Abstract
Background:
MiaoMiao (MM) is a Bluetooth transmitter, which when paired with a smart phone/device, converts the Abbott FreeStyle Libre flash glucose monitoring system into a Do-It-Yourself (DIY) continuous glucose monitor (CGM). Families are increasingly adopting DIY CGM solutions, but little is known about parent and child experiences with these add-on technologies. We aimed to explore experiences of families using MM-CGM including challenges faced and their advice to others who may choose to use the technology.
Methods:
Between May and July 2019, we conducted 12 semistructured interviews (in person or via video conference) with parents of children (aged ≤16 years) with type 1 diabetes using MM-CGM. Interviews were audio recorded; professionally transcribed and key themes were identified through thematic analysis.
Results:
Overall, parents used MM-CGM to proactively manage their child’s blood glucose. In all participants, this led to a perceived decrease in frequency of hypoglycemia. Participants reported that the visibility and easy access to blood glucose readings, glucose trends, and customized alarms on parent’s phones decreased their disease burden and improved their sleep quality. Common barriers to using MM-CGM included difficulty of the setting up process, connectivity issues, and lack of support from medical teams.
Conclusion:
This study highlights the potential feasibility of using a DIY CGM system like MM-CGM, which could be an empowering and cost-effective tool for enabling remote monitoring of blood glucose in real time.
Keywords: continuous glucose monitoring, Do-It-Yourself, glycemic control, sleep quality, type 1 diabetes mellitus
Introduction
Management of childhood type 1 diabetes mellitus (T1DM) incurs significant treatment burden for both patients and their caregivers.1 While intensive management reduces long-term complication risk,2,3 in the diabetes control and complications trial (DCCT), it resulted in a threefold increase in severe hypoglycemia, with much of this occurring during sleep.4 However, the incidence of severe hypoglycemia has decreased over the last two decades with implementation of the results of the DCCT by diabetes teams, insulin analog therapy, and the use of advanced technology.5 Complications of severe hypoglycemia include seizures, coma, or rarely death.6 Fear of severe hypoglycemia is common among parents of children with T1DM, with resultant suboptimal glycemic control.5-9
Glucose monitoring is a vital part of both hypoglycemia prevention and intensive diabetes management, and is often the responsibility of the caregiver for children and youth with T1DM, particularly overnight.10 Each additional capillary glucose measurement up to six daily is associated with a clinically significant reduction of 5 mmol/mol in HbA1c.11,12 Continuous glucose monitoring (CGM) systems that measure interstitial glucose levels every five minutes may offer a range of advantages over traditional capillary glucose monitoring. These systems provide additional information on glucose trends and reduce the need for frequent finger pricks. In addition, with the ability to set glucose threshold alarms (both for hypo- and hyperglycemia), the requirement for overnight testing may also be reduced.13 Frequent use of CGM translates to improved glycemic control including reduced hypoglycemia.14 Flash glucose monitoring (FGM), also known as intermittently scanned CGM, measures interstitial glucose and is an alternative CGM device. FGM has the advantage of not requiring capillary glucose testing for calibration; but although it continuously measures interstitial glucose, these readings are only available when the user swipes their reader across the sensor. Therefore, the currently available models are unable to utilize glucose threshold alarms or remote monitoring. Despite these limitations, FGM is now used by 1.5 million people worldwide15 with its relative popularity arguably attributable to its lower price (with FGM costing approximately $US1600/year vs CGM at $US3200-6400/year). Cost is an important barrier to access/uptake, and while FGM and CGM are funded in some countries, both remain unfunded in New Zealand.
A third-party device (MiaoMiao, MM) has recently entered the market.16 In combination with FGM, it has the potential to offer many of the benefits of commercial CGM at a fraction of the price (MM can be purchased online for $US139 as a one-off cost). MiaoMiao, along with other similar devices, converts FGM into a Do-It-Yourself continuous glucose monitor (MM-CGM). MiaoMiao is placed over the standard FGM sensor, uses near-field communication to read raw data from the FGM sensor, and then transmits this via Bluetooth to a paired smart device, bypassing the official algorithm. The raw data are then processed by an algorithm in a nonofficial CGM app: either a proprietary third-party CGM app, Glimp17 or Tomato,18 or an open source CGM app developed by the #WeAreNotWaiting movement, Spike, or xDrip+.16,19 #WeAreNotWaiting is a patient-led diabetes technology innovation and advocacy movement that developed in response to the slow rate of development of patient-centered digital diabetes technologies.20 As DIY projects, users are responsible for setting up their own system with limited online support provided by the wider #WeAreNotWaiting community. Whereas FGM is factory calibrated and unable to receive finger prick calibrations, finger prick calibration is a requirement of these systems, which is anecdotally a positive feature.
