Abstract
This review aimed to evaluate the impact of obesity on the onset, exacerbation, and mortality of coronavirus disease 2019 (COVID‐19); and compare the effects of different degrees of obesity. PubMed, EMBASE, and Web of Science were searched to find articles published between December 1, 2019, and July 27, 2020. Only observational studies with specific obesity definition were included. Literature screening and data extraction were conducted simultaneously by two researchers. A random‐effects model was used to merge the effect quantity. Sensitivity analysis, subgroup analysis, and meta‐regression analysis were used to deal with the heterogeneity among studies. Forty‐one studies with 219,543 subjects and 115,635 COVID‐19 patients were included. Subjects with obesity were more likely to have positive SARS‐CoV‐2 test results (OR = 1.50; 95% CI: 1.37–1.63, I 2 = 69.2%); COVID‐19 patients with obesity had a higher incidence of hospitalization (OR = 1.54, 95% CI: 1.33–1.78, I 2 = 60.9%); hospitalized COVID‐19 patients with obesity had a higher incidence of intensive care unit admission (OR = 1.48, 95% CI: 1.24–1.77, I 2 = 67.5%), invasive mechanical ventilation (OR = 1.47, 95% CI: 1.31–1.65, I 2 = 18.8%), and in‐hospital mortality (OR = 1.14, 95% CI: 1.04–1.26, I 2 = 74.4%). A higher degree of obesity also indicated a higher risk of almost all of the above events. The region may be one of the causes of heterogeneity. Obesity could promote the occurrence of the whole course of COVID‐19. A higher degree of obesity may predict a higher risk. Further basic and clinical therapeutic research needs to be strengthened.
Keywords: COVID‐19, hospitalization, ICU admission, in‐hospital mortality, invasive mechanical ventilation, obesity, positive SARA‐CoV‐2 test result
1. INTRODUCTION
The coronavirus disease 2019 (COVID‐19)
 first appeared in December 2019 and has been confirmed to be caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2).
1
COVID‐19 is an infectious disease that could involve almost all the vital organs of the body. As of September 16, 2020, there have been more than 32 million COVID‐19 patients and 940,000 related deaths worldwide.
2
first appeared in December 2019 and has been confirmed to be caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2).
1
COVID‐19 is an infectious disease that could involve almost all the vital organs of the body. As of September 16, 2020, there have been more than 32 million COVID‐19 patients and 940,000 related deaths worldwide.
2
Studies have shown that obesity, as a worldwide epidemic, is associated with the severity and prognosis of COVID‐19. 3 , 4 If the effects mentioned above do exist, they would hinder the prevention and control of COVID‐19, cause more harm to COVID‐19 patients, and deserve health policymakers’ attention in various countries.
Some studies have systematically evaluated the impact of obesity on the whole process of COVID‐19. 5 , 6 However, whether the influences of various degrees of obesity are different, especially for the positive diagnosis of COVID‐19 and hospitalization, have not been involved in current systematic reviews.
The aim of this systematic review and meta‐analysis of observational studies is to evaluate the impact of obesity on the positive SARS‐CoV‐2 test result of subjects, hospitalization of COVID‐19 patients, and intensive care unit (ICU) admission, invasive mechanical ventilation (IMV), and in‐hospital mortality of hospitalized COVID‐19 patients, and to compare the effects of different body mass index (BMI) ranges.
2. MATERIALS AND METHODS
We reported this systematic review and meta‐analysis based on the PRISMA statement and the MOOSE checklist. 7 , 8 Our research protocol has been registered in PROSPERO—the international prospective register of systematic reviews (CRD42020203399). We carried out the project according to the research protocol.
2.1. Search strategy
Two authors with medical doctorates (Jun Yang and Ying Chen) conducted a systemic literature search on July 27, 2020. PubMed, EMBASE, and Web of Science databases were searched to find all the publications between December 1, 2019, and July 27, 2020, in English. The following keywords were used without other restrictions: (“overweight” OR “obesity” OR “obese” OR “BMI” OR “body mass index”) AND (“COVID‐19” OR “coronavirus disease 2019” OR “2019‐nCoV” OR “SARS‐CoV‐2” OR “2019 novel coronavirus”). We also searched the reference lists of included articles to find missing literature (Jun Yang and Ying Chen). The positive SARS‐CoV‐2 test result refers to a positive SARS‐CoV‐2 nucleic acid test result in nasopharyngeal swabs. COVID‐19 patients refer to subjects with positive SARS‐CoV‐2 test results.
2.2. Study selection
After importing the downloaded titles and abstracts into EndnoteX9 software, the duplicate documents were removed using the software's de‐duplication function and manual reading of the authors, titles, and journals (Jun Yang and Ying Chen). Clinical studies concerning COVID‐19 were screened out by two authors with medical postgraduate education background through reading the titles and abstracts. Those studies concerning the relationship between obesity and COVID‐19(both positive and negative) were selected by further reading the full text (Hongyu Chi and Ying Chen). If the screening results of the two researchers were inconsistent, it would be solved through negotiation.
The final included studies should be observational ones with targeted outcome indicators, including positive SARS‐CoV‐2 test results, hospitalization, ICU admission, invasive mechanical ventilation, and in‐hospital mortality. These papers should also include the odds ratio (OR) and 95% confidence intervals (CIs) of outcome indicators of obesity compared to those without. For in‐hospital mortality, the value could be OR, risk ratio (RR), or hazard ratio (HR). Those studies with the following characteristics would be excluded: cases overlap with other larger ones, have less than 20 cases, or without a clear definition of obesity (for non‐Asian: BMI ≥ 30 kg/m2; for Asian: BMI ≥ 28 kg/m2) or overweight (for non‐Asian: 30 kg/m2>BMI ≥ 25 kg/m2; for Asian: 28 kg/m2>BMI ≥ 24 kg/m2). In this process, if two studies overlap in both source hospital and collection time, we consider the overlap of cases. In this case, we would include the larger one.
We have contacted 11 authors by E‐mail who had not mentioned the exact definition of obesity in their papers. Six of them have responded with the detailed diagnostic criteria for obesity. One has provided data that were not published in the original article. The other five studies were not included in the final analysis.
2.3. Data extraction
We established an information extraction table. Two researchers with medical postgraduate education background (Jun Yang and Congmin Tian) independently extracted the literature information. If there were any differences, it would be solved through negotiation and discussion.
