Abstract
SARS‐CoV‐2 is the novel coronavirus behind the COVID‐19 pandemic. Since its emergence, the global scientific community has mobilized to study this virus, and an overwhelming effort to identify COVID‐19 treatments is currently ongoing for a variety of therapeutics and prophylactics. To better understand these efforts, we compiled a list of all COVID‐19 vaccines undergoing preclinical and clinical testing using the WHO and ClinicalTrials.gov database, with details surrounding trial design and location. The most advanced vaccines are discussed in more detail, with a focus on their technology, advantages and disadvantages, as well as any available recent clinical findings. We also cover some of the primary challenges, safety concerns and public responses to COVID‐19 vaccine trials, and consider what this can mean for the future. By compiling this information, we aim to facilitate a more thorough understanding of the extensive COVID‐19 clinical testing vaccine landscape as it unfolds, and better highlight some of the complexities and challenges being faced by the joint effort of the scientific community in finding a prophylactic against COVID‐19.
Keywords: antiviral agents, coronavirus, coronavirus infections, COVID‐19, COVID‐19 drug treatment, immunosuppressive agents, pandemics, SARS virus, severe acute respiratory syndrome coronavirus 2, vaccines
1. INTRODUCTION
At the end of 2019, a pneumonia of unknown aetiology emerged in Wuhan, China. Most patients presented to hospital with acute respiratory failure, and some developed severe complications. 1 The pathogen behind this disease (COVID‐19, coronavirus disease 2019) was later identified as the novel coronavirus SARS‐CoV‐2 (severe acute respiratory syndrome coronavirus 2). 2 SARS‐CoV‐2 transmits primarily via air‐borne respiratory droplets, and its spread is exacerbated by the prevalence of asymptomatic carriers. 3 On 11 March 2020, the World Health Organization (WHO) declared COVID‐19 a pandemic, and as of 25 November 2020, 59 481 313 cases have been confirmed across 220 countries, with 1 404 542 confirmed deaths. 4
SARS‐CoV‐2 is a zoonotic member of the positive‐sense single‐stranded RNA (+ssRNA) coronavirus (CoV) family. Named for their crown‐like surface spike glycoprotein, these viruses, which are capable of cross‐species transmission, are genetically sub‐divided into 4 genera; αCoV, βCoV, γCoV, and δCoV. 5 Of the 7 zoonotic coronaviruses, 4 lead only to mild respiratory infection (accounting for 30% of common cold cases) 6 while the other 3 (SARS‐CoV, MERS‐CoV [MERS, Middle Eastern respiratory syndrome] and SARS‐CoV‐2 [COVID‐19]) have claimed international notoriety in the last 2 decades for their severe, sometimes fatal pathogenicity.
Although most COVID‐19 cases are asymptomatic or accompanied only by mild, flu‐like symptoms, certain populations—primarily in the elderly and individuals with underlying medical conditions—can develop acute lung injury and acute respiratory distress syndrome. 7 Severely ill patients suffer not from viral cytopathogenicity, but as a result of cytokine storms, indicated by persistently high fevers, hypoxia and pneumonia, despite declining viral titres. 8 As COVID‐19 cases continue to rise and treatments limited to symptom management and supportive care, there is an undeniable need for a prophylactic solution capable of preventing or minimizing the severity of SARS‐CoV‐2 9 (Figure 1).
FIGURE 1.
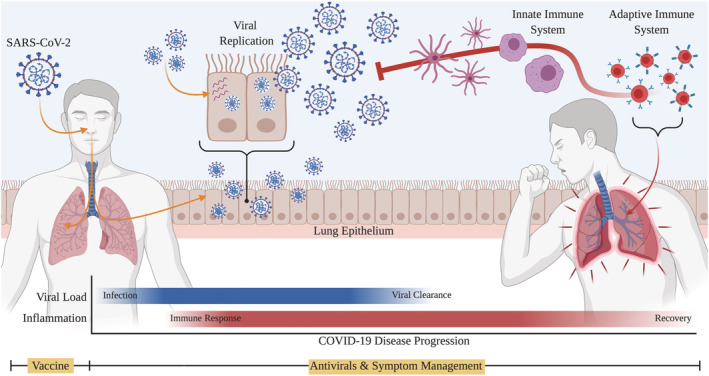
COVID‐19 treatment strategies are dictated by disease progression. While antivirals, steroids, plasma therapy, and other drugs exist for the management of symptoms after infection, vaccines are only effective if administered prophylactically
In this review, we introduce the accelerated vaccine development pipeline, and consolidate the current state of COVID‐19 vaccine trials with a focus on the most promising candidates. We discuss the advantages and disadvantages of different vaccine platforms. Finally, we address some of the challenges precipitated by the pandemic and what we can learn from them.
2. UNDERSTANDING THE COVID‐19 VACCINE DEVELOPMENT LANDSCAPE
Vaccine development is not a straightforward task; of the roughly 30 major human viral pathogens, vaccines have been approved for only 17. 10 , 11 Viral biology, including genetic diversity, mutagenic rate, incubation period, and immunogenicity, can impede a vaccine's ability to produce neutralizing antibodies. 12 , 13 Fortunately, SARS‐CoV‐2 is a good vaccine candidate, as most COVID‐19 patients develop antibodies targeting the receptor binding domain (RBD) of the viral spike protein following infection, which are capable of neutralizing SARS‐CoV‐2. 14 , 15 Therefore, all COVID‐19 vaccines have been designed to target the viral spike RBD.
Immunization begins with the innate immune system, which recognizes viral surface proteins via pattern recognition Toll‐like receptors (TLRs) on dendritic cells, macrophages, fibroblasts, and endothelial cells. 16 Once activated, these cells endocytose and disassemble the pathogen into smaller fragments (antigens), 17 presenting them to T‐cells and B‐cells. 16 Antibodies raised against these antigens are released into circulation, where they both bind and neutralize the target pathogen and enhance viral clearance by phagocytic cells.
Vaccine safety and efficacy is often inversely correlated; vaccines that resemble their viral target can more effectively activate the immune system to produce antibodies, but are also more likely to retain some level of viral cytopathogenicity. 11 To enhance the efficacy of vaccines, nonviral components termed adjuvants are often incorporated. Adjuvants can consist of aluminium salts, bacterial lipids or nucleic acids, and work by binding TLRs to trigger a robust immune response. 18 Therefore, the majority of mild, self‐resolving side effects associated with vaccination, including local inflammation, fever and nausea, are a natural consequence of immunization. In rare instances, certain adjuvant formulations can over‐activate the immune system, leading to severe adverse effects. For example, build‐up of aluminium‐based adjuvants in the tissue may trigger allergy‐like symptoms (by activiatingTh2 and eosinophils), or chronic inflammation. 19 It is therefore important to identify the right vaccine formulation via preclinical and clinical trials.
Many mild adverse effects are considered acceptable risks to vaccination, but severe reactions that enhance pathogen cytopathogenicity, like antibody‐dependent enhancement and vaccine‐associated enhanced respiratory disease (VAERD), must be avoided. Antibody‐dependent enhancement occurs when a vaccine generates antibodies that bind but fail to neutralize its target pathogen. Upon postvaccination infection, these non‐neutralizing antibodies end up facilitating targeted infection of phagocytic immune cells, which respond by producing excessive amounts of proinflammatory cytokines, leading to severe inflammation. 20 VAERD was first clinically observed during a vaccine trial for respiratory syncytial virus (RSV), where vaccinated children developed an accumulation of neutrophils and lymphocytes in the lung upon RSV infection, and were therefore more likely to be hospitalized compared to unvaccinated children. 21 While not mechanistically understood, VAERD correlates with inadequate neutralizing antibody production, inefficient cytotoxic T‐cell activation, and inappropriate activation of Th2 and eosinophils in the lung. Since VAERD is primarily seen in infants and young children, it is commonly attributed to low vaccine efficacy and immune system immaturity. 22 Clinical trials must be carefully conducted to avoid these possible effects.
Traditionally, a vaccine may take at least 10 years from conception to implementation. Candidate vaccines are first tested preclinically with in vitro and animal models, to determine immunogenicity and toxicity. Then, they must complete 3 phases of clinical testing before being licensed: Phase I, which confirms vaccine immunogenicity; Phase II, which identifies appropriate dosing and immunization schedules; and Phase III, which tests for efficacy and safety in a larger pool of participants. To catch both short‐term and long‐term effects, each phase can last multiple years, and further pharmacovigilance (Phase IV) is conducted even after a vaccine enters the market. However, when the need for a vaccine is immediate, this process is accelerated by conducting different phases in parallel and shortening the length of each phase. 23 In response to COVID‐19, the American Food and Drug Association (FDA) released acceptable trial design guidelines for accelerated vaccine development, 24 making it possible for promising vaccine candidates to apply for Emergency Use Authorization (EUA). 25 Unlike full licensing, completion of Phase III testing is not required for EUA application, and instead the FDA can actively adjust vaccine implementation strategies in response to supplemental Phase III/IV results. A vaccine is considered successful if it has sufficient interim Phase III safety and efficacy data to show that it reduces the likelihood of developing COVID‐19 by ≥50% and “the known and potential benefits of the vaccine outweigh the known and potential risks” 25 (comparatively, the efficacy of the seasonal flu vaccine in the USA fluctuated between 19 and 60% between 2009 and 2019 26 ). As it is both dangerous and unethical to inoculate trial participants with SARS‐CoV‐2, vaccine efficacy is instead determined by comparing the number of COVID‐19 cases in placebo‐treated participants against that of vaccinated participants.
To consolidate the COVID‐19 vaccine landscape, we extracted the list of preclinical and clinical vaccine trials from both the WHO database as well as ClinicalTrials.gov (Table S1) and noted the participant pool's size, age, and geographic region (Figure S1). In total, we identified 221 candidate vaccines being developed or tested for SARS‐CoV‐2 (Figure 2), and those in the most advanced stages of human trials are discussed in further detail (Figure 3). These vaccines can be broadly classified into 4 groups based on their components: traditionally inactivated or weakened viruses; viral vectors; nucleic acids; and viral proteins. In addition, we introduce some current efforts to repurpose existing vaccines for COVID‐19.
FIGURE 2.
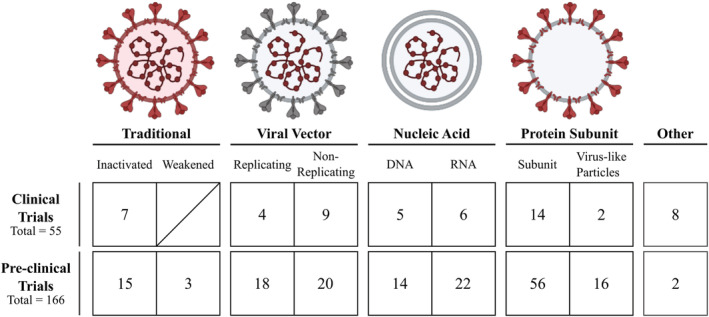
COVID‐19 vaccines currently under development. A total of 221 vaccines are currently in active development, with 55 in clinical trials and the other 166 in preclinical evaluation. They are displayed here grouped by vaccine type. In the schematic for each vaccine design, any SARS‐CoV‐2 material present in the vaccine is shown in red to better highlight the similarities and differences of each vaccination approach
FIGURE 3.
