Abstract
Background
Severe coronavirus disease 2019 (COVID‐19) is characterized by an increased risk of thromboembolic events, with evidence of microthrombosis in the lungs of deceased patients.
Objectives
To investigate the mechanism of microthrombosis in COVID‐19 progression.
Patients/Methods
We assessed von Willebrand factor (VWF) antigen (VWF:Ag), VWF ristocetin‐cofactor (VWF:RCo), VWF multimers, VWF propeptide (VWFpp), and ADAMTS13 activity in a cross‐sectional study of 50 patients stratified according to their admission to three different intensity of care units: low (requiring high‐flow nasal cannula oxygenation, n = 14), intermediate (requiring continuous positive airway pressure devices, n = 17), and high (requiring mechanical ventilation, n = 19).
Results
Median VWF:Ag, VWF:RCo, and VWFpp levels were markedly elevated in COVID‐19 patients and increased with intensity of care, with VWF:Ag being 268, 386, and 476 IU/dL; VWF:RCo 216, 334, and 388 IU/dL; and VWFpp 156, 172, and 192 IU/dL in patients at low, intermediate, and high intensity of care, respectively. Conversely, the high‐to‐low molecular‐weight VWF multimers ratios progressively decreased with increasing intensity of care, as well as median ADAMTS13 activity levels, which ranged from 82 IU/dL for patients at low intensity of care to 62 and 55 IU/dL for those at intermediate and high intensity of care.
Conclusions
We found a significant alteration of the VWF‐ADAMTS13 axis in COVID‐19 patients, with an elevated VWF:Ag to ADAMTS13 activity ratio that was strongly associated with disease severity. Such an imbalance enhances the hypercoagulable state of COVID‐19 patients and their risk of microthrombosis.
Keywords: ADAMTS13 Protein, COVID‐19, Microvasculature, Severe acute respiratory syndrome coronavirus 2, Thrombosis, von Willebrand factor
Essentials
-
•
Microthrombosis has been reported in patients with severe coronavirus disease 2019 (COVID‐19).
-
•
We evaluated the VWF‐ADAMTS13 axis in 50 COVID‐19 patients admitted to a major Italian hospital.
-
•
Increased VWF antigen to ADAMTS13 activity ratio was strongly associated with COVID‐19 severity.
-
•
Such an imbalance increases the hypercoagulable state and the risk of microthrombosis in COVID‐19 patients.
Alt-text: Unlabelled Box
1. INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) is the cause of the ongoing pandemic of coronavirus disease 2019 (COVID‐19), which has spread around the world, causing more than 1.1 million deaths and a global health care, social, and economic crisis of unprecedented proportions.1 COVID‐19 is characterized by a wide variety of clinical manifestations, ranging from mild symptoms such as fever and cough to severe forms of pneumonia, potentially leading to acute respiratory distress, multiorgan failure, and death.2 The identification of biomarkers associated with variable disease severity, besides providing valuable insights into the disease mechanisms in place, are pivotal to help stratifying patients’ risk and developing the most efficacious therapies.
We, among others, have shown that marked hypercoagulability and perturbation of the endothelium are hallmarks of COVID‐disease, in which a high rate of venous thromboembolism has been consistently reported, particularly in patients with critical illness requiring intensive care.3., 4., 5., 6., 7. More recently, the hypothesis of pulmonary microvascular thrombosis as a driver of disease worsening has also emerged.8., 9., 10. Cardiopulmonary findings from 10 autopsies performed on COVID‐19 patients showed diffuse alveolar damage, with CD4+ aggregates around thrombosed small vessels and significant associated hemorrhage. Interestingly, evidence of thrombotic microangiopathy in the lungs was found and the pulmonary arteries at the hilum of each lung were free of thromboemboli. In all cases, small platelet‐rich thrombi were present within small vessels of the peripheral parenchyma and alveolar capillaries, highlighted by CD61 and von Willebrand factor (VWF) immunostaining.9
The role of VWF in primary hemostasis is essential, mediating platelet adhesion and aggregation at the sites of vascular injury.11 VWF is also a marker of endothelium activation, being massively released after inflammation‐mediated vascular damage.12 VWF multimers are regulated in size by ADAMTS13 (A Disintegrin And Metalloprotease with ThromboSpondin 1 repeats, number 13), the severe deficiency of which (activity below 10 IU/dL) is diagnostic for thrombotic thrombocytopenic purpura (TTP), a severe and life‐threatening thrombotic microangiopathy caused by the accumulation of hyperactive ultra‐large VWF multimers.13., 14. Reduced levels of this metalloprotease have been reported to be a risk factor for thrombosis in patients with ischemic stroke and myocardial infarction.15., 16. In line with this, TTP survivors with reduced ADAMTS13 activity during disease remission have a higher risk of TTP‐unrelated stroke,17 and congenital TTP patients treated with prophylactic ADAMTS13 replacement therapy have a reduced risk of ischemic stroke.18 In addition, the imbalance between high molecular weight VWF multimers and ADAMTS13 could cause a prothrombotic state in inflammatory‐induced conditions, as demonstrated in sepsis and overt disseminated intravascular coagulation.19., 20., 21.
Previous work from our group demonstrated an elevated level of VWF in COVID‐19 patients, increasing with the severity of the disease.4., 22. The aim of the present study was to focus on the VWF‐ADAMTS13 axis to better understand the pathophysiology of microthrombosis in COVID‐19. To do so, we assessed the VWF multimeric pattern, the VWF propeptide, and ADAMTS13 levels in patients with different degrees of disease severity.
2. PATIENTS AND METHODS
2.1. Patients and sample collection
We performed a cross‐sectional study of 50 COVID‐19 patients referred to the Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan (Italy), between the beginning of March and mid‐April 2020 and enrolled in the COHERENT study of our institution (“COVID‐19: Hemostasis, Immune Response, Endothelial Perturbation and Complement [COHERENT]”, Ethics Committee approval by Comitato Etico Area 2, Milano (no. 360_2020), according to the Helsinki Declaration of 1964 revised in 2013)22. Patients were clinically diagnosed with COVID‐19 and tested positive for SARS‐CoV‐2 by real‐time reverse transcriptase polymerase chain reaction of nasopharyngeal swabs. Patients were stratified based on their admission to three hospital units of different intensity of care and the type of ventilation support needed, as follows: 14 patients at low intensive care on high‐flow nasal cannula oxygen therapy; 17 patients at intermediate sub‐intensive care on continuous positive airway pressure; 19 patients at high intensive care, who needed intubation and mechanical ventilation. Samples were collected at a median time after admission at the hospital emergency room of 13 days (interquartile range [IQR] 6 to 18), ranging from 7 days for patients at intermediate intensity of care to 15 days for patients at low and high intensity of care.