Information extracted from each of the included studies included: (1) basic information of the article (the first author and title); (2) characteristics of the survey (country, the period of participation, the cut‐off point of BMI, outcome indicators, study type); (3) characteristics of subjects (source of subjects, caseload, number of males, age); (4) summary measures: OR, RR, or HR as mentioned above. We would prioritize the adjusted values provided in the original text rather than the unadjusted ones calculated based on binary variables.
2.4. Statistical analysis
Stata16.0 software was used to merge effect indicators and calculate other related values. I 2 was used to calculate the heterogeneity among studies. It would be considered low, medium, high, and very high in the range of ≤25%, 25%–50%, 50%–75%, and ≥75%. 9 The advantage of this approach relies on its independence of the number of studies included. When the heterogeneity was high or very high, we would find possible sources by sensitivity analysis, subgroup analysis, and meta‐regression analysis. These potential sources include region, caseload, age, study type, and type of value. When the heterogeneity was very high, we would not carry out a meta‐analysis but just conduct a systematic review. As the heterogeneity among studies could not be entirely measured by I 2, we used the random‐effects model to merge the effect indicators. This method would take into account both intra‐study and inter‐study variation.
2.5. Risk of bias assessment
To assess the risk of bias in the included literature, two researchers (Jun Yang and Congmin Tian) independently used the Newcastle‐Ottawa Quality Assessment Scale (NOS) to score the quality of each research. 10 Most of the included studies scored 7 or above, indicating that the overall quality was high. The inconsistency was solved through negotiation and discussion.
We drew a funnel plot and made a preliminary judgment from the visual symmetry to assess the risk of bias among the included articles. Also, Egger's test was conducted, and p < .05 indicated that the existence of publication bias could not be rejected.
3. RESULTS
3.1. General study characteristics
The PubMed, EMBASE, and Web of Science databases were searched for a total of 1913 records. The included literature references were explored in the later stage, and two pieces of literature were further included. A total of 917 articles remained after de‐duplication, 696 of which were not clinical observational studies and were further removed. Full‐text browsing led to the elimination of 180 pieces, and 41 papers were finally included in the systematic review (Figure 1). Two pairs of studies with overlapping cases were included in the meta‐analysis but belonged to different outcome indicators. 3 , 13
Figure 1.
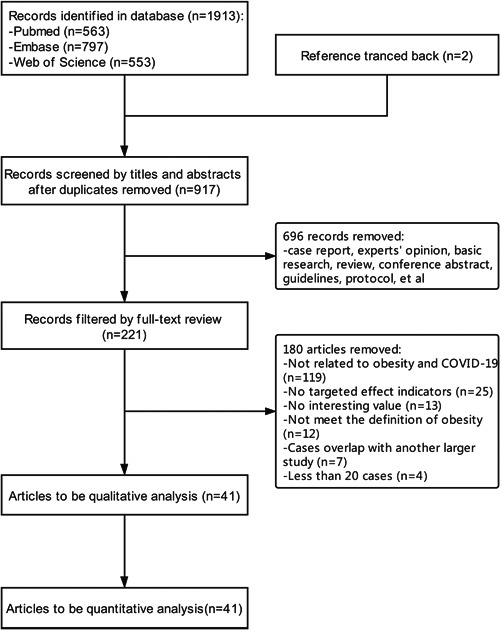
Flow chart of literature screening
The included studies were mainly conducted in the USA and Europe, including 23, 5, 3, 2, 2, 2, 1, 1, 1, and 1 from the USA, Italy, France, Spain, the UK, China, Mexico, Greece, Brazil, and international cooperation among the USA, Italy, and Spain. The 41 studies included 219,543 subjects receiving the SARS‐CoV‐2 test and 115,635 confirmed COVID‐19 patients. The number of patients included in a single survey ranged from 46 to 51,633. Most studies included more male patients, and those older than 60 years are also in the majority. All the studies were conducted and published in 2020. The detailed information of each paper is shown in Table 1. The research quality scores based on the NOS are shown in Table S1.
Table 1.
Characteristics of included studies
| First author | Region | Patient source and period | N (M) | Age (average/median) | Outcome | Studytype‐1 | Studytype‐2 |
|---|---|---|---|---|---|---|---|
| Argenziano MG | USA |
Presbyterian/Columbia University Irving Medical Center Mar 1st–Apr 5th, 2020 |
1000 (596) | 63 |
Hospitalization ICU admission |
Retrospective | Case‐control study |
| Argyropoulos KV | USA |
Emergency department in Manhattan, New York Mar 12th–18th, 2020 |
205 (108) |
Nonhospitalized: 45 Hospitalized: 60 |
Hospitalization | Retrospective | Case‐control study |
| Barbero P | Spain |
Hospital Universitario 12 de Octubre, Madrid Mar 3rd–May 31st, 2020 |
91 (0) | ‐ | Hospitalization | Retrospective | Case‐control study |
| Bello‐Chavolla OY | Mexico |
The General Directorate of Epidemiology of Mexican Ministry of Health As to May 18th, 2020 |
Positive: 51,633 (29,803) Negative: 98,567 (47,177) |
Positive: 46.65 Negative: 42.25 |
Positive test Hospitalization ICU admission IMV In‐hospital mortality |
Retrospective | Case‐control study |
| Busetto L | Italy |
Padova University Hospital Mar 23rd–Apr 11th, 2020 |
92 (57) | 70.5 |
ICU admission IMV In‐hospital mortality |
Prospective | Cohort study |
| Cai QX | China |
The Third People's Hospital of Shenzhen Jan 11th–Feb 16th, 2020 followed until Mar 26th, 2020 |
383 (184) |
Normal: 50 Underweight: 35.5 Overweight: 50 Obesity: 48 |
ICU admission IMV |
Prospective | Cohort study |
| Cariou B | France |
53 French centers Mar 10th–31st, 2020 |
1317 (855) | 69.8 | In‐hospital mortality | Prospective | Case‐control study |
| Caussy C | France |
Lyon University Hospital Feb 27th–Apr 8th, 2020 |
291 | ‐ | IMV | Prospective | Cohort study |
| Chao JY | USA |
The Children's Hospital at Montefiore in New York City Mar 15th–Apr 13th, 2020 |
46 (31) |
Non‐ICU: 3.6 ICU: 14.8 |
ICU admission | Retrospective | Case‐control study |
| Ciceri F | Italy |
San Raffaele Hospital in Milano Feb 25th–Mar 24th |
410 (299) | 65 | In‐hospital mortality | Prospective | Case‐control study |
| Cravedi P |
USA Italy Spain |
