Abstract
Background:
A growing number of people with diabetes are choosing to adopt do-it-yourself artificial pancreas system (DIYAPS) despite a lack of approval from the US Food and Drug Administration.
We describe patients’ experiences using DIYAPS, and patient and diabetes providers’ perspectives on the use of such technology.
Methods:
We distributed surveys to patients and diabetes providers to assess each group’s perspectives on the use of DIYAPS. The patient survey also assessed glycemic control and impact on sleep. The patient survey was distributed in February 2019 via Facebook and Twitter (n = 101). The provider survey was distributed via the American Association of Diabetes Educators’ e-mail newsletter in April 2019 and the Pediatric Endocrine Society membership e-mail list in May 2019 (n = 152).
Results:
Patients overwhelmingly described improvements in glycemic control and sleep quality: 94% reported improvement in time in range, and 64% reported improvement in all five areas assessed. Eighty-nine percent of patients described DIYAPS as “Safe” or “Very Safe,” compared to only 27% of providers. Most felt encouraged by their diabetes provider to continue using DIYAPS, but few described providers as knowledgeable regarding its use. Providers cited a lack of experience with such systems and an inability to troubleshoot them as their most significant challenges.
Conclusions:
Despite evidence that DIYAPS usage is increasing, our surveys suggest that patients’ adoption of this technology and trust in it is outpacing that of diabetes providers. Providers must be aware of this growing population of patients and familiarize themselves with DIYAPS to support patients using this technology.
Keywords: artificial pancreas, closed loop, do-it-yourself, insulin pump, continuous glucose monitor, time in range
Introduction
Diabetes technology companies have been actively developing closed-loop insulin delivery systems and components. Efforts from Medtronic, Tandem, Insulet, and Bigfoot Biomedical, among others, are in varying stages of development and US Food and Drug Administration (FDA) approval. Meanwhile, the many years required for this process led to a parallel effort to develop similar systems outside of the traditional industry pathway. These efforts were led by individuals affected by diabetes who were eager to benefit from more advanced technology.1 The resulting “do-it-yourself artificial pancreas system” (DIYAPS) relies upon an array of online instructions and peer support for its implementation but lacks provider training programs, formal regulatory approval, and coverage by health insurance companies.
Three DIYAPS platforms currently exist: OpenAPS,2 Loop,3 and AndroidAPS.4 All three allow for algorithm-based hybrid closed-loop insulin dosing. Patients are tasked with building the system independently, relying upon open-source computer code, instructions, and a network of internet-based peer support. They are also responsible for sourcing the necessary hardware: an insulin pump, a continuous glucose monitor (CGM), and a compatible smartphone. Most configurations also require a small hardware component that serves to issue commands to the insulin pump through radio frequency communication.5
The technical and labor-intensive nature of this technology has necessitated the development of a robust peer-to-peer support network online, organized largely through Facebook. The development of such groups has in some ways supplanted the provider’s role in educating patients in supporting their use of diabetes technology, and is consistent with patient usage of diabetes online communities more broadly.6,7 The rapid pace of diabetes technological development sometimes outpaces that of new therapy approvals, and has created a complicated environment that provides patients with many choices, but impedes efforts to stay abreast of new developments for patients and providers.8
Given the open-source and heterogeneous nature of this technology, accurate estimates regarding the number of patients currently using DIYAPS are lacking, as are large formal efficacy and safety studies. Some smaller studies, however, suggest improvement in glycemic control after patients adopt DIYAPS.9,10 These systems lack FDA approval and the support and warranty traditionally provided by diabetes technology companies.
Despite these limitations, use of these systems appears to be increasing. The “Looped” Facebook group is a focal point for organization, and as of January 2020, contained over 20 000 members from 99 different countries. This is a marked increase from 5700 members just 15 months earlier. These members include patients actively using DIYAPS, their caregivers, those potentially interested in adopting it, and medical providers, among others. Discussions also take place on Twitter, organized by hashtags including #WeAreNotWaiting, #OpenAPS, and #Loop. A 2019 analysis showed that tweets regarding this technology were sent dozens of times per week and originated from 92 different countries.11
Increasing use of DIYAPS is likely due to a combination of factors. DIYAPS allows for a high degree of customization, including limiting the number of unnecessary alarms patients receive and allowing for flexible and aggressive blood glucose targets. Much of this flexibility is, at least in part, related to the lack of regulations imposed upon the design of such systems. DIYAPS provides consistent closed-loop functionality in situations where commercially available systems may not. Conversely, patients struggled to remain in Auto Mode using the first commercially available hybrid closed-loop system, the Medtronic Minimed 670G.12 Development of commercially available automated insulin dosing (AID) systems continues to advance, but barriers to access imposed by the FDA approval process and limited coverage by health insurers further compel some patients to pursue DIY solutions.