Historical controls included 274 healthy volunteers recruited between 2006 and 2014 among friends and nonconsanguineous relatives of patients tested for thrombophilia at the Hemophilia and Thrombosis Center of Milan. Historical controls were recruited and tested in the frame of a different study.23
Peripheral blood samples of COVID‐19 patients were collected into 3.2% buffered sodium citrate solution‐containing evacuated tubes and centrifuged for 20 minutes at 3000g at controlled room temperature. Hematology testing of VWF antigen (VWF:Ag) and VWF ristocetin‐cofactor (VWF:RCo) activity was performed on freshly centrifuged capped evacuated tubes on a fully automated coagulation analyzer. The remaining plasma was aliquoted, nitrogen‐frozen, and stored at −80° until use.
2.2. Laboratory measurements
VWF:Ag and VWF:RCo were measured on an ACL TOP 700 analyzer (Werfen, Bedford, MA, USA) by means of immunoturbidimetric latex‐based assays using the commercial kits HemosIL von Willebrand Factor Antigen assay (HemosIL VWF:Ag, Werfen) and HemosIL von Willebrand Factor Ristocetin Cofactor Activity assay (HemosIL VWF:RCo, Werfen), which uses ristocetin and latex particles coated with a wild‐type recombinant glycoprotein Ibα instead of platelets. VWF propeptide (VWFpp) levels were determined with the anti‐human VWFpp MW1939 antibody pair and Tool Set 2 (Sanquin, Amsterdam, The Netherlands) according to the manufacturer's instructions.24 ADAMTS13 activity was measured using the FRETS‐VWF73 assay, as previously described.25 For all of these assays, results were expressed as IU/dL with reference to a pooled normal plasma calibrated with the relative World Health Organization (WHO) international standard (NIBSC codes 07/316 and 12/252).
The VWF multimeric pattern was assessed with the Hydragel 11 von Willebrand multimers kit (H11VWM) using the semiautomated HYDRASYS 2 instrumentation (Sebia, Lisses, France), as previously described.26 Agarose gel electrophoresis was performed using precast 2% agarose gels, direct immunofixation, and visualization with peroxidase‐labeled antibody and a specific substrate. Because of the high levels of the VWF:Ag, samples were tested at high dilutions to a final VWF:Ag concentration of 10 IU/dL. Densitometry was performed by Hydrasys 2 GelScan. The percentage of low molecular weight multimers, intermediate molecular weight multimers, and high molecular weight multimers was assessed using the Phoresis 8.6.3 software (Sebia) with peaks 1 through 3 designated as low molecular weight multimers, peaks 4 through 7 as intermediate molecular weight multimers, and peaks above 7 as high molecular weight multimers, according to the manufacturer's recommendations. Pooled normal plasma was included on each gel as reference sample.
2.3. Statistical analysis
Categorical variables were expressed as counts and percentages and continuous variables as medians and ranges. Study groups were compared using the nonparametric Kruskal‐Wallis H test. In case of statistical significance, the post hoc Dunn's test for pairwise comparisons followed, and Bonferroni‐adjusted P values were reported. To further evaluate the relationship between the analyzed laboratory parameters and the degree of intensive care as an ordinal variable, a Spearman's rank‐order correlation was run. Median differences with 95% confidence intervals were estimated using the Hodges‐Lehmann method to compare the results of VWF:Ag and ADAMTS13 activity in COVID‐19 patients and historical controls. The relationship between these markers and case‐control status was also studied by logistic regression analysis before and after adjusting for age and sex.
All statistical analyses were performed by SPSS, release 26.0 (IBM Corp.).
3. RESULTS
The baseline characteristics of the 50 COVID‐19 patients are reported in Table 1 The majority of patients were men (64% overall). Median age was 59 years, similar in all intensity care groups. Almost one‐half of the patients had at least one comorbidity among hypertension, diabetes, active cancer, or obesity. Among these, hypertension (28%) was the most prevalent, followed by obesity (22%).
Table 1.
Baseline characteristics of study subjects
| Overall (n = 50) | Intensity of Care |
|||
|---|---|---|---|---|
| Low (n = 14) | Intermediate (n = 17) | High (n = 19) | ||
| Male, n (%) | 32 (64) | 7 (50) | 12 (71) | 13 (68) |
| Age, y, median (range) | 59 (27‐85) | 58 (27‐85) | 60 (28‐79) | 59 (40‐71) |
| BMI, median (range) | 27 (18‐43) | 27 (18‐43) | 28 (18‐35) | 25 (24‐43) |
| Comorbidities, n (%)a | 22 (44) | 7 (50) | 8 (47) | 7 (37) |
| Hypertension, n (%) | 14 (28) | 6 (43) | 5 (29) | 3 (16) |
| Diabetes, n (%) | 4 (8) | 1 (7) | 2 (12) | 1 (5) |
| Active cancer, n (%) | 2 (4) | 0 (0) | 2 (12) | 0 (0) |
| Obesity, n (%) | 11 (22) | 4 (29) | 3 (18) | 4 (21) |
Abbreviations: BMI, body mass index.
At least one comorbidity among hypertension, diabetes, active cancer, and obesity.
VWF:Ag levels were markedly increased in COVID‐19 patients compared with historical controls (median difference 245 IU/dL) (Table 2 ). Conversely, ADAMTS13 activity levels were reduced (median difference −34 IU/dL), resulting in an even more remarkable increase in the VWF:Ag to ADAMTS13 activity ratio (median difference with historical controls 4.69). The association of these markers with case‐control status were maintained after adjusting by age and sex, as tested by logistic regression analysis (Table 2).
Table 2.