12 centers in the international TANGO consortium Mar 2nd–May 15th, 2020 |
144 (94) | 62 | In‐hospital mortality | Retrospective | Case‐control study |
| de Lusignan S | UK |
Oxford RCGP Research and Surveillance Center network Jan 28th–April 4th, 2020 |
3802 (1612) |
Men: 58.0 Women: 51.5 |
Positive test | Retrospective | Case‐control study |
| Docherty AB | UK |
208 acute care hospitals in England, Wales, and Scotland Feb 6th–Apr 19th, 2020 |
20,133 (12,068) | 72.9 | In‐hospital mortality | Prospective | Case‐control study |
| Duanmu Y | USA |
Stanford Health Care in Santa Clara County Mar 4th–23rd, 2020 |
100 (56) | 45 | Hospitalization | Prospective | Case‐control study |
| Ebinger JE | USA |
Cedars‐Sinai Health System in Los Angeles, California Mar 8th–21st, 2020 |
442 (256) | 52.72 |
Hospitalization ICU admission IMV |
Retrospective | Case‐control study |
| Giacomelli A | Italy |
Luigi Sacco Hospital in Milan Feb 21st–Mar 19th, 2020 |
233 (161) | 61 | In‐hospital mortality | Prospective | Case‐control study |
| Goyal P | USA |
Weill Cornell Medicine in Manhattan, New York Mar 5th–27th, 2020 |
1687 (1004) | 66.5 | In‐hospital mortality | Retrospective | Cohort study |
| Gupta S | USA |
ICUs at 65 hospitals across the US Mar 4th–Apr 4th, 2020 |
2215 (1436) | 60.5 | In‐hospital mortality | Prospective | Case‐control study |
| Hajifathalian K | USA |
New York ‐ Presbyterian Hospital and Weill Cornell Medical Center in New York Mar 4th–Apr 9th, 2020 |
770 (468) | 64 |
ICU admission IMV In‐hospital mortality |
Retrospective | Cohort study |
| Halasz G | Italy |
Guglielmo da Saliceto Hospital in Piacenza February–April 2020 |
242 (194) | 64 | In‐hospital mortality | Retrospective | Case‐control study |
| Halvatsiotis P | Greece |
8 hospitals in Greece Mar 10th–Apr 13th, 2020 |
90 (72) | 65.5 | In‐hospital mortality | Retrospective | Case‐control study |
| Hashemi N | USA |
Single healthcare system in Massachusetts Mar 11th–Apr 2nd, 2020 |
363 (201) | 63.4 |
ICU admission IMV In‐hospital mortality |
Retrospective | Case‐control study |
| Hur K | USA |
Northwestern‐affiliated healthcare centers in Chicago, Illinois Mar 1st–Apr 8th, 2020 |
486 (271) | 59 | IMV | Retrospective | Case‐control study |
| Kalligeros M | USA |
Rhode Island Hospital, The Miriam Hospital, or Newport Hospital in Rhode Island Feb 17th–April 5th, 2020 |
103 (63) | 60 | ICU admission | Retrospective | Case‐control study |
| Killerby ME | USA |
Hospitalized in Metropolitan Atlanta, Georgia Mar 1st–30th, 2020 |
531 (228) |
Nonhospitalized: 45 Hospitalized: 61 |
Hospitalization | Retrospective | Case‐control study |
| Kim L | USA |
154 acute care hospitals in 74 counties in 13 states Mar 1st–May 2nd, 2020 |
2491 (1326) | 62 | ICU admission | Retrospective | Case‐control study |
| Klang E | USA |
5 hospital campuses in Mount Sinai, New York Mar 1st–May 17th, 2020 |
3406 (1961) |
Age ≤ 50: Survivors 40.0 Non‐survivors 46.5 Age > 50: Survivors 68.0 Non‐survivors 76.0 |
IMV In‐hospital mortality |
Retrospective | Case‐control study |
| Lighter J | USA |
NYU Langone Health Mar 4th–Apr 4th, 2020 |
3615 | ‐ |
Hospitalization ICU admission |
Retrospective | Case‐control study |
| Mani VR | USA |
Harlem Hospital in Manhattan, New York March–April 2020 |
184 (111) | 64.72 | IMV | Retrospective | Case‐control study |
| Nakeshbandi M | USA |
SUNY Downstate Health Sciences University in New York Mar 10th–Apr 13th, 2020 |
504 (263) | 68 |
Intubation In‐hospital mortality |
Retrospective | Cohort study |
| Petrilli CM | USA |
NYU Langone Health Mar 1st–Apr 8th, 2020 |
Positive: 5279 (2615) Hospitalization: 2741 (1678) |
Positive: 54 |
Positive test Hospitalization In‐hospital mortality |
Prospective | Case‐control study |
| Pettit NN | USA |
University of Chicago Medical Center Mar 1st–Apr 18th, 2020 |
238 (113) | 58.5 |
ICU admission IMV In‐hospital mortality |
Retrospective | Cohort study |
| Rottoli M | Italy |
Sant'Orsola Hospital Mar 1st–Apr 20th, 2020 |
482 (302) | 66.2 |
ICU admission In‐hospital mortality |
Retrospective | Cohort study |
| Salacup G | USA |
Henry Ford Health System in metropolitan Detroit, Michigan Mar 9th–27th, 2020 |
242 (123) | 66 | In‐hospital mortality | Retrospective | Case‐control study |
| Shah P | USA |
Phoebe Putney Memorial Hospital, Albany, Georgia Mar 2nd–May 6th, 2020 |
522 (218) | 63 | In‐hospital mortality | Retrospective | Case‐control study |
| Simonnet A | France |
Roger Salengro Hospital Feb 27th–Apr 5th, 2020 |
124 (90) | 60 | IMV | Retrospective | Case‐control study |
| Soares R | Brazil |
The State Health Secretariat page from Esp'ırito Santo state Last updated on June 11th, 2020 |
10,713 (4804) | < 60 |
Hospitalization In‐hospital mortality |
Retrospective | Case‐control study |
| Suleyman G | USA |
Henry Ford Health System in metropolitan Detroit, Michigan Mar 9th–27th, 2020 |
463 (204) | 57.5 |
Hospitalization ICU admission |
Retrospective | Case‐control study |
| Toussie D | USA |
Mount Sinai Hospital in New York city Mar 10th–26th, 2020 |
338 (210) | 39 |
Hospitalization IMV |
Retrospective | Case‐control study |
| Urra JM | Spain |
University Hospital of Ciudad Real Mar 1st–Apr 15th, 2020 |
172 (104) | ‐ | ICU admission | Retrospective | Case‐control study |
| Zhang F | China |
Tongji Hospital and Wuhan Pulmonary Hospital Feb 7th–Mar 27th, 2020 |
53 | ‐ | In‐hospital mortality | Retrospective | Case‐control study |
Abbreviations: Apr, April; Feb, February; ICU, Intensive Care Unit; IMV, invasive mechanical ventilation; Jan, January; M, male; Mar, March; N, number; Studytype‐1, retrospective/prospective; Studytype‐2, cohort study/case‐control study; UK, United Kingdom; USA, United States of America.