Patients and their families are adopting MM-CGM as an affordable CGM solution. However, this product/system has no regulatory approval or safety data and the literature on the patient/family experience in using a MM-CGM system is lacking. To date, there has been no study investigating the experience of those families who were early adopters of using this technology, usually without any recommendation from their medical team. Therefore, we aimed to explore experiences of families using MM-CGM, including reasons for choosing MM-CGM over other glucose monitoring approaches, the broad impacts this technology has on children and adolescents with T1DM and their families, challenges while using this technology, and families’ recommendations to others who may choose to use DIY CGM solutions.
Methods
Recruitment
The study was conducted from May to July 2019. This study was approved by the University of Otago Human Ethics Committee (reference number: H19/002). The inclusion criteria included having a child ≤16 years diagnosed with T1DM, with current or recent use of MiaoMaio (MiaoMiao Smart Reader, BL512, Shanghai High Brilliant Health Technology Co. Ltd., China), English fluency, and willingness to participate with no other restriction. Participants were recruited using two approaches, initially from Southern District Health Board diabetes clinics (n = 4) and then advertisements posted to New Zealand T1DM Facebook groups (n = 8). This enabled recruitment of those who were broadly representative of parents who have chosen to use MM-CGM in New Zealand.
Study Design
Twelve semistructured interviews were conducted with the parents. The final sample size was determined when data saturation was reached, in that, for interviews 10 to 12, no new information or perspectives regarding the main themes became apparent. Before conducting the study, an interview guide was developed based on a literature review and consensus between investigators with expertise in child health, pediatric endocrinology, and qualitative research.
Data Collection and Analysis
All participants completed a demographic questionnaire to collect basic demographic criteria including age, gender, ethnicity, parent’s and child’s age, and duration of MM-CGM use. Other clinical information such as recent HbA1c, duration of diabetes, and method of insulin administration was collected from the primary diabetes care physician after getting parental consent. The Diabetes Treatment Satisfaction Questionnaires (DTSQs)21 were also used for better understanding and quantitative evaluation of the level of satisfaction these participants experience. The DTSQs were analyzed according to the scoring analysis instructions from the health psychology research unit.21 One of the research team investigators (SB or ME) carried out the interviews, with (BW, SB, and ME) all involved in interview 1.
The interviews were conducted face to face (n = 3) or via Zoom (Zoom Video communications, San Jose, California, United States; n = 9) in accordance with participants’ preference. Interviews lasted 50 to 75 minutes and were digitally recorded. Recordings were transcribed verbatim, then checked for their accuracy and de-identified.
The transcripts were coded thematically22 using a framework organized by the study objectives (ie, exploring parents’ perceptions of wanting a CGM system, learning about MM-CGM, setting up MM-CGM, and impacts and experiences after using the system) in NVivo software (NVivo 12, QRS International Pty Ltd., VIC, Australia). All transcripts were independently coded according to the framework by two investigators (ME and SB). Following this, members of the research team (ME, SB, HC, and BW) discussed codes and themes, achieving consensus that all key experiences had been captured. The frequency for each theme and subtheme was quantified to aid determination of thematic saturation, as well as to demonstrate the common experiences among families.
Results
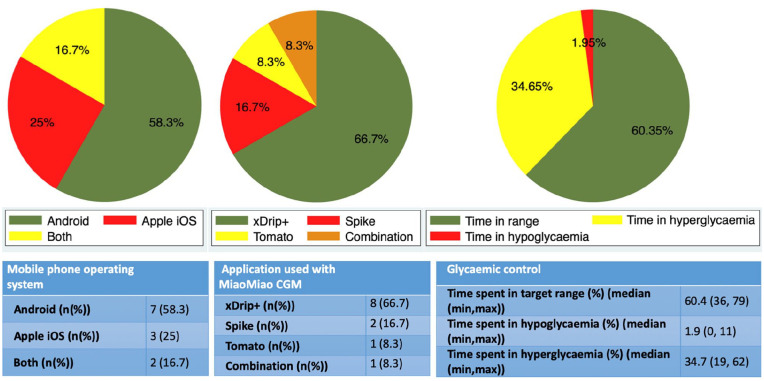
The demographic details of 12 participating families are shown in Table 1. Details of the MM-CGM system, the applications used by participants, and the number of people using follower functions are shown in Table 1 and Figure 1.
Table 1.