VWF and ADAMTS13 results in COVID‐19 patients and historical controls
| Cases (n = 50) | Controls (n = 274) | Median Difference (95% CI) | OR1 (95% CI) | OR2 (95% CI) | |
|---|---|---|---|---|---|
| VWF:Ag, IU/dL | 372 (281‐485) | 115 (88‐144) | 245 (212‐282) | 1.51 (1.33‐1.72) | 1.56 (1.34‐1.81) |
| ADAMTS13 activity, IU/dL | 65 (53‐80) | 100 (87‐113) | ‐34 (−40‐−28) | 0.43 (0.34‐0.54) | 0.46 (0.36‐0.58) |
| VWF:Ag to ADAMTS13 activity ratio | 6.07 (3.62‐8.59) | 1.14 (0.89‐1.48) | 4.69 (3.74‐5.61) | 12.75 (5.70‐28.55) | 20.59 (7.04‐60.19) |
Note
Results are expressed as median and interquartile range.
Median differences with 95% confidence intervals are reported, as well as results of logistic regression analysis before and after adjusting for age and sex. Estimated ORs for 10 (VWF:Ag and ADAMTS13 activity) and 1 (VWF:Ag to ADAMTS13 ratio) unit increase of the independent variable. Median difference with 95% confidence intervals estimated with the Hodges‐Lehmann method.
Abbreviations: CI, confidence interval; OR, odds ratio; OR1: unadjusted; OR2: adjusted for age and sex.
The results of the laboratory measurements stratified by the degree of care intensity are reported in Table 3 , Figure 1 , and Figure S1. As previously shown in COVID‐19 patients recruited at our institution in the frame of the COHERENT study,22 median platelet counts were within the normal range (130‐400 × 109/L) in all study groups. However, platelet counts of patients at intermediate and high intensity of care were increased to a similar extent in comparison with those of patients at low‐intensity care. Of patients with levels above the upper limit of the normal range there was only one in the low group (10%) versus six in the intermediate‐ (35%) and seven in the high‐intensity group (37%).
Table 3.
Laboratory results in COVID‐19 patients by intensity of care
| Intensity of Care |
Kruskal‐Wallis H Test P Value | Spearman Rho Correlation Coefficient, P Value | |||
|---|---|---|---|---|---|
| Low (n = 14) | Intermediate (n = 17) | High (n = 19) | |||
| Platelet count, ×109/La | 234 (173‐293) | 362 (304‐483) | 358 (299‐467) | 0.021 | 0.27, 0.065 |
| VWF:Ag, IU/dL | 268 (225‐309) | 386 (305‐468) | 476 (380‐537) | <0.001 | 0.61, <0.001 |
| VWF:RCo, IU/dL | 216 (188‐262) | 334 (257‐407) | 388 (328‐438) | <0.001 | 0.58, <0.001 |
| VWF:RCo to VWF:Ag ratio | 0.88 (0.76‐0.93) | 0.87 (0.79‐0.93) | 0.81 (0.79‐0.85) | 0.118 | −0.28, 0.049 |
| VWF propeptide, IU/dL | 156 (109‐175) | 172 (155‐274) | 192 (153‐261) | 0.052 | 0.321, 0.023 |
| VWF propeptide to antigen ratio | 0.60 (0.50‐0.74) | 0.54 (0.42‐0.70) | 0.45 (0.38‐0.59) | 0.068 | −0.331, 0.019 |
| HMW VWF multimers, % | 50.5 (47.0‐52.5) | 48 (42‐49) | 46 (39‐48) | 0.021 | −0.38, 0.006 |
| IMW VWF multimers, % | 33.5 (30.8‐37) | 36 (34.5‐40.0) | 36 (34‐39) | 0.117 | 0.27, 0.062 |
| LMW VWF multimers, % | 15.5 (13.8‐20.0) | 17 (14.5‐20.0) | 19 (17.0‐22‐0) | 0.089 | 0.31, 0.027 |
| HMW to IMW VWF multimer ratio | 1.50 (1.29‐1.75) | 1.35 (1.04‐1.47) | 1.28 (1.03‐1.39) | 0.034 | −0.36, 0.011 |
| HMW to LMW VWF multimer ratio | 3.18 (2.54‐3.80) | 2.76 (2.33‐3.38) | 2.30 (1.70‐3.06) | 0.035 | −0.37, 0.008 |
| ADAMTS13 activity, IU/dL | 82 (71‐101) | 62 (54‐67) | 55 (42‐68) | 0.001 | −0.50, <0.001 |
| VWF:Ag to ADAMTS13 activity ratio | 3.42 (1.98‐3.92) | 6.77 (4.62‐8.25) | 8.33 (5.49‐11.61) | <0.001 | 0.61, <0.001 |
Results are expressed as median and interquartile range.
Abbreviations: HMW, high molecular weight; IMW, intermediate molecular weight; LMW, low molecular weight; VWF:Ag, VWF antigen; VWF:RCo, von Willebrand ristocetin‐cofactor.
Available in 10 of 14 patients at low intensity of care.
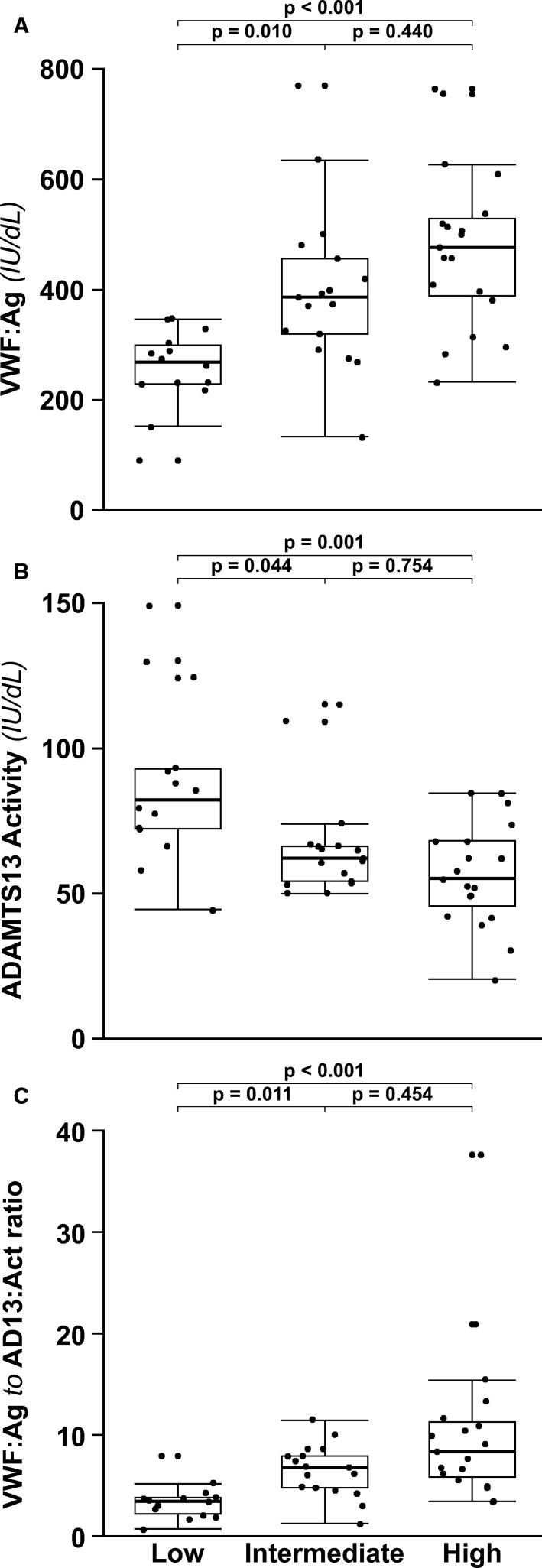
Figure 1.