3.2. Positive SARS‐CoV‐2 test result
This section included three studies, which were from the USA, 11 Mexico, 14 and the UK, 15 respectively. A total of 164,622 subjects were tested for SARS‐CoV‐2 nucleic acid, and 57499 were positive. The positive rate ranged from 15.4% to 49.7% among included studies.
Pooled analysis showed that subjects with obesity had a higher incidence of positive test results than those without (OR = 1.50, 95% CI: 1.37–1.63, I 2 = 69.2%, Figure 2A). The trend of the pooled results did not change after each study was removed (Figure S1). Due to the small number of included studies, no subsequent subgroup analysis, meta‐regression, or funnel plot were conducted.
Figure 2.
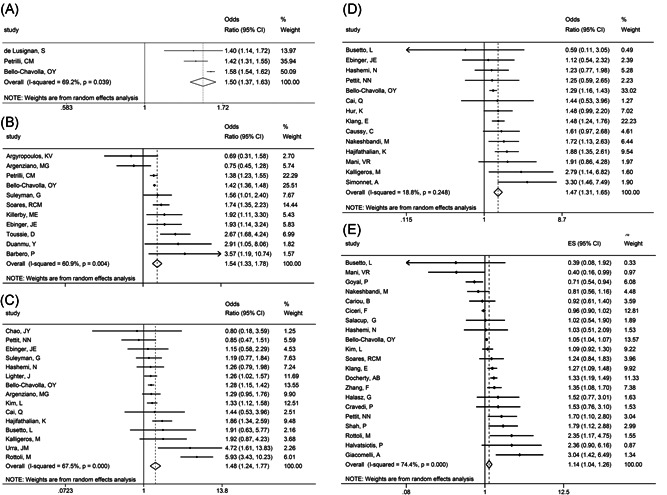
Meta‐analysis of effect indicators in subjects with obesity compared to those without. A, Positive SARS‐CoV‐2 test result. B, Hospitalization. C, ICU admission. D, Invasive mechanical ventilation. E, In‐hospital mortality
We further compared the possibility of positive test results among subjects receiving the SARS‐CoV‐2 test with different BMI ranges. The results showed that a higher BMI indicates a higher possibility of positive test result (25 ≤ BMI < 30 vs. BMI < 25: OR = 1.58, 95% CI: 1.44–1.72, I 2 = 0.0%; 30 ≤ BMI < 40 vs. BMI < 25: OR = 1.86, 95% CI: 1.69–2.04, I 2 = 0.0%; BMI ≥ 40 vs. BMI < 25: OR = 1.85, 95% CI: 1.28–2.66, I 2 = 58.6%; Figure S2).
3.3. Hospitalization
A total of 11 studies were included in this section, including eight from the USA 11 , 13 , 16 , 17 , 18 , 19 , 20 , 21 and the remaining three from Brazil, 22 Mexico, 14 and Spain. 23 Of the 70795 confirmed patients included, 25,403 were hospitalized. The hospitalization rate ranged from 10.8% to 85.0% among included studies. All the research studies were case‐control studies.
Pooled analysis showed that COVID‐19 patients with obesity had a higher incidence of hospitalization than those without (OR = 1.54, 95% CI: 1.33–1.78, I 2 = 60.9%, Figure 2B). The trend of this result did not change after each study was excluded (Figure S3). We conducted subgroup analysis and meta‐regression analysis on all included studies. We found no confounding factors causing heterogeneity among studies (Tables S2 and S5).
We further compared the possibility of hospitalization among COVID‐19 patients with different BMI ranges. The results showed that a higher BMI would predict higher possibility of hospitalization (25 ≤ BMI < 30 vs. BMI < 25: OR = 1.30, 95% CI: 1.09–1.57, I 2 = 0.0%; 30 ≤ BMI < 40 vs. BMI < 25: OR = 2.09, 95% CI: 1.34–3.26, I 2 = 51.5%; BMI ≥ 40 vs BMI < 25: OR = 2.76, 95% CI: 1.76–4.32, I 2 = 25.8%; Figure S4).
3.4. ICU admission
A total of 15 studies were included, 10 from the USA, 3 , 13 , 16 , 19 , 24 , 25 , 26 , 27 , 28 , 29 two from Italy, 30 , 31 and the remaining three from China, 32 Mexico, 14 and Spain, 33 respectively. Of all the 30,268 inpatients from 15 studies, 4086 in 29905 from 14 studies involving the exact number of patients requiring ICU admission, with the remaining one just provide OR value. The ICU admission rate of inpatients ranged from 9.1% to 44.3% among the 14 studies.
Pooled analysis showed that hospitalized COVID‐19 patients with obesity had a higher incidence of ICU admission than those without (OR = 1.48, 95% CI: 1.24–1.77, I 2 = 67.5%, Figure 2C). The trend of this result did not change after each study was excluded (Figure S5). We conducted subgroup analysis and meta‐regression analysis on all included studies and found region to be the possible confounding factor causing heterogeneity among studies (Tables S3 and S6).
We further compared the possibility of ICU admission among hospitalized patients with different BMI ranges. The results showed that patients with a higher BMI may have a higher trend of ICU admission, though they were not significant (25 ≤ BMI < 30 vs. BMI < 25: OR = 1.90, 95% CI: 0.89–4.07, I 2 = 0.0%; 30 ≤ BMI < 40 vs. BMI < 25: OR = 1.44, 95% CI: 0.67–3.09, I 2 = 0.0%; BMI ≥ 40 vs. BMI < 25: OR = 2.19, 95% CI: 0.51–9.35, I 2 = 63.8%; Figure S6).