The purpose of this study was to assess perspectives of US patients and diabetes providers on the safety and efficacy of DIYAPS, to better describe how diabetes providers are approaching this technology as it grows in popularity, and to describe how the internet-based peer support network has impacted the patient–provider relationship in patients using DIYAPS.
Methods
We developed a web-based patient survey and provider survey through an iterative process (Supplemental Appendix 1). The patient survey included 30 multiple-choice and closed-ended questions and an open comment section. It was designed to assess patients’ clinical outcomes, perception of the systems’ safety and reliability, and the diabetes teams’ role in the patient’s adoption of DIYAPS. Responses were accepted from patients or caregivers located in the United States and over 14 years old. The survey was distributed in February 2019 via the “Looped” Facebook group and via Twitter by tagging the post with #WeAreNotWaiting, #Loop, and #OpenAPS.
The provider survey included 21 multiple-choice, close-ended, and rating questions, and an open comment section. It was designed to assess the level of familiarity diabetes practitioners had with DIYAPS, their approach to patients interested in adopting it, and what they perceived as the most significant barriers to supporting patients with it. The survey was distributed via the American Association of Diabetes Educators’ e-mail newsletter in April 2019 (12 659 members) and the Pediatric Endocrine Society membership e-mail listserv in May 2019 (1280 members).
Study data were collected using REDCap electronic data capture tools hosted at the Center for Research Informatics at the University of Chicago.13,14
Data from patient and provider surveys were analyzed and summarized to describe and compare the perspectives of providers and patients on the adoption of DIYAPS for multiple-choice items. Descriptive statistics were calculated using Microsoft Excel software. Differences in the two groups’ self-reported computer literacy and safety perceptions were compared using Fisher’s exact test with Stata 15 software. Responses were scaled 1-5 for each analysis, with “I don’t know” assigned a value of three. Open-ended comments were analyzed for common themes.
Results
Survey Demographics
The patient survey had 101 respondents representing 34 states (Table 1). California (22 responses) and New York (7 responses) were the most commonly represented. Eighteen percent of patients were under the age of 18. Eighty-nine percent of respondents reported having at least a college degree.
Table 1.
Survey Respondent Characteristics.
| Patient survey | Provider survey | ||
|---|---|---|---|
| Responses | n = 101 | Responses | n = 152 |
| States represented | 34 | States represented | 36 |
| Patient age (years) | Provider type | ||
| Less than 10 | 6 (6%) | Physician | 105 (69%) |
| 10-17 | 12 (12%) | Nurse | 26 (17%) |
| 18-34 | 29 (29%) | NP or PA | 7 (5%) |
| 35-49 | 29 (29%) | Dietitian | 12 (8%) |
| 50+ | 25 (25%) | ||
| Patient population | |||
| DIYAPS | Pediatric | 111 (73%) | |
| Loop | 74 (73%) | Adult | 25 (16%) |
| OpenAPS | 18 (18%) | Adult + Pediatric | 12 (8%) |
| AndroidAPS | 8 (8%) | Other | 4 (3%) |
| Duration of DIYAPS use (years) | Number of patients using DIYAPS | ||
| Less than 1 | 44 (44%) | 0 | 99 (65%) |
| 1-2 | 35 (35%) | 1-10 | 47 (31%) |
| 2-3 | 18 (18%) | Greater than 10 | 6 (4%) |
| Greater than 3 | 4 (4%) | ||
| Computer literacy | Computer literacy | ||
| Minimal | 13 (13%) | Minimal | 19 (13%) |
| Limited | 31 (31%) | Limited | 91 (60%) |
| Moderate | 31 (31%) | Moderate | 37 (24%) |
| Extensive | 6 (6%) | Extensive | 4 (3%) |
| Expert | 20 (20%) | Expert | 1 (0%) |
Computer literacy is defined as Minimal (limited mostly to web browsing and basic application usage), Limited (experience with applications more complex than basic web and office programs, but no significant experience with computer coding), Moderate (some limited experience with computer coding and/or command-line inputs), Extensive (extensive experience with computer coding), and Expert (formally trained in computer coding and perform high-level functions frequently).