Box plots of (A) VWF antigen, (B) ADAMTS13 activity, and (C) VWF antigen to ADAMTS13 activity ratio stratified by disease severity. Bonferroni‐adjusted P values of the post hoc Dunn's test for pairwise comparisons are reported. Black dots represent single values. Black dots above or below the range‐depicting horizontal lines represent outliers
VWF:Ag and VWF:RCo were higher than normal in the vast majority of patients, with values progressively increasing across the different levels of intensity of care (Spearman Rho coefficient 0.61 and 0.58, respectively). The increase of VWF:Ag was more pronounced than that of VWF:RCo activity and median VWF:RCo to VWF:Ag ratios resulted to be similar (low 0.88, intermediate 0.87) or slightly reduced (high 0.81). As expected from the antigen levels, VWFpp showed a gradual increase according to the level of care intensity (Spearman Rho coefficient 0.32). However, this increase was not proportional to that of VWF:Ag, leading to VWFpp to VWF:Ag median ratios decreasing from 0.60 to 0.54 and 0.45 (Spearman Rho coefficient −0.33).
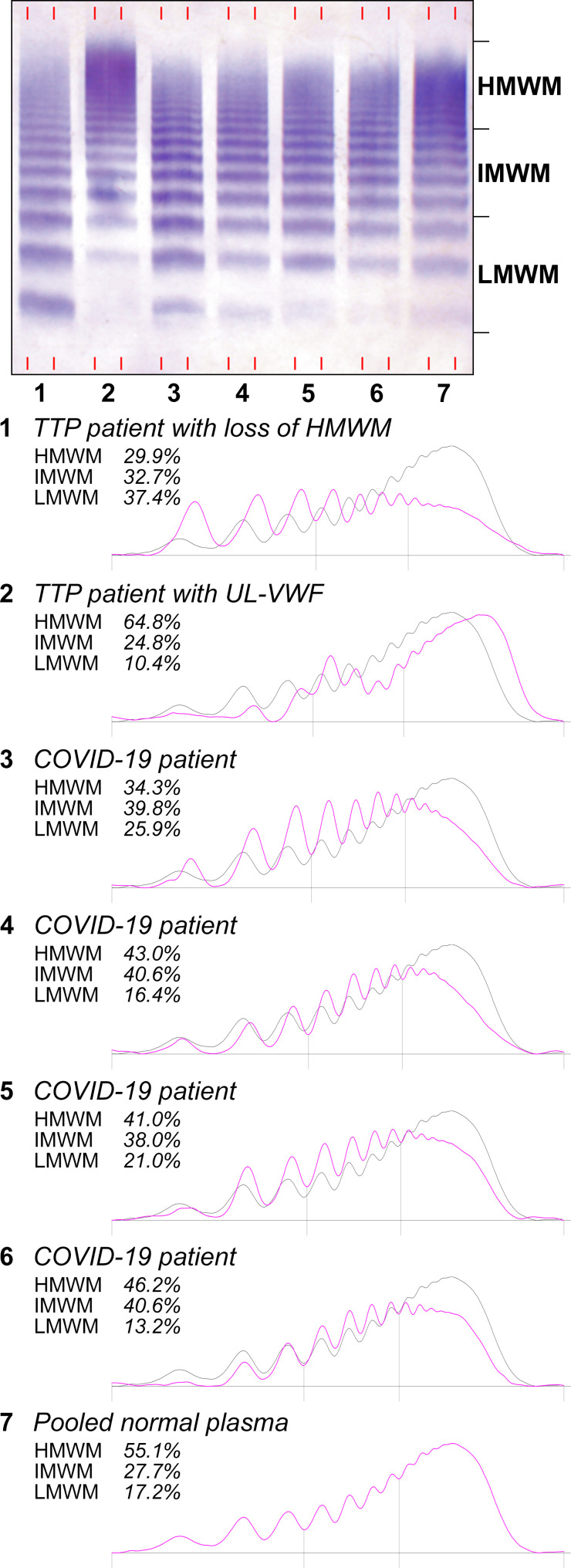
With regards to the VWF multimer pattern, the densitometric analysis revealed a progressively lower proportion of HMW VWF multimers with increasing intensity of care (Figure 2 ), as demonstrated by the decreased ratios of HMW to both IMW and LMW multimers in patients’ plasma. No evidence of ultra‐large VWF multimers was found.
Figure 2.