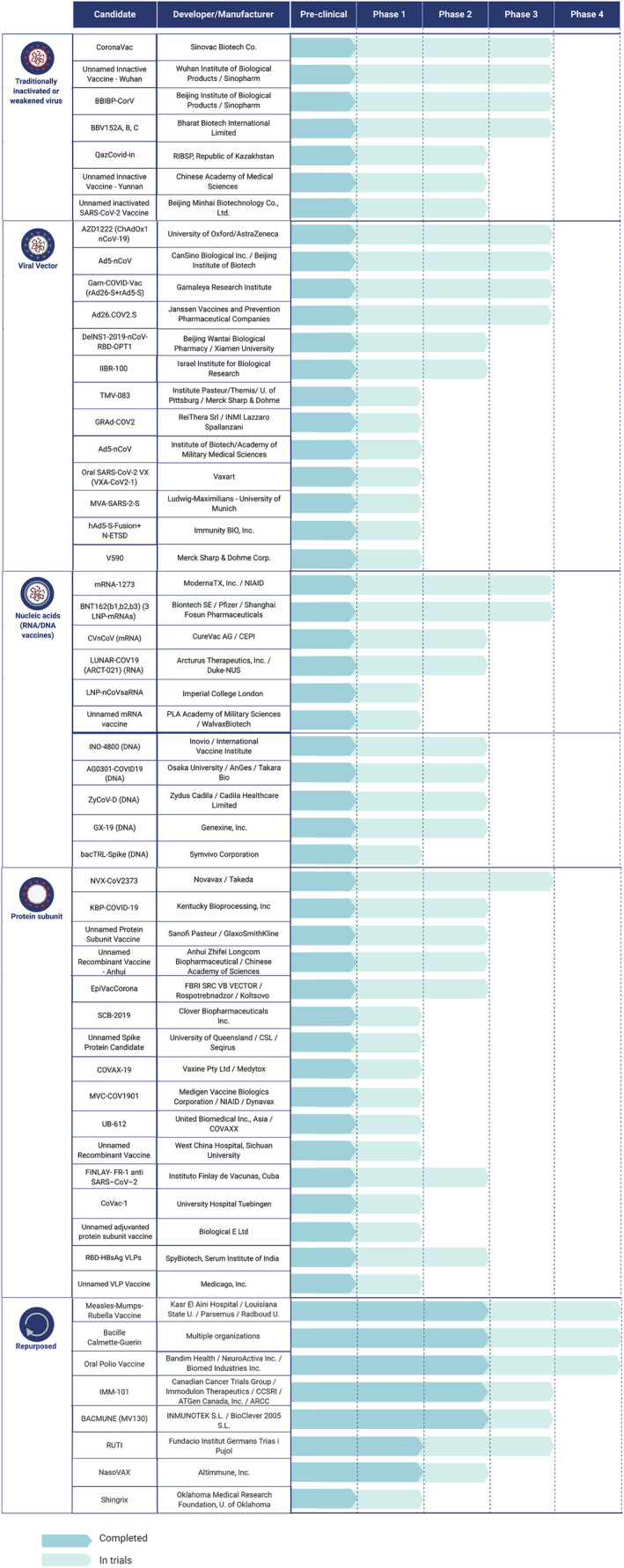
The current landscape of clinical trials for the 50 most advanced vaccines for COVID‐19. Of the 2021 vaccines currently being studied, 166 are in preclinical stages and 55 are in ongoing human trials. Showcased in this figure are the 55 most promising vaccines in different clinical stages under 5 different categories, based on the technology used for vaccine development. Shown in light blue are the current ongoing trials and shown in darker blue are the completed preclinical or clinical trials
2.1. Live attenuated or weakened virus vaccines
Traditionally, vaccines licensed for human use consisted of viruses that were inactivated or weakened via mutagenesis or neutralizing chemical treatments (e.g. formaldehyde, β‐propiolactone). 27 , 28 , 29 Since these vaccines physically resemble live virus, they can elicit strong, protective immune responses. However, care must be taken to fully eliminate viral cytopathogenicity before use.
To manufacture inactivated viral vaccines en masse, large amounts of infectious material is needed. Live viral samples are usually collected from multiple patients and replicated in Vero cells, an African green monkey cell line that is susceptible to infection. 30 The strain with the best proliferative ability and lowest mutagenesis rate is then isolated, further proliferated, and inactivated to generate the vaccine. There are currently 7 inactivated SARS‐CoV‐2 vaccines undergoing advanced clinical trials (Figure 3).
2.1.1. CoronaVac (previously PiCoVacc)
Beijing‐based pharmaceutical company Sinovac Biotech's CoronaVac vaccine combines a SARS‐CoV‐2 sample sourced from a Chinese hospitalized patient with aluminium adjuvant. 31 In initial animal studies, CoronaVac was found to induce the production of neutralizing antibodies against SARS‐CoV‐2 in mice and rats. 31 Triple 3‐ or 6‐μg doses of the vaccine also protected against subsequent viral challenge in rhesus macaques, a nonhuman primate capable of developing age‐dependent pneumonia in response to SARS‐CoV‐2. 31 , 32 No immunopathological exacerbations were observed. In Phase I/II trials, results showed that 2 3‐μg doses administered either 14 or 28 days apart resulted in seroconversion in 92.4 and 97.4% of the participants when measured 2 and 3 weeks later, respectively. 33 In addition, neutralizing antibody titres decreased with participant age (18–59 y), and both doses were well tolerated. 33 A Phase III clinical trial enrolling frontline healthcare professionals launched in Brazil on 2 July 2020 (PROFISCOV, ClinicalTrials.gov NCT04456595) followed by trials in Indonesia (NCT04508075) and Turkey (NCT04582344). In China, CoronaVac is already approved for emergency use by essential workers and high risk individuals, 34 and Phase I/II trials are underway to determine its safety and immunogenicity in both children over 3 years and adults over 60 years (ClinicalTrials.gov NCT04551547, NCT04383574, NCT04617483).
2.1.2. Unnamed inactive vaccine—Wuhan
The Chinese pharmaceutical company Sinopharm is developing an inactivated vaccine with the Wuhan Institute of Biological Products. Currently unnamed, this vaccine uses a SARS‐CoV‐2 strain isolated from a patient at Wuhan's Jinyintan Hospital, and is enhanced with aluminium adjuvant. 35 It was also the first inactivated vaccine to initiate Phase III testing.
Phase I trials in which 72 participants (18–59 y) received either triple doses of 2.5 μg, 5 μg, or 10 μg vaccine reported 97% seroconversion 2 weeks after the final injection. 35 Interim analysis from a Phase II trial conducted in parallel showed that 2 doses of 5 μg administered across 14 or 21 days achieved similar immunogenicity outcomes (41 out of 42 vaccinated participants, 97.6%). 35 Both trials reported a low rate of mild adverse reactions, with the most common being pain at the site of injection and fever. 35 In June, Phase III trials began with 15 000 participants in the United Arab Emirates (UAE; Chinese Clinical Trial Registry, ChiCTR2000034780), and has since expanded to Jordan, Bahrain (ClinicalTrials.gov, NCT04510207), and Morocco (ChiCTR2000039000) for an estimated total of 45 600 participants. On 14 September 2020, the UAE approved the use of this vaccine as an emergency prophylaxis for healthcare workers. 36
2.1.3. BBIBP‐CorV
Sinopharm is also working with the Beijing Institute of Biological Products to create a second inactivated vaccine with aluminium adjuvant, BBiBP‐CorV. 37 In preclinical trials, 2 2‐μg doses induced sufficient levels of neutralizing antibody in 6 mammalian species, including rhesus macaques, and protected against subsequent SARS‐CoV‐2 challenge. 37 A total of 192 participants in Phase I trials received 2 doses of 2, 4 or 8 μg vaccine, which were well‐tolerated and triggered only dosage‐dependent mild adverse reactions. 38 While all participants had neutralizing antibodies by 28 days, larger doses resulted in earlier seroconversion, and the vaccine's immunogenicity did not drop with participant age. 38 Subsequent Phase II trials tested either a single 8‐μg dose or 2 4‐μg doses administered either 14 days or 28 days apart. Those who received 2 doses 28 days apart had the highest antibody titres after 3 weeks compared to the other treatment groups. 38 Phase III trials are underway in the UAE, Jordan, Egypt, and Bahrain alongside the unnamed Wuhan vaccine mentioned above (ClincalTrials.gov NCT04510207), and a separate Phase III trial for 3000 participants began 23 September in Argentina (NCT04560881).
2.2. Replicating or nonreplicating viral vector vaccines
While live attenuated vaccines are made with intact viruses, viral vector vaccines consist of an replication‐deficient or attenuated replication‐competent human or chimpanzee adenovirus containing the DNA of the target pathogen's surface protein. 27 In the case of COVID‐19, the spike protein gene is the only component of SARS‐CoV‐2 incorporated into these vaccines. While only a few viral‐vectored vaccines have been approved for viral infections, this platform has been extensively investigated for cancer treatment. Unlike inactivated vaccines, viral vector vaccines have lower cytotoxicity risk (as even nonattenuated adenoviruses only cause mild cold or flu) but are still recognized as viruses by TLRs. 39 However, certain populations, especially in developing countries, may already be immune to these viruses. For example, natural seroprevalence for the commonly used human adenovirus 5 (Ad5) and chimpanzee adenovirus (ChAd), is between 60 and 90%, 40 and 1 and 19% 41 , 42 respectively, with consistently higher rates in Asian and African populations. For these reasons, the efficacy of viral vector vaccines may vary between different ethnic groups.
2.2.1. ChAdOx1 nCoV‐19 (AZD‐1222)
Developed by Oxford University and AstraZeneca, ChAdOx1 nCoV‐19 is made by fusing the SARS‐CoV‐2 spike gene to the replication defective chimpanzee adenovirus ChAdOx1. 43 This design is based on recent Phase I results with ChAdOx1‐based vaccines for tuberculosis (TB) and MERS. 44 , 45 A single dose generated neutralizing antibodies in mice and rhesus macaques that reduced disease severity and viral load in the lower respiratory tract after subsequent SARS‐CoV‐2 challenge, but did not prevent infection. 46
Phase I/II trials were conducted with 2 doses of 5 × 1010 viral particles across 28 days. 91% of participants seroconverted after the first dose, and all participants had neutralizing antibodies after the second. 47 The vaccine was well‐tolerated. 47 Following these findings, Phase III trials were approved in the USA in August (ClinicalTrials.gov NCT04516746) for 30 000 participants. Phase II/III trials are also being held in the UK (NCT04400838), South America (NCT04516746, NCT04536051), Japan (NCT04568031), and South Africa (NCT04444674), where the vaccine's efficacy will be tested in human immunodeficiency virus‐positive participants.
On 6 September, all trials were paused after a participant allegedly developed transverse myelitis, 48 , 49 a rare but severe neurological condition in which inflammation along the spinal cord results in pain, muscle weakness and paralysis. Transverse myelitis has been linked to vaccines for hepatitis B, measles, and diphtheria in rare instances, 50 and 1 case was reported in 2009 in response to the H1N1 vaccine. 51 While news of the initial hold was seen as evidence of scientifically rigorous testing, 48 AstraZeneca's decision to restart trials in the UK on 14 September without disclosing results from the safety investigation triggered calls for transparency and fuelled growing suspicions over vaccine safety in the USA and UK. 52 , 53 After reviewing all safety data, the FDA re‐authorized ChAdOx1 nCov‐19 trials in the USA on 23 October, marking the complete resumption of all AstraZeneca trials globally. 54
On 23 November, AstraZeneca announced Phase III interim analysis results, in which the vaccine was reported to be 90% effective when administered in 2 doses of 2.5 × 1010 and 5 × 1010 viral particles at least 1 month apart, and 62% efficacy when administered as 2 doses of 5 × 1010 viral particles at least 1 month apart. 55 Of the 131 COVID‐19 cases identified, vaccinated participants with COVID‐19 did not experience severe symptoms or require hospitalization. The report also claimed that no vaccine‐triggered severe adverse events have been reported. As with most vaccines, ChAdOx1 nCoV‐19 can be stored and transported at regular refrigeration temperatures (2–8°C) and last at least 6 months. 55
2.2.2. Ad5‐nCoV
The Chinese vaccine company CanSino Biologics is developing a viral vector vaccine using a replication‐defective form of the human adenovirus Ad5 and the SARS‐CoV‐2 spike gene. During preclinical trials, Ad5‐nCov sufficiently protected ferrets and mice from SARS‐CoV‐2 challenge. 56 In subsequent Phase I trials, the vaccine was tested at 3 different doses (5 × 1010, 1 × 1011 and 1.5 × 1011 viral particles), and dose‐dependent titres of neutralizing antibodies were detected by day 14. 57 Since those who received the highest dose experienced higher reactogenicity including reports of severe fever, fatigue, dyspnoea, muscle pain and joint pain, 57 Phase II trials focused on the 2 lower doses, and confirmed that both could induce seroconversion and were well‐tolerated. 58 This vaccine is currently undergoing Phase II testing in children ≥6 years and adults ≥56 years in China (NCT04566770), and Phase III trials in Russia (NCT04540419) and Pakistan (NCT04526990).