DIYAPS, do-it-yourself artificial pancreas; NP, nurse practitioner; PA, physician assistant.
In this survey, Loop was by far the most common DIYAPS platform in use (73%), followed by OpenAPS (18%) and AndroidAPS (8%). Most patients had been using DIYAPS for less than two years (78%), and respondents skewed toward recent adopters, with almost half of the respondents reporting to have started within the past year.
The provider survey had 152 respondents from 36 states. California (16 responses), Massachusetts (12 responses), and New York (11 responses) were the most commonly represented. The majority of respondents (69%) were physicians, and most reported treating exclusively pediatric patients (73%). The majority did not treat any patients using a DIYAPS (65%). Of those providers who do treat such patients, 89% reported treating ten or fewer.
Computer Literacy
Both patients/caregivers and providers were asked to select one of five options to describe their level of experience with computer coding. Baseline computer literacy was significantly higher among patient respondents than providers: “Expert” (20% vs 0.4%), “Extensive” (6% vs 3%), “Moderate” (31% vs 24%), “Limited” (31% vs 60%), and “Minimal” (13% vs 13%) (Table 1), P < .001.
Clinical Impact
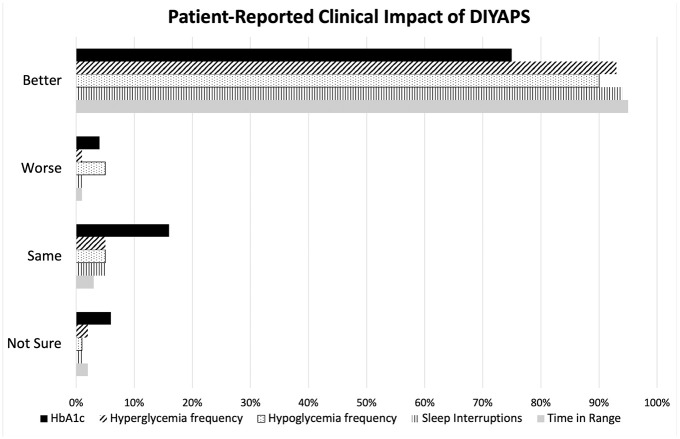
Patient-reported clinical impact of DIYAPS is shown in Figure 1. Seventy-three percent of respondents had transitioned from using an insulin pump and CGM combination prior to DIYAPS, and 18% had been using multiple daily injections and CGM. After transitioning to DIYAPS, respondents reported improvements in the following:
Figure 1.
Patients were asked how each of these five measures have changed since transitioning to a do-it-yourself artificial pancreas system.
Black solid line = HbA1c; angled line pattern = hyperglycemia frequency; dot pattern = hypoglycemia frequency; vertical line pattern = sleep interruptions; gray solid = time in range.
HbA1c, hemoglobin A1c.
- time in range (94%);
- diabetes-related sleep interruptions (93%);
- hyperglycemia frequency (92%);
- hypoglycemia frequency (89%);
- hemoglobin A1c (74%).
Sixty-four percent of patients reported improvement in all five areas.
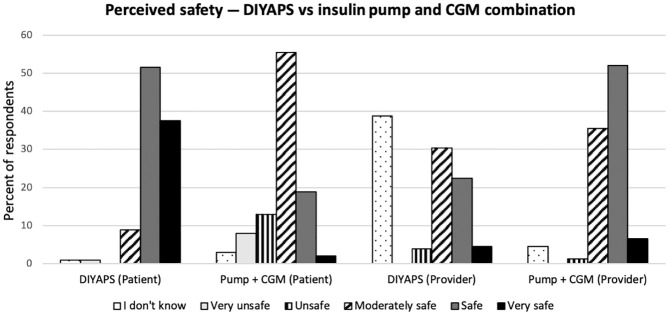
Safety
Both surveys asked respondents to select one of five choices to describe the safety of DIYAPS and that of a traditional insulin pump and CGM combination (Figure 2). Patients were significantly more likely than providers to view positively the safety profile of DIYAPS: “Very Safe” (38% vs 5%), “Safe” (52% vs 22%), “Moderately Safe” (9% vs 30%), “Unsafe” (0% vs 4%), and “Very Unsafe” (1% vs 0%), P < .001. Providers’ most common choice regarding its safety was “I don’t know” (1% of patients and 39% of providers).