Representative multimeric structures of plasma VWF and densitometric analysis in COVID‐19 patients. Samples from TTP patients are included as pathologic reference. Lane 1: TTP patient at the first acute episode with loss of HMW VWF multimers; lane 2: TTP patient with undetectable ADAMTS13 activity during disease remission with ultra‐large VWF multimers (UL‐VWF); lanes 3 to 6: COVID‐19 patients with different degrees of HMW VWF multimers depletion; lane 7: pooled normal plasma. Low molecular weight multimers (LMWM, peaks 1 to 3), intermediate molecular weight multimers (IMWM, peaks 4 to 7) and high molecular weight multimers (HMWM, peaks 8 and above) are indicated on the right side of the blot image. The solid gray line and the pink line depict densitograms of pooled normal plasma and patient plasmas, respectively
A negative association of ADAMTS13 activity levels with the intensity of care was observed (Spearman Rho coefficient −0.50), with median values decreasing from 82 IU/dL (low) to 62 IU/dL (intermediate) to 55 IU/dL (high), and overall minimum‐maximum values ranging between 20 IU/dL and 149 IU/dL. The increase of VWF:Ag was paralleled by the decrease of ADAMTS13 activity, which yielded markedly elevated VWF:Ag to ADAMTS13 activity ratios that were three times higher than normal in the low‐intensity care patient group (median 3.42 vs 1.14) and further increased in patients at intermediate (median 6.77) and high intensity of care (median 8.33) (Tables 2 and 3, Figure 1).
4. DISCUSSION
Italy was the first European country to be hit by the COVID‐19 pandemic. Our hospital, one of the major hospitals in Lombardy, became a COVID‐19 hub for the management of patients. A high incidence of venous thromboembolic events in COVID‐19 patients, between 20% to 50% depending on the intensity of care and the severity of the clinical manifestations, was observed.5., 6., 7., 27., 28., 29., 30. In agreement with others, we found a state of hypercoagulability driven by high factor VIII levels, and a perturbation of the endothelium, with elevated VWF and complement activation.3., 4., 22. We thus decided to evaluate in more depth the role of VWF in COVID‐19 by assessing multiple biomarkers involved in a high shear microvessel environment, such as platelets, VWF antigen, VWF ristocetin‐cofactor activity, VWF multimeric pattern, VWF propeptide, and ADAMTS13, the VWF‐cleaving protease. We found a significant alteration in the platelet‐VWF‐ADAMTS13 axis in COVID‐19 patients, with extremely high VWF levels coexisting with normal to high platelet counts and an important increase of three‐ to seven‐fold of the VWF antigen to ADAMTS13 activity ratio associated with the severity of disease.
Under high shear stress conditions, VWF plays a key role in mediating platelet adhesion at the sites of vascular injury.11 However, under normal conditions, VWF and platelets circulate in blood without apparent interactions. This fine balance is regulated by several elements such as the VWF concentration and the size of its molecules, the number of circulating platelets, the changes of blood flow rate (shear stress),31 and the proteolytic activity of the plasma protease ADAMTS13. Alterations of these elements might result into hemorrhagic (acquired von Willebrand syndrome) or thrombotic (TTP) manifestations. The high levels of VWF found in COVID‐19 patients enhance its spontaneous interaction with circulating platelets. This interaction, mediated by the VWF A1 domain and the platelets receptor glycoprotein Ibα (GPIbα), is further supported by the elevated number of platelets (>400 x 109/L) present in 35% of the most severe COVID‐19 patients. This shifted equilibrium between platelets and VWF toward spontaneous binding may also increase the affinity and cleavage of ADAMTS13, which should control this interaction. Indeed, VWF A1‐GPIbα binding induces a conformational change in the adjacent VWF A2 domain (target of ADAMTS13 catalytic activity), increasing the cleavage efficiency by ADAMTS13.32
The severe deficiency of ADAMTS13 is the hallmark of TTP, a rare thrombotic microangiopathy characterized by acute episodes of thrombocytopenia, microangiopathic hemolytic anemia, and disseminated microvascular thrombosis. In TTP, mutations in the ADAMTS13 gene or a dysregulated autoimmune response cause a severe reduction of the enzyme activity, which is typically undetectable in plasma. This in turn causes the accumulation of ultra‐large VWF multimers, which, in the presence of high shear stress, spontaneously aggregate platelets leading to uncontrolled thrombus formation in the microcirculation.14 In the peripheral blood of our COVID‐19 patients, we observed a mild to moderate reduction of ADAMTS13 activity levels compared with historical, SARS‐CoV‐2‐negative controls. No patient presented a severe deficiency of ADAMTS13, with the lowest level being 20 IU/dL. However, a moderate ADAMTS13 reduction was observed in the more severe COVID‐19 cases, with about one‐third of patients in the high‐intensity care unit presenting ADAMTS13 activity levels below 50 IU/dL and VWF antigen levels above 150 IU/dL, thereby confirming an important prothrombotic status. In line with a reduced but functional enzyme, we found no evidence of ultra‐large VWF multimers in the peripheral plasma of COVID‐19 patients. Conversely, we observed a slight decrease of high molecular weight multimers with a corresponding relative increase of intermediate and low molecular weight VWF multimers, more pronounced in the most severe cases, in line with the reduced ratio between the VWF ristocetin‐cofactor activity and VWF antigen. These findings may be explained by an early increase of VWF proteolysis by ADAMTS13, which must overcome the massive release of VWF multimers by the activated endothelium as a consequence of local inflammation. Hypothetically, ADAMTS13 is ultimately consumed in the frame of this process, reaching the aforementioned reduced levels in the most severe cases. The decrease of high molecular weight multimers may also be explained by the formation of VWF‐platelet aggregates which results in the consumption of larger VWF multimers and platelets, as seen in TTP patients at presentation of a first acute event (Figure 2).33 In this regard, we did not see any evidence of thrombocytopenia in our patients, not even in the most severe cases in contrast to what was previously described.34 Nevertheless, it is reasonable to speculate that platelets and VWF spontaneously interact in these patients who have extremely elevated levels of VWF antigen and platelet counts, which, despite being normal in the majority of patients, tend to exceed or exceed the normal range upper limit in the most severe cases.
