JH. Stent thrombosis in patients with atrial fibrillation undergoing coronary stenting in the AUGUSTUS trial. Circulation 2020;141:781–783. [DOI] [PubMed] [Google Scholar]
- 33. Hammwohner M, Goette A. Kidney diseases and NOAC therapy: is there a light at the end of the tunnel? Int J Cardiol 2017;236:162–163. [DOI] [PubMed] [Google Scholar]
- 34. Andò G, Capranzano P. Non-vitamin K antagonist oral anticoagulants in atrial fibrillation patients with chronic kidney disease: a systematic review and network meta-analysis. Int J Cardiol 2017;231:162–169. [DOI] [PubMed] [Google Scholar]
- 35. Kimachi M, Furukawa TA, Kimachi K, Goto Y, Fukuma S, Fukuhara S. Direct oral anticoagulants versus warfarin for preventing stroke and systemic embolic events among atrial fibrillation patients with chronic kidney disease. Cochrane Database Syst Rev 2017;11:CD011373.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Andreu-Cayuelas JM, Pastor-Pérez FJ, Puche CM, Mateo-Martínez A, García-Alberola A, Flores-Blanco PJ, Valdés M, Lip GYH, Roldán V, Manzano-Fernández S. Impact of variations in kidney function on nonvitamin K oral anticoagulant dosing in patients with atrial fibrillation and recent acute heart failure. Rev Esp Cardiol (Engl Ed) 2016;69:134–140. [DOI] [PubMed] [Google Scholar]
- 37. Wang SY, Giugliano RP. Non-vitamin K antagonist oral anticoagulant for atrial fibrillation in obese patients. Am J Cardiol 2020;127:176–183. [DOI] [PubMed] [Google Scholar]
- 38. Lip GYH, Merino JL, Banach M, de Groot JR, Maier LS, Themistoclakis S, Boriani G, Jin J, Melino M, Winters SM, Goette A. Impact of body mass index on outcomes in the edoxaban versus warfarin therapy groups in patients underwent cardioversion of atrial fibrillation (from ENSURE-AF). Am J Cardiol 2019;123:592–597. [DOI] [PubMed] [Google Scholar]
- 39. Eikelboom JW, Connolly SJ, Brueckmann M, Granger CB, Kappetein AP, Mack MJ, Blatchford J, Devenny K, Friedman J, Guiver K, Harper R, Khder Y, Lobmeyer MT, Maas H, Voigt J-U, Simoons ML, Van de Werf F; RE-ALIGN Investigators. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med 2013;369:1206–1214. [DOI] [PubMed] [Google Scholar]
- 40. Shah M, Avgil Tsadok M, Jackevicius CA, Essebag V, Eisenberg MJ, Rahme E, Humphries KH, Tu JV, Behlouli H, Guo H, Pilote L. Warfarin use and the risk for stroke and bleeding in patients with atrial fibrillation undergoing dialysis. Circulation 2014;129:1196–1203. [DOI] [PubMed] [Google Scholar]
- 41. Konigsbrugge O, Ay C. Atrial fibrillation in patients with end-stage renal disease on hemodialysis: magnitude of the problem and new approach to oral anticoagulation. Res Pract Thromb Haemost 2019;3:578–588. [DOI] [PMC free article] [PubMed] [Google Scholar]