Characteristics of the Participants.
| Parents | |
|---|---|
| Sex, female n (%) | 11 (91.7) |
| Age (y), (median (min, max)) | 39 (29, 47) |
| European ethnicity, n (%) | 12 (100) |
| Married n (%)/partnered n (%) | 11 (91.7)/1 (8.3) |
| Education level—tertiary n (%)/high school n (%) | 8 (66.7)/4 (33.3) |
| Working status | |
| Working full-time, n (%) | 4 (33.3) |
| Working part-time, n (%) | 6 (50) |
| Full-time carer, n (%) | 2 (16.7) |
| NZDep13 indexa, median (median (min, max)) | 2 (1, 8) |
| Children | |
| Age (y), (median (min, max)) | 6.65 (1.2, 14) |
| Sex, female n (%) | 8 (66.7) |
| Diabetes/MM | |
| Duration of diabetes (months) (median (min, max)) | 24.5 (3, 144) |
| Insulin pumpb n (%)/MDI n (%) | 9 (75)/3 (25) |
| Duration of using MM-CGM (months), (median (min, max)) | 11.5 (1.5, 18) |
| Applicationc used for remote following, one application n (%)/Combination n (%) | 5 (41.7)/7 (58.3) |
| Number of people following, (median (min, max)) | 2 (1, 5) |
| Using smart watch, n (%) | 4 (33.3) |
| HbA1cd (mmol/mol), (median (min, max)) | 55 (40, 65) |
| DTSQs | |
| Diabetes treatment satisfactione (median (min, max)) | 45 (39, 51) |
Abbreviations: DTSQs, Diabetes Treatment Satisfaction Questionnaires; MDI, multiple daily injections; MM, MiaoMiao; MM-CGM, MiaoMiao continuous glucose monitor.
NZDep13 index is a deprivation index based on household address with one being the least deprived and ten being the most deprived.
No artificial pancreas/closed loop users.
Applications used for the “follower” function; this included Nightscout, Nightguard, xDrip+, and Tomato applications.
HbA1c is the most recent haemoglobin A1c obtained from the participants’ medical team after getting informed consent.
DTSQs treatment satisfaction maximum and minimum scores are 60 and 0, respectively; status version for parents was used.
Figure 1.
Glycemic control, used applications, and type of mobile phone used by participants.
Self-reported used mobile device to pair with MiaoMiao continuous glucose monitor (left), used application to remotely follow blood glucose of the child (center), and time spent in target glucose range as reported by the application paired with MiaoMiao continuous glucose monitor over previous two weeks (right).
Overall, participants described their experience of using MM-CGM as positive, expressing general satisfaction with the device. This was confirmed by the results of DTSQs (Table 1). All participants noted positive aspects of remote monitoring and safety alarms with predictive trend arrows and graphs and associated increased awareness of hypo/hyperglycemia. Results are presented in relation to the five key study objectives (detailed in Tables 2 and 3).
Table 2.
Reasons for Choosing MiaoMiao Continuous Glucose Monitor and the Setting Up Process: Representative Quotes.
| Themes/subtheme (frequency)a | Quotes (participant’s description) |
|---|---|
| Reasons for choosing MM-CGM | |
| Affordability (9/12) | (1) “That’s why we came to the MiaoMiao because it was the cost
effectiveness of it and it still gave you the same sort of
things as a Dexcom would.” Participant 07 (2) “Ah, because we couldn’t afford the other one. It’s like Dexcom or something but we couldn’t afford that.” Participant 12 |
| Size of MM-CGM (7/12) | (3) “Blucon, we sort of looked into that but it didn’t float my
boat because it sat too high on top of the Libre. . ..we wanted
something a wee bit more flatter” Participant 02 (4) “the smaller profile of it for him to wear.” Participant 11 |
| Personal experience with other DIY CGM devices (4/12) | (5) “We used that because that came out first, so we used that
first of all but the Blucon was much bigger, it sticks out a lot
further and the Bluetooth connectivity is terrible with it. So,
we were always having to take it off and reset it and put it
back on again” Participant 05 (6) “The inserts for the G4 were beginning to get quite sore for her I think she realized how big the needle was. So we decided to trial the Libre again because we could get MiaoMiao” Participant 02 |
| Guidance from their medical team (3/12) | (7) “xxxxx told us about the various options that were available. He didn’t push us either way at all. He just gave us the information and said ‘You might want to have a look at this’. This is another option.” Participant 04 |
| Popularity of MM (2/12) | (8) “We were told about a number of Facebook pages and its
seemed that MiaoMiao was the most common.” Participant
01 (9) “I spent a long time asking questions on Facebook or trying to follow peoples thinking, everyone loves this MiaoMiao, I was pretty sure we wanted to buy one.” Participant 03 |
| Knowing someone already using MM-CGM (2/12) | (10) “We also had a friend whose daughter has got Type 1 and they had bought the MiaoMiao so we saw it in action.” Participant 01 |
| Initiation, setup process, and other negative issues | |
| Online resources (9/12) | (11) “Actually the developers of the programs are actually on
these Facebook groups so they can talk you through. Quite often
they’ll actually have frequently asked questions in the top of
the page that you can go read. . .” Participant
07 (12) “But in saying that, when I got to having done a whole lot of this reading I actually went back to the MiaoMiao website because someone pointed me back to that and if I’d just gone there straight away it would have been really simple. So there is some good information on the MiaoMiao website about setting it up.” Participant 01 |
| Getting help from IT experts (5/12) | (13) “I did ask my brother in law, he is a computer programmer”
Participant 05 (14) “I got my friend involved and she helped me set up with Nightscout to get it all set up. That’s her job. She codes” Participant 08 |
| Difficulty with setup process (10/12) | (15) “I am relatively simple. I can read the instructions but it
need to be plug and play sort of thing” Participant
01 (16) “Then I said to my husband, this sounds really hard, we have to follow instructions it’s not as user friendly as the fully paid up licensed apps that we are otherwise used to.” Participant 03 (17) “But it’s definitely not that easy. Definitely not easy to set up. If you don’t know technology you’d be absolutely lost.” Participant 09 |
| Suggestions to make the process easier | (18) “Probably having Spike as just an app. . ., Yeah just
having it as an app available that you can just download.”