2.2.3. Ad26.COV2.S
Johnson & Johnson is developing the viral vector vaccine Ad26.COV2.S using Ad26, a human adenovirus that has lower natural seroprevalence rates than Ad5 (43–68% in African populations). 41 , 59 The vaccine effectively protected rhesus macaques and hamsters from SARS‐CoV‐2 challenge in preclinical trials. 60 , 61 Phase I/II trials identified single doses of 5 × 1010 or 1 × 1011 viral particles sufficient to trigger neutralizing antibodies in 92% of participants a month after vaccination. 62 Two Phase III trials are being conducted; the first, which began on 10August, is testing single doses of 1 × 1011 viral particles on 60 000 participants within the USA, South America, Southeast Asia, South Africa and parts of Europe (ENSEMBLE, ClinicalTrials.gov NCT04505722), while a second is testing a 2‐dose regimen in the UK, France, the USA and South Africa (ENSEMBLE2, NCT04614948).
On 12 October, Johnson & Johnson placed a temporary pause on all clinical trials in response to an unexplained illness in a study participant. This pause was lifted on 23 October with FDA approval, after an independent investigation found no connection between it and the vaccine. 63 , 64 At time of writing, no details have been released regarding the nature of the illness.
2.2.4. Gam‐COVID‐Vac (sputnik V)
Gam‐COVID‐Vac is a COVID‐19 vaccine being developed by Russia's Gamaleya Research Institute of Epidemiology and Microbiology. Unlike other viral vector vaccines, Gam‐COVID‐Vac is a heterologous formulation consisting of 2 human adenovirus vectors, Ad26 and Ad5, both carrying the SARS‐CoV‐2 spike gene. Phase I/II trials from June found that a single dose of 1 × 1011 viral particles triggered seroconversion in all 76 participants by 42 days postinjection. 65 Despite doubt from the scientific community, the vaccine gained regulatory approval in Russia on 11 August. 66 In September, Phase III trials began in Russia with 40 000 participants (RESIST, ClinicalTrials.gov NCT04530396) in which the vaccine was administered in 2 doses 21 days apart, and on October 27th, Gam‐COVID‐Vac became the first COVID‐19 vaccine submitted to the WHO for approval. 67 In an interim report published on 22 November, Gamaleya claimed its vaccine was 92% effective after the first dose based on 20 COVID‐19 cases across 16 000 participants. 68 By 24 November, updated reports describe the vaccine as being 91.% and 95% effective at 7 and 21 days respectively, and after both doses have been administered, based on a total of 39 confirmed COVID‐19 cases. 69 Both reports note the absence of unexpected adverse effects, and Gamaleya intends to release a third interim analysis once 78 COVID‐19 cases have been recorded. 68 , 69 These results have yet to be formally published.
2.3. Nucleic‐acid vaccines (DNA/RNA)
Gene‐based approaches to vaccine development represent a new way of approaching immunization. 70 By directly introducing fragments of viral genomic material in the form of DNA or RNA into host cells, these vaccines provide cells with the information necessary to express and present viral antigens to the immune system. Since only the viral genetic sequence is required, nucleic acid vaccines are cheap, safe, and relatively easy to design and manufacture. However, this strategy comes with its own set of unique challenges. Although the structure of DNA contributes to its stability, RNA is chemically reactive and therefore prone to self‐cleavage and degradation by ribonucleases, and while these vaccines are stabilized inside lipid nanoparticles, 70 RNA vaccines remain much more vulnerable compared to vaccines containing DNA, protein or whole viral particles. 71 For this reason, the temperature and storage conditions of RNA vaccines needs to be determined empirically, and more regulation will probably be necessary for distribution and administration. DNA vaccines are also uniquely complicated; unlike other vaccines, DNA vaccines must enter the cell nucleus to drive antigen expression. Since the nuclear membrane is less porous than the plasma membrane, these vaccines must be administered with a gene gun, 27 which also must be tested to ensure efficacy and safety.
While recombinant plasmid DNA has been explored as a vaccine platform for decades, the possibility of using RNA for human vaccine development is rather new. 71 , 72 , 73 To date, only a few RNA‐based treatments are in use, including the small interfering RNA (siRNA) therapies Givlaari (givosiran) for acute hepatic porphyria 74 and Onpattro (patisiran) for polyneuropathy of hereditary TTR‐mediated amyloidosis. 75 While nucleic acid vaccines hold promise, the relative lack of existing DNA/RNA prophylactics mean that relatively less is known about its safety profile, reliability, and feasibility compared to other vaccine platforms.
2.3.1. BNT162b2 vaccine
BioNTech, a German pharmaceutical company, is currently pursuing the development of its nucleoside‐modified mRNA vaccine BNT162b2 in partnership with Pfizer. BNT162b2 consists of the full‐length SARS‐CoV‐2 spike protein mRNA encased in a lipid nanoparticle. In Phase I, this vaccine triggered neutralizing antibodies titres 1.8–2.8 times higher than titres seen in recovered COVID‐19 patients. 76 In addition, both Phase I and II trials revealed only mild to moderate side effects, including fever, chills and fatigue, and identified a 2‐dose regimen across 21 days as the most appropriate for subsequent trials. 77 Phase III trials began in Germany in May (ClinicalTrials.gov NCT04537949) followed by additional Phase II/III trials in the USA, parts of South America and South Africa (NCT04365728). In August, Phase I trials were approved in China (NCT04523571). On 12 October, Pfizer announced that Phase III trials in the USA were being expanded to include children as young as 12 years, making it the first US/European‐based COVID‐19 vaccine tested in individuals younger than 18 years. 78 Although COVID‐19 hospitalization and mortality rates are lowest in this age group, 10.7% of US COVID‐19 cases occur in children, making them significant sources of viral spread. 79
On 9 November, Pfizer announced initial Phase III interim reports describing its vaccine as having up to 90% efficacy against SARS‐CoV‐2. 80 By 18 November, the company officially concluded its Phase III trial 81 and reported its vaccine as being 95% effective. A total of 170 COVID‐19 cases were identified in 43 661 participants tested 7 days after completing the treatment or placebo. Of these, 162 cases belonged to the placebo group, and 8 from vaccinated participants. Furthermore, of the 10 severe COVID‐19 cases observed, 9 occurred in the placebo group. 81 The report also stated a lack of severe safety concerns across its ethnically diverse participant pool, and consistent vaccine efficacy across sex and race demographics. The vaccine was similarly effective in participants ranging from 18 to 85 years. 81 As BNT162b2 was empirically determined to be most stable at −70°C, 81 the company is currently working to establish reliable cold‐chain distribution and storage methods. This report does not discuss the vaccine's efficacy in individuals with comorbidities. As of time of writing, Pfizer has applied for EUA with the FDA and is preparing to manufacture 50 million doses of this vaccine by the end of 2020. 81
2.3.2. mRNA‐1273
American biotech company Moderna is home to mRNA‐1273, which consists of the SARS‐CoV‐2 spike protein's mRNA encapsulated in a lipid nanoparticle. 82 , 83 Designed only 45 days after the SARS‐CoV‐2 genome was sequenced, mRNA‐1273 elicited neutralizing antibodies in a dose‐dependent fashion within 2 weeks of vaccination in Phase I trials. 83 Three immunization regiments were tested: 2 injected doses of 25 or 100 μg, and a single 250‐μg dose. While no serious vaccine‐related adverse events were reported, ≥50% of participants experienced mild events including fatigue, chills, headache, myalgia and pain at the injection site, and the single concentrated dose correlated with more moderate adverse events. Subsequent Phase II testing determined that 2 doses of 100 μg was most appropriate (ClinicalTrials.gov NCT04405076) and Phase III trials, which began recruiting on 14 July across the USA, prioritized high‐risk individuals including healthcare workers and those with underlying medical conditions (COVE, NCT04470427).
On 16 November, and with 30 000 participants enrolled, Moderna's mRNA vaccine became the second of its kind to release interim Phase III analysis. 84 In total, 95 COVID‐19 cases were identified in participants tested 2 weeks after the second injection. Of these, 90 came from the placebo group, and 5 from the vaccinated group. Importantly, 11 cases were described as being severe, all of which came from the placebo group. Based on these results, Moderna concluded its vaccine to be 94.5% effective. Up to now, there have been no reports of severe adverse effects, and no safety concerns have been raised. 84 This report also emphasized the ethnic and medical diversity of the participants tested, of which 37% belonged to communities of colour, and 42% were high‐risk due to existing comorbidities. 84 The vaccine appears to be stable for up to a month in a standard refrigerator (2–8°C), and can be stored for up to 6 months at standard freezer temperatures (−25 to −15°C), 85 making it less challenging to administer compared to Pfizer's mRNA vaccine. The trial intends to be maintained until at least 151 COVID‐19 cases are identified, and participants will be followed up with continued testing until 2 months post‐treatment.
2.3.3. DNA‐based vaccines
Of the 4 DNA‐based COVID‐19 vaccines in clinical trials, none have advanced to Phase III trials (Figure 3) and little is known about their efficacy. For example, INO‐4800, which contains the spike protein gene, is currently being developed by US biotechnology company Inovio Pharmaceuticals. Preclinical results in mice and guinea pigs examined the vaccine's immunogenicity. 86 As a proof of concept, a separate group independently created a DNA vaccine with the same genetic material, and reported that it could elicit neutralizing antibody production in rhesus macaque which protected against (but did not prevent) subsequent SARS‐CoV‐2 challenge. 87 Interim Phase I results reported by the company stated neutralizing antibodies were induced in 94% of participants (40 total), with no significant safety concerns. 88 On 16 November, Phase II/III trials were announced to further test the safety and efficacy of both INO‐4800 as well as the gene gun being used to administer this vaccine directly through the skin, but as of writing, recruitment has not yet begun. 89
2.4. Protein‐based: Protein subunits or virus‐like particles
In direct contrast to other vaccine approaches, protein‐based vaccines contain only viral proteins without any genomic material, and therefore run no risk of becoming infectious. 90 These vaccines are made either with protein subunits (fragments of viral surface protein) or virus‐like particles (nanostructures that resemble the outer viral envelope).
Protein subunit vaccines contain recombinant viral surface proteins packaged in a way that mimics the viral target. For these vaccines, the protein is made and purified from nonmammalian systems using laboratory methods and assembled in vitro. While this design permits more control over the shape and density of the viral proteins presented to the immune system, these vaccines are also more sensitive to improper epitope conformations. 91 , 92 Previously, protein‐based vaccines containing the spike protein RBD from both SARS‐CoV‐1 and MERS‐CoV showed promising seroconversion of animal models. Currently, there are 14 COVID‐19 subunit vaccines in clinical trials (Figure 3), and 54 other candidates under preclinical development, making this the most commonly studied vaccine platform for COVID‐19.
While also assembled in vitro, virus‐like particles (VLPs) differ in that they consist of multiple different viral structural proteins. In the case of SARS‐CoV‐2, VLPs contain the spike protein coassembled with the M, E and sometimes N coronavirus envelope proteins, all purified from eukaryotic expression systems. 91 , 92 Several approved commercial vaccines, including those for human papillomavirus and hepatitis B, are based on VLPs, so its biology and safety profiles are well‐established, and they can be safely and easily mass‐produced. 93 To date, only 2 COVID‐19 VLP vaccines are in clinical testing: RBD‐HBsAg VLP and an Unnamed VLP vaccine, in Phase I/II and Phase I respectively.