3.5. Invasive mechanical ventilation
A total of 14 studies were included, including nine from the USA, 19 , 25 , 26 , 27 , 29 , 34 , 35 , 36 , 37 two from France, 4 , 38 and the remaining three from China, 32 Mexico 14 and Italy, 30 respectively. Of all the 25,945 hospitalized patients from 14 studies, 2789 in 22,176 patients from 12 papers had received IMV with a detailed description. The remaining two just provide OR values. The IMV rate of inpatients ranged from 9.1% to 68.5% among the 12 studies.
Pooled analysis showed that hospitalized COVID‐19 patients with obesity had a higher incidence of receiving IMV than those without (OR = 1.47, 95% CI: 1.31–1.65, I 2 = 18.8%, Figure 2D).
We further compared the possibility of IMV among hospitalized patients with different BMI ranges. The results showed that a higher BMI may indicate a higher possibility of IMV (25 ≤ BMI < 30 vs. BMI < 25: OR = 1.60, 95% CI: 0.97–2.65, I 2 = 0.0%; 30 ≤ BMI < 40 vs. BMI < 25: OR = 2.02, 95% CI: 1.23–3.30, I 2 = 0.0%; BMI ≥ 40 vs. BMI < 25: OR = 3.73, 95% CI: 1.86–7.50, I 2 = 30.9%; Figure S7).
3.6. In‐hospital mortality
A total of 23 studies were included in this section, including 11 from the USA, 11 , 12 , 25 , 26 , 28 , 29 , 35 , 37 , 39 , 40 , 41 five from Italy, 30 , 31 , 42 , 43 , 44 and the remaining seven from Brazil, 22 China, 45 France, 46 Greece, 47 Mexico, 14 UK, 48 and international cooperation among the USA, Italy and Spain, 49 respectively. Of all the 54938 patients from 23 studies, 8259 in 51330 inpatients from 19 studies involved a specific death toll. The remaining four just provide OR values. The in‐hospital mortality rate ranged from 10.1% to 43.5% among the 19 studies.
Pooled analysis showed that hospitalized COVID‐19 patients with obesity had a higher incidence of in‐hospital mortality than those without (OR = 1.14, 95% CI: 1.04–1.26, I 2 = 74.4%, Figure 2E). The direction of this result did not change after each study was excluded (Figure S8). We conducted subgroup analysis and meta‐regression analysis on all included studies. We found no confounding factor causing heterogeneity among the included studies (Tables S4 and S7).
We further compared the possibility of in‐hospital mortality among COVID‐19 patients with different BMI ranges. The results showed that patients with BMI ≥ 40 have a higher possibility of in‐hospital mortality (25 ≤ BMI < 30 vs. BMI < 25: OR = 1.06, 95% CI: 0.79–1.42, I 2 = 0.0%; 30 ≤ BMI < 35 vs. BMI < 25: OR = 1.01, 95% CI: 0.74–1.39, I 2 = 0.0%; 35 ≤ BMI < 40 vs. BMI < 25: OR = 1.32, 95% CI: 0.88–1.96, I 2 = 0.0%; BMI ≥ 40 vs. BMI < 25: OR = 1.56, 95% CI: 1.07–2.26, I 2 = 0.0%; Figure S9).
3.7. Risk of bias
We have drawn funnel charts for the effect indicators of the included literature concerning hospitalization, ICU admission, IMV, and in‐hospital mortality. Preliminary judgments have shown that the figures’ points are all in symmetrical distribution (Figure 3). We further conducted the Egger's test and found all the p‐values were larger than 0.05, indicating no evidence of publication bias (Table S8).
Figure 3.
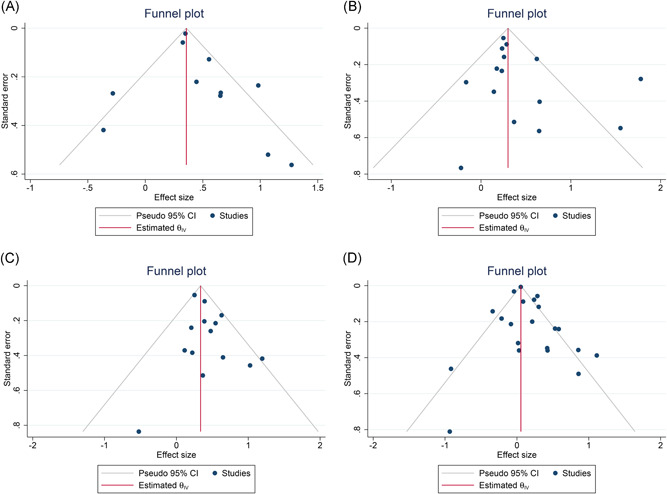
Funnel plots of the literature concerning effect indicators. A, Hospitalization. B, ICU admission. C, Invasive mechanical ventilation. D, In‐hospital mortality
4. DISCUSSION
This systematic review and meta‐analysis found that subjects with obesity were more likely to show positive results in the detection of SARS‐CoV‐2. Obese COVID‐19 patients were more likely to be hospitalized than those without. Hospitalized COVID‐19 patients with obesity were more likely to receive ICU admission, invasive mechanical ventilation, and die than those without. A higher degree of obesity also indicates a higher risk of occurrence for the above events.
Ten systematic reviews have assessed the relationship between obesity and COVID‐19. Tamara et al. 50 have included three retrospective cohort studies and found that obesity could significantly increase the risk of severe conditions in COVID‐19 patients. Our research team conducted the first meta‐analysis on this topic at almost the same time. 51 We have found that the BMI of COVID‐19 patients with severe conditions was significantly higher than those with mild conditions. The risk of developing severe conditions in COVID‐19 patients with obesity was significantly higher than those without. Zhou et al., 52 Sales‐Peres et al., 53 and Malik et al. 6 have come to almost the same conclusion as us. Pranata et al. 54 looked at the association between higher BMI and the risk of composite adverse endpoints, death, and critical illness. They found that a higher BMI was associated with increased risk of these events. However, most studies’ BMI ranges were not consistent, which may lead to a decline in the reliability and extrapolation of the results. Hussain et al have found a significant correlation between BMI > 25 kg/m2 and increased mortality, needs for respiratory support, and critical illness in COVID‐19 patients. 55 Földi et al. 56 found that obesity was a risk factor for ICU admission and IMV therapy. They have also compared the risk of receiving IMV in different BMI ranges of COVID‐19 patients and found that a higher BMI indicates a higher risk of receiving IMV. Malik et al. 57 have observed the prevalence of COVID‐19 to be 0.60 among patients with BMI < 25 kg/m2 compared to 0.34 among patients with BMI > 25 kg/m2. However, as they included a small sample of retrospective case‐control studies, such results’ credibility needs to be further validated. Most of these studies adopt composite endpoints, which have made it difficult to assess the impact of obesity on the risk of specific single endpoint events. During this paper's writing, a meta‐analysis published in the latest issue of Obesity Reviews had systematically evaluated outcome indicators of COVID‐19 patients with obesity. 5 Unlike current studies, this one has set a strict definition of obesity and BMI segmentation points for comparing the risk of five outcome events in subjects with different BMI ranges. In this study, we used the index of in‐hospital mortality rather than overall mortality to reduce the impact of other factors on mortality. This paper also tries to find the heterogeneity source and finds that region may be an essential factor.