Participant 08 (19) “if they had a video just showing someone doing it.” Participant 10 |
| Lag period between MM-CGM reading and finger pricking value (7/12) | (20) “It’s got this really big lag and sometimes it gets really
stuck.” Participant 03 (21) “It might be 5 to 10 minutes the lag time so that’s if the blood glucose is changing really rapidly the interstitial glucose will be a little bit behind. It normally takes five to 10 minutes for it to catch up.” Participant 05 (22) “There’s a time lapse between the two, the blood ones that now reading and the Libre, MiaoMiao is more like 15 minutes ago reading so as I said it depends on how fast her blood sugars are changing.” Participant 06 |
| Temporary losing connection (9/12) | (23) “So if she’s out of Wi-Fi range then you can’t follow.”
Participant 01 (24) “Sometimes we just find it’s disconnected, and we don’t really know why. I should not blame the MiaoMiao. When I am talking about the MiaoMiao, I do not necessarily mean the little product you buy from China. I mean, the whole setup of Nightscout and Spike, and I don’t necessarily know exactly where the problem is, or what bit to blame or what bit to fix” Participant 03 (25) “When she’s playing sport and her phone is quite far away obviously it’s not communicating the blood sugars in those situations. Maybe 40 minutes a day we might not be receiving the data.” Participant 06 |
Abbreviations: DIY CGM, Do-It-Yourself continuous glucose monitor; MM, MiaoMiao; MM-CGM, MiaoMiao continuous glucose monitor.
Number of participants describing each theme/subtheme.
Table 3.
Glycemic Control, Positive Impacts, and Recommendations: Representative Quotes.
| Themes/subtheme (frequency)a | Quotes (participant’s description) |
|---|---|
| Glycemic control and growing confidence with MM-CGM | |
| Decreased frequency of hypoglycemia (12/12) | (26) “Technically he doesn’t really get lows anymore because we
can actually jump on and say, Hey you’re going low. Have some
carbohydrate.” Participant 07 (27) “He still does have his lows, but we catch them earlier so he’s not going as low as what he used to.” Participant 09 (28) “I’d say less hypoglycaemia because at night time we would have that alarm to stop it from happening so if she got to a 4 then we could either decide to stay up for a little while.” Participant 10 |
| Depending on MM for making treatment decision only when in range (12/12) | (29) “When she gets to the extremes, we would always do a finger
prick anyway.” Participant 01 (30) “We trust the MiaoMiao a lot more than anything else and so if he’s within a target of 4 to 15, we just use what’s on the MiaoMiao.” Participant 04 (31) “We don’t often act on that number although in saying that our daughter would because she’s a teenager and she’s lazy and she looks at the watch that receives MiaoMiao data and so when she’s at school she doesn’t want to get anything out of her bag, because she would rely on the MiaoMiao for her lunchtime bolus.” Participant 06 (32) “We do usually finger prick if we’re going to do a correction, we’ll finger prick for a high but for a low not so much.” Participant 07 (33) “If she goes under 4 I will still finger prick her” Participant 12 |
| Accuracy and reliability of MM-CGM (9/12) | (34) “To be honest I find the MiaoMiao more accurate than the
scanner (Freestyle Libre) sometimes. The scanner is not very
accurate with anything under say four and a half and anything
over 10.” Participant 02 (35) “I would say it’s like it’s 90% reliable.” Participant 08 (36) “I actually find the MiaoMiao is probably more accurate to the finger prick than the Libre.” Participant 09 |
| Calibration and the decreased burden of finger pricking (12/12) | (37) “So he didn’t have to have as many finger pricks but we
finger pricked every morning so that we could do the
calibration.” Participant 04 (38) “We always calibrate when we start a sensor. We will calibrate if we notice that it has got out, we will do a recalibration.” Participant 05 (39) “Definitely with the MiaoMiao you get a more, because you can calibrate to your app, so you get a more precise reading.” Participant 07 (40) “I would say when we change his sensor we probably do three or four finger pricks and then if we need to check maybe one or two over the next couple of weeks but he’s pretty anti finger pricking so we try and minimise it as much as possible.” Participant 09 |
| Positive impacts, better quality of life, and better sleep quality | |
| Positive impact on child’s life (12/12) | (41) “It gives her a break from the disease without actually
there being any difference in what we’re doing or controlling.