2.4.1. NVX‐CoV2373
NVX‐CoV2373 is a protein subunit vaccine developed by Novavax, consisting of the SARS‐CoV‐2 spike protein, and is the most advanced COVID‐19 vaccine with this platform. The vaccine is formulated with Novavax's patented Matrix‐M adjuvant, a saponin‐based adjuvant that stimulates entry of antigen‐presenting cells into the injection site to enhance immune system activation 94 , 95 to produce stronger, long‐lasting immune responses. Novavax recently announced results from Phase I/II trials showing that the vaccine was well‐tolerated and that 2 doses of 5 μg was sufficient to induce neutralizing antibody titres exceeding those found in convalescent serum from symptomatic Covid‐19 patients. 94 , 96 In October, Novavax began Phase III clinical trials in the UK with healthy adult participants (ClinicalTrials.gov NCT04583995), and in November, additional Phase III trials were initiated in the USA and Mexico to recruit high‐risk participants (NCT04611802).
2.5. Repurposed
Alongside global efforts to develop and test COVID‐19 vaccines, various groups have been exploring the possibility of repurposing existing vaccines for COVID‐19. These efforts are built upon prior knowledge of vaccine nonspecific effects (NSEs).
NSE is a phenomenon by which the administration of 1 vaccine improves or impairs one's response to unrelated pathogens, probably because the vaccine modified components of the immune system. 97 Vaccine NSEs were first recognized in the 1970s, when the introduction of a live attenuated measles vaccine to low‐income countries led to reductions in mortality in vaccinated children that could not be explained by protection against measles infection alone. 98 Since then, numerous observational studies in children from both low‐income and high‐income settings show that immunization with live attenuated vaccines, such as those against measles, smallpox, TB and polio can have broad beneficial NSEs while immunization using nonlive vaccines, including inactivated vaccines for polio, diphtheria–tetanus–pertussis, hepatitis B and seasonal influenza, do not. 98 In some cases, childhood immunization with nonlive vaccines has been shown to actually increase susceptibility to unrelated infections in girls; however, the same effect have not been found in boys. 98 In cases where live and nonlive vaccines were administered in combination, the vaccine administered last dictated NSEs, and individuals with prior immunity to the targeted pathogen can still experience NSEs upon re‐vaccination. 98 Based on these findings, clinical trials are underway to determine if existing live‐attenuated vaccines can protect against SARS‐CoV‐2. Since these vaccines are already FDA‐approved, have known safety profiles, and are generally accessible as they are routinely administered around the world, they can be easily repurposed as temporary prophylactics if a COVID‐19 vaccine is unavailable.
2.5.1. Measles–mumps–rubella vaccine
The measles–mumps–rubella (MMR) vaccine, is a cocktail comprised of live‐attenuated strains of the 3 highly contagious RNA viruses that, while not incurable, can trigger a host of serious health complications. 99 Developed in the 1970s, the MMR is the main vaccine provided worldwide to protect against these viruses. In the USA, the CDC recommends the MMR vaccine to be administered to children before age 6 years, as well as to high‐risk adults (i.e. health care workers). Studies of children in the USA, 100 Italy, 101 Denmark 102 , 103 and the Netherlands 104 show that children who were most recently vaccinated with MMR had reduced rates of hospital admissions for other respiratory illnesses, including RSV, compared to children who were most recently vaccinated with nonlive vaccines.
The SARS‐CoV‐2 spike protein is 20% identical to that of the rubella virus, and COVID‐19 patients with higher circulating rubella antibodies reportedly experience lower SARS‐CoV‐2 viral burden. 105 Additionally, 2 unreviewed epidemiological studies suggest a negative correlation between MMR immunization rates and COVID‐19 rates and symptom severity, both globally and across 37 North American and European countries. 106 , 107 The same trends were not found between COVID‐19 and diphtheria–tetanus–pertussis immunization rates. 107 The Washington University School of Medicine is leading the effort to determine if MMR protects against COVID‐19 infection through a Phase III trial (CROWN CORONA, ClinicalTrial.gov NCT04333732) in healthcare workers.
2.5.2. Bacille Calmette–Guerin vaccine
The Bacille Calmette–Guerin (BCG) vaccine is a live‐attenuated vaccine containing the bacteria Mycobacterium bovis, which was isolated in 1900 from the milk of a cow with bovine TB and attenuated by repeated passaging on potatoes. 108 Today, it remains the main prophylactic treatment for human TB. The BCG vaccine is usually administered intradermally on the upper arm, where it leaves behind a small scar. Unlike MMR, the efficacy of BCG against TB is disputable, and has been reported to fall anywhere from 0 to >80% 109 due to the heterogenous nature of TB as well as variations between different attenuated M. bovis cultures around the world. 110 For this reason, BCG vaccine administration policies differ significantly between regions; in most of Asia, Africa and South America, BCG is universally administered to newborns, while in Australia, as well as parts of Europe and North America, it is only recommended for at‐risk communities. 110
The broad beneficial NSE of BCGs have been well documented worldwide, as immunization correlates with lower neonatal mortality rates than can be expected from protection against TB alone. 111 , 112 , 113 Furthermore, BCG appears to confer nonspecific, potentially long‐lasting protection against lower respiratory tract infections. 114 , 115 However, given that BCG is usually not administered to adults, it is unclear if these benefits exist in later life. Epidemiological analysis of socially similar European countries and communities in Japan identified a negative correlation between BCG vaccination levels and COVID‐19 mortality. 116 , 117 Globally, BCG‐vaccinated countries appears to have lower COVID‐19 prevalence rates compared to countries without a BCG vaccination programme. 118 , 119 To determine if BCG protects against COVID‐19 infection in adults, Phase III studies with healthcare workers are currently underway in multiple countries, including the Netherlands (BRACE, ClinicalTrials.gov NCT04327206) and Australia (BCG‐CORONA, NCT04328441).
2.5.3. Oral polio vaccine
The oral polio vaccine (OPV) is made by combining attenuated versions of the 3 poliovirus serotypes behind poliomyelitis. 120 Vaccination for the poliovirus, which, like SARS‐CoV‐2, is a +ssRNA virus, can occur at any age and communities in polio‐prone regions often receive regular booster doses. 121 Unfortunately, while OPV is effective against poliomyelitis, it has been known to de‐attenuate and create a vaccine‐generated poliovirus that, over time, can trigger a polio outbreak similar to that created by regular poliovirus. Because of this, proper eradication of the poliovirus requires a 2‐pronged approach; an initial treatment with the attenuated OPV, followed by secondary treatment using an inactivated, injectable polio vaccine to eliminate the potential development of vaccine‐generated poliovirus. As OPV is cheaper and easier to produce, transport and administer compared to injectable polio vaccine, it is much more accessible, which is 1 of the reasons why polio continues to persist in certain developing countries. 121 Similar to the other live‐attenuated vaccines introduced above, the NSEs of OPV are largely beneficial, ranging from general reduction in infant mortality, 122 , 123 , 124 to protection against childhood diarrhea 125 and ear infections. 126 Phase III trials testing the effect of OPV on COVID‐19 rates are currently underway in the USA (OPV‐NA831, ClinicalTrials.gov NCT04540185) and Guinea‐Bissau (NCT04445428).
3. THINKING AHEAD: LESSONS FOR THE POST‐COVID SCIENTIST
In the past year, our understanding of the SARS‐CoV‐2 virus has expanded at a truly unprecedented rate. While the vaccines summarized here represent only a small fraction of the scientific accomplishments achieved by research groups working around the world—and around the clock—the strain this pandemic has brought to our global community sheds light on certain cracks in our system that must be addressed.
3.1. Pandemics end, coronaviruses do not
SARS‐CoV‐2 shares about 80–90% sequence identity to SARS‐CoV‐1 (SARS), 1 , 127 and about 50% sequence identity to MERS‐CoV (MERS). 1 In addition, both SARS‐CoV‐1 and SARS‐CoV‐2 infect host cells by binding to the angiotensin‐converting enzyme 2 receptor via highly similar spike proteins. This level of conservation within members of the coronavirus family has allowed much of the knowledge gained during the 2002 SARS epidemic to be applied in the design of COVID‐19 prophylactics and treatments. However, when SARS disappeared suddenly in the summer of 2003, interest and funding for further development of a vaccine or reliable treatment were redirected into other channels. Additionally, despite emerging in 2012 and being serious health problem for certain parts of the world, there are still no vaccines or specialized treatments for MERS, possibly due to the lack of global interest. Given what is known about coronavirus cross‐species transmission 128 and the simple fact that 3 of the 7 zoonotic coronaviruses to date are capable of significantly reducing our quality of life, it would be imprudent not to prepare for future coronavirus epidemics. Further research into coronaviruses and efforts to develop therapies must persist even after COVID‐19 elimination so that communities may be more prepared to respond. Efforts to track the biology and evolution of other coronavirus strains should be made so that we may predict—and minimize—future animal‐to‐human transmission. Indeed, predictions of a second zoonotic SARS‐CoV virus emerged as early as 2007, 129 but were largely ignored. With the right knowledge, preparation and support from scientifically driven policies, future coronavirus‐based epidemic scan and should be mitigated.
3.2. To stop viral spread, countries need to work together
Given the speed of SARS‐CoV‐2 transmission around the globe despite lockdown and travel restrictions, it is clear that no country will be safe from infection without concerted efforts worldwide. The success or failure of virus eradication policies depend largely on vaccine accessibility and distribution. For many low‐income countries, the lack of infrastructure and funds necessary to acquire, transport, and administer vaccines can prevent disease eradication. For example, while the WHO‐funded campaign to eradicate polio, which was launched in 1985, helped eliminate the disease within the Americas and Europe by 1994 and 2002 respectively, countries including Afghanistan, Pakistan, Nigeria and the Philippines continue to suffer outbreaks today. 130 In the case of COVID‐19, this disparity will be even more serious, as developed countries have already established heavy monopolies on existing SARS‐CoV‐2 vaccine candidates. The necessity of a coordinated global effort to control viral spread cannot be over‐stated, therefore, conversations must be held about SARS‐CoV‐2 in developing regions, and infrastructure to fund and monitor COVID‐19 vaccination will be needed in these communities to facilitate timely global recovery.
This pandemic has also amplified existing challenges involving other viral pathogens. Thanks to the combination of viral persistence in developing countries and a souring attitude towards vaccination in certain developed countries, measles—which was eliminated from the USA in 2000—has since re‐emerged. Last year, 1276 USA cases were reported, and cases are projected to rise in the coming years due to COVID‐19 disrupting regular childhood vaccination schedules around the world. 131 , 132 A similar narrative is predicted for polio, which, prior to the pandemic, was on track for eradication by 2021. 133 While not all viruses can be feasibly eradicated due to the presence of nonhuman reservoirs, long latency periods, or the lack of a vaccine conferring life‐long immunity, 134 this is not the case for measles, polio and several other viral diseases. Therefore, to minimize the risk of what are entirely avoidable epidemics, we must take disease eradication seriously where it is applicable, and to do so, effort should be made to address vaccination policies both internationally and at home.
3.3. To combat misinformation and facilitate public trust, increased scientific transparency is needed
Alongside the pandemic, communities around the world are being overwhelmed by misinformation regarding COVID‐19, ranging from trivialization of health risks to downright fraudulent claims of cures and prophylactics. 135 , 136 While we have been focusing our efforts on treating the pandemic, this infodemic has gone unchecked. Science communication and transparency is crucial to maintaining public trust, especially now, when each new COVID‐19 report or finding is heavily scrutinized by the public eye. Currently, the relative obscurity and unusual speed by which drug companies and their academic partners complete trials, as well as concerns over possible political pressure on scientific judgement has both degraded the public's trust in treatments and vaccines and fuelled further misunderstanding. This can lead to noncompliance when a vaccine is eventually released, which can delay or prevent COVID‐19 elimination.
Undoubtedly, there is a need for clearer communication during clinical trials. When AstraZeneca's viral vector vaccine ChAdOx1 nCov‐19 was temporarily halted, this was reported to be due to neurological symptoms in a participant. 48 , 49 However, when the pause was lifted in the USA, the only announcement made was that the FDA had reviewed the safety data and found it satisfactory. 54 Similarly, Johnson & Johnson's Phase III trial for Ad26.COV2.S was paused for about a week in October with no public report explaining either the reason for the hold or why it was lifted. The absence of official reports can fuel misinformation and mistrust, and while companies need to maintain the confidentiality of trial participants, they must also learn to be more proactive, transparent, and consistent with the public by announcing and explaining major decisions.
