Abstract
The role of extracorporeal membrane oxygenation (ECMO) in the management of critically ill COVID‐19 patients remains unclear. Our study aims to analyze the outcomes and risk factors from patients treated with ECMO. This retrospective, single‐center study includes 17 COVID‐19 patients treated with ECMO. Univariate and multivariate parametric survival regression identified predictors of survival. Nine patients (53%) were successfully weaned from ECMO and discharged. The incidence of in‐hospital mortality was 47%. In a univariate analysis, only four out of 83 pre‐ECMO variables were significantly different; IL‐6, PCT, and NT‐proBNP were significantly higher in non‐survivors than in survivors. The Respiratory Extracorporeal Membrane Oxygenation Survival Prediction (RESP) score was significantly higher in survivors. After a multivariate parametric survival regression, IL‐6, NT‐proBNP and RESP scores remained significant independent predictors, with hazard ratios (HR) of 1.069 [95%‐CI: 0.986‐1.160], P = .016 1.001 [95%‐CI: 1.000‐1.001], P = .012; and .843 [95%‐CI: 0.564‐1.260], P = .040, respectively. A prediction model comprising IL‐6, NT‐proBNP, and RESP score showed an area under the curve (AUC) of 0.87, with a sensitivity of 87.5% and 77.8% specificity compared to an AUC of 0.79 for the RESP score alone. The present study suggests that ECMO is a potentially lifesaving treatment for selected critically ill COVID‐19 patients. Considering IL‐6 and NT‐pro‐BNP, in addition to the RESP score, may enhance outcome predictions.
Keywords: Acute respiratory distress syndrome, COVID‐19, critical care, extra corporeal membrane oxygenation, risk factors, SARS‐CoV‐2
1. INTRODUCTION
COVID‐19, caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection, is a global health crisis with over 46 million infections and approximately 1.2 million deaths as of November 1, 2020. 1 Most COVID‐19 patients present with no or mild symptoms. 2 , 3 However, in the early Chinese experience, 14% of patients were severely ill, and 5% developed critical respiratory failure, shock, and multi‐organ failure. 3 , 4 The mortality rate in critically ill patients is high: between 26% and 61%. 2 , 5 Using extracorporeal membrane oxygenation (ECMO) in these patients remains controversial, 6 , 7 , 8 and its beneficial effect in non‐COVID‐19 induced acute respiratory distress syndrome (ARDS) has been questioned. 9 , 10 , 11 Recently, veno‐venous (VV) ECMO support in ARDS has shown better outcomes and decreased mortality rates. 9 , 10 The current understanding of COVID‐19 pathophysiology is narrow, and knowledge on the utility of ECMO in COVID‐19 patients remains limited. 12 , 13 , 14 There is an urgent need for in‐depth analyses of ECMO use in COVID‐19 patients. We aimed to present our initial experience and outcomes of critically ill COVID‐19 patients supported with ECMO. Moreover, we intended to identify factors that affect the survival of COVID‐19 patients treated with ECMO and develop a potential predictive model.
2. PATIENTS AND METHODS
Heinsberg district, in the German state of North Rhine‐Westphalia, was one of the first regions presenting with a severe but localized outbreak of COVID‐19. The majority of critically ill COVID‐19 patients from the region were transferred to RWTH Aachen University Hospital, which serves as a tertiary care center for the area. Our center prepared for the SARS‐CoV‐2 pandemic by increasing ICU capacity; as such, triage was never undertaken based on ICU or mechanical ventilation availability.
This single‐center, retrospective observational study included all adult inpatients (≥18 years old) from March 1, 2020, to April 20, 2020, who were diagnosed with COVID‐19, according to the WHO interim guidance, 15 who developed severe COVID‐19 disease with ARDS, requiring ECMO support. The study was approved by the local ethics commission of RWTH University Hospital (EK 093/20). Due to the retrospective nature of the study, the ethical board waived informed consent.
2.1. Data collection
Demographics, medical history, treatment regimes, laboratory, mechanical ventilation parameters, and ECMO settings throughout the hospital stay, and outcomes were extracted from our patient data management systems (Philips IntelliSpace Critical Care and Anesthesia and Siemens Medico). Complications occurring post‐ECMO implantation, including multi‐organ failure and hemocompatibility‐related adverse events (HRAE), including bleeding and thromboembolic events, were recorded and analyzed.
2.2. ECMO indication and setting
Critically ill COVID‐19 patients, who presented with commonly accepted ECMO indications as suggested by the Extracorporeal Life Support Organization (ELSO), 16 in whom all other treatments options have been exhausted: lung protective invasive mechanical ventilation (MV); prone positioning; neuro‐muscular blockade; and inhaled nitric oxide (iNO) rescue therapy, were considered for ECMO treatment.
Our center’s standard operating procedure includes an evaluation by the ECMO‐team consisting of intensivist physicians, cardiothoracic surgeons, and pneumologists during daily ICU rounds. The ECMO‐team made the decision on the initiation of ECMO support after bedside assessment of the patient.
Our standard approach for the treatment of isolated respiratory failure is VV ECMO utilization. Percutaneous cannulation, using the Seldinger technique, 17 was our technique of choice for VV ECMO. Depending on the desired flow rate, and whenever possible, bi‐caval single‐site cannulation, using a dual‐lumen cannula (27 to 31 Fr), was preferentially performed over two‐site cannulation (femoral‐jugular or femoral‐femoral) with 19 to 25 Fr cannula. The decision whether single‐site double‐lumen cannulation or two sites cannulation was to be performed depends on many factors. Briefly, in general, when a patient with high BSA (2.2‐2.5 m2), a 25 Fr venous cannula as a drainage cannula is necessary, and for venous‐return, a 17‐19 Fr cannula will be appropriate to achieve enough flow with adequate carbon dioxide clearance and oxygenation. In our experience, the double‐lumen cannula 27‐31 Fr will not provide enough flow and adequate gas exchange in such settings. The double‐lumen cannula’s insertion and positioning are more complex and require more experience and the ability to perform precise transesophageal echocardiography guidance. Therefore, in emergent cases or when performing cannulation in an external hospital, the two sites’ cannulation (femoral‐ jugular) is more accessible, safer, and faster. Another factor in choosing the cannulae was the cannula’s availability during the COVID‐19 pandemic.
The positioning of the cannulae was performed under transesophageal echocardiographic control. The anticoagulation management for VV ECMO is to achieve and maintain a targeted activated partial thromboplastin time (aPTT) of 40–50 seconds (1 to 1.5 times above the normal range [20‐35 seconds]), using unfractionated heparin. During our study period, an increased risk of thromboembolic events in critically ill COVID‐19 patients was reported. Therefore, by 01/04, we altered anticoagulation in ECMO patients to aPTT of 50–60 seconds or an activated clotting time (ACT) of 170–180 seconds. As recommended by ELSO, 18 careful examinations of the whole ECMO circuit, using a flashlight, were performed twice per day to detect white platelet/fibrin thrombi and clots, usually identified as dark non‐moving areas on the surfaces. Pre and post membrane pressures are continuously monitored. Clotting in the oxygenator is represented by increasing membrane lung pressure gradient. 18
2.3. Laboratory analysis
Detailed laboratory analyses were performed daily and included complete blood count electrolytes; measures of hemostasis; hemolysis markers; biochemical tests of cardiac, renal, and liver function; NT‐pro‐brain natriuretic peptide (NT‐proBNP); interleukin 6 (IL‐6); procalcitonin (PCT); plasma C‐reactive protein (CRP); fibrinogen; and D‐dimers. Blood gas analyses were performed in intervals of 1‐2 hours.