So, that’s been a good benefit. I did not expect. It wasn’t the
primary reason buying it.” Participant 03 (42) “Ability to monitor (child’s name) closely but not be in her world so much and not have her notice and I don’t know if other parents find this but I would like diabetes to play a smaller part in her life because I don’t want her to think of herself as “the diabetic.” Participant 03 (43) “So we didn’t have to irritate her with this constant ‘What’s happening, what’s happening?’ I think it definitely helps stopping them getting worn down by it.” Participant 06 (44) “We can feel quite confident sending him to a play date for an afternoon even for a full day knowing that we can monitor him ourselves from afar.” Participant 07 (45) “She can go on school trips now confidently.” Participant 08 (46) “Whenever he wants he can go to a friend’s place and stay the night and I can monitor him from home.” Participant 09 |
| Impact on quality of life and disease burden in the family (12/12) | (47) “A huge reliance on it now and definitely much less worry
and anxiety because. We’re getting that information and we’re
keeping better blood sugars” Participant 05 (48) “It has definitely. Over-exceeded my expectation to be honest. It has taken a lot of stress out of it a lot of worry a lot of thinking out of it because we can actually get real-time information especially if he is at school and we are at work. it’s definitely helped us lead a more normal life” Participant 07 (49) “90% or 100% of my day revolves around the MiaoMiao.” Participant 08 (50) “MiaoMiao just you know it made my life a lot easier not having to disrupt the balance.” Participant 10 (51) “Well I was able to go to the movies last night and so that’s why my husband downloaded the app, so we swapped it over to his phone and I went to the movies and I didn’t have to stress.” Participant 12 |
| Better sleep quality and night time care (10/12) | (52) “What it enables me to do is when I do wake up, I can just
roll over have a quick look and go back to sleep. So you’re
getting up less, possibly waking more, but that’s just a habit
that you get into I guess and you’re not actually getting up out
of bed.” Participant 02 (53) “We were sleeping better because we had that safety and more comfort” Participant 11 (54) “Since having MiaoMiao she hasn’t been pricked overnight at all. More sleep for both of us.” Participant 12 |
| Peace of mind (5/12) | (55) “Visibility and the alarms, the peace of mind and
reassurance.” Participant 01 (56) “Peace of mind’s a great thing with this with type one, you know, it just gives us so much peace of mind.” Participant 02 (57) “It’s just peace of mind and you know just if anyone, if anyone God forbid had the same issue then yeah Libre and MiaoMiao would be an absolute must.” Participant 09 |
| Continued use of MM-CGM and recommendation for others | |
| Continue using MM-CGM in the future and considering purchasing a second one (7/12) | (58) “We’ve just bought another one. So, that if the one we’ve
got fails. I think we’d be lost now without it.” Participant
01 (59) “I think it is fantastic. Wouldn’t be without it.” Participant 02 (60) “No, I don’t want to change it. I’m pretty sure that Dexcom and all those other ones will still have connectivity issues and things like that but in the meantime it works for us from a financial point of view as well which is important.” Participant 04 (61) “I don’t know of anything else that could replace it at the moment. I wouldn’t stop using it” Participant 08 |
| Changing my mind in the future and using a different device (5/12) | (62) “If the Medtronic system can come up with a similar
solution where it bends out to an app that allows us to see
what’s going on, I would consider changing to that even though
it’s more expensive you still get the advantage of the glucose
suspend functions.” Participant 01 (63) “Changing pump technology or if the Libre just developed its own transmit function.” Participant 03 (64) “it will just depend on what the news around the Dexcom G6 at the time it gets released because there’s no point spending money on a new MiaoMiao if we’re going to change to the Dexcom a few months later.” Participant 11 |
| Recommending MM-CGM for other families (12/12) | (65) “I would recommend it but I would always say to them that I
would explain that the setup that is required and I would
explain that it’s not just a product that comes from a company
that you can then call for backup.” Participant
05 (66) “Definitely yes, also well being funded would be awesome and also having them being brought in by a New Zealand company and sold by a New Zealand company so there’s customer service in New Zealand and if there’s anything that goes wrong with it you don’t need to contact overseas, we’ve got a New Zealand contact for it.” Participant 07 (67) “Definitely because it’s just like buying peace of mind. I would recommend it for sure.” Participant 09 |
Abbreviations: MM, MiaoMiao; MM-CGM, MiaoMiao continuous glucose monitor.
Number of participants describing each theme/subtheme.