One way to prevent misinformation and foster trust is to make details regarding treatment design and clinical trials available to the broader community. So far, Moderna, Pfizer, AstraZeneca, and Johnson & Johnson have all published their Phase III trial protocols, and the FDA has announced its intention to do the same with its scientific review process for COVID‐19 EUAs. 137 These documents include details on data collection, participant pool size, known side effects, and how vaccine efficacy was determined. EU regulators are also making an effort to improve transparency around COVID‐19 treatments. In particular, the European Medicines Agency is publishing all clinical data in support of applications for COVID‐19 treatments, along with the full risk management plan generated by the European Medicines Agency's scientific committees' assessment which powers subsequent commission decision of treatment licensing. 138 Policies such as this foster public trust and promote science literacy over time. We therefore encourage other pharmaceutical companies and research groups to follow suit, and call for continued data transparency in the post‐COVID‐19 world.
3.4. For future vaccine development, could mRNA be the answer?
Interim Phase III results have so far reflected a triumph for mRNA vaccines. To date, the Pfizer BNT162b2 and the Moderna mRNA‐1273 vaccines are reported to be 95 and 94% effective, respectively, which is significantly higher than the minimal 50% efficacy required by the FDA. If commercialized, these would be the first approved vaccines using RNA. There are clear benefits to nucleic acid vaccines beyond their immunogenicity; unlike all other vaccine platforms, the nucleic acid vaccine production does not rely on live vaccine samples or other biological systems. Genomic material can be quickly and cheaply reproduced in a test tube, and only knowledge about its sequence is necessary for vaccine design. In theory, this minimalistic approach should also allow the same development pipeline to be applied to a large variety of pathogen targets, making this platform suitable for future vaccine development.
While the relative instability and inefficient delivery of nucleic material had previously limited the development of RNA vaccines, recent technological advances in lipid nanoparticle formulation have largely removed the latter barrier. 71 Improvements to nanoparticle design and cold‐chain distribution infrastructure are currently being made to address stability concerns, but unless significant progress is made, this vulnerability will probably limit the platform's ability to be used in most developing countries. Hopefully, future technological developments and efforts to concentrate RNA vaccine immunogenicity into a single dose 139 will negate some of these administrative challenges in the coming years. DNA vaccines are similarly inhibited by challenges in nuclear delivery, and efforts to improve gene gun technologies as part of COVID‐19 DNA vaccine development are expected to significantly impact the future prospects of this platform.
Since RNA vaccines have not been commercialized before, little is known about their safety profile and long‐term efficacy. So far, interim Phase III safety data have been largely positive, but these trials are unlikely to identify rare adverse effects due to limits in participant size and can only provide information on short‐term effects observed in the last few months. Since the half‐life of RNA material in the body is only a matter of hours, and these vaccines do not contain any other viral material, these treatments are unlikely to trigger unexpected long‐term adverse effects. However, little is known about the duration of its conferred immunity in the absence of long‐term data. As it becomes more and more likely that an RNA vaccine for COVID‐19 will be approved for emergency use, cautious vigilance must be maintained to determine the long‐term efficacy and safety of these vaccines over the next months and years in order to determine the suitability of this platform for future vaccine development.
3.5. Regulate accelerated vaccine development, maximize trial data output
When the FDA announced guidelines for accelerated vaccine development, a number of decisions, including study demographic groups, trial end points, and even the definition of mild vs severe COVID‐19 cases were left to the discretion of pharmaceutical companies. This has resulted in small variations in trial design between companies, including the ages and health status of participants, and when to test for COVID‐19 after treatment. While these variations do not negate the results from each individual study, they prevent different vaccines from being compared against each other, which is required to determine if certain vaccines are more suitable for certain populations. Normally, additional trials comparing different therapies using the same protocol are conducted, but during a pandemic, we lack the benefit of time.
One solution to this problem is to organize a single massive Phase III trial to simultaneously test various vaccines against COVID‐19 and each other, such as that proposed by the WHO for the EU. 140 While this Solidarity Trial is theoretically beneficial and require fewer enrolled participants overall (as all vaccines share the same placebo group), the level of coordination required by all participating parties has led to undesirable delays. To make the most out of the limited efficacy and safety data that can be obtained during accelerated vaccine development, without obstructing testing, we propose that certain terms, expected outcomes, and important participant groups should be defined in advance by regulators. Since different therapies necessarily variate in dosing strategies and target populations, pharmaceutical companies should be allowed to opt out of certain defined study groups or endpoints that are inapplicable for their vaccine. Improving this infrastructure will help to facilitate accelerated vaccine development during a pandemic, ensure some level of comparability between different candidates, and help regulatory bodies make informed decisions.
4. CONCLUSION
In this review, we summarized the current vaccine landscape for COVID‐19 and provided an updated snapshot of this rapidly developing field. Understanding the mechanisms by which these vaccines function help us to not only better grasp the significance of these clinical findings, but also appreciate the complexity of the efforts. As our global community will doubtlessly go on to face similar viral challenges in the future, we also identified a number of issues that need to be addressed so that we may be better prepared to respond to the next global viral challenge. The degree by which the scientific community has mobilized to study and develop effective treatments for COVID‐19 has been remarkable. While it is our role as scientists to confront current challenges, we must also strive to protect our communities from future risks.
4.1. Nomenclature of targets and ligands
Wherever applicable, the nomenclature in this work follows the guidelines set by IUPHAR/BPS. 141 Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20. 141
COMPETING INTERESTS
There are no competing interests to declare.
Supporting information
TABLE S1 List of all COVID‐19 vaccines currently under development. A list of all 221 vaccines that are in active development, with 55 in clinical trials and the other 166 in preclinical evaluation. Each vaccine is categorized based on its platform, developer, current stage of clinical evaluation and regulatory status, and clinical trial numbers. List was developed using both ClinicalTrials.gov and WHO datasets. Ad5: Adenovirus type 5; Ad26: Adenovirus type 26; MVA: modified vaccinia virus Ankara; LNP: lipid nanoparticles; RBD: Receptor binding domain; S‐2P: spike ectodomain with 2 proline substitutions; HLA‐DR: human leukocyte antigen–DR isotype; saRNA: self‐amplifying RNA; VSV: vesicular stomatitis virus; VLP: virus‐like particle; HBsAg: hepatitis B surface antigen; MMR: measles–mumps–rubella; IPV: inactivated polio vaccine; JE: Japanese encephalitis; HAV: hepatitis A; ZIKV: Zika virus; FMD: foot and mouth disease; SIV: simian immunodeficiency virus; RSV: respiratory syncytial virus; DENV: Dengue virus; EBOV: Ebola virus; MARV: Marburg marburgvirus; HIV: human immunodeficiency virus; CHIKV: Chikungunya; RVF: Rift Valley fever; HBV: hepatitis B virus; VEE: Venezuelan equine encephalitis; MERS: Middle East respiratory syndrome; VZV: varicella zoster virus; HSV: herpes simplex virus; HEV: hepatitis E virus; NiV: Nipah virus; CCHFV: Crimean–Congo haemorrhagic fever virus, H7N9: avian influenza A; NSCLC: nonsmall cell lung cancer; CMV: cytomegalovirus; GBM: glioblastoma
FIGURE 1 Summary of clinical trial size, age, and region for all vaccines. Details on all completed and ongoing Phase I/II/III clinical trials for COVID‐19 vaccines under development. Vaccines are categorized based on platform. List was developed using both ClinicalTrials.gov and WHO datasets.
ACKNOWLEDGEMENTS
The authors would like to thank Drs Ron Liem and Yinghui Mao (Columbia University, department of Pathobiology and Cell Biology) as well as Dr Marcella O'Reilly (Columbia University, Cardiometabolic Genomics Center) for providing valuable feedback on an earlier version of this manuscript. We also thank Dr Serge Cremers (Columbia University, department of Pathology) and Dr Muredach Reilly (Columbia University, Cardiometabolic Genomics Center) for their encouragement and advice. All diagrams in this work were created using BioRender.com.
Calvo Fernández E, Zhu LY. Racing to immunity: Journey to a COVID‐19 vaccine and lessons for the future. Br J Clin Pharmacol. 2021;87(9):3408–3424. 10.1111/bcp.14686
Ester Calvo Fernández and Lucie Y. Zhu, contributed equally to this work
REFERENCES
- 1. Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565‐574. 10.1016/S0140-6736(20)30251-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jiang S, Shi Z, Shu Y, et al. A distinct name is needed for the new coronavirus. Lancet. 2020;395(10228):949. 10.1016/S0140-6736(20)30419-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus‐infected pneumonia. N Engl J Med. 2020;382(13):1199‐1207. 10.1056/NEJMoa2001316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. WHO . COVID‐19 Data Dashboard. COVID‐19 Reported Deaths: Using Shewhart Control Charts to Understand Variation. Boston: Institute for Healthcare Improvement. Published 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019. Accessed May 27, 2020. [Google Scholar]
- 5. Modrow S, Falke D, Truyen U, Schätzl H. Viruses with Single‐Stranded, Positive‐Sense RNA Genomes. In: Molecular Virology. Springer Berlin Heidelberg; 2013:185‐349. 10.1007/978-3-642-20718-1_14 [DOI] [Google Scholar]
- 6. Myint SH. Human coronaviruses: A brief review. Rev Med Virol. 1994;4(1):35‐46. 10.1002/rmv.1980040108 [DOI] [Google Scholar]
- 7. Singhal T. A Review of Coronavirus Disease‐2019 (COVID‐19). Indian J Pediatr. 2020;87(4):281‐286. 10.1007/s12098-020-03263-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Perlman S, Netland J. Coronaviruses post‐SARS: Update on replication and pathogenesis. Nat Rev Microbiol. 2009;7(6):439‐450. 10.1038/nrmicro2147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Thanh Le T, Andreadakis Z, Kumar A, et al. The COVID‐19 vaccine development landscape. Nat Rev Drug Discov. 2020;19(5):305‐306. 10.1038/d41573-020-00073-5 [DOI] [PubMed] [Google Scholar]
- 10. Kaslow DC. Biological feasibility of developing prophylactic vaccines for viral pathogens: Incubation period as a critical parameter. Hum Vaccin. 2007;3(1):1‐7. 10.4161/hv.3.1.3519 [DOI] [PubMed] [Google Scholar]
- 11. Kaslow DC. Certainty of success: three critical parameters in coronavirus vaccine development. Npj Vaccines. 2020;5(1):42. 10.1038/s41541-020-0193-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sanjuán R, Domingo‐Calap P. Mechanisms of viral mutation. Cell Mol Life Sci. 2016;73(23):4433‐4448. 10.1007/s00018-016-2299-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tannock GA, Kim H, Xue L. Why are vaccines against many human viral diseases still unavailable; an historic perspective? J Med Virol. 2020;92(2):129‐138. 10.1002/jmv.25593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kirkcaldy RD, King BA, Brooks JT. COVID‐19 and Postinfection Immunity: Limited Evidence, Many Remaining Questions. JAMA ‐ J am Med Assoc. 2020;323(22):2245‐2246. 10.1001/jama.2020.7869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Premkumar L, Segovia‐Chumbez B, Jadi R, et al. The receptor binding domain of the viral spike protein is an immunodominant and highly specific target of antibodies in SARS‐CoV‐2 patients. Sci Immunol. 2020;5(48):eabc8413. 10.1126/sciimmunol.abc8413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Buonaguro L, Pulendran B. Immunogenomics and systems biology of vaccines. Immunol Rev. 2011;239(1):197‐208. 10.1111/j.1600-065X.2010.00971.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hato T, Dagher PC. How the innate immune system senses trouble and causes trouble. Clin J Am Soc Nephrol. 2015;10(8):1459‐1469. 10.2215/CJN.04680514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Coffman RL, Sher A, Seder RA. Vaccine adjuvants: Putting innate immunity to work. Immunity. 2010;33(4):492‐503. 10.1016/j.immuni.2010.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Petrovsky N. Comparative Safety of Vaccine Adjuvants: A Summary of Current Evidence and Future Needs. Drug Saf. 2015;38(11):1059‐1074. 10.1007/s40264-015-0350-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Iwasaki A, Yang Y. The potential danger of suboptimal antibody responses in COVID‐19. Nat Rev Immunol. 2020;20(6):339‐341. 10.1038/s41577-020-0321-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fulginiti VA, Eller JJ, Sieber OF, Joyner JW, Minamitani M, Meiklejohn G. Respiratory virus immunization: A field trial of two inactivated respiratory virus vaccines; an aqueous trivalent paratnfluenza virus vaccine and an alum‐precipitated respiratory syncytial virus vaccine. Am J Epidemiol. 1969;89(4):435‐448. 10.1093/oxfordjournals.aje.a120956 [DOI] [PubMed] [Google Scholar]
- 22. Acosta PL, Caballero MT, Polack FP. Brief History and Characterization of Enhanced Respiratory Syncytial Virus Disease. Clin Vaccine Immunol. 2016;23(3):189‐195. 10.1128/CVI.00609-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. WHO . Coronavirus Update #37: What we know about COVID‐19 vaccine development. WHO.int. Published 2020. https://www.who.int/docs/default-source/coronaviruse/risk-comms-updates/update37-vaccine-development.pdf?sfvrsn=2581e994_6. Accessed November 21, 2020. [Google Scholar]
- 24. FDA . Development and Licensure of Vaccines to Prevent COVID‐19: Guidance for Industry.; 2020. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/development-and-licensure-vaccines-prevent-covid-19. Accessed November 21, 2020.