Figure 2.
Safety is defined as “Very Unsafe” (it is unpredictable, unreliable, requires constant monitoring, and carries an excessively high risk of hyper- or hypoglycemia or equipment failure), “Unsafe” (it is at times unpredictable and/or unreliable despite frequent monitoring and carries a high risk of hyper- or hypoglycemia or equipment failure), “Moderately Safe” (the system is mostly predictable and reliable, but still carries a risk of fairly frequent hyper- or hypoglycemic episodes, and/or a relatively high risk of equipment failure), “Safe” (the system is almost always predictable and reliable, occasional hyper- and hypoglycemic episodes may occur despite frequent monitoring, but the risk of equipment failure is low), and “Very Safe” (I trust the system to always act predictably and to maintain a safe glucose level, even with only occasional monitoring, with no exceptions, and a minimal chance of equipment failure).
White dotted bar = “I don’t know”; light gray bar = “Very Unsafe”; vertical line pattern bar = “Unsafe”; angled line pattern bar = “Moderately Safe”; dark gray bar = “Safe”; Black bar = “Very Safe.”
CGM, continuous glucose monitoring; DIYAPS, do-it-yourself artificial pancreas system.
Conversely, patients were significantly more likely than providers to view negatively the safety profile of a traditional insulin pump and CGM combination: “Very Safe” (2% vs 7%), “Safe” (19% vs 52%), “Moderately Safe” (55% vs 36%), “Unsafe” (13% vs 1%), and “Very Unsafe” (8% vs 0%), P < .001. Three percent of patients and 5% of providers selected “I don’t know.”
Taken together, patients were far more likely than providers to describe DIYAPS as at least as safe as traditional therapy with an insulin pump and CGM. Ninety-seven percent of patients described DIYAPS as equally safe or safer than traditional therapy, compared to only 61% of diabetes providers, excluding anyone who selected “I don’t know.”
Diabetes Provider Experiences
Patients were asked to describe their diabetes provider’s experience with DIYAPS and how supportive they have been in its use. Seventy-four percent reported being the sole patient in the practice using DIYAPS. The majority of patients felt that their diabetes providers encouraged their use of DIYAPS, but few providers were perceived as knowledgeable regarding its use.
Patients were given four choices to best describe their provider’s role in supporting them:
- “They understand the system well and encourage me to continue using it” (20%).
- “They do not understand the system well but encourage me to continue using it” (60%).
- “They do not understand the system well and do not encourage me to continue using it” (16%).
- “They refuse to support me using this system and/or I’ve had to change providers” (4%).
Patients were asked to select the most important resource for maintaining and troubleshooting their DIYAPS:
- social media including Twitter and Facebook (45%);
- DIYAPS official documentation (36%);
- chat rooms dedicated to DIYAPS (12%);
- another patient that you know personally (9%);
- clinician through endocrinologist office (0%);
- insulin pump or CGM official documentation (0%);
- insulin pump or CGM customer support (0%);
- other (0%).
Diabetes providers were asked to select any resources that they have utilized to learn about DIYAPS:
- articles published in peer-reviewed medical journals (40%);
- articles published elsewhere, including medical news, popular press, and blogs (34%);
- social media groups for these systems (30%);
- consultation with endocrinologists with specific experience regarding DIY systems (26%);
- official software documentation via Loop, OpenAPS, and AndroidAPS websites (20%);
- none of the above (16%);
- other (29%).
Provider respondents who selected “Other” most commonly described learning about it directly from patients. Other avenues included attending conferences (both ADA and AADE conferences were mentioned), podcasts, and the respondent’s own personal use of DIYAPS.
Providers were asked to choose the best description of their “primary concern regarding patients implementing a DIY artificial pancreas system”:
- inadequate experience with these systems in my practice and/or a limited ability to troubleshoot them (52%);
- safety: risk of equipment malfunction leading to severe hyper- or hypoglycemia (25%);
- lack of FDA approval and/or lack of insurance coverage for these systems (10%);
- lack of efficacy data (4%);
- liability concerns (3%);
- other (6%).