Reasons for Choosing MM-CGM
Families relied on Facebook groups (12/12) and wider internet searches to look for full CGM alternatives to FGM. Two families had tried commercial CGM, but for most families, the price of commercial CGM was reported as prohibitive (9/12). During these internet searches, families reported that MM was the most frequently recommended device for building DIY CGM. Beyond price, key reasons reported for choosing MM-CGM included its perceived reasonable size (7/12) as compared to other DIY CGM and Dexcom G4 CGM, with some families (4/12) reporting the previous negative experiences with other CGM systems, relating to bulky size, poor connectivity (quote 5), or painful insertion (quote 6).
MiaoMiao Continuous Glucose Monitor Initiation, Setting Up Process, and Other Negative Issues
The majority of participants (10/12) found the initial setup process challenging. The most difficult challenges were related to setting up Nightscout and sharing CGM data between iOS and android devices. iOS users (n = 5) reported difficulties with the open source CGM app, Spike. While the majority of parents (10/12) were able to set up the system using online resources and support via Facebook groups, two parents sought technical assistance from someone other than their own partner (quotes 13 and 14). Participants expressed a desire for a “plug and play” alternative to the multistep process that was required (quote 15).
Some families (n = 3) reported that the transmission of the data from the primary collecting device to follower (caregivers) devices was more stable when connected on Wi-Fi as compared to connection on mobile data. The majority (n = 9) noticed temporary signal loss when the primary collecting device was not in the Bluetooth range of the MM (quotes 23, 24, and 25). Many (7/12) noted a lag period between MM CGM readings and actual capillary blood glucose levels (quotes 20, 21, and 22). In addition, five participants (5/12) reported that the first MM transmitter they used was faulty or cracked and they had to replace it. None of the families currently used the adhesive stickers that were enclosed in the MM package directly applied to the skin as they were noted to cause skin reactions. They reported only using these stickers between MM and FGM sensors. Most of them (11/12) used additional adhesive sport tapes.
Glycemic Control and Growing Confidence With MM-CGM
All families depended on MM-CGM to inform proactive use of corrective insulin dosing or carbohydrate consumption to minimize out-of-target glucose levels. They found having these data available on their phones in real time to be more useful and informative than glucose readings obtained from finger pricking or FGM scanning. They reported more stable blood glucose levels which were reflected by increased self-reported time spent in range (Figure 1) and decreased self-reported frequency of hypoglycemia (12/12). The system was also reported as facilitating learning about diabetes, with families reporting growing capability in making treatment decisions in response to glycemic fluctuations and trend arrow readings. Most families used “low” alerts/alarms (set between 3.8 and 6.0 mmol/L) and “high” alerts (set between 8 and 24.0 mmol/L). In all cases, these low and high alerts/alarms were reported as being set by families without input from their diabetes teams. Furthermore, none of our participants reported receiving any guidance from their medical teams regarding safety alerts nor the frequency of calibration in order to get accurate results from the system. Therefore, while all participants calibrated, calibration frequency ranged from five times a day as mentioned by one family to only two times per week.
Nearly all parents (11/12) primarily used MM-CGM adjunctively with confirmatory capillary finger pricks when managing hypo/hyperglycemia. One teenage girl was reported to only use MM-CGM data (no adjunctive capillary input) to make treatment decisions at school (quote 31). Nine families described MM-CGM to be accurate and reliable, some reported that the device was more accurate than the FGM (n = 5). However, one family reported trusting FGM more.
Positive Impacts, Improved Quality of Life, and Better Sleep Quality
All of our participants reported a better quality of life after using the device with reduced parental stress and anxiety. Some allowed their children to have more social activities, such as being out with their friends and having a sleepover, as remote monitoring alleviated their previous concerns relating to hypoglycemia. Additionally, MM-CGM was noted to contribute to improved parental sleep quality/quantity (n = 10). This was due to decreased frequency and necessity of nocturnal blood glucose monitoring. Nearly all the parents noticed that the burden of multiple waking every night to check glucose levels, which was reported as ranging from two to five times per night, was dramatically reduced using MM-CGM. However, MM-CGM did not eliminate all T1DM-related sleep disruption. One family had decided to use the system only during the night to utilize out-of-range alerts. Another family reported that their adolescent decided to switch off the alarms at school because the child did not want to draw attention and the parents did not follow the blood glucose levels during school time.
Continued Use of MM-CGM and Recommendations for Others
All of our participants (12/12) planned to continue to use MM-CGM and recommended it to other families because of its affordability and functionality as a reliable remote CGM. A perceived benefit regarding the rechargeable long-life battery of the device was reported by the majority of participating families (11/12), which can last two to four weeks. The only ongoing cost was the cost of the FGM sensors. Some (3/12) suggested others would be able to set up the system provided they followed setup instructions in a logical manner. The remainder suggested that the difficulty of the setup process was a barrier to potential users. Parents hoped that commercial CGM devices would get funded by the government. Some (3/12) would prefer to be able to purchase the MM transmitter from a New Zealand-based company, so they would get after-sale customer service. Also, some specifically wished for more involvement and guidance from their medical teams (n = 5), both in setup and reviewing data and safety alerts; none of our participants reported any guidance from their medical teams before purchase. Two families reported that their medical teams were not familiar with MM-CGM.