- 25. FDA . Emergency Use Authorization for Vaccines to Prevent COVID‐19. Guidance for Industry; 2020. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/emergency-use-authorization-vaccines-prevent-covid-19. Accessed November 21, 2020. [Google Scholar]
- 26. CDC . CDC Seasonal Flu Vaccine Effectiveness Studies. https://www.cdc.gov/flu/vaccines-work/effectiveness-studies.htm
- 27. Clem AS. Fundamentals of vaccine immunology. J Glob Infect Dis. Wolters Kluwer ‐Medknow Publications; 2011;3:73‐78 10.4103/0974-777X.77299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shang W, Yang Y, Rao Y, Rao X. The outbreak of SARS‐CoV‐2 pneumonia calls for viral vaccines. Npj Vaccines. 2020;5(1):18. 10.1038/s41541-020-0170-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bonnafous P, Nicolaï MC, Taveau JC, et al. Treatment of influenza virus with Beta‐propiolactone alters viral membrane fusion. Biochim Biophys Acta ‐ Biomembr. 2014;1838(1 PARTB):355‐363. 10.1016/j.bbamem.2013.09.021 [DOI] [PubMed] [Google Scholar]
- 30. Murray J, Todd KV, Bakre A, et al. A universal mammalian vaccine cell line substrate. Jin D‐Y. PLoS ONE. 2017;12(11):e0188333. 10.1371/journal.pone.0188333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gao Q, Bao L, Mao H, et al. Rapid development of an inactivated vaccine for SARS‐CoV‐2. bioRxiv. Published online April 19, 2020. 2020;04(17):046375. 10.1101/2020.04.17.046375 [DOI] [Google Scholar]
- 32. Yu P, Qi F, Xu Y, et al. Age‐related rhesus macaque models of COVID‐19. Anim Model Exp Med. 2020;3(1):93‐97. 10.1002/ame2.12108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang Y, Zeng G. Immrunogenicity and Safety of a SARS‐CoV‐2 Inactivated Vaccine in Healthy Adults Aged 18–59 years: Report of the Randomized, Double‐blind, and Placebo‐controlled Phase 2 Clinical Trial. medRxiv. Published online August 10, 2020: 2020.07.31.20161216. 10.1101/2020.07.31.20161216 [DOI]
- 34. Reuters . Sinovac coronavirus vaccine offered by Chinese city for emergency use costs $60. Reuters. Published 2020. https://www.reuters.com/article/us-health-coronavirus-china-vaccine/sinovac-coronavirus-vaccine-offered-by-chinese-city-for-emergency-use-costs-60-idUSKBN2710UQ. Accessed October 16, 2020.
- 35. Xia S, Duan K, Zhang Y, et al. Effect of an Inactivated Vaccine Against SARS‐CoV‐2 on Safety and Immunogenicity Outcomes. JAMA. 2020;324(10):951‐960. 10.1001/jama.2020.15543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Reuters Staff . UAE announces emergency approval for use of COVID‐19 vaccine|Reuters. Reuters. https://www.reuters.com/article/us-health-coronavirus-emirates-vaccine/uae-announces-emergency-approval-for-use-of-covid-19-vaccine-idUSKBN2652OM. Accessed October 6 2020.
- 37. Wang H, Zhang Y, Huang B, et al. Development of an Inactivated Vaccine Candidate, BBIBP‐CorV, with Potent Protection against SARS‐CoV‐2. Cell. 2020;182(3):713‐721.e9. 10.1016/j.cell.2020.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Xia S, Zhang Y, Wang Y, et al. Safety and immunogenicity of an inactivated SARS‐CoV‐2 vaccine, BBIBP‐CorV: a randomised, double‐blind, placebo‐controlled, phase 1/2 trial. Lancet Infect Dis. 2020;0(0). 10.1016/S1473-3099(20)30831-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wold W, Toth K. Adenovirus Vectors for Gene Therapy, Vaccination and Cancer Gene Therapy. Curr Gene Ther. 2014;13(6):421‐433. 10.2174/1566523213666131125095046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yu B, Zhou Y, Wu H, et al. Seroprevalence of neutralizing antibodies to human adenovirus type 5 in healthy adults in China. J Med Virol. 2012;84(9):1408‐1414. 10.1002/jmv.23325 [DOI] [PubMed] [Google Scholar]
- 41. Zhang S, Huang W, Zhou X, Zhao Q, Wang Q, Jia B. Seroprevalence of neutralizing antibodies to human adenoviruses type‐5 and type‐26 and chimpanzee adenovirus type‐68 in healthy Chinese adults. J Med Virol. 2013;85(6):1077‐1084. 10.1002/jmv.23546 [DOI] [PubMed] [Google Scholar]
- 42. Xiang Z, Li Y, Cun A, et al. Chimpanzee adenovirus antibodies in humans, sub‐Saharan Africa. Emerg Infect Dis. 2006;12(10):1596‐1599. 10.3201/eid1210.060078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. van Doremalen N, Lambe T, Spencer A, et al. ChAdOx1 nCoV‐19 vaccine prevents SARS‐CoV‐2 pneumonia in rhesus macaques. Nature. Published online July. 2020;30(7830):1‐5. 10.1038/s41586-020-2608-y [DOI] [Google Scholar]
- 44. Wilkie M, Satti I, Minhinnick A, et al. A phase I trial evaluating the safety and immunogenicity of a candidate tuberculosis vaccination regimen, ChAdOx1 85A prime – MVA85A boost in healthy UK adults. Vaccine. 2020;38(4):779‐789. 10.1016/j.vaccine.2019.10.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Folegatti PM, Bittaye M, Flaxman A, et al. Safety and immunogenicity of a candidate Middle East respiratory syndrome coronavirus viral‐vectored vaccine: a dose‐escalation, open‐label, non‐randomised, uncontrolled, phase 1 trial. Lancet Infect Dis. 2020;20(7):816‐826. 10.1016/S1473-3099(20)30160-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. van Doremalen N, Lambe T, Spencer A, et al. ChAdOx1 nCoV‐19 vaccination prevents SARS‐CoV‐2 pneumonia in rhesus macaques. bioRxiv Prepr Serv Biol. Published online May 13. 2020: 2020.05.13.093195. 10.1101/2020.05.13.093195 [DOI] [Google Scholar]
- 47. Folegatti PM, Ewer KJ, Aley PK, et al. Safety and immunogenicity of the ChAdOx1 nCoV‐19 vaccine against SARS‐CoV‐2: a preliminary report of a phase 1/2, single‐blind, randomised controlled trial. Lancet. 2020;396(10249):467‐478. 10.1016/S0140-6736(20)31604-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Phillips N, Cyranoski D, Mallapaty S. A leading coronavirus vaccine trial is on hold: scientists react. Nature. Published online September. 2020;9. 10.1038/d41586-020-02594-w [DOI] [PubMed] [Google Scholar]
- 49. Cohen E, Bonifield J. Internal AstraZeneca safety report sheds light on neurological condition suffered by vaccine trial participant ‐ CNN. CNN. https://www.cnn.com/2020/09/17/health/astrazeneca-vaccine-trial-document/index.html. Accessed September 28, 2020. [Google Scholar]
- 50. Agmon‐Levin N, Kivity S, Szyper‐Kravitz M, Shoenfeld Y. Transverse myelitis and vaccines: A multi‐analysis. Lupus. 2009;18(13):1198‐1204. 10.1177/0961203309345730 [DOI] [PubMed] [Google Scholar]
- 51. Akkad W, Salem B, Freeman JW, Huntington MK. Longitudinally extensive transverse myelitis following vaccination with nasal attenuated novel influenza A(H1N1) vaccine. Arch Neurol. 2010;67(8):1018‐1020. 10.1001/archneurol.2010.167 [DOI] [PubMed] [Google Scholar]
- 52. Cyranoski D, Mallapaty S. Scientists relieved as coronavirus vaccine trial restarts ‐ but question lack of transparency. Nature. 2020;585(7825):331‐332. 10.1038/d41586-020-02633-6 [DOI] [PubMed] [Google Scholar]
- 53. AstraZeneca . COVID‐19 vaccine AZD1222 clinical trials resumed in the UK. AstraZeneca. Published 2020. https://www.astrazeneca.com/content/astraz/media-centre/press-releases/2020/covid-19-vaccine-azd1222-clinical-trials-resumed-in-the-uk.html. Accessed November 16, 2020. [Google Scholar]
- 54. AstraZeneca . FDA authorises restart of the COVID‐19 AZD1222 vaccine US Phase III trial. Published 2020. https://www.astrazeneca.com/content/astraz/media-centre/press-releases/2020/fda-authorises-restart-of-the-covid-19-azd1222-vaccine-us-phase-iii-trial.html. Accessed November 16, 2020.
- 55. AstraZeneca . AZD1222 vaccine met primary efficacy endpoint in preventing COVID‐19. Published 2020. https://www.astrazeneca.com/media-centre/press-releases/2020/azd1222hlr.html. Accessed November 23, 2020.
- 56. Wu S, Zhong G, Zhang J, et al. A single dose of an adenovirus‐vectored vaccine provides complete protection of the upper and lower respiratory tracts against SARS‐CoV‐2 challenge. Published online 2020. 10.21203/rs.3.rs-35891/v1 [DOI] [PMC free article] [PubMed]
- 57. Zhu FC, Li YH, Guan XH, et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type‐5 vectored COVID‐19 vaccine: a dose‐escalation, open‐label, non‐randomised, first‐in‐human trial. Lancet. 2020;395(10240):1845‐1854. 10.1016/S0140-6736(20)31208-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zhu FC, Guan XH, Li YH, et al. Immunogenicity and safety of a recombinant adenovirus type‐5‐vectored COVID‐19 vaccine in healthy adults aged 18 years or older: a randomised, double‐blind, placebo‐controlled, phase 2 trial. Lancet. 2020;396(10249):479‐488. 10.1016/S0140-6736(20)31605-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Barouch DH, Kik SV, Weverling GJ, et al. International seroepidemiology of adenovirus serotypes 5, 26, 35, and 48 in pediatric and adult populations. Vaccine. 2011;29(32):5203‐5209. 10.1016/j.vaccine.2011.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mercado NB, Zahn R, Wegmann F, et al. Single‐shot Ad26 vaccine protects against SARS‐CoV‐2 in rhesus macaques. Nature. Published online July. 2020;30(7830):1‐6. 10.1038/s41586-020-2607-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tostanoski LH, Wegmann F, Martinot AJ, et al. Ad26 vaccine protects against SARS‐CoV‐2 severe clinical disease in hamsters. Nat Med. Published online September. 2020;3(11):1‐7. 10.1038/s41591-020-1070-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sadoff J, le Gars M, Shukarev G, et al. Safety and immunogenicity of the Ad26.COV2.S COVID‐19 vaccine candidate: interim results of a phase 1/2a, double‐blind, randomized, placebo‐controlled trial. medRxiv. Published online September 25. 2020;2020.09.23.20199604. 10.1101/2020.09.23.20199604 [DOI] [Google Scholar]
- 63. Johnson & Johnson . Johnson & Johnson Temporarily Pauses All Dosing in Our Janssen COVID‐19 Vaccine Candidate Clinical Trials. https://www.jnj.com/our-company/johnson-johnson-temporarily-pauses-all-dosing-in-our-janssen-covid-19-vaccine-candidate-clinical-trials. Accessed October 15 2020.