2.4. Confirmation of SARS‐CoV‐2
Throat‐swabs, tracheal secretions, or bronchoscopic alveolar lavage were obtained for SARS‐CoV‐2 testing from each patient immediately at admission. COVID‐19 infection was confirmed by real‐time reverse‐transcription‐polymerase‐chain‐reaction (RT‐PCR) assays.
2.5. Statistical analysis
Categorical variables are presented as absolute numbers and percentages. Continuous variables were tested for normal distribution with the Kolmogorov‐Smirnov test and presented as the median and interquartile range (IQR) for non‐normally distributed variables and mean ± standard deviation (SD) for normally distributed data. Comparison between survivors and non‐survivors was accomplished through univariate analyses using a Mann‐Whitney U test or t test, where appropriate. Categorical variables were analyzed using Fisher’s exact test. To identify predictors of in‐hospital mortality and calculate the hazard ratio (HR) with a 95%‐confidence interval (95%‐CI), a multivariate parametric survival regression analysis was performed. The entry criteria for the multivariate analysis was a P value < .05 in the univariate analysis. We examined the receiver‐operator characteristic (ROC) curve and area under the curve (AUC) from each independent predictor, and all predictors combined, as a prediction model. Kaplan–Meier survival curves were generated, and the log‐rank test was used for a linear trend. All statistical comparisons were two‐sided. P values < .05 were considered significant. Parametric survival regression and ROC curves analyses were performed with STATA (Release 16, StataCorp, College Station, TX, USA). All other analyses were performed using R (Version 3.6, Vienna, Austria) and the Jamovi project (Version 1.2, https://www.jamovi.org).
3. RESULTS
3.1. Pre ECMO phase
During the study period, 17 COVID‐19 patients were treated with ECMO. Table 1 depicts the characteristics and laboratory data before ECMO implantation. 35% of the patients were female, the median age was 57 years (range 39‐73 yrs), with a median BMI of 28.2 kg/m2 (IQR 24.7, 31.1). Eleven patients (64%) presented with typical COVID‐19 symptoms at hospital admission (fever, dyspnea, diarrhea). The median body temperature at hospital admission was 38.6°C (37.3°C, 38.8°C).
TABLE 1.
Demographics and clinical course before ECMO initiation
| Total (n = 17) | Survivors (n = 9) | Non‐survivors (n = 8) | P value | |
|---|---|---|---|---|
| Age years | 57.0 (53.0, 62.0) | 57.0 (53.0, 60.0) | 57.5 (52.5, 62.0) | .89 |
| Female | 6 (35%) | 2 (22%) | 4 (50%) | .28 |
| BMI Kg/m2 | 28.2 (24.7, 31.1) | 28.2 (25.7, 30.5) | 30.1 (24.6, 36.9) | .58 |
| LVEF % | 55.0 (50.0, 55.0) | 55.0 (54.0, 55) | 52.5 (50.0, 57.5) | .89 |
| aHT | 6 (35%) | 4 (44.4%) | 2 (25%) | .40 |
| CAD | 1 (6%) | 1 (11%) | 0 (0%) | .33 |
| DM | 6 (35%) | 3 (33%) | 3 (38%) | .86 |
| KD | 14 (82%) | 8 (89%) | 6 (75%) | .45 |
| PAD | 1 (6%) | 0 | 1 (12%) | .27 |
| CVD | 0 | 0 | 0 | ‒ |
| Nicotine use | 4 (24%) | 2 (22%) | 2 (25%) | .89 |
| Prior pneumonia | 5 (29%) | 2 (22%) | 3 (38%) | .61 |
| COPD | 3 (18%) | 2 (22%) | 1 (12%) | .60 |
| HPL | 2 (12%) | 1 (11%) | 1 (12%) | .93 |
| AF | 6 (35%) | 4 (44%) | 2 (25%) | .40 |
| History of RHF | 4 (24%) | 1 (11%) | 3 (38%) | .20 |
| Prior MI | 1 (6%) | 1 (11%) | 0 (0%) | .33 |
| Prior PCI | 1 (6%) | 1 (11%) | 0 (0%) | .33 |
| PHT | 1 (6%) | 1 (11%) | 0 (0%) | .33 |
| Prior CS | 1 (6%) | 0 (0%) | 1 (12%) | .27 |
| Prior Lung Surgery | 1 (6%) | 1 (11%) | 0 (0%) | .33 |
| History of malignancy | 1 (6%) | 1 (11%) | 0 (0%) | .33 |
| Immunosuppressive agents | 1 (6%) | 0 (0%) | 1 (12%) | .27 |
| Typical symptoms | 17 (100%) | 9(100%) | 8(100%) | ‒ |
| Fever at admission | 9 (53%) | 6 (67%) | 3 (38%) | .23 |
| Temperature °C | 38.6 (37.3, 38.8) | 38.7 (37.9, 38.9) | 37.7 (36.3, 38.7) | .16 |
| Cough | 10 (58.8%) | 4 (44.4%) | 6 (75.0%) | .20 |
| Dyspnea | 12 (70.6%) | 6 (66.7%) | 6 (75.0%) | .71 |
| Diarrhea | 3 (17.6%) | 3 (33.3%) | 0 (0.0%) | .072 |
| Pre ECMO‐LOS days | 5.0 (4.0, 16.0) | 4.0 (3.0, 5.0) | 12.0 (5.0, 18.5) | .11 |
| Mechanical ventilation settings pre ECMO implantation | ||||
| MV pre‐ECMO days | 3 (3, 15) | 3 (3, 4) | 8.5 (2, 17) | .39 |
| iNO inhalation | 8 (47%) | 5 (56%) | 3 (38%) | .46 |
| FiO2 % | 80 (65, 100) | 80 (60, 100) | 75 (67.5, 97.5) | .96 |
| Pinsp mbar | 28 (25, 30) | 26 (25, 28) | 29 (28, 31) | .062 |
| PEEP mbar | 14 (12, 15) | 14 (10, 15) | 13.5 (12.5, 14.5) | .69 |
| VTe mL | 320 (280, 454) | 347 (295, 454) | 299 (219, 410) | .47 |
| Vf min−1 | 24 (22, 30) | 24 (22, 26) | 24 (23, 30.5) | .35 |
| CVP mmHg | 14.0 (11.0, 15.0) | 15.0 (11.0, 16.0) | 13.5 (11.0, 14.5) | .49 |
| mPAP mmHg | 30.0 (25.0, 33.0) | 29.0 (25.0, 32.0) | 32.5 (26.5, 34.0) | .53 |
| Antiviral treatment | 3 (18%) | 2 (22.2%) | 1 (12%) | 1.00 |
| Inotropes | 12 (70.6%) | 7 (77.8%) | 5 (62.5%) | .61 |
| Vasopressor | 15 (88.2%) | 8 (88.9%) | 7 (88%) | 1.00 |
| Blood gas and laboratory tests | ||||
| pO2 mmHg | 77.0 (67.0, 93.0) | 79.0 (67.0, 103.0) | 76.5 (60.5, 87.0) | .41 |
| pCO2 mmHg | 66.1 (47.7, 77.2) | 65.5 (35.4, 72.8) | 70.7 (55.0, 78.7) | .29 |
| pH | 7.3 (7.2, 7.4) | 7.3 (7.2, 7.4) | 7.3 (7.2, 7.3) | .70 |
| BE mmol/L | 1.8 (‐3.7, 6.5) | 1.8 (‐3.5, 2.3) | 3.1 (‐4.6, 7.8) | .47 |
| HCO3 mmol/L | 26.4 (24.7, 34.4) | 25.6 (22.3, 30.8) | 29.2 (24.9, 36.1) | .15 |