Many factors may participate in the aggravation of obesity on COVID‐19. Obesity could increase the expression of ACE2‐ and CD147‐related genes in the bronchus and blood, while the latter two are receptors for SARS‐CoV‐2 to invade. 58 This would make obese subjects more susceptible to infection and may explain the higher positive test rate of SARS‐CoV‐2 to some extent. Obesity and concomitant metabolic syndrome may bring about potential damages to organ function, making lung, kidney and other organs more prone to the state of dysfunction. 59 Adipose tissue may become the site of virus retention due to the increased expression of ACE2 in obese subjects, which could slow down virus clearance and aggravate the infection. 60 The low‐level inflammation caused by obesity may also damage the immune system and make it abnormal in the infection of SARS‐Cov‐2. Obesity itself may lead to increased chest and abdominal pressure, limiting the expansion of the lungs. 61 In the case of COVID‐19, obese patients would be more prone to acute respiratory distress. These factors could largely explain why obese patients are more likely to be infected with SARS‐Cov‐2 and be more severe after infection.
By integrating the results of different original studies, this study aims to avoid the limitation of the applicability of conclusions caused by the insufficient number of cases in a single research study and the single source of patients. Nevertheless, there are inevitable limitations in this study. The included studies may have different implementation standards for hospitalization, ICU admission, IMV, and other COVID‐19 patients’ treatments, impacting the final meta‐analysis results’ reliability. Second, the risk of bias in the included studies is inconsistent. The types of studies are mainly retrospective, including case‐control studies and cohort studies, which may further affect the conclusions’ reliability. Third, we only selected literature in English, which may lead to the omission of related non‐English literature and the lack of the final conclusion's reliability.
In this systematic review and meta‐analysis, we have systematically retrieved the existing literature and restricted the diagnosis of obesity and other BMI section cut‐off points. The results have indicated a promoting role of obesity for subjects in COVID‐19 diagnosis, for COVID‐19 patients in hospitalization, and for hospitalized COVID‐19 patients in ICU admission, invasive mechanical ventilation, and in‐hospital mortality. Simultaneously, subjects with a higher degree of obesity may have a greater risk of developing the above adverse outcomes. These findings suggest that obesity would bring about severe challenges to the prevention and control of this epidemic. It would also cause more pain and harm to COVID‐19 patients and deserves health policymakers' attention in various countries. To combat this potential impact in the context of the COVID‐19, we should maintain healthy diets and exercise habits while wearing masks frequently and keeping distance from others. Obesity is a risk factor for many diseases, and eating healthily and exercising regularly should be a program to be adhered to by people worldwide. Also, the pathophysiological characteristics of obese COVID‐19 patients need further study. When writing this paper, a newly published multicenter study further suggested the relationship between obesity and mortality in COVID‐19 patients, which further confirmed our conclusion. 62 This also indicates the importance of high‐quality observational studies and basic research.
5. CONCLUSIONS
Obesity could promote the occurrence of positive SARS‐Cov‐2 test results, hospitalization of COVID‐19 patients, ICU admission, invasive mechanical ventilation therapy, and in‐hospital mortality of hospitalized COVID‐19 patients. Subjects with a higher degree of obesity may have a greater risk of developing the adverse outcomes mentioned above. Further basic and clinical therapeutic research concerning this aggravation needs to be strengthened.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Jun Yang and Chunyan Zhu conceived the plan for this study. Jun Yang, Ying Chen, Congmin Tian, and Hongyu Chi conducted literature retrieval, screening, and data extraction. Jun Yang and Jiahao Li carried out statistical analysis. Congmin Tian and Jun Yang carried out the risk of bias scoring. Jun Yang and Ying Chen interpreted the findings; Jun Yang and Congmin Tian made figures. Jun Yang and Ying Chen made tables. Jun Yang wrote the first draft. Jun Yang, Ying Chen, Congmin Tian, Hongyu Chi, Jiahao Li, and Chunyan Zhu checked and revised the manuscript together. Jun Yang conducted the final examination and submission. The corresponding author (Jun Yang) gaurantees that all listed authors meet the author's criteria. No other authors who meet the criteria are omitted. We are grateful to Professor Omar Yaxmehen Bello‐Chavolla for his generous contribution to the unpublished data and Professor Na Lin for her help in forming the research team. The authors, who belong to the Institute of Chinese Materia Medica, China Academy of Chinese Medical Sciences, were funded by the Special project for training outstanding young scientific and technological talents (innovative type) of necessary scientific research business expenses of the China Academy of Chinese Medical Sciences (ZZ13‐YQ‐051).
Supporting information
Supporting information.
Yang J, Tian C, Chen Y, Zhu C, Chi H, Li J. Obesity aggravates COVID‐19: an updated systematic review and meta‐analysis. J Med Virol. 2021;93:2662–2674. 10.1002/jmv.26677
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Gupta A, Madhavan MV, Sehgal K, et al. Extrapulmonary manifestations of COVID‐19. Nat Med. 2020;26(7):1017‐1032. [DOI] [PubMed] [Google Scholar]
- 2. https://www.worldometers.info/coronavirus/. Accessed August 20, 2020.