- 64. Johnson & Johnson Services I . Johnson & Johnson Prepares to Resume Phase 3 ENSEMBLE Trial of its Janssen COVID‐19 Vaccine Candidate in the U.S. Johnson & Johnson. Published 2020. https://www.jnj.com/our-company/johnson-johnson-prepares-to-resume-phase-3-ensemble-trial-of-its-janssen-covid-19-vaccine-candidate-in-the-us. Accessed November 16, 2020. [Google Scholar]
- 65. Logunov DY, Dolzhikova IV, Zubkova OV, et al. Safety and immunogenicity of an rAd26 and rAd5 vector‐based heterologous prime‐boost COVID‐19 vaccine in two formulations: two open, non‐randomised phase 1/2 studies from Russia. Lancet. 2020;396(10255):887‐897. 10.1016/S0140-6736(20)31866-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Paul Ehrlich Institute . REGULATORY APPROVAL IN RUSSIA OF A COVID‐19 VACCINE DEVELOPED BY GAMALEYA INSTITUTE. Published 2020. https://www.pei.de/SharedDocs/Downloads/EN/newsroom-en/dossiers/approval-vaccine-russia.pdf?__blob=publicationFile&v=2 [Google Scholar]
- 67. Russian Direct Investment Fund . Russian application of Sputnik V vaccine for WHO vaccine prequalification among the first applications submitted. Published 2020. https://rdif.ru/Eng_fullNews/5963/. Accessed November 16, 2020.
- 68. Gamaleya . The first interim data analysis of the Sputnik V vaccine against COVID‐19 phase III clinical trials in the Russian Federation demonstrated 92% efficacy. Published 2020. https://sputnikvaccine.com/newsroom/pressreleases/the-first-interim-data-analysis-of-the-sputnik-v-vaccine-against-covid-19-phase-iii-clinical-trials-/. Accessed November 23, 2020.
- 69. Gamaleya . Second interim analysis of clinical trial data showed a 91.4% efficacy for the Sputnik V vaccine on day 28 after the first dose; vaccine efficacy is over 95% 42 days after the first dose|Official website vaccine against COVID‐19 Sputnik V. Published 2020. https://sputnikvaccine.com/newsroom/pressreleases/second-interim-analysis-of-clinical-trial-data-showed-a-91-4-efficacy-for-the-sputnik-v-vaccine-on-d/. Accessed November 25, 2020.
- 70. Amanat F, Krammer F. SARS‐CoV‐2 Vaccines: Status Report. Immunity. 2020;52(4):583‐589. 10.1016/j.immuni.2020.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines‐a new era in vaccinology. Nat Rev Drug Discov. 2018;17(4):261‐279. 10.1038/nrd.2017.243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Fynan EF, Lu S, Robinson HL. One Group's Historical Reflections on DNA Vaccine Development. Hum Gene Ther. 2018;29(9):966‐970. 10.1089/hum.2018.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Jackson NAC, Kester KE, Casimiro D, Gurunathan S, DeRosa F. The promise of mRNA vaccines: a biotech and industrial perspective. Npj Vaccines. 2020;5(1):11. 10.1038/s41541-020-0159-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Scott LJ. Givosiran: First Approval. Drugs. 2020;80(3):335‐339. 10.1007/s40265-020-01269-0 [DOI] [PubMed] [Google Scholar]
- 75. Hoy SM. Patisiran: First Global Approval. Drugs. 2018;78(15):1625‐1631. 10.1007/s40265-018-0983-6 [DOI] [PubMed] [Google Scholar]
- 76. Mulligan MJ, Lyke KE, Kitchin N, et al. Phase 1/2 Study to Describe the Safety and Immunogenicity of a COVID‐19 RNA Vaccine Candidate (BNT162b1) in Adults 18 to 55 Years of Age: Interim Report . medRxiv. Published online July 1, 2020:2020.06.30.20142570. 10.1101/2020.06.30.20142570 [DOI]
- 77. Rego GNA, Nucci MP, Alves AH, et al. Current clinical trials protocols and the global effort for immunization against sars‐cov‐2. Vaccine. 2020;8(3):1‐44. 10.3390/vaccines8030474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Pfizer . Study to Describe the Safety, Tolerability, Immunogenicity, and Efficacy of RNA Vaccine Candidates Against COVID‐19 in Healthy Individuals. https://www.pfizer.com/science/find-a-trial/nct04368728-0
- 79. Academy Pediatric . A. Children and COVID‐19: State Data Report 30 July 2020. AAP. Published online 2020. https://services.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/children-and-covid-19-state-level-data-report/. Accessed October 15 2020.
- 80. Pfizer . Pfizer and BioNTech Announce Vaccine Candidate Against COVID‐19 Achieved Success in First Interim Analysis from Phase 3 Study. Published 2020. https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-announce-vaccine-candidate-against. Accessed November 17, 2020.
- 81. Pfizer. PFIZER AND BIONTECH CONCLUDE PHASE 3 STUDY OF COVID‐19 . VACCINE CANDIDATE, MEETING ALL PRIMARY EFFICACY ENDPOINTS. Published 2020. https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-conclude-phase-3-study-covid-19-vaccine. Accessed November 18, 2020.
- 82. Corbett KS, Flynn B, Foulds KE, et al. Evaluation of the mRNA‐1273 Vaccine against SARS‐CoV‐2 in Nonhuman Primates. N Engl J Med. 2020;383(16):1544‐1555. 10.1056/nejmoa2024671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Jackson LA, Anderson EJ, Rouphael NG, et al. An mRNA Vaccine against SARS‐CoV‐2 — Preliminary Report. N Engl J Med. Published online July 14. 2020:NEJMoa2022483. 10.1056/nejmoa2022483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Moderna . Moderna Announces Positive Interim Phase 1 Data for its mRNA Vaccine (mRNA‐1273) Against Novel Coronavirus. 2020;5184:2‐4. http://www.modernatx.com. Accessed June 24, 2020.
- 85. Moderna . Moderna Announces Longer Shelf Life for its COVID‐19 Vaccine Candidate at Refrigerated Temperatures|Moderna, Inc. Published 2020. https://investors.modernatx.com/news-releases/news-release-details/moderna-announces-longer-shelf-life-its-covid-19-vaccine. Accessed November 20, 2020.
- 86. Smith TRF, Patel A, Ramos S, et al. Immunogenicity of a DNA vaccine candidate for COVID‐19. Nat Commun. 2020;11(1):2601. 10.1038/s41467-020-16505-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Yu J, Tostanosk LH, Peter L, et al. DNA vaccine protection against SARS‐CoV‐2 in rhesus macaques. Science (80‐). 2020;369(6505):806‐811. 10.1126/science.abc6284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. INOVIO . INOVIO Announces Positive Interim Phase 1 Data For INO‐4800 Vaccine for COVID‐19 . Published 2020. http://ir.inovio.com/news-releases/news-releases-details/2020/INOVIO-Announces-Positive-Interim-Phase-1-Data-For-INO-4800-Vaccine-for-COVID-19/default.aspx. Accessed November 17, 2020.
- 89. Inovo Pharmaceuticals . INOVIO Announces Initiation of Phase 2 Segment of its Phase 2/3 Clinical Trial for its COVID‐19 DNA Vaccine Candidate, INO‐4800; Trial Will Be Funded by the U.S. Department of Defense. Published 2020. http://ir.inovio.com/news-releases/news-releases-details/2020/INOVIO-Announces-Initiation-of-Phase-2-Segment-of-its-Phase-23-Clinical-Trial-for-its-COVID-19-DNA-Vaccine-Candidate-INO-4800-Trial-Will-Be-Funded-by-the-U.S.-Department-of-Defense/default.aspx. Accessed November 17, 2020.
- 90. Jeong H, Seong BL. Exploiting virus‐like particles as innovative vaccines against emerging viral infections. J Microbiol. 2017;55(3):220‐230. 10.1007/s12275-017-7058-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Oscherwitz J. The promise and challenge of epitope‐focused vaccines. Hum Vaccin Immunother. 2016;12(8):2113‐2116. 10.1080/21645515.2016.1160977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Du L, He Y, Zhou Y, Liu S, Zheng BJ, Jiang S. The spike protein of SARS‐CoV ‐ A target for vaccine and therapeutic development. Nat Rev Microbiol. 2009;7(3):226‐236. 10.1038/nrmicro2090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Donaldson B, Lateef Z, Walker GF, Young SL, Ward VK. Virus‐like particle vaccines: immunology and formulation for clinical translation. Expert Rev Vaccines. 2018;17(9):833‐849. 10.1080/14760584.2018.1516552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Novavax . Novavax Announces Positive Phase 1 Data for its COVID‐19 Vaccine Candidate . https://ir.novavax.com/news-releases/news-release-details/novavax-announces-positive-phase-1-data-its-covid-19-vaccine. Accessed August 13 2020.
- 95. Novavax . Our Unique Technology. https://novavax.com/our-unique-technology. Accessed August 13, 2020.