In severe COVID‐19, microthrombi may form onto the membrane of endothelial cells, where a significant amount of local inflammation leads to endothelial activation and massive release of VWF, altering the local relative concentration of VWF, platelets, and ADAMTS13.10., 35., 36. The results of autopsies performed by Fox and colleagues support this potential mechanism, because all 10 of their patients presented with evidence of thrombosis and microangiopathy in the small vessels and capillaries of the lungs, but only three had antemortem a low platelet count (80, 123, and 128 × 109/L, normal range 130‐400 × 109/L).9 This hypothesis is in line with emerging evidence of the crucial role of pulmonary endothelial cells in the initiation and evolution of severe COVID‐19.36., 37. This process may extend to other endothelial districts by means of systemic inflammation, leading to microthrombosis in other organs and promoting multiple organ failure, which may be observed in severe COVID‐19 patients.2
The increase of VWF antigen paralleled by decreased ADAMTS13 activity resulted in a three‐ to seven‐fold higher VWF to ADAMTS13 ratio than in historical controls. The ratio of 8.3 observed by us in the most severe patients is very similar to that of 8.5 reported by Huisman and Sikma in 12 patients with severe COVID‐19 disease requiring mechanical ventilation. The authors found reduced ADAMTS13 activity levels (mean 48 IU/dL) in the presence of very high VWF antigen levels (mean 408 IU/dL).38 A reduced ADAMTS13 activity (mean activity 49 IU/dL) was also reported by Bazzan et al in 88 Italian COVID‐19 patients (mean ADAMTS13 activity 49 IU/dL),39 with ADAMTS13 activity levels being significantly lower in nonsurvivors than survivors (32 IU/dL vs 51 IU/dL). Furthermore, nonsurvivors had higher VWF antigen levels (396 IU/dL vs 296 IU/dL) and lower platelet counts (140 vs 268 × 109/L) than survivors. At variance with the aforementioned studies and ours, Escher et al reported markedly elevated VWF but normal levels of ADAMTS13 activity in COVID‐19 patients, but their sample size was limited (only three patients, two of whom requiring high‐intensity care and intubation).40 Finally, Tiscia and colleagues reported a normal median level of ADAMTS13 activity of 70 IU/dL in another Italian cohort of 77 COVID‐19 patients, with patients with ADAMTS13 activity below 70 IU/dL showing a significantly lower survival at Kaplan‐Meier analysis.41 The type of patient populations and the severity of disease could play an important role in the different results across studies.
To further investigate the central role of the endothelium in the progression into severe COVID‐19, we also measured the VWF propeptide that, not being consumed by platelet aggregation or influenced by blood group,42 represents a more accurate marker of endothelial activation. Furthermore, because of the equimolar secretion but differential half‐lives of the two polypeptides,43 the VWF propeptide to VWF antigen ratio has been used as a tool to distinguish between acute or chronic vascular perturbation (ie, increase of both markers as seen in acute thrombotic microangiopathy or sepsis vs increase in VWF antigen alone as seen in diabetes)24 and to evaluate VWF clearance.41 We found increased levels of the VWF propeptide across all patient groups (overall mean 199 IU/dL vs 103 IU/dL in historical controls)44. However, the propeptide increase was less pronounced than that of VWF antigen, resulting in ratios lower than normal, especially in patients at high intensity of care (overall mean 0.54 vs 0.95 in historical controls)44. These results suggest both an acute ongoing stimulus to the endothelium and a diminished clearance of VWF, perhaps from the clogging of the related catabolic pathways.
This study has limitations. First, the sample size was limited, yet sufficient to identify significant differences across patients at three different intensity of care. Second, the study design was cross‐sectional, with tests performed on a single sample differentially taken during the course of hospitalization. Studies on larger cohorts of patients followed longitudinally after hospital admission are warranted to establish a causal role of the VWF‐ADAMTS13 axis in COVID‐19 progression and to estimate the risk for poor outcomes associated with each analyzed variable. To date, it is unclear whether the high levels of VWF are somehow drivers of COVID‐19 progression to its most severe forms or an epiphenomenon of the systemic inflammation characterizing the disease. Notwithstanding these limitations, we identified new potential markers of disease severity and provided further evidence supporting inflammatory microthrombogenesis in patients with severe COVID‐19.
In conclusion, our data do not suggest a dysfunctional VWF‐ADAMTS13 axis but rather a quantitatively imbalance between the substrate and the enzyme, with a seven‐fold increased VWF antigen to ADAMTS13 activity ratio associated with severe COVID‐19 that required high‐intensity care and mechanical ventilation. Based on these laboratory data, COVID‐19 microangiopathy does not resemble TTP, but rather a microangiopathy secondary to sepsis (with functional ADAMTS13, perhaps reduced from consumption), needed to tackle huge VWF plasma levels. The VWF‐ADAMTS13 imbalance further increases the hypercoagulable state promoted by COVID‐19 disease and the risk of microthrombosis in these patients.
CONFLICT OF INTEREST
Dr. Mancini received honoraria for participating as a speaker at educational meetings organized by Instrumentation Laboratory and Sanofi‐Genzyme, outside the submitted work. Dr. Novembrino received honoraria for lectures from Instrumentation Laboratory, Roche, Bayer, Novonordisk, and Sobi, outside the submitted work. Dr. Gualtierotti reports personal fees from Biomarin and Takeda, outside the submitted work. Dr. Aliberti reports personal fees from Bayer Healthcare, Grifols, Astra Zeneca, Zambon, GlaxoSmithKline, Menarini, and ZetaCube Srl; grants from Fisher & Paykel; and grants and personal fees from Chiesi and INSMED, outside the submitted work. Dr. Grasselli received payment for lectures and travel/congress registration support from Getinge, Draeger Medical, Biotest, Fisher & Paykel, Thermofisher, and MSD, outside the submitted work. Dr. Blasi reports personal fees from Guidotti, Grifols, Menarini, Mundipharma, Novartis, and Zambon; grants from Bayer; and grants and personal fees from Astrazeneca, Chiesi, GlaxoSmithKline, INSMED, and Pfizer, outside the submitted work. Dr. Peyvandi is a scientific advisory member of Bioverativ, Roche, Sanofi‐Genzyme, and Sobi, outside the submitted work. The other authors do not have any conflict of interests to disclose.