Provider respondents who chose “Other” generally described concerns related to patient misuse of DIYAPS. One adult and pediatric diabetes nurse described concerns related to patients’ “lack of pattern management knowledge” and inability to make adjustments to settings. A pediatric endocrinologist further expressed concern that “Over time, there is a tendency for patients/families to stop paying attention.”
Providers were also asked to select the option that best describes their practice’s policy regarding DIYAPS:
- “I support everyone using a DIY system who wishes to, provided they maintain appropriate glucose control” (16%).
- “I support certain trusted patients implementing a DIY system” (46%).
- “I recommend against it but am willing to continue treating patients who insist on using a DIY system” (30%).
- “I will not treat patients who use one of the current DIY systems” (8%).
Patient and Provider Comments
The patient survey concluded with a field for respondents to choose three words to describe DIYAPS. Themes that emerged included improvements in quality of life, improvements in sleep quality, and a belief that DIYAPS is superior to other available options (Table 2). Twenty-six percent of respondents chose independently to describe the system as “life-changing,” “life-saving,” or another related description of improved quality or duration of life. Twenty-one percent of respondents described how DIYAPS favorably compares to other options. Two respondents chose to voice reservations, describing “some frustration” and “much experimentation required.” There were many less specific but positive endorsements, describing DIYAPS as a “dream come true,” the “best thing ever,” “amazing,” and even “my best friend.” No respondents described concerns about safety or reliability.
Table 2.
Patient and Provider Comments.
| Patient survey | Provider survey |
|---|---|
|
Improved quality of life
“Life-changing” “Life-saving” “I’ll live longer” “Better relationship with my teen” “I feel normal” “Much less work” |
Patients have a right to try new technology
“Patients have a right to these advances” “Providers have very little control over how patients actually use their insulin” “We cannot hold people back because we may not understand something” “Diabetes is not without risk no matter what the treatment” |
|
Improved sleep quality
“We all sleep!” “Sleep is amazing” “Much more sleep” |
Risks related to patient operation of do-it-yourself artificial pancreas system
Concern regarding “Patients using it without understanding it” Some patients “don’t know how to troubleshoot or make adjustments” |
|
Improvement over other options
“Light years ahead” “Better than 670 g” “Never going back” “Closest to a cure” |
Providers need further training
“I wish we had more training on DIY” “Need more tools for providers to understand & troubleshoot” “This is the first I have actually heard of this” “Need more education on the system” |
|
Resource-intensive setup process
“Great, some frustration” “Much experimentation required” |
Adoption is limited to only certain patients
“These systems are very expensive” “The T1D patients I know who are using them are tech savvy and sophisticated in their self-care” “In the community I work my patients don’t have the intellect needed to understand and manage these systems.” “It’s limited to those with the financial means and self-education to do it” |
|
Generally positive
“Community-driven miracle” “Amazing” “Best thing ever” “Our greatest blessing” |
Patients were asked: “In three words, please describe your artificial pancreas system.” Providers were asked: “Please describe any other thoughts you have regarding patients with diabetes implementing do-it-yourself closed loop artificial pancreas systems.” These comments are representative examples and do not include all responses received.
The provider survey also included an unstructured field for comments. Themes that emerged included lack of provider knowledge regarding DIYAPS, concern that patients do not fully understand the technology, opposition to pediatric use, and belief that patients have an inherent right to try new technology regardless of regulatory approval (Table 2).
Discussion
The use of DIYAPS in United States seems to be expanding, based on the rapid rise in members of DIYAPS social media groups, and in the relatively recent adoption of this technology by most patients surveyed here. Patients we surveyed overwhelmingly reported improvements in glycemic control, and they are enthusiastically embracing this technology as seen in their passionate comments. The two resources most commonly cited for maintaining and troubleshooting these systems are official documentation provided online by the three platforms and social media groups, rather than traditional sources of support like the provider’s office and diabetes technology company representatives.
Unlike patients, most providers responded that an insulin pump and CGM combination is safer than DIYAPS. Despite citing limited experience with these systems as their primary concern, only a small minority of diabetes providers report having read the official documentation for DIYAPS or having joined social media communities founded to support its use. These resources are essential for patients building and understanding these systems, and this discrepancy suggests that providers should be made aware of these resources to narrow this knowledge gap and better support patients.