Discussion
To our knowledge, this is the first study to explore any type of DIY CGM. The main findings highlight the generally positive self-reported experiences of parents of children with T1DM using MM-CGM, including reductions in fear of hypoglycemia, improvements in parental and child sleep quality, glycemic understanding and control, and decreased overall disease burden. Negative aspects were also reported, including the burden of setup, signal loss, lack of after-purchase customer service, and their wish to have more support and involvement from diabetes health professionals. Clearly, while MM-CGM holds promise, there remains a need for improvement and further research.
Importantly, a reduction in overall frequency of hypoglycemia and fear of hypoglycemia was reported by all families. These benefits regarding hypoglycemia translated for many families into improved peace of mind and reduced anxiety, especially at night. While this needs confirmation from well-designed trials, it is notable that the i-HART CGM trial reported potential benefits of real-time CGM over FGM in reducing the time spent in hypoglycemia and in improving fear of hypoglycemia.23 Several parents reported better sleep quality/quantity while using MM-CGM. This is supported by the general CGM literature.24,25 Similarly, our results suggest that improved parental sleep may also improve sleep quality for children.
In addition to benefits around hypoglycemia, as with the previous CGM studies,26-28 these participants reported better glycemic control. The benefits of remote monitoring of glucose levels were frequently mentioned and contributed to a parental feeling of improved safety. The literature on CGM is mixed, with recent data supporting improvements in parental psychological measures including fear of hypoglycemia,13 although negative aspects have also been reported, including heightened worry and anxiety as a result of increased awareness of their child’s glucose measurements,25 which was reported by one family in our study. The ability to take timely action on receiving a preset alarm seemed to provide reassurance to our participants.
Being a DIY diabetes technology, there were barriers facing users of MM-CGM, especially during the initial setup which was described as a multistep, complex process. Although the MM hardware came fully built, setup of software on collecting and follower devices was undertaken by users without input from their medical team or the manufacturer. Instead, online resources and peer support from others within the DIY diabetes community facilitated the setting up process and ongoing use. Furthermore, the participants reported neither receiving support from their medical team regarding setting alarms for glycemic excursions nor receiving education on best calibration practices. Both alarm settings and calibration practices varied significantly among families. Advice from clinicians regarding alarms and calibration practices may assist future users to minimize alarm fatigue29 and maximize the accuracy of their CGM.30
Although the variation in calibration practices may appear concerning, nearly all of our participants (apart from one teenager) reported using MM-CGM as an adjunctive device for out-of-range readings and regularly performed confirmatory capillary glucose tests. Similarly, the connectivity issues which participants experienced were partially mitigated by the open-source CGM apps being designed to cope with failure, with their ability to set alarms for signal loss. Parents trusted the device and the open-source software that they used alongside the device. This brings into light the importance of examining healthcare professionals’ perceptions of this new open resource “nonapproved” CGM technology and their views for best practice.31
Generalizing the results of our study to other families considering DIY CGM remains uncertain. These participants were highly motivated and most of them were highly educated; they were also from a less deprived socioeconomic position and all were of New Zealand European ethnicity. In addition, as with other CGM data, overall parental technology satisfaction (as measured by DTSQs) was also seen with the use of MM-CGM,32-35 although DTSQs prior to use were not measured for comparison. This could be another limitation point as well as the self-reported improvement in the glycemic control. The findings of this study represent parental self-reported experience after using MM-CGM and clearly more objective trial data are required. Strengths of this study include the in-depth examination of the parental experience of using MM-CGM. Though the interview guide was predefined, the interviewers could explore new insights as they arose, aiding our understanding of parental experiences. Although the sample size was small, no new significant insights emerged during the final three interviews, suggesting that the key experiences of caregivers using the system were identified.
Conclusion
In conclusion, this study confirms that families impacted by T1DM are using DIY CGM systems, and in this sample reported generally positive experiences, and recommended these systems to other families. The use of DIY CGM is primarily driven by the affordability of the system compared to commercial CGM. Important benefits appear to cluster around perceptions of improved safety, quality of life, reduced parental anxiety, improved night time diabetes care, and importantly decreased frequency of severe hypoglycemia. Negatives with this DIY CGM solution do exist and awareness of these are important for future users and diabetes health professionals so all are fully informed before beginning MM-CGM use. These findings highlight the potential feasibility of using a DIY CGM system; but clearly before healthcare professionals can readily recommend these systems, further research building on the findings of this study is needed to confirm the efficacy, safety, and reliability of DIY CGM systems.
Acknowledgments
The authors wish to thank the participants for their generous involvement in this study.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was funded by the Department of Women’s and Children’s Health, Dunedin School of Medicine, University of Otago, New Zealand.