- 3. Lighter J, Phillips M, Hochman S, et al. Obesity in patients younger than 60 years is a risk factor for Covid‐19 hospital admission. Clin Infect Dis. 2020;71(15):896‐897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Simonnet A, Chetboun M, Poissy J, et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) requiring invasive mechanical ventilation. Obesity. 2020;28(7):1195‐1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Popkin BM, Du S, Green WD, et al. Individuals with obesity and COVID‐19: a global perspective on the epidemiology and biological relationships. Obes Rev. 2020;21(11):e13128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Malik P, Patel U, Patel K, et al. Obesity a predictor of outcomes of COVID‐19 hospitalized patients—a systematic review and meta‐analysis [online ahead of print]. J Med Virol. 2020. 10.21002/jmv.26555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stroup DF. Meta‐analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283(15):2008‐2012. [DOI] [PubMed] [Google Scholar]
- 9. Sun X, Li Y, Cai L, Wang Y. Effects of physical activity interventions on cognitive performance of overweight or obese children and adolescents: a systematic review and meta‐analysis [online ahead of print]. Pediatr Res. 2020. 10.1038/s41390-41020-40941-41393 [DOI] [PubMed] [Google Scholar]
- 10. Kashyap S, Moher D, Fung MF, Rosenwaks Z. Assisted reproductive technology and the incidence of ovarian cancer: a meta‐analysis. Obstet Gynecol. 2004;103(4):785‐794. [DOI] [PubMed] [Google Scholar]
- 11. Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Salacup G, Lo KB, Gul F, et al. Characteristics and clinical outcomes of COVID‐19 patients in an underserved‐inner city population: a single tertiary center cohort [online ahead of print]. J Med Virol. 2020. 10.21002/jmv.26252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Suleyman G, Fadel RA, Malette KM, et al. Clinical characteristics and morbidity associated with coronavirus disease 2019 in a series of patients in metropolitan Detroit. JAMA Netw Open. 2020;3(6): e2012270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bello‐Chavolla OY, Bahena‐López JP, Antonio‐Villa NE, et al. Predicting mortality due to SARS‐CoV‐2: a mechanistic score relating obesity and diabetes to COVID‐19 outcomes in Mexico. J Clin Endocrinol Metab. 2020;105(8):2752‐2761. 10.1210/clinem/dgaa1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. de Lusignan S, Dorward J, Correa A, et al. Risk factors for SARS‐CoV‐2 among patients in the Oxford Royal College of General Practitioners Research and Surveillance Centre primary care network: a cross‐sectional study. Lancet Infect Dis. 2020;20(9):1034‐1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Argenziano MG, Bruce SL, Slater CL, et al. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. BMJ. 2020;369:m1996. 10.1136/bmj.m1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Argyropoulos KV, Serrano A, Hu J, et al. Association of initial viral load in severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) patients with outcome and symptoms. Am J Pathol. 2020;190(9):1881‐1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Duanmu Y, Brown IP, Gibb WR, et al. Characteristics of emergency department patients with COVID‐19 at a single site in Northern California: clinical observations and public health implications. Acad Emerg Med. 2020;27(6):505‐509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ebinger JE, Achamallah N, Ji H, et al. Pre‐existing traits associated with Covid‐19 illness severity. PLOS One. 2020;15(7):e0236240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Killerby ME, Link‐Gelles R, Haight SC, et al. Characteristics associated with hospitalization among patients with COVID‐19—Metropolitan Atlanta, Georgia, March–April 2020. Morb Mortal Wkly Rep. 2020;69(25):790‐794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Toussie D, Voutsinas N, Finkelstein M, et al. Clinical and chest radiography features determine patient outcomes in young and middle age adults with COVID‐19. Radiology. 2020;297(1):E197‐E206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Soares RCM, Mattos LR, Raposo LM. Risk factors for hospitalization and mortality due to COVID‐19 in Espírito Santo State, Brazil. Am J Trop Med Hyg. 2020;103(3):1184‐1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Barbero P, Mugüerza L, Herraiz I, et al. SARS‐CoV‐2 in pregnancy: characteristics and outcomes of hospitalized and non‐hospitalized women due to COVID‐19 [online ahead of print]. J Matern‐Fetal Neonat Med. 2020:1‐7. 10.1080/14767058.14762020.11793320 [DOI] [PubMed] [Google Scholar]
- 24. Chao JY, Derespina KR, Herold BC, et al. Clinical characteristics and outcomes of hospitalized and critically ill children and adolescents with coronavirus disease 2019 (COVID‐19) at a tertiary care medical center in New York City. J Pediatr. 2020;223:14‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hajifathalian K, Kumar S, Newberry C, et al. Obesity is associated with worse outcomes in COVID‐19: analysis of early data from New York City. Obesity. 2020;28(9):1606‐1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hashemi N, Viveiros K, Redd WD, et al. Impact of chronic liver disease on outcomes of hospitalized patients with COVID‐19: a multicenter United States experience. Liver Int. 2020;40:2515‐2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kalligeros M, Shehadeh F, Mylona EK, et al. Association of obesity with disease severity among patients with COVID‐19. Obesity. 2020;28(7):1200‐1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kim L, Garg S, O'Halloran A, et al. Risk factors for intensive care unit admission and in‐hospital mortality among hospitalized adults identified through the U.S. coronavirus disease 2019 (COVID‐19)‐associated hospitalization surveillance network (COVID‐NET). Clin Infect Dis 2020:ciaa1012. 10.1093/cid/ciaa1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pettit NN, MacKenzie EL, Ridgway JP, et al. Obesity is associated with increased risk for mortality among hospitalized patients with COVID‐19. Obesity. 2020;28(10):1806‐1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Busetto L, Bettini S, Fabris R, et al. Obesity and COVID‐19: an Italian snapshot. Obesity. 2020;28(9):1600‐1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rottoli M, Bernante P, Belvedere A, et al. How important is obesity as a risk factor for respiratory failure, intensive care admission and death in hospitalised COVID‐19 patients? Results from a single Italian centre. Eur J Endocrinol. 2020;183(4):389‐397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cai Q, Chen F, Wang T, et al. Obesity and COVID‐19 severity in a designated hospital in Shenzhen, China. Diabetes Care. 2020;43(7):1392‐1398. [DOI] [PubMed] [Google Scholar]