| Na+ mmol/L | 145.0 (142.0, 151.0) | 145.0 (144.0, 148.0) | 144.0 (139.5, 152.0) | .81 |
| K+ mmol/L | 4.40 (3.80, 4.60) | 4.50 (3.90, 4.60) | 4.30 (3.80, 4.65) | .88 |
| Hb g/dL | 9.3 (7.9, 9.9) | 9.4 (7.8, 9.9) | 9.2 (8.2, 11.1) | .81 |
| WBC /nL | 14.0 ± 6.9 | 14.9 ± 9.3 | 12.9 ± 3.0 | .54 |
| Platelets /nL | 226 (205, 389) | 226 (205, 290) | 243.3 (179.5, 458.5) | .96 |
| Creatinine mg/dL | 1.1 (0.8, 1.6) | 1.4 (0.8, 1.8) | 1.1 (0.9, 1.4) | .74 |
| BUN mg/dL | 64.0 (43.0, 111.0) | 62.0 (35.0, 77.0) | 67.5 (48.5, 132.5) | .25 |
| GLDH U/L | 8.6 (3.9, 12.0) | 4.3 (3.2, 8.6) | 11.0 (6.7, 13.0) | .10 |
| CK U/L | 234.0 (73.0, 598.0) | 234.0 (167.0, 715.0) | 338.5 (43.5, 597.0) | .39 |
| CK‐MB U/L | 21.0 (18.0, 27.0) | 23.0 (20.0, 27.0) | 18.0 (15.0, 40.0) | .36 |
| TnT pg/mL | 22.0 (12.0, 52.0) | 23.0 (10.0, 52.0) | 21.0 (15.0, 57.5) | .63 |
| Bilirubin mg/dL | 0.5 (0.4, 0.8) | 0.5 (0.4, 0.7) | 1.1 (0.4, 2.2) | .25 |
| ALT U/L | 36.5 (31.0, 45.0) | 32.0 (27.0, 39.0) | 44.0 (34.8, 83.0) | .092 |
| AST U/L | 66 (38, 126) | 61 (37, 91) | 79.5 (54, 154) | .370 |
| fpHb mg/L | 36.0 (22.0, 44.0) | 25.0 (20.0, 36.0) | 43.5 (29.5, 55.0) | .11 |
| LDH U/L | 403.0 (314.0, 480.0) | 403.0 (314.0, 480.0) | 369.5 (313.5, 560.0) | 1.00 |
| PTT seconds | 33.0 (28.6, 36.8) | 33.0 (28.6, 36.8) | 33.1 (28.2, 36.0) | .92 |
| INR | 1.3 (1.2, 1.3) | 1.3 (1.2, 1.3) | 1.2 (1.1, 1.3) | .65 |
| IL6 pg/mL | 255 (112, 404) | 112.4 (80, 287) | 422 (267.4, 1120) | .013 |
| CRP mg/L | 186.5 (120.0, 280.0) | 276.0 (120.0, 280.0) | 174.2 (127.5, 249.2) | .70 |
| PCT ng/mL | 5.1 (0.56, 6.9) | 1.13 (0.56, 2.7) | 6.7 (5.5, 9.1) | .026 |
| D‐dimer µg/dL | 454.7 (174.5, 1204.6) | 658.5 (376.4, 1874.4) | 283.8 (171.9, 829.6) | .34 |
| Fibrinogen mg/dL | 656.0 (432.0, 717.0) | 672.0 (462.0, 717.0) | 464.0 (414.5, 704.5) | .47 |
| NT‐proBNP pg/mL | 1765.0 (605.0, 4122.0) | 706.9 (438.0, 1765.0) | 4097.0 (1765.7, 8065.0) | .043 |
Abbreviations: AF, atrial fibrillation; ALT, alanine transaminase; AST, aspartate transaminase; aHT, arterial hypertension; BE, base excess; BMI, body mass index; BUN, blood urea nitrogen; CAD, coronary artery disease; CK, creatine kinase; CK‐MB, creatine kinase myocardial band; COPD, chronic obstructive pulmonary disease; CS, cardiac surgery; CVD, cerebrovascular disease; CVP, central venous pressure; DM, diabetes mellitus; FiO2, fraction of inspired oxygen; ECMO, extracorporeal membrane oxygenation; fpHb, free plasma hemoglobin; GLDH, glutamate dehydrogenase; Hb, hemoglobin; HCO3, hydrogen carbonate; HLP, hyperlipoproteinemia; K+, potassium; KD, kidney disease; iNO, inhaled nitric oxide; ICU, intensive care unit; IL‐6, interleukin‐6; LDH, lactate dehydrogenase; LOS, length of stay; LVEF, left ventricular ejection fraction; MI, myocardial infarction; mPAP, mean pulmonary artery pressure; MV, mechanical ventilation; Na+, sodium; NT‐proBNP, N‐terminal prohormone of brain natriuretic peptide; PAD, peripheral arterial disease; pCO2, partial pressure of carbon dioxide; pCRP, plasma C‐reactive protein; PCT, procalcitonin; PHT, pulmonary hypertension; PCI, percutaneous coronary intervention; pO2, partial pressure arterial oxygen; PTT, partial thromboplastin time; RHF, right heart failure; PEEP, positive end‐expiratory pressure; Pinsp, maximal inspiratory pressure; Vf, ventilation frequency; VTe, expiratory tidal volume; WBC, white blood cells; TnT: Troponin T.
One patient had a history of cardiac surgery; one patient had coronary artery disease, with a history of myocardial infarction and percutaneous coronary intervention; four patients (24%) were active smokers; five patients (29%) had pneumonia in their medical history; and one patient had a history of lung surgery, due to adenocarcinoma; one patient was on immunosuppressive medication because of kidney transplantation. All patients had a PaO2/FiO2 ratio < 100 before ECMO initiation (range 53‐75). The median duration of MV before ECMO was three days (3, 15). Eight patients (47%) had iNO before ECMO support.
Three patients (18%) received antiviral medication, all 17 patients required antibiotics for bacterial superinfection, and all patients had prone position treatment and initial neuromuscular blockade. In the last blood gas analysis prior to ECMO, the median pO2 was 77 mmHg (67, 93) and the median pCO2 was 66 mmHg (47, 77). Prior to ECMO implantation, the mean leukocyte number was 14 ± 6.9 /nL, median IL‐6 level was 255 pg/nL (112, 404), median PCT was 5.1 µg/L (0.56, 6.9), median C‐reactive protein was 186 ng/mL (120, 280), NT‐proBNP was 1765 pg/mL (605, 4122), and creatine kinase‐MB was 21 U/L (18, 27). Further details are presented in Table 1.
3.2. ICU scoring system
Details of all ICU scores just before ECMO initiation and on the last day of ECMO support are presented in Table 2. Glasgow Coma Score (GCS) at hospital admission was 13.8 ± 0.8. Prior to ECMO initiation, mean scores were as follows: Sequential Organ Failure Assessment score (SOFA) 11.9 ± 9.4 9; Richmond Agitation Sedation Scale (RASS) −2.9 ± 5.1; Simplified Acute Physiology Score II (SAPS II) 46.1 ± 11.8; Respiratory Extracorporeal Membrane Oxygenation Survival Prediction (RESP) score −1.0 ± 2.7; predicted survival probability ranged from 15% to 75%.
TABLE 2.