AUTHOR CONTRIBUTIONS
Ilaria Mancini contributed to design the study collected samples, analyzed the data, interpreted the results, and wrote the manuscript; Luciano Baronciani contributed to design the study, interpreted the results, and critically reviewed the manuscript; Andrea Artoni enrolled patients and critically reviewed the manuscript; Paola Colpani collected samples and performed experiments, contributed to data analysis, interpreted the results, and critically reviewed the manuscript; Marina Biganzoli, Giovanna Cozzi, Cristina Novembrino, and Massimo Boscolo performed experiments and critically reviewed the manuscript; Valentina de Zan enrolled patients, collected clinical and laboratory data, and critically reviewed the manuscript; Maria Teresa Pagliari performed experiments on historical controls and critically reviewed the manuscript; Roberta Gualtierotti enrolled patients and critically reviewed the manuscript; Stefano Aliberti, Mauro Panigada, Giacomo Grasselli, and Francesco Blasi enrolled patients and critically reviewed the manuscript; Flora Peyvandi designed the study, enrolled patients, interpreted the results, and critically reviewed the manuscript. All authors approved the final version of the manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. L.F. Ghilardini for his help in preparing the figures.
This work was partially supported by the Italian Ministry of Health ‐ Bando Ricerca Corrente and partially financed by Italian fiscal contribution "5x1000" 2017 devolved to Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico.
Italian fiscal contribution "5x1000" 2017 devolved to Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico
Italian Ministry of HealthBando Ricerca Corrente
Footnotes
Manuscript handled by: Jill Johnsen
Final decision: Jill Johnsen, 13 November 2020
Supporting Information
Figure S1
REFERENCES
- 1.WHO. Who weekly epidemiological update – 20 October 2020. Data as received by WHO from national authorities, as of 18 October 2020, 10 am CEST, 2020. https://www.who.int/publications/m/item/weekly‐epidemiological‐update–‐20‐october‐2020. Accessed October 24, 2020.
- 2.Zaim S., Chong J.H., Sankaranarayanan V., Harky A. COVID‐19 and multiorgan response. Curr Probl Cardiol. 2020;45:100618. doi: 10.1016/j.cpcardiol.2020.100618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cugno M., Meroni P.L., Gualtierotti R., et al. Complement activation in patients with COVID‐19: a novel therapeutic target. J Allergy Clin Immunol. 2020;146:215–217. doi: 10.1016/j.jaci.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Panigada M., Bottino N., Tagliabue P., et al. Hypercoagulability of COVID‐19 patients in intensive care unit. a report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost. 2020;18:1738–1742. doi: 10.1111/jth.14850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., et al. Incidence of thrombotic complications in critically ill ICU patients with COVID‐19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Middeldorp S., Coppens M., van Haaps T.F., et al. Incidence of venous thromboembolism in hospitalized patients with COVID‐19. J Thromb Haemost. 2020;18(8):1995–2002. doi: 10.1111/jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poissy J., Goutay J., Caplan M., et al. Pulmonary embolism in COVID‐19 patients: awareness of an increased prevalence. Circulation. 2020;142:184–186. doi: 10.1161/CIRCULATIONAHA.120.047430. [DOI] [PubMed] [Google Scholar]
- 8.Ciceri F., Beretta L., Scandroglio A.M., et al. Microvascular COVID‐19 lung vessels obstructive thromboinflammatory syndrome (MicroCLOTS): an atypical acute respiratory distress syndrome working hypothesis. Crit Care Resusc. 2020;22(2):95–97. doi: 10.51893/2020.2.pov2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fox S.E., Akmatbekov A., Harbert J.L., Li G., Quincy Brown J., Vander Heide R.S. Pulmonary and cardiac pathology in African American patients with COVID‐19: an autopsy series from New Orleans. Lancet Respir Med. 2020;8:681–686. doi: 10.1016/S2213-2600(20)30243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ackermann M., Verleden S.E., Kuehnel M., et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in covid‐19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruggeri Z.M. Von Willebrand factor, platelets and endothelial cell interactions. J Thromb Haemost. 2003;1:1335–1342. doi: 10.1046/j.1538-7836.2003.00260.x. [DOI] [PubMed] [Google Scholar]
- 12.Lip G.Y., Blann A. von Willebrand factor: a marker of endothelial dysfunction in vascular disorders? Cardiovasc Res. 1997;34:255–265. doi: 10.1016/s0008-6363(97)00039-4. [DOI] [PubMed] [Google Scholar]
- 13.Scully M., Cataland S., Coppo P., et al. Consensus on the standardization of terminology in thrombotic thrombocytopenic purpura and related thrombotic microangiopathies. J Thromb Haemost. 2017;15:312–322. doi: 10.1111/jth.13571. [DOI] [PubMed] [Google Scholar]
- 14.Sadler J.E. Pathophysiology of thrombotic thrombocytopenic purpura. Blood. 2017;130:1181–1188. doi: 10.1182/blood-2017-04-636431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sonneveld M.A.H., de Maat M.P.M., Portegies M.L.P., et al. Low ADAMTS13 activity is associated with an increased risk of ischemic stroke. Blood. 2015;126:2739–2746. doi: 10.1182/blood-2015-05-643338. [DOI] [PubMed] [Google Scholar]
- 16.Maino A., Siegerink B., Lotta L.A., et al. Plasma ADAMTS‐13 levels and the risk of myocardial infarction: an individual patient data meta‐analysis. J Thromb Haemost. 2015;13:1396–1404. doi: 10.1111/jth.13032. [DOI] [PubMed] [Google Scholar]
- 17.Upreti H., Kasmani J., Dane K., et al. Reduced ADAMTS13 activity during TTP remission is associated with stroke in TTP survivors. Blood. 2019;134:1037–1045. doi: 10.1182/blood.2019001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alwan F., Vendramin C., Liesner R., et al. Characterization and treatment of congenital thrombotic thrombocytopenic purpura. Blood. 2019;133:1644–1651. doi: 10.1182/blood-2018-11-884700. [DOI] [PubMed] [Google Scholar]
- 19.Ono T., Mimuro J., Madoiwa S., et al. Severe secondary deficiency of von Willebrand factor‐cleaving protease (ADAMTS13) in patients with sepsis‐induced disseminated intravascular coagulation: its correlation with development of renal failure. Blood. 2006;107:528–534. doi: 10.1182/blood-2005-03-1087. [DOI] [PubMed] [Google Scholar]