ORCID iDs: Mona Elbalshy  https://orcid.org/0000-0002-7772-7756
https://orcid.org/0000-0002-7772-7756
Sara Boucher  https://orcid.org/0000-0002-8358-881X
https://orcid.org/0000-0002-8358-881X
Martin I. de Bock  https://orcid.org/0000-0003-0454-6679
https://orcid.org/0000-0003-0454-6679
References
- 1. Macaulay GC, Boucher SE, Yogarajah A, Galland BC, Wheeler BJ. Sleep and night-time caregiving in parents of children and adolescents with type 1 diabetes mellitus–a qualitative study [published online ahead of print 1 August 2019]. Behav Sleep Med. 2019:1-15. 10.1080/15402002.2019.1647207 [DOI] [PubMed] [Google Scholar]
- 2. The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977-986. [DOI] [PubMed] [Google Scholar]
- 3. Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353(25):2643-2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. The Diabetes Control and Complications Trial Research Group. Epidemiology of severe hypoglycemia in the diabetes control and complications trial. The DCCT research group. Am J Med. 1991;90(4):450-459. [PubMed] [Google Scholar]
- 5. Abraham MB, Jones TW, Naranjo D, et al. ISPAD clinical practice consensus guidelines 2018: assessment and management of hypoglycemia in children and adolescents with diabetes. Pediatr Diabetes. 2018;19 Suppl 27:178-192. [DOI] [PubMed] [Google Scholar]
- 6. Kalra S, Mukherjee JJ, Venkataraman S, et al. Hypoglycemia: the neglected complication. Indian J Endocrinol Metab. 2013;17(5):819-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Herbert LJ, Monaghan M, Cogen F, Streisand R. The impact of parents’ sleep quality and hypoglycemia worry on diabetes self-efficacy. Behav Sleep Med. 2015;13(4):308-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Leiter LA, Yale J, Chiasson J, Harris S, Kleinstiver P, Sauriol L. Assessment of the impact of fear of hypoglycemic episodes on glycemic and hypoglycemia management. Can J Diabetes. 2005;29(3):186-192. [Google Scholar]
- 9. Monaghan MC, Hilliard ME, Cogen FR, Streisand R. Nighttime caregiving behaviors among parents of young children with type 1 diabetes: associations with illness characteristics and parent functioning. Fam Syst Health. 2009;27(1):28. [DOI] [PubMed] [Google Scholar]
- 10. Feeley CA, Clougherty M, Siminerio L, Charron-Prochownik D, Allende AL, Chasens ER. Sleep in caregivers of children with type 1 diabetes. Diabetes Educ. 2018:45(1):80-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Anderson B, Ho J, Brackett J, Finkelstein D, Laffel L. Parental involvement in diabetes management tasks: relationships to blood glucose monitoring adherence and metabolic control in young adolescents with insulin-dependent diabetes mellitus. J Pediat. 1997;130(2):257-265. [DOI] [PubMed] [Google Scholar]
- 12. Ziegler R, Heidtmann B, Hilgard D, et al. Frequency of SMBG correlates with HbA1c and acute complications in children and adolescents with type 1 diabetes. Pediatr Diabetes. 2011;12(1):11-17. [DOI] [PubMed] [Google Scholar]
- 13. Burckhardt MA, Roberts A, Smith GJ, Abraham MB, Davis EA, Jones TW. The use of continuous glucose monitoring with remote monitoring improves psychosocial measures in parents of children with type 1 diabetes: a randomized crossover trial. Diabetes Care 2018;41(12):2641-2643. [DOI] [PubMed] [Google Scholar]
- 14. Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group, Bode B, Beck RW, et al. Sustained benefit of continuous glucose monitoring on A1C, glucose profiles, and hypoglycemia in adults with type 1 diabetes. Diabetes Care. 2009;32(11):2047-2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Abbott to hike production of lower-cost glucose monitors as diabetes soars. https://www.reuters.com/article/us-abbott-diabetes/abbott-to-hike-production-of-lower-cost-glucose-monitors-as-diabetes-soars-idUSKCN1UB0Z3. Accessed September 11, 2019.
- 16. MiaoMiao, Freestyle Libre Reader. Continuous glucose readings every 5 minutes straight to your device. 2019. https://miaomiao.cool/. Accessed August 26, 2019.
- 17. How to use MiaoMiao with Glimp? https://miaomiao.cool/pages/how-to-use-miaomiao-with-glimp. Accessed September 11, 2019.
- 18. The smarter way to get a CGM for diabetics! http://tomato.cool/. Accessed September 11, 2019.
- 19. Get the most out of your CGM transmitter. 2019. https://spike-app.com/. Accessed August 26, 2019.
- 20. The #Wearenotwaiting diabetes DIY movement. 2019. https://www.healthline.com/health/diabetesmine/innovation/we-are-not-waiting#9. Accessed