- 33. Urra JM, Cabrera CM, Porras L, Ródenas I. Selective CD8 cell reduction by SARS‐CoV‐2 is associated with a worse prognosis and systemic inflammation in COVID‐19 patients. Clin Immunol. 2020;217:108486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hur K, Price CPE, Gray EL, et al. Factors associated with intubation and prolonged intubation in hospitalized patients with COVID‐19. Otolaryngol Head Neck Surg. 2020;163(1):170‐178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Klang E, Kassim G, Soffer S, Freeman R, Levin MA, Reich DL. Morbid obesity as an independent risk factor for COVID‐19 mortality in hospitalized patients younger than 50. Obesity. 2020;28(9):1595‐1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mani VR, Kalabin A, Valdivieso SC, Murray‐Ramcharan M, Donaldson B. At the epicenter of the American Coronavirus outbreak—New York inner city hospital COVID‐19 experience and current data: a retrospective analysis. J Med Internet Res. 2020;22(9):e20548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nakeshbandi M, Maini R, Daniel P, et al. The impact of obesity on COVID‐19 complications: a retrospective cohort study. Int J Obes. 2020;44(9):1832‐1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Caussy C, Wallet F, Laville M, Disse E. Obesity is associated with severe forms of COVID‐19. Obesity. 2020;28(7):1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Goyal P, Ringel JB, Rajan M, et al. Obesity and COVID‐19 in New York City: a retrospective cohort study. Ann Intern Med. 2020:M20‐2730. 10.7326/M2720-2730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gupta S, Hayek SS, Wang W, et al. Factors associated with death in critically ill patients with coronavirus disease 2019 in the US. JAMA internal medicine. 2020;180(11):1‐12. 10.1001/jamainternmed.2020.3596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shah P, Owens J, Franklin J, et al. Demographics, comorbidities, and outcomes in hospitalized COVID‐19 Patients in rural Southwest Georgia. Ann Med. 2020;52(7):354‐360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ciceri F, Castagna A, Rovere‐Querini P, et al. Early predictors of clinical outcomes of COVID‐19 outbreak in Milan, Italy. Clin Immunol. 2020;217:108509. 10.1016/j.clim.2020.108509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Giacomelli A, Ridolfo AL, Milazzo L, et al. 30‐day mortality in patients hospitalized with COVID‐19 during the first wave of the Italian epidemic: a prospective cohort study. Pharmacol Res. 2020;158:104931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Halasz G, Leoni ML, Villani GQ, Nolli M, Villani M. Obesity, overweight and survival in critically ill patients with SARS‐CoV‐2 pneumonia: is there an obesity paradox? Preliminary results from Italy [online ahead of print]. Eur J Prev Cardiol. 2020. 10.1177/2047487320939675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhang F, Xiong Y, Wei Y, et al. Obesity predisposes to the risk of higher mortality in young COVID‐19 patients [online ahead of print]. J Med Virol. 2020. 10.21002/jmv.26039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cariou B, Hadjadj S, Wargny M, et al. Phenotypic characteristics and prognosis of inpatients with COVID‐19 and diabetes: the CORONADO study. Diabetologia. 2020;63(8):1500‐1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Halvatsiotis P, Kotanidou A, Tzannis K, et al. Demographic and clinical features of critically ill patients with COVID‐19 in Greece: the burden of diabetes and obesity. Diabetes Res Clin Pract. 2020;166:108331. 10.1016/j.diabres.2020.108331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Docherty AB, Harrison EM, Green CA, et al. Features of 20 133 UK patients in hospital with COVID‐19 using the ISARIC WHO Clinical Characterisation Protocol: Prospective observational cohort study. BMJ. 2020;369m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cravedi P, Mothi SS, Azzi Y, et al. COVID‐19 and kidney transplantation: results from the TANGO International Transplant Consortium. Am J Transplant. 2020;20:3140‐3148. 10.11111/ajt.16185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tamara A, Tahapary DL. Obesity as a predictor for a poor prognosis of COVID‐19: a systematic review. Diabetes Metab Syndr. 2020;14(4):655‐659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yang J, Hu J, Zhu C. Obesity aggravates COVID‐19: a systematic review and meta‐analysis [online ahead of print]. J Med Virol. 2020. 10.21002/jmv.26237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhou Y, Yang Q, Chi J, et al. Comorbidities and the risk of severe or fatal outcomes associated with coronavirus disease 2019: a systematic review and meta‐analysis. Int J Infect Dis. 2020;99:47‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sales‐Peres SHC, de Azevedo‐Silva LJ, Bonato RCS, Sales‐Peres MC, Pinto ACdS, Santiago Junior JF. Coronavirus (SARS‐CoV‐2) and the risk of obesity for critically illness and ICU admitted: meta‐analysis of the epidemiological evidence. Obes Res Clin Pract. 2020;14(5):389‐397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pranata R, Lim MA, Yonas E, et al. Body mass index and outcome in patients with COVID‐19: a dose‐response meta‐analysis [online ahead of print]. Diabetes Metab. 2020. 10.1016/j.diabet.2020.1007.1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hussain A, Mahawar K, Xia Z, Yang W, El‐Hasani S. Obesity and mortality of COVID‐19. Meta‐analysis. Obes Res Clin Pract. 2020;14(4):295‐300. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 56. Földi M, Farkas N, Kiss S, et al. Obesity is a risk factor for developing critical condition in COVID‐19 patients: a systematic review and meta‐analysis. Obes Rev. 2020;21(10):e13095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Malik VS, Ravindra K, Attri SV, Bhadada SK, Singh M. Higher body mass index is an important risk factor in COVID‐19 patients: a systematic review and meta‐analysis. Environ Sci Pollut Res Int. 2020;27:42115‐42123. 10.1007/s11356-11020-10132-11354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Radzikowska U, Ding M, Tan G, et al. Distribution of ACE2, CD147, CD26 and other SARS‐CoV‐2 associated molecules in tissues and immune cells in health and in asthma, COPD, obesity, hypertension, and COVID‐19 risk factors. Allergy. 2020;75:2829‐2845. 10.1111/all.14429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Shah D, Romero F, Guo Z, et al. Obesity‐induced endoplasmic reticulum stress causes lung endothelial dysfunction and promotes acute lung injury. Am J Respir Cell Mol Biol. 2017;57(2):204‐215. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 60. Csige I, Ujvárosy D, Szabó Z, et al. The impact of obesity on the cardiovascular system. J Diabetes Res. 2018;2018:3407306‐3407312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gheblawi M, Wang K, Viveiros A, et al. Angiotensin‐converting enzyme 2: SARS‐CoV‐2 Receptor and regulator of the renin‐angiotensin system. Circ Res. 2020;126(10):1456‐1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tartof SY, Qian L, Hong V, et al. Obesity and mortality among patients diagnosed with COVID‐19: results from an integrated health care organization. Ann Intern Med. 2020:M20‐3742. 10.7326/M3720-3742 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