- 20.Martin K., Borgel D., Lerolle N., et al. Decreased ADAMTS‐13 (a disintegrin‐like and metalloprotease with thrombospondin type 1 repeats) is associated with a poor prognosis in sepsis‐induced organ failure. Crit Care Med. 2007;35:2375–2382. doi: 10.1097/01.ccm.0000284508.05247.b3. [DOI] [PubMed] [Google Scholar]
- 21.Kremer Hovinga J.A., Zeerleder S., Kessler P., et al. ADAMTS‐13, von Willebrand factor and related parameters in severe sepsis and septic shock. J Thromb Haemost. 2007;5:2284–2290. doi: 10.1111/j.1538-7836.2007.02743.x. [DOI] [PubMed] [Google Scholar]
- 22.Peyvandi F., Artoni A., Novembrino C., et al. Hemostatic alterations in COVID‐19. Haematologica. 2020 doi: 10.3324/haematol.2020.262634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pagliari M.T., Boscarino M., Cairo A., et al. ADAMTS13 activity, high VWF and FVIII levels in the pathogenesis of deep vein thrombosis. Thromb Res. 2020;197:132–137. doi: 10.1016/j.thromres.2020.10.037. [DOI] [PubMed] [Google Scholar]
- 24.van Mourik J.A., Boertjes R., Huisveld I.A., et al. von Willebrand factor propeptide in vascular disorders: A tool to distinguish between acute and chronic endothelial cell perturbation. Blood. 1999;94:179–185. [PubMed] [Google Scholar]
- 25.Lotta L.A., Valsecchi C., Pontiggia S., et al. Measurement and prevalence of circulating ADAMTS13‐specific immune complexes in autoimmune thrombotic thrombocytopenic purpura. J Thromb Haemost. 2014;12:329–336. doi: 10.1111/jth.12494. [DOI] [PubMed] [Google Scholar]
- 26.Bowyer A.E., Goodfellow K.J., Seidel H., et al. Evaluation of a semi‐automated von Willebrand factor multimer assay, the Hydragel 5 von Willebrand multimer, by two European Centers. Res Pract Thromb Haemost. 2018;2:790–799. doi: 10.1002/rth2.12141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Helms J., Tacquard C., Severac F., et al. High risk of thrombosis in patients with severe SARS‐CoV‐2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cui S., Chen S., Li X., Liu S., Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18:1421–1424. doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nahum J., Morichau‐Beauchant T., Daviaud F., et al. Venous thrombosis among critically ill patients with Coronavirus disease 2019 (COVID‐19) JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.10478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Llitjos J.‐.F., Leclerc M., Chochois C., et al. High incidence of venous thromboembolic events in anticoagulated severe COVID‐19 patients. J Thromb Haemost. 2020;18:1743–1746. doi: 10.1111/jth.14869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frankel D.S., Meigs J.B., Massaro J.M., et al. Von Willebrand factor, type 2 diabetes mellitus, and risk of cardiovascular disease: the Framingham Offspring Study. Circulation. 2008;118:2533–2539. doi: 10.1161/CIRCULATIONAHA.108.792986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nishio K., Anderson P.J., Zheng X.L., Sadler J.E. Binding of platelet glycoprotein Ibalpha to von Willebrand factor domain A1 stimulates the cleavage of the adjacent domain A2 by ADAMTS13. Proc Natl Acad Sci U S A. 2004;101:10578–10583. doi: 10.1073/pnas.0402041101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lotta L.A., Lombardi R., Mariani M., et al. Platelet reactive conformation and multimeric pattern of von Willebrand factor in acquired thrombotic thrombocytopenic purpura during acute disease and remission. J Thromb Haemost. 2011;9:1744–1751. doi: 10.1111/j.1538-7836.2011.04428.x. [DOI] [PubMed] [Google Scholar]
- 34.Lippi G., Plebani M., Henry B.M. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID‐19) infections: a meta‐analysis. Clin Chim Acta. 2020;506:145–148. doi: 10.1016/j.cca.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goshua G., Pine A.B., Meizlish M.L., et al. Endotheliopathy in COVID‐19‐associated coagulopathy: evidence from a single‐centre, cross‐sectional study. Lancet Haematol. 2020;7(8):e575–e582. doi: 10.1016/S2352-3026(20)30216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Varga Z., Flammer A.J., Steiger P., et al. Endothelial cell infection and endotheliitis in COVID‐19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Teuwen L.‐.A., Geldhof V., Pasut A., Carmeliet P. COVID‐19: the vasculature unleashed. Nat Rev Immunol. 2020;20:389–391. doi: 10.1038/s41577-020-0343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huisman A., Beun R., Sikma M., Westerink J., Kusadasi N. Involvement of ADAMTS13 and von Willebrand factor in thromboembolic events in patients infected with SARS‐CoV‐2. Int J Lab Hematol. 2020;42(5):e211–e212. doi: 10.1111/ijlh.13244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bazzan M., Montaruli B., Sciascia S., Cosseddu D., Norbiato C., Roccatello D. Low ADAMTS 13 plasma levels are predictors of mortality in COVID‐19 patients. Intern Emerg Med. 2020;15(5):861–863. doi: 10.1007/s11739-020-02394-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Escher R., Breakey N., Lämmle B. ADAMTS13 activity, von Willebrand factor, factor VIII and D‐dimers in COVID‐19 inpatients. Thromb Res. 2020;192:174–175. doi: 10.1016/j.thromres.2020.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tiscia G.L., Favuzzi G., De Laurenzo A., et al. Reduction of ADAMTS13 levels predicts mortality in SARS‐CoV‐2 patients. TH Open. 2020;4:e203–e206. doi: 10.1055/s-0040-1716379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nossent A.Y., van Marion V., van Tilburg N.H., et al. von Willebrand factor and its propeptide: the influence of secretion and clearance on protein levels and the risk of venous thrombosis. J Thromb Haemost. 2006;4:2556–2562. doi: 10.1111/j.1538-7836.2006.02273.x. [DOI] [PubMed] [Google Scholar]
- 43.Borchiellini A., Fijnvandraat K., ten Cate J.W., et al. Quantitative analysis of von Willebrand factor propeptide release in vivo: effect of experimental endotoxemia and administration of 1‐deamino‐8‐D‐arginine vasopressin in humans. Blood. 1996;88:2951–2958. [PubMed] [Google Scholar]
- 44.Stufano F., La Marca S., Pontiggia S., Musallam K.M., Peyvandi F. von Willebrand factor propeptide to antigen ratio in acquired thrombotic thrombocytopenic purpura. J Thromb Haemost. 2012;10:728–730. doi: 10.1111/j.1538-7836.2012.04642.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1