In DIYAPS, patients have found an insulin delivery system that is clinically effective and preferred by many over currently available FDA-approved options. Alternate controller-enabled insulin pumps, interoperable CGMs, and AID systems may soon revolutionize how insulin is administered for most patients. The high regulatory burden on these systems, however, may mean people with diabetes still have years to wait. Free of such oversight, DIYAPS can target more aggressive glucose targets and can be revised and upgraded more quickly than future commercially available alternatives are likely to be.
The comments provided in the patient survey suggest that DIYAPS users do not see this technology as merely a logical evolution in existing technology, but a transformative advancement producing real quality-of-life improvements. It is essential then that diabetes providers become more familiar with this technology that is only likely to grow in popularity.
Study Limitations
The high level of educational attainment and computer literacy among patient survey respondents may make their experiences difficult to generalize to a broader population. The patient survey used self-reported data to gage clinical outcomes, so there may be response bias present in the reported measures related to glycemic control and sleep quality. Improvements in these areas may also have been due to other unrelated interventions.
The patient survey was sent through social media channels to users of DIYAPS, so it is unlikely to have captured patients who used the system and have since discontinued it, potentially selecting for more successful patients. Since the study was conducted in early 2019, the responses reflect patients primarily using Loop or OpenAPS with Medtronic insulin pumps. Since then, Loop expanded to include Insulet insulin pumps, and the experiences of users utilizing an Insulet Omnipod may differ from those described here. Because our patient survey was distributed to the “Looped” Facebook group, we may have oversampled Loop in relation to AndroidAPS and OpenAPS users, since other, albeit smaller, Facebook groups exist that focus primarily on those platforms. Surveys were only distributed to people in the United States, and results may not be generalizable to patients and providers elsewhere given differences in device availability, insurance coverage, and provider perspectives.
Our survey was distributed to AADE and PES, so adult providers were underrepresented. While the opinions of certified diabetes educators, especially related to safety, will generally reflect the policies and opinions of their practices more broadly, this is an imperfect proxy for describing the opinions of adult diabetes providers. In May 2019, after this survey was distributed, the FDA released a statement specifically warning medical providers against patients using “unauthorized diabetes management devices,” which include DIYAPS,15 and attitudes toward the technology may thus have subsequently changed.
Conclusion
Use of DIYAPS is growing, and our data suggest that patients are having positive clinical outcomes after adopting it. Patients surveyed commonly described these systems as “life-saving” or “amazing,” showing enthusiastic adoption despite its lack of formal FDA approval and even a recent FDA warning against the use of “unauthorized diabetes management devices.” The enthusiasm for and trust in this technology among this small group of patients has outpaced that of most diabetes providers surveyed.
Diabetes providers will benefit from utilizing online resources like official DIYAPS documentation and guides for clinicians,16,17 Facebook (“Looped” and related groups), and Twitter (#WeAreNotWaiting, #Loop, #OpenAPS), as well as a growing body of peer-reviewed literature and resources, to better familiarize themselves with this technology. Moving away from the typical device approval pathway marks a significant change in the patient–provider relationship and diabetes providers may need to reassess how they approach shared decision-making with patients. A closed-loop insulin delivery system is now commercially available and FDA-approved in the United States with more on the horizon. However, it remains to be seen whether these systems will allow patients the same flexibility to achieve tight glycemic control as DIYAPS. Patients with diabetes are not waiting until a more competitive closed-loop insulin delivery system is widely available, and the number choosing to use DIYAPS is only likely to increase in coming years.
Supplemental Material
Supplemental material, Appendix_1_-_Patient_and_Provider_Survey_Text for Using a Do-It-Yourself Artificial Pancreas: Perspectives from Patients and Diabetes Providers by Walter Palmer, Siri Atma W. Greeley, Lisa R. Letourneau-Freiberg and Rochelle N. Naylor in Journal of Diabetes Science and Technology
Acknowledgments
A portion of the patient survey data was presented previously at ADA Scientific Sessions 2019 (San Francisco, CA, USA).
We would like to thank all of the patients, caretakers, and medical providers who completed our surveys, as well as the American Association of Diabetes Educators and the Pediatric Endocrine Society for distributing them.