- 96. Keech C, Albert G, Cho I, et al. Phase 1–2 Trial of a SARS‐CoV‐2 Recombinant Spike Protein Nanoparticle Vaccine. N Engl J Med. Published online September. 2020;383:2320‐2332. 10.1056/nejmoa2026920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Benn CS, Netea MG, Selin LK, Aaby P. A small jab – a big effect: nonspecific immunomodulation by vaccines. Trends Immunol. 2013;34(9):431‐439. 10.1016/J.IT.2013.04.004 [DOI] [PubMed] [Google Scholar]
- 98. Benn CS, Fisker AB, Rieckmann A, Sørup S, Aaby P. Vaccinology: time to change the paradigm? Lancet Infect Dis. 2020;20(10):e274‐e283. 10.1016/S1473-3099(19)30742-X [DOI] [PubMed] [Google Scholar]
- 99. Stratton K, Ford A, Rusch E, Clayton EW. Adverse Effects of Vaccines: Evidence and Causality. National Academies Press (US); 2012. 10.17226/13164. [DOI] [PubMed] [Google Scholar]
- 100. Bardenheier BH, McNeil MM, Wodi AP, McNicholl JM, DeStefano F. Risk of nontargeted infectious disease hospitalizations among US children following inactivated and live vaccines, 2005‐2014. Clin Infect Dis. 2017;65(5):729‐737. 10.1093/cid/cix442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. La Torre G, Saulle R, Unim B, et al. The effectiveness of measles‐mumps‐rubella (MMR) vaccination in the prevention of pediatric hospitalizations for targeted and untargeted infections: A retrospective cohort study. Hum Vaccin Immunother. 2017;13(8):1879‐1883. 10.1080/21645515.2017.1330733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Sørup S, Benn CS, Poulsen A, Krause TG, Aaby P, Ravn H. Live vaccine against measles, mumps, and rubella and the risk of hospital admissions for nontargeted infections. JAMA ‐ J am Med Assoc. 2014;311(8):826‐835. 10.1001/jama.2014.470 [DOI] [PubMed] [Google Scholar]
- 103. Sørup S, Benn CS, Stensballe LG, Aaby P, Ravn H. Measles‐mumps‐rubella vaccination and respiratory syncytial virus‐associated hospital contact. Vaccine. 2015;33(1):237‐245. 10.1016/j.vaccine.2014.07.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Tielemans SMAJ, de Melker HE, Hahné SJM, et al. Non‐specific effects of measles, mumps, and rubella (MMR) vaccination in high income setting: Population based cohort study in the Netherlands. BMJ. 2017;358:j3862. 10.1136/bmj.j3862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Franklin R, Young A, Neumann B, et al. Homologous protein domains in SARS‐CoV‐2 and measles, mumps and rubella viruses: preliminary evidence that MMR vaccine might provide protection against COVID‐19. medRxiv. Published online 2020. 10.1101/2020.04.10.20053207 [DOI] [Google Scholar]
- 106. Gold JE. MMR Vaccine Appears to Confer Strong Protection from COVID‐19: Few Deaths from SARS‐CoV‐2 in Highly Vaccinated Populations MMR Vaccine Appears to Confer Strong Protection from COVID‐19: Few Deaths from SARS‐CoV‐2 in Highly Vaccinated Populations. Vol 7; 2020. 10.13140/RG.2.2.32128.25607 [DOI]
- 107. Guven R, Ikbal Sasmaz M, Eyupoglu G, Yilmaz Semerci S. Do Childhood Measles and DTaP Vaccination Decrease the Mortality Rate Caused by COVID‐19 in OECD Countries? ClinicalTrials. Published online 2020. 10.21203/rs.3.rs-48106/v1 [DOI] [Google Scholar]
- 108. Oettinger T, Jørgensen M, Ladefoged A, Hasløv K, Andersen P. Development of the Mycobacterium bovis BCG vaccine: Review of the historical and biochemical evidence for a genealogioal tree. Tuber Lung Dis. 1999;79(4):243‐250. 10.1054/tuld.1999.0206 [DOI] [PubMed] [Google Scholar]
- 109. Vaudry W. “To BCG or not to BCG, that is the question!”. The challenge of BCG vaccination: Why can't we get it right? Paediatr Child Health (Oxford). 2003;8(3):141‐144. 10.1093/pch/8.3.141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Zwerling A, Behr MA, Verma A, Brewer TF, Menzies D, Pai M. The BCG world atlas: A database of global BCG vaccination policies and practices. PLoS Med. 2011;8(3):e1001012. 10.1371/journal.pmed.1001012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Biering‐Sørensen S, Aaby P, Lund N, et al. Early BCG‐Denmark and Neonatal Mortality among Infants Weighing ≤2500g: A Randomized Controlled Trial. Clin Infect Dis. 2017;65(7):1183‐1190. 10.1093/cid/cix525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Aaby P, Gustafson P, Roth A, et al. Vaccination scars associated with better survival for adults. An observational study from Guinea‐Bissau. Vaccine. 2006;24(29–30):5718‐5725. 10.1016/j.vaccine.2006.04.045 [DOI] [PubMed] [Google Scholar]
- 113. Rieckmann A, Villumsen M, Sørup S, et al. Vaccinations against smallpox and tuberculosis are associated with better long‐term survival: A Danish case‐cohort study 1971‐2010. Int J Epidemiol. 2017;46(2):695‐705. 10.1093/ije/dyw120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. De Castro MJ, Pardo‐Seco J, Martinón‐Torres F. Nonspecific (heterologous) protection of neonatal BCG vaccination against hospitalization due to respiratory infection and sepsis. Clin Infect Dis. 2015;60(11):1611‐1619. 10.1093/cid/civ144 [DOI] [PubMed] [Google Scholar]
- 115. Stensballe LG, Nante E, Jensen IP, et al. Acute lower respiratory tract infections and respiratory syncytial virus in infants in Guinea‐Bissau: A beneficial effect of BCG vaccination for girls: Community based case‐control study. Vaccine. 2005;23(10):1251‐1257. 10.1016/j.vaccine.2004.09.006 [DOI] [PubMed] [Google Scholar]
- 116. Escobar LE, Molina‐Cruz A, Barillas‐Mury C. BCG vaccine protection from severe coronavirus disease 2019 (COVID‐19). Proc Natl Acad Sci U S A. 2020;117(30):17720‐17726. 10.1073/pnas.2008410117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Kinoshita M, Tanaka M. Impact of routine infant BCG vaccination in young generation on prevention of local COVID‐19 spread in Japan: BCG vaccination prevent COVID‐19 spread. J Infect. 2020;81(4):625‐633. 10.1016/j.jinf.2020.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Madan M, Pahuja S, Mohan A, et al. TB infection and BCG vaccination: are we protected from COVID‐19? Public Health. 2020;185:91‐92. 10.1016/j.puhe.2020.05.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Ozdemir C, Kucuksezer UC, Tamay ZU. Is BCG vaccination affecting the spread and severity of COVID‐19? Allergy Eur J Allergy Clin Immunol. 2020;75(7):1824‐1827. 10.1111/all.14344 [DOI] [PubMed] [Google Scholar]
- 120. Baicus A. History of polio vaccination. World J Virol. 2012;1(4):108‐114. 10.5501/wjv.v1.i4.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. John J. Role of injectable and oral polio vaccines in polio eradication. Expert Rev Vaccines. 2009;8(1):5‐8. 10.1586/14760584.8.1.5 [DOI] [PubMed] [Google Scholar]
- 122. Lund N, Andersen A, Hansen ASK, et al. The Effect of Oral Polio Vaccine at Birth on Infant Mortality: A Randomized Trial. Clin Infect Dis. 2015;61(10):1504‐1511. 10.1093/cid/civ617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Benn CS, Jacobsen LH, Fisker AB, et al. Campaigns with oral polio vaccine may lower mortality and create unexpected results. Vaccine. 2017;35(8):1113‐1116. 10.1016/j.vaccine.2016.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Andersen A, Fisker AB, Rodrigues A, et al. National Immunization Campaigns with Oral Polio Vaccine Reduce All‐Cause Mortality: A Natural Experiment within Seven Randomized Trials. Front Public Health. 2018;6:13. 10.3389/fpubh.2018.00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Upfill‐Brown A, Taniuchi M, Platts‐Mills JA, et al. Nonspecific Effects of Oral Polio Vaccine on Diarrheal Burden and Etiology Among Bangladeshi Infants. Clin Infect Dis. 2017;65(3):414‐419. 10.1093/cid/cix354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Seppälä E, Viskari H, Hoppu S, et al. Viral interference induced by live attenuated virus vaccine (OPV) can prevent otitis media. Vaccine. 2011;29(47):8615‐8618. 10.1016/j.vaccine.2011.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Chen Y, Liu Q, Guo D. Emerging coronaviruses: Genome structure, replication, and pathogenesis. J Med Virol. 2020;92(4):418‐423. 10.1002/jmv.25681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Hulswit RJG, de Haan CAM, Bosch BJ. Coronavirus Spike Protein and Tropism Changes. In: Advances in Virus Research. Vol. 96 Academic Press; 2016:29‐57. 10.1016/bs.aivir.2016.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Cheng VCC, Lau SKP, Woo PCY, Kwok YY. Severe acute respiratory syndrome coronavirus as an agent of emerging and reemerging infection. Clin Microbiol Rev. 2007;20(4):660‐694. 10.1128/CMR.00023-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. WHO . GPEI – Global Polio Eradication Initiative. http://polioeradication.org/. Accessed November 22 2020.
- 131. Mahase E. Measles: 142 000 people died in 2018, mostly aged under 5. BMJ. 2019;367:l6830. 10.1136/bmj.l6830 [DOI] [PubMed] [Google Scholar]
- 132. Mulholland K, Kretsinger K, Wondwossen L, Crowcroft N. Action needed now to prevent further increases in measles and measles deaths in the coming years. Lancet. Published online November. 2020;12. 10.1016/S0140-6736(20)32394-1 [DOI] [PubMed] [Google Scholar]
- 133. Din M, Asghar M, Ali M. Delays in polio vaccination programs due to COVID‐19 in Pakistan: a major threat to Pakistan's long war against polio virus. Public Health. 2020;189:1‐2. 10.1016/j.puhe.2020.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. de Quadros CA. Introduction. In: Considerations for Viral Disease Eradication: Lessons Learned and Future Strategies. 1st ed. National Academies Press (US); 2002. https://www.ncbi.nlm.nih.gov/books/NBK98114/ [PubMed] [Google Scholar]
- 135. Galvão J. COVID‐19: the deadly threat of misinformation. Lancet Infect Dis. 2020. 10.1016/S1473-3099(20)30721-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. FDA. Fraudulent Coronavirus Disease 2019 . (COVID‐19) Products . Published 2020. https://www.fda.gov/consumers/health-fraud-scams/fraudulent-coronavirus-disease-2019-covid-19-products. Accessed November 25, 2020.
- 137. FDA . COVID‐19 Update: FDA's Ongoing Commitment to Transparency for COVID‐19 EUAs. Published 2020. https://www.fda.gov/news-events/press-announcements/covid-19-update-fdas-ongoing-commitment-transparency-covid-19-euas. Accessed November 22, 2020.
- 138. Cohen AF, van Gerven J, Burgos JG, et al. COVID‐19 vaccines: the importance of transparency and fact‐based education. Br J Clin Pharmacol. 2020;86(11):2107‐2110. 10.1111/bcp.14581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Abbasi J. COVID‐19 and mRNA vaccines ‐ First large test for a new approach. JAMA ‐ J am Med Assoc. 2020;324(12):1125‐1127. 10.1001/jama.2020.16866 [DOI] [PubMed] [Google Scholar]
- 140. WHO . WHO R&D Blueprint: Novel Coronavirus ‐ An International Randomised Trial of Candidate Vaccines against COVID‐19.; 2020. https://www.who.int/blueprint/priority-diseases/key-action/Outline_CoreProtocol_vaccine_trial_09042020.pdf. Accessed November 22, 2020.
- 141. Alexander SPH, Kelly E, Mathie A, et al. The Concise Guide to PHARMACOLOGY 2019/20: Introduction and Other Protein Targets. Br J Pharmacol. 2019;176(S1):S1‐S20. 10.1111/bph.14747 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TABLE S1 List of all COVID‐19 vaccines currently under development. A list of all 221 vaccines that are in active development, with 55 in clinical trials and the other 166 in preclinical evaluation. Each vaccine is categorized based on its platform, developer, current stage of clinical evaluation and regulatory status, and clinical trial numbers. List was developed using both ClinicalTrials.gov and WHO datasets. Ad5: Adenovirus type 5; Ad26: Adenovirus type 26; MVA: modified vaccinia virus Ankara; LNP: lipid nanoparticles; RBD: Receptor binding domain; S‐2P: spike ectodomain with 2 proline substitutions; HLA‐DR: human leukocyte antigen–DR isotype; saRNA: self‐amplifying RNA; VSV: vesicular stomatitis virus; VLP: virus‐like particle; HBsAg: hepatitis B surface antigen; MMR: measles–mumps–rubella; IPV: inactivated polio vaccine; JE: Japanese encephalitis; HAV: hepatitis A; ZIKV: Zika virus; FMD: foot and mouth disease; SIV: simian immunodeficiency virus; RSV: respiratory syncytial virus; DENV: Dengue virus; EBOV: Ebola virus; MARV: Marburg marburgvirus; HIV: human immunodeficiency virus; CHIKV: Chikungunya; RVF: Rift Valley fever; HBV: hepatitis B virus; VEE: Venezuelan equine encephalitis; MERS: Middle East respiratory syndrome; VZV: varicella zoster virus; HSV: herpes simplex virus; HEV: hepatitis E virus; NiV: Nipah virus; CCHFV: Crimean–Congo haemorrhagic fever virus, H7N9: avian influenza A; NSCLC: nonsmall cell lung cancer; CMV: cytomegalovirus; GBM: glioblastoma
FIGURE 1 Summary of clinical trial size, age, and region for all vaccines. Details on all completed and ongoing Phase I/II/III clinical trials for COVID‐19 vaccines under development. Vaccines are categorized based on platform. List was developed using both ClinicalTrials.gov and WHO datasets.