Intensive care unit calculated risk scores
| Total (n = 17) | Survivors (n = 9) | Non‐survivors (n = 8) | P value | |
|---|---|---|---|---|
| GCS at hospital admission | 13.9 ± 0.8 | 13.8 ± 0.8 | 14.1 ± 0.8 | .97 |
| Pre‐ECMO implantation | ||||
| RESP score | −1.0 ± 2.7 | 0.2 ± 2.0 | −2.6 ± 2.8 | .046 |
| NEMS | 38.9 ± 10.6 | 41.8 ± 4.8 | 35.6 ± 14.4 | .21 |
| NEMS+SAS | 42.2 ± 10.6 | 44.8 ± 4.2 | 39.4 ± 14.8 | .41 |
| RASS | −2.9 ± 5.1 | −4.1 ± 0.2 | −1.6 ± 7.5 | .80 |
| SAPS II | 46.1 ± 11.8 | 46.7 ± 8.7 | 45.4 ± 15.2 | .88 |
| SAS | 2.9 ± 2.3 | 2.5 ± 0.7 | 3.3 ± 3.4 | .84 |
| SOFA | 11.9 ± 9.4 | 9.8 ± 2.3 | 12.5 ± 7.9 | .85 |
| Core‐10‐TISS | 17.4 ± 4.7 | 16.7 ± 3.7 | 18.1 ± 5.7 | .63 |
| Last day on ECMO / pre‐explantation | ||||
| NEMS | 41.5 ± 11.2 | 45.9 ± 4.4 | 36.6 ± 14.7 | .059 |
| NEMS+SAS | 45.1 ± 11.9 | 48.6 ± 4.9 | 41.1 ± 16.2 | .29 |
| RASS | −1.88 ± 7.99 | −3.75 ± 0.46 | 0.00 ± 11.34 | .91 |
| SAPSII | 46.1 ± 8.7 | 47.5 ± 10.1 | 44.8 ± 7.4 | .71 |
| SAS | 3.6 ± 4.7 | 2.5 ± 0.8 | 4.8 ± 6.6 | .57 |
| SOFA | 14.8 ± 6.3 | 13.0 ± 2.1 | 16.9 ± 8.8 | .28 |
| Core‐10‐TISS | 18.6 ± 10.0 | 21.6 ± 10.4 | 15.4 ± 9.1 | .33 |
Abbreviations: RESP, Respiratory Extracorporeal Membrane Oxygenation Survival Prediction score; NEMS, Nine Equivalents of Nursing Manpower use score; SAS, Riker Sedation‐Agitation Scale; RASS, Richmond Agitation‐Sedation Scale; SAPS II, Simplified Acute Physiology Score II; SOFA, Sequential Organ Failure Assessment score; Core‐10‐TISS, Therapeutic Intervention Scoring System.
3.3. ECMO initiation
Details of ECMO settings and clinical progression, during and after ECMO, are shown in Table 3. Sixteen (94%) patients had VV ECMO, and one patient received VA ECMO. Nine patients (53%) had one‐site cannulation, with a dual‐lumen cannula in the right internal jugular vein (IJV). Seven patients (41%) received two‐site cannulations in the internal femoral artery and IJV. One patient had two‐site cannulations using the femoral artery and vein. One patient, who initially received VV ECMO with two site cannulations, suffered myocardial infarction during ECMO‐support, decompensated, and developed cardiogenic shock with predominant severe right heart failure and required a switch to VA ECMO using femoral‐artery and vein cannulation. Nine patients (53%) were assessed, cannulated, and retrieved by our mobile ECMO‐team from a peripheral hospital. The mean ECMO flow was 4.5 ± 1 L/min. Hemoperfusion for cytokine adsorption using the CytoSorb (Cytosorbents, Monmouth Junction, NJ, USA) or HA‐380 cartridges (Jafron Biomedical, Zhuhai City, China) was applied to eight patients (47%).
TABLE 3.
ECMO settings and clinical progression
| Total (n = 17) | Survivors (n = 9) | Non‐survivors (n = 8) | P value | |
|---|---|---|---|---|
| VV ECMO | 16 | 8 (88.9%) | 8 (100%) | 1.00 |
| VA ECMO | 1 (6%) | 1 (11.1%) | 0 | 1.00 |
| Configuration switch | 1 (6%) | 0 | 1 (6%) | 1.00 |
| FV‐IJV | 7 (41%) | 2 (22.2%) | 5 (62.5%) | .23 |
| FV‐FA | 1 (6%) | 1 (11.1%) | 0 | 1.00 |
| DLC | 9 (53%) | 6 (66.7%) | 3 (37.5%) | .47 |
| CytoSorb use | 8 (47%) | 5 (55.6%) | 3 (37.5%) | .78 |
| ECMO duration days | 16 (11, 21) | 16 (11, 19) | 14.5 (9.5, 22) | .74 |
| ECMO flow L/min | 5.0 (4.0, 5.7) | 4.6 (4.0, 5.5) | 4.5 (4.0, 5.8) | .78 |
| RHF | 7 (41%) | 4 (44%) | 3 (38%) | .77 |
| iNO inhalation | 8 (47%) | 5 (56%) | 3 (38%) | .46 |
| iNO duration days | 2.0 (0.0, 4.0) | 2.0 (0.0, 4.0) | 2.0 (0.0, 4.5) | 1.00 |
| Septic shock | 10 (59%) | 5 (56%) | 5 (62%) | .77 |
| Sepsis | 15 (88%) | 8 (89%) | 7 (88%) | .93 |
| Dialysis | 15 (88%) | 9 (100%) | 6 (75%) | .11 |
| Delirium | 4 (24%) | 3 (33%) | 1 (12%) | .31 |
| DIC | 4 (24%) | 1 (11%) | 3 (38%) | .20 |
| MI | 1 (6%) | 0 (0%) | 1 (12%) | .27 |
| Total PRBC units | 32.0 (24.0, 41.0) | 36.0 (32.0, 51.0) | 25.0 (19.5, 35.5) | .16 |
| PRBC during ECMO units | 25.0 (16.0, 33.5) | 25.0 (12.0, 35.5) | 25.0 (19.5, 33.5) | .71 |
| Total PC units | 0.0 (0.0, 7.0) | 0.0 (0.0, 1.0) | 3.5 (0.0, 11.0) | .33 |
| Total albumin units | 85.0 (6.0, 251.0) | 121.0 (6.0, 167.0) | 43.0 (4.5, 320.0) | .92 |
| Total LOS days | 24.0 (17.0, 55.0) | 55.0 (36.0, 64.0) | 14.0 (4.0, 20.0) | .005 |
| ICU stay days | 24.0 (14.7, 54.0) | 53.0 (36.0, 57.0) | 19.9 (11.1, 23.9) | .083 |
| Total MV days | 46.2 (22.1, 57.0) | 57.0 (47.0, 58.0) | 23.1 (13.3, 38.5) | .007 |
Abbreviations: DIC, disseminated intravascular coagulation; DLC, double‐lumen cannula in the right internal jugular vein; ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit; IJV, internal jugular vein; LOS, length of stay; MI, myocardial infarction; PRBC, packed red blood cell; PC, platelet concentration; FA, femoral artery; FV, femoral vein; MV, mechanical ventilation; RHF, right heart failure; VA, veno‐arterial; VV, veno‐venous.
Median ECMO support lasted 16 (11, 21) days. On average, each ECMO circuit required one exchange of the oxygenators. We had 20 exchanges in 17 ECMO circuits. In three patients with prolonged ECMO‐support, replacement of the oxygenator was required twice. As of June 1, 2020, nine patients (53%) have been weaned successfully from ECMO and discharged, and eight patients (47%) died while on ECMO support. Septic shock with vasoplegia and multi‐organ failure was the leading cause of death (88%). Seven patients (41%) developed right heart failure, while on ECMO‐support, and eight patients (47%) received iNO during ECMO. The median duration of iNO treatment was two days (0, 4). One patient suffered a myocardial infarction while on VV ECMO support. The supplementary Table S1 demonstrates a comparison between double‐lumen and two sites cannulation. There were no significant differences between the two different cannulation approaches in blood flow, survival, ICU stays, or adverse event incidence.
Details about HRAE are presented in Table 4. The incidence of ischemic stroke was 12%, and hemorrhagic stroke was 29%. Five patients had a pulmonary embolism (29%). The incidence of peripheral thromboembolic events was 29%. Eight patients (47%) had airway bleeding requiring transfusion of packed red blood cells (PRBCs). The median length of ICU stay was 24 days (14, 54) days, and the total length of hospital stay was 24 days (17, 55).
TABLE 4.
Hemocompatibility‐related adverse events
| Total (n = 17) | Survivors (n = 9) | Non‐survivors (n = 8) | P value | |
|---|---|---|---|---|
| Total HRAE | 12 (71%) | 6 (67%) | 6 (75%) | .71 |
| Total TEE | 7 (41%) | 5 (56%) | 2 (25%) | .20 |
| Bleeding events | 10 (59%) | 4 (44%) | 6 (75%) | .20 |
| GI bleeding | 2 (12%) | 1 (11%) | 1 (12%) | .93 |
| Airway bleeding | 8 (47%) | 3 (33%) | 5 (62%) | .23 |
| Hemothorax | 1 (6%) | 0 (0%) | 1 (12%) | .27 |
| Pericardial tamponade | 1 (6%) | 0 | 1 (12%) | .93 |
| Hemorrhagic stroke | 5 (29%) | 3 (33%) | 2 (25%) | .71 |
| Ischemic stroke | 2 (12%) | 1 (11%) | 1 (12%) | .93 |
| Pulmonary artery embolism | 5 (29%) | 3 (33%) | 2 (25%) | .71 |
| Embolic event | 1 (6%) | 1 (11%) | 0 (0%) | .33 |
| Thrombosis | 4 (24%) | 3 (33%) | 1 (12%) | .31 |
| Bleeding from cannulation sites | ||||
| Requiring ≥ 2 PRBC | 9 (53%) | 5 (56%) | 4 (50%) | .934 |
| Requiring intervention | 1 (6%) | 1 (11%) | 0 | .33 |
Abbreviations: GI, gastrointestinal; HRAE, Hemocompatibility related adverse events; PRBC, packed red blood cell; TEE, thromboembolic events.
3.4. Survivors versus non‐survivors
Table 1 presents a comparison of patients’ characteristics stratified by hospital survival status (survivors vs. non‐survivors). Prior to ECMO initiation, the median NT‐proBNP value was significantly higher in non‐survivors: 4097 pg/mL (1765, 8065) versus 706 pg/mL (438, 1765), P = .045. IL‐6 and PCT were also significantly higher in non‐survivors: 112 pg/mL (80, 287) versus 422 pg/mL (267, 1120), P = .013, and 6.7 ng/mL (5.5, 9.1) versus 1.1 ng/mL (0.5, 2.7), P = .026, respectively. All other characteristics and laboratory data, prior to ECMO support, did not differ by survival status (Table 1).
Of the ICU scores, the RESP score was significantly higher in survivors; 0.2 ± 2.0 versus −2.6 ± 2.8, P = .046. All other scores did not differ significantly between the two groups (Table 2). Pre‐ECMO SOFA scores did not differ between survivors and non‐survivors (Table 2). However, within each group, the SOFA score increased during ECMO support. It was significantly higher at last before ECMO‐cessation within each group compared to pre‐ECMO SOFA score (survivors: 9.8 ± 2.23 vs. 13.0 ± 2.1, P = .007) and (non‐survivors: 12.5 ± 7.9 vs. 16.9 ± 8.8, P = .004).
Three non‐surviving patients (38%) and two surviving patients (22%) had a history of pneumonia (NS, P > .05). The cannulation site and ECMO configuration did not differ between survivors and non‐survivors (Table 3). Median ECMO support time for non‐survivors was 14.5 days (9.5, 22), and the maximum duration was 61 days. The median survival time for the whole cohort was 62 days (Figure 1). For the survivors’ group, the median ECMO support time was 16 days (11, 19), and the maximum duration was 27 days.
FIGURE 1.
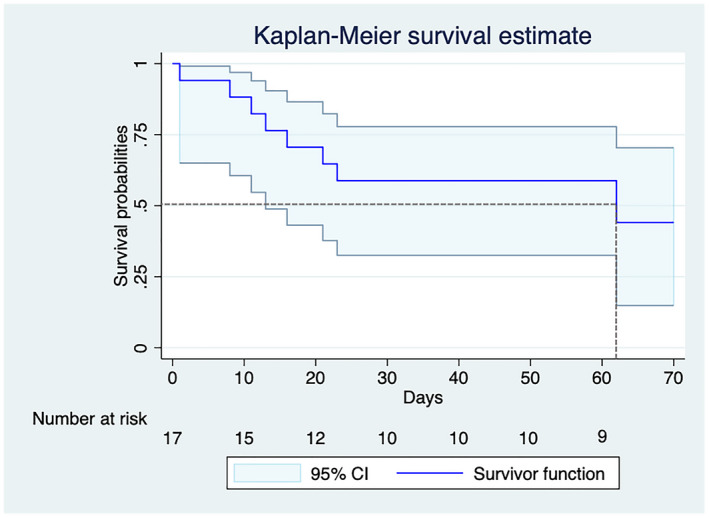
Kaplan–Meier survival curves for COVID‐19 patients treated with extracorporeal membrane oxygenation. The gray dotted lines show the median survival time, and the light blue indicates the 95%‐confidence interval [Color figure can be viewed at wileyonlinelibrary.com]
Patients in the survivors’ group had 544 days at risk, compared to 155 days among non‐survivors. The in‐hospital mortality rate was 0.01 patients per day, with log‐rank testing showing a significant difference (P < .001) between the Kaplan‐Meier survival curves of the two groups (Figure 1).
The time courses of selected biomarkers are presented in Figure 2. During ECMO support, all measured laboratory parameters, including inflammatory and infection biomarkers, and hemolysis markers, were not significantly different between survivors and non‐survivors. Time courses of NT‐proBNP and D‐dimer concentrations were also comparable in both groups. Troponin T (TnT) increased in both groups during ECMO support, compared to levels prior to ECMO initiation (NS, P > .05).
FIGURE 2.
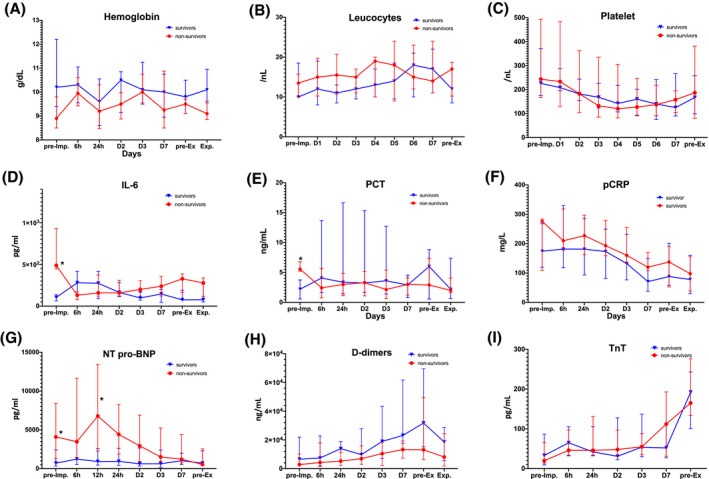
Time‐course of selected laboratory parameters. Hb: hemoglobin; IL‐6: interleukin‐6; NT‐proBNP: N‐terminal prohormone of brain natriuretic peptide; PCT: procalcitonin; pCRP: plasma C‐reactive protein; TnT: Troponin T [Color figure can be viewed at wileyonlinelibrary.com]
3.5. Sub‐analysis of cytokine adsorber effect
Supplementary Table S2 shows the time course of laboratory parameters comparing eight patients treated with ECMO plus cytokine adsorber and nine patients who received ECMO without cytokine adsorber. Only CRP levels decreased significantly in the hemoperfusion group, starting at day three of ECMO support. They remained markedly lower for seven days (Figure S1). IL‐6 and PCT did not differ between the groups (Figure S1). Three patients (37%) in the ECMO + hemoperfusion group and five patients (55.5%) in the ECMO only group did not survive to hospital discharge (NS log‐rank test, P > .05) (Figure S1).
3.6. Multivariate parametric survival regression
Eighty‐one variables from Tables 1 and 2 were included in the univariate analysis; only four variables were independent factors with P < .05. Pre‐ECMO NT‐proBNP levels were significantly higher in non‐survivors: 4097 pg/mL (1765, 8065) versus 706 (438, 1765), P = .043. Non‐surviving patients also had significantly higher pre‐ECMO IL‐6 (P = .013) and PCT values (P = .026). The RESP score was significantly lower in non‐survivors (P = .046); the estimated survival from the RESP score was 29% ± 14% for non‐survivors and 51% ± 12% for survivors. We entered RESP scores, NT‐proBNP, IL‐6, and PCT values into the multivariate parametric survival regression (Table 5). All variables, except PCT, remained independent predictors of in‐hospital mortality for critically ill COVID‐19 patients treated with ECMO.
TABLE 5.
Multivariate parametric survival regression
| HR | Std. Err. | P value | 95% ‐ CI | Interval | |
|---|---|---|---|---|---|
| RESP score | 0.843 | 0.173 | .040 | 0.564 | 1.260 |
| IL‐6 pg/mL | 1.069 | 0.044 | .016 | 0.968 | 1.160 |
| PCT ng/mL | 1.007 | 0.002 | .108 | 1.002 | 1.011 |
| NT‐proBNP pg/mL | 1.001 | 0.000 | .012 | 1.000 | 1.001 |
Abbreviations: CI, Confidence interval; HR, Hazard ratio; IL‐6, Interleukin‐6; NT‐proBNP, N‐terminal prohormone of brain natriuretic peptide; PCT, procalcitonin; RESP, respiratory extracorporeal membrane oxygenation survival prediction score; Std. Err, Standard error.
The RESP score had an HR of 0.843 [95%‐CI: 0.564‐1.260], P = .04; IL‐6 had an HR of 1.069 [95%‐CI: 0.986‐1.160], P < .023; and NT‐proBNP had an HR of 1.001 [95%‐CI: 1.000‐1.001], P = .012 (Table 4). Using only the RESP score to predict mortality gave an AUC of 0.79 with 62.5% sensitivity and 100% specificity (Figure 3). We tested a prediction model for in‐hospital mortality in COVID‐19 patients treated with ECMO, with RESP scores and pre‐ECMO IL‐6 and NT‐proBNP values. The model showed an AUC of 0.87 with 87.5% sensitivity and 77.8% specificity (Figure 3). IL‐6 alone had an AUC of 0.70 and, with a cut‐off of 122 pg/mL, a sensitivity of 88.8% and specificity of 50%. NT‐proBNP had an AUC of 0.74, 88% sensitivity, and 55% specificity, with a 814 pg/mL cut‐off.
FIGURE 3.

Receiver operator curves and area under the curves of in‐hospital mortality predictors. AUC: area under the curve; IL‐6: interleukin‐6; NT‐proBNP: N‐terminal prohormone of brain natriuretic peptide; PCT: procalcitonin; RESP: respiratory extracorporeal membrane oxygenation survival prediction score [Color figure can be viewed at wileyonlinelibrary.com]
4. DISCUSSION
ECMO support is recommended for critically ill COVID‐19 patients who do not improve despite optimal ARDS standard therapies, including prone position, protective MV with high end‐expiratory pressure, and low tidal volume. 15 , 16 However, its exact role and benefits in COVID‐19 patients remain uncertain.
In the present study, the outcomes and risk factor analyses of ECMO‐support in 17 critically ill COVID‐19 patients are reported. The key findings are summarized as follows. First, the rate of successful weaning from ECMO and survival to hospital discharge was 53%, demonstrating a reasonable outcome comparable to recently published data from the ELSO registry and the Paris–Sorbonne University Hospital Network. 8 , 19
Second, our analyses demonstrated that the RESP score is a reliable predictor of survival of COVID‐19 patients treated with ECMO. Third, we identified high levels of IL‐6 and NT‐proBNP as independent predictors of in‐hospital mortality. By adding IL‐6 and NT‐proBNP values to the RESP score, we demonstrated a superior prediction capability.
Patients who are successfully weaned from a prolonged ECMO‐support and survive severe COVID‐19 disease often require MV and have disability leading to a prolonged hospital stay and making the “awake ECMO” not the appropriate approach. Crotti et al. 20 clearly demonstrated that patients awaiting lung transplants and patients with COPD responded excellently to spontaneous breathing ECMO, while 50% of ARDS patients failed to tolerate “awake ECMO.”
In this study, nine patients were discharged from the hospital after a prolonged weaning from MV and intensive physiotherapy treatment. Nevertheless, the majority were transferred to rehabilitation facilities (2 to 3 weeks) to continue recovery. These findings underline the need for future studies focusing on the long‐term outcomes of these patients.
Another important finding in our study is the high rate of hemorrhagic stroke in COVID‐19 patients treated with ECMO (29%) compared to the reported incidence of 1.8%–10.9% in non‐COVID‐19 ARDS patients. 21 , 22 The high rate of intracranial hemorrhage in COVID‐19 has been reported in recently published studies. 23 , 24 A possible explanation for the higher incidence of intracranial bleeding is the vasculopathy and anticoagulation disorder induced by COVID‐19. 25
The successful weaning rate from ECMO in our study is greater than previously reported rates. 2 , 12 Jacobs et al. 12 reported on 32 COVID‐19 patients from nine different hospitals; at the time of analysis, 17 patients (53%) were still alive on ECMO, and only five (15.6%) had been weaned from ECMO and MV. 12 Yang et al. 2 describe the clinical course of 52 critically ill COVID‐19 patients from Wuhan, China, six required ECMO support, and mortality reached 83%. Conversely, Marullo et al. 13 analyzed data from 333 COVID‐19 patients using the ELSO registry and reported an overall mortality of only 17%. However, at the time of the analysis, the actual ECMO weaning rate was 18%, with the remaining patients still alive on ECMO support. The patient selection might significantly contribute to the differences in these survival rates.
4.1. IL‐6 and cytokine adsorption as a possible rescue tool
It is of utmost importance to identify risk factors and survival predictors to refine ECMO indications and offer it to COVID‐19 patients most likely to benefit. Recent studies suggest that COVID‐19 mortality is associated with virus‐activated “cytokine storm syndrome”. 26 , 27 The cytokine storm describes a hyperactive immune response, defined by the release of interleukins, interferons, tumor necrosis factors, chemokines, and various mediators. 27 Previous clinical ARDS trials have confirmed that ARDS’s hyper‐inflammatory phenotype, characterized by increased pro‐inflammatory cytokines, is associated with worse outcomes. 28 , 29 The characteristics of this phenotype are most likely to correspond to those in cytokine storms.
Marullo et al. 13 speculated that the use of VA ECMO might offer better lung protection, as it can decrease IL‐6 levels in the pulmonary circulatory system by bypassing the pulmonary circulation. Following recent studies, 26 , 30 we found that higher levels of IL‐6 before ECMO initiation increased mortality by 6%. Higher levels of IL‐6 in ARDS patients have also been shown to increase the risk of mortality. 31 , 32 However, the underlying mechanism, and the role of IL‐6 in ARDS pathogenesis, has not yet been thoroughly investigated. During ECMO support, we did not detect a significant difference in IL‐6 levels between survivors and non‐survivors. One possible explanation for this is that both COVID‐19 and ECMO support generate an extensive inflammatory response. 33 , 34 Therefore, any significant difference between the groups in IL‐6 levels before ECMO initiation vanished as soon as ECMO started. Risens et al. 33 also suggested IL‐6 levels may predict the outcome of patients treated with ECMO. They analyzed 22 patients, including neonates and adults, and found that survivors had lower IL‐6 levels than non‐survivors. 33 With this in mind, we can speculate that another possible tool to eliminate the damaging effects of IL‐6 and improve patients’ outcomes might be the use of a cytokine adsorber while on ECMO support. Recent studies have suggested the use of hemoperfusion is beneficial in critically ill COVID‐19 patients treated with ECMO who developed acute renal failure. 35 , 36 In our study, we used hemoperfusion with a cytokine adsorber in eight patients (47%). However, we did not detect any significant changes in IL‐6 levels or outcomes when using the cytokine adsorber; only CRP levels decreased significantly in the hemoperfusion group.
4.2. Risk factors and mortality predictors
Given the limited resources for ECMO therapy, a better understanding of contraindications to this treatment in severe COVID‐19 patients is essential for patient selection. Marullo et al. and Gupta et al. 13 , 37 found that patient age (>60 years) was a major risk factor for COVID‐19 patients treated with ECMO. In an analysis of >2000 COVID‐19 patients, Gupta et al. 37 found that age (>80 years) and gender (male) are independent risk factors for mortality. However, in our analysis, we could not identify an effect of age or gender on survival. The median age in our study was 57 years (compared to 60.5 ± 14.5 years in 37 ) and ranged from 39 to 73 years, with only five patients (29%) aged over 60 years. This might explain our findings’ inconsistency and those from Marullo et al. and Gupta et al. 13 , 37
Similar to other studies, 12 , 13 we could not detect specific demographic or clinical variables to predict the outcome of COVID‐19 patients treated with ECMO.
We found that the RESP score is reliable in predicting survival in COVID‐19 patients treated with ECMO. This is in agreement with Yang et al, 38 who have used the RESP score to help decisions on ECMO treatment for COVID‐19 patients. They chose RESP’s predicted survival of <40%, and age >65 years as cut‐offs to initiate ECMO support, giving excellent outcomes of 14% mortality rate, compared to reported mortality rates of 23%–61%. 2 , 14
Besides IL‐6 and RESP scores, we also found a negative correlation between high levels of pre‐ECMO NT‐proBNP and survival. Similar to our findings, recently published studies 2 , 4 , 39 have linked myocardial injury, as indicated by increased BNP, Troponin T, or CK‐MB, with poor clinical outcomes.
4.3. Limitations
We acknowledge important limitations to our study, namely the retrospective design and small sample size represent the major limitations. Second, our patients were treated in a high‐volume tertiary academic hospital; this might limit our findings’ generalizability. As a reason for our cohort’s limited size, biases in patient selection and indication might have existed. Another limitation is the inability to estimate cut‐off values for the variables included in the prediction model. One more important limitation is that we report only short‐term outcomes from a small cohort. Thus, we cannot underline any definitive conclusions regarding patients’ selection and predictors of survival. Given these limitations, validation of our findings in larger patient sample sizes and long‐term follow‐up is required.
5. CONCLUSION
The present study suggests that ECMO is a valuable lifesaving treatment for selected critically ill COVID‐19 patients. It may guide further investigations to optimize patient selection and identify predictors of in‐hospital mortality.
Our study results suggest adding IL‐6 and NT‐proBNP to the RESP score for better prediction accuracy. Further knowledge and analysis are required to optimize patient selection criteria and improve outcomes of critically ill COVID‐19 patients.
Conflicts of Interest
The authors declare that they have no conflicts of interest with the contents of this article.
Author Contributions
Zayat and Kalverkamp equally as co‐first authors.
Concept and design: Autschbach, Spillner, Marx, Marx, Dreher
Acquisition and analysis: Zayat, Kalverkamp, Kersten, Durak
Interpretation of data: Kersten, Spillner, Zayat, Kalverkamp
Visualization of the Results: Zayat, Durak, Kersten
Writing the first version of the manuscript: Zayat and Kalverkamp
Drafting of the manuscript: Kalverkamp and Zayat
Critical revision of the manuscript for intellectual content and approval for submission: All Authors
Statistical analysis: Zayat, Durak, Autschbach
Administrative, technical, or material support: Kersten, Marx, Marx, Autschbach, Dreher
Supervision: Kersten, Spillner, Autschbach, Marx, Marx, Autschbach, Dreher
Supporting information
Supplementary Material
Zayat R, Kalverkamp S, Grottke O, et al. Role of extracorporeal membrane oxygenation in critically Ill COVID‐19 patients and predictors of mortality. Artif Organs.2021;45:E158–E170. 10.1111/aor.13873
Rashad Zayat and Sebastian Kalverkamp are the authors contributed equally to this study.
Funding information
This study did not receive any external funding.
References
- 1. Papazian L, Aubron C, Brochard L, Chiche J‐D, Combes A, Dreyfuss D, et al. Formal guidelines: management of acute respiratory distress syndrome. Ann Intensive Care. 2019;9:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72314 cases from the chinese center for disease control and prevention. JAMA 2020;323:1239–42. [DOI] [PubMed] [Google Scholar]
- 4. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet (London, England). 2020;395:1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS‐CoV‐2 admitted to ICUs of the Lombardy Region. Italy. JAMA. 2020;323:1574–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lang Y, Zheng Y, Li T. The treatment of extracorporeal organ support for critical ill patients with coronavirus disease 2019: a brief perspective from the front line. Artif Organs. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hong X, Xiong J, Feng Z, Shi Y. Extracorporeal membrane oxygenation (ECMO): does it have a role in the treatment of severe COVID‐19? Int J Infect Dis. 2020;94:78–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schmidt M, Hajage D, Lebreton G, Monsel A, Voiriot G, Levy D, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome associated with COVID‐19: a retrospective cohort study. The Lancet Respiratory Medicine. 2020;8:1121–1131. 10.1016/s2213-2600(20)30328-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Combes A, Hajage D, Capellier G, Demoule A, Lavoué S, Guervilly C, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med. 2018;378:1965–75. [DOI] [PubMed] [Google Scholar]
- 10. Munshi L, Walkey A, Goligher E, Pham T, Uleryk EM, Fan E. Venovenous extracorporeal membrane oxygenation for acute respiratory distress syndrome: a systematic review and meta‐analysis. Lancet Respir Med. 2019;7:163–72. [DOI] [PubMed] [Google Scholar]
- 11. Goligher EC, Tomlinson G, Hajage D, Wijeysundera DN, Fan E, Jüni P, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome and posterior probability of mortality benefit in a post hoc bayesian analysis of a randomized clinical trial. JAMA 2018;320:2251–9. [DOI] [PubMed] [Google Scholar]
- 12. Jacobs JP, Stammers AH, St Louis J, Hayanga JWA, Firstenberg MS, Mongero LB, et al. Extracorporeal membrane oxygenation in the treatment of severe pulmonary and cardiac compromise in coronavirus disease 2019: experience with 32 patients. ASAIO J. 1992;2020(66):722–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Marullo AG, Cavarretta E, Biondi Zoccai G, Mancone M, Peruzzi M, Piscioneri F, et al. Extracorporeal membrane oxygenation for critically ill patients with coronavirus‐associated disease 2019: an updated perspective of the European experience. Minerva Cardioangiologica. 2020;68:5. 10.23736/s0026-4725.20.05328-1. [DOI] [PubMed] [Google Scholar]
- 14. Yang X, Cai S, Luo Y Zhu F, Hu M, Zhao Y, et al. Extracorporeal membrane oxygenation for coronavirus disease 2019‐induced acute respiratory distress syndrome: a multicenter descriptive study. Crit Care Med. 2020;48:1289–95. [DOI] [PubMed] [Google Scholar]
- 15. WHO . Clinical Management of COVID‐19 Interim Guidance. Geneva, Switzerland: WHO Global; 2020. https://www.who.int/publications/i/item/clinical‐management‐of‐covid‐19. [Google Scholar]
- 16. Bartlett RH, Ogino MT, Brodie D, McMullan DM, Lorusso R, MacLaren G, et al. Initial ELSO guidance document: ECMO for COVID‐19 patients with severe cardiopulmonary failure. ASAIO J. 1992;2020(66):472–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pranikoff T, Hirschl RB, Remenapp R, Swaniker F, Bartlett RH. Venovenous extracorporeal life support via percutaneous cannulation in 94 patients. Chest 1999;115:818–22. [DOI] [PubMed] [Google Scholar]
- 18. Shekar K, Badulak J, Peek G, Boeken U, Dalton HJ, Arora L, et al. Extracorporeal life support organization coronavirus disease 2019 interim guidelines: a consensus document from an international group of interdisciplinary extracorporeal membrane oxygenation providers. ASAIO Journal. 2020;66:707–21. 10.1097/mat.0000000000001193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Barbaro RP, MacLaren G, Boonstra PS, Iwashyna TJ, Slutsky AS, Fan E, et al. Extracorporeal membrane oxygenation support in COVID‐19: an international cohort study of the Extracorporeal Life Support Organization registry. Lancet (London, England). 2020;396:1071–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Crotti S, Bottino N, Ruggeri GM, Spinelli E, Tubiolo D, Lissoni A, et al. Spontaneous breathing during extracorporeal membrane oxygenation in acute respiratory failure. Anesthesiology. 2017;126:678–87. [DOI] [PubMed] [Google Scholar]
- 21. Fletcher‐Sandersjöö A, Thelin EP, Bartek J Jr, Broman M, Sallisalmi M, Elmi‐Terander A, et al. Incidence, outcome, and predictors of intracranial hemorrhage in adult patients on extracorporeal membrane oxygenation: a systematic and narrative review. Front Neurol. 2018;9:548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Muellenbach RM, Kilgenstein C, Kranke P, Küstermann J, Kredel M, Roewer N, et al. Effects of venovenous extracorporeal membrane oxygenation on cerebral oxygenation in hypercapnic ARDS. Perfusion. 2014;29:139–41. [DOI] [PubMed] [Google Scholar]
- 23. Masur J, Freeman CW, Mohan S. A double‐edged sword: neurologic complications and mortality in extracorporeal membrane oxygenation therapy for COVID‐19‐related severe acute respiratory distress syndrome at a tertiary care center. AJNR Am J Neuroradiol 2020. 41(11):2009–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Usman AA, Han J, Acker A, Olia SE, Bermudez C, Cucchiara B, et al. A case series of devastating intracranial hemorrhage during venovenous extracorporeal membrane oxygenation for COVID‐19. J Cardiothor Vasc An. 2020;34:3006–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ, et al. COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet (London, England). 2020;395:1033–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sinha P, Matthay MA, Calfee CS. Is a “Cytokine Storm” relevant to COVID‐19? JAMA Intern Med 2020;180:1152–4. [DOI] [PubMed] [Google Scholar]
- 28. Calfee CS, Delucchi K, Parsons PE, Thompson BT, Ware LB, Matthay MA, et al. Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. Lancet Respir Med. 2014;2:611–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Famous KR, Delucchi K, Ware LB, Kangelaris KN, Liu KD, Thompson BT, et al. Acute respiratory distress syndrome subphenotypes respond differently to randomized fluid management strategy. Am J Respir Crit Care Med 2017;195:331–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID‐19 based on an analysis of data of 150 patients from Wuhan, China. Inten Care Med. 2020;46:846–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Goldman JL, Sammani S, Kempf C, Saadat L, Letsiou E, Wang T, et al. Pleiotropic effects of interleukin‐6 in a “two‐hit” murine model of acute respiratory distress syndrome. Pulm Circ. 2014;4:280–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Meduri GU, Headley S, Kohler G, Stentz F, Tolley E, Umberger R, et al. Persistent elevation of inflammatory cytokines predicts a poor outcome in ARDS. Plasma IL‐1 beta and IL‐6 levels are consistent and efficient predictors of outcome over time. Chest 1995;107:1062–73. [DOI] [PubMed] [Google Scholar]
- 33. Risnes I, Wagner K, Ueland T, Mollnes T, Aukrust P, Svennevig J. Interleukin‐6 may predict survival in extracorporeal membrane oxygenation treatment. Perfusion. 2008;23:173–8. [DOI] [PubMed] [Google Scholar]
- 34. Millar JE, Fanning JP, McDonald CI, McAuley DF, Fraser JF. The inflammatory response to extracorporeal membrane oxygenation (ECMO): a review of the pathophysiology. Crit Care. 2016;20:387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rajagopal K, Keller SP, Akkanti B, Bime C, Loyalka P, Cheema FH, et al. Advanced pulmonary and cardiac support of COVID‐19 patients: emerging recommendations from ASAIO‐A “Living Working Document”. ASAIO J. 1992;2020(66):588–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Swol J, Lorusso R. Additive treatment considerations in COVID‐19—the clinician’s perspective on extracorporeal adjunctive purification techniques. Artif Organs. 2020;44:918–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gupta S, Hayek SS, Wang W, Chan L, Mathews KS, Melamed ML, et al. Factors associated with death in critically Ill patients with coronavirus disease 2019 in the US. JAMA Intern Med. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yang Y, Rali AS, Inchaustegui C, Alakbarli J, Chatterjee S, Herlihy JP, et al. Extracorporeal membrane oxygenation in coronavirus disease 2019‐associated acute respiratory distress syndrome: an initial US experience at a high‐volume centre. Cardiac Failure Rev. 2020;6:e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zochios V, Brodie D, Charlesworth M, Parhar KK. Delivering extracorporeal membrane oxygenation for patients with COVID‐19: what, who, when and how? Anaesthesia 2020;75:997–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