Footnotes
Author Contributions: WP, RN, SG, and LL contributed to conception and design of the study, and acquisition, analysis, and interpretation of the data. WP contributed to drafting of the manuscript and revising the manuscript critically for important intellectual content. RN (guarantor), SG, and LL contributed to revising the manuscript critically for important intellectual content.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by a grant from the National Institutes of Health National Center for Advancing Translational Sciences (CTSA UL1TR000430).
ORCID iDs: Walter Palmer  https://orcid.org/0000-0002-0864-5722
https://orcid.org/0000-0002-0864-5722
Lisa R. Letourneau-Freiberg  https://orcid.org/0000-0001-9465-4870
https://orcid.org/0000-0001-9465-4870
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Barnard KD, Ziegler R, Klonoff DC, et al. Open source closed-loop insulin delivery systems: a clash of cultures or merging of diverse approaches? J Diabetes Sci Technol. 2018;12:1223-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. OpenAPS. Welcome to OpenAPS’s documentation. https://openaps.readthedocs.io/. Accessed October 24, 2019.
- 3. Loop. Welcome to Loop. https://www.loopdocs.org/. Accessed October 24, 2019.
- 4. AndroidAPS. AndroidAPS documentation. https://androidaps.readthedocs.io/. Accessed October 24, 2019.
- 5. Crabtree TSJ, McLay A, Wilmot EG. DIY artificial pancreas systems: here to stay? Prac Diabetes. 2019;36:63-68. [Google Scholar]
- 6. Crocket H. Peer mentoring in the do-it-yourself artificial pancreas system community [published online ahead of print October 24, 2019]. J Diabetes Sci Technol. doi: 10.1177/1932296819883876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Litchman ML, Walker HR, Ng AH, et al. State of the science: a scoping review and gap analysis of diabetes online communities. J Diabetes Sci Technol. 2019;13:466-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Barnard KD, Breton MD. Diabetes technological revolution: winners and losers? J Diabetes Sci Technol. 2018; 12:1227-1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Petruzelkova L, Soupal J, Plasova V, et al. Excellent glycemic control maintained by open-source hybrid closed-loop AndroidAPS during and after sustained physical activity. Diabetes Technol Ther. 2018;20:744-750. [DOI] [PubMed] [Google Scholar]
- 10. Choi SB, Hong ES, Noh YH. Open artificial pancreas system reduced hypoglycemia and improved glycemic control in patients with type 1 diabetes. Diabetes. 2018;67:964. [Google Scholar]
- 11. Litchman ML, Lewis D, Kelly LA, Gee PM. Twitter analysis of #OpenAPS DIY artificial pancreas technology use suggests improved A1C and quality of life. J Diabetes Sci Technol. 2019;13:164-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goodwin G, Waldman G, Lyons J, Oladunjoy A, Steil G. OR14-5 Challenges in implementing hybrid closed loop insulin pump therapy (Medtronic 670G) in a ‘real world’ clinical setting. Journal of the Endocrine Society. 2019;3 10.1210/js.2019-OR14-5. [DOI] [Google Scholar]
- 13. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)-A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. U. S. Food and Drug Administration (May 17, 2019). FDA Warns People with Diabetes and Health Care Providers Against the Use of Devices for Diabetes Management Not Authorized for Sale in the United States: FDA Safety Communication [Press Release]; 2019. https://www.fda.gov/medical-devices/safety-communications/fda-warns-people-diabetes-and-health-care-providers-against-use-devices-diabetes-management-not.
- 16. OpenAPS. For clinicians – a general introduction and guide to OpenAPS. http://openaps.readthedocs.io/en/latest/docs/Resources/clinician-guide-to-OpenAPS.html. Accessed October 25, 2019.
- 17. AndroidAPS. For clinicians – a general introduction and guide to AndroidAPS. http://androidaps.readthedocs.io/en/latest/EN/Resources/clinician-guide-to-AndroidAPS.html. Accessed October 25, 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Appendix_1_-_Patient_and_Provider_Survey_Text for Using a Do-It-Yourself Artificial Pancreas: Perspectives from Patients and Diabetes Providers by Walter Palmer, Siri Atma W. Greeley, Lisa R. Letourneau-Freiberg and Rochelle N. Naylor in Journal of Diabetes Science and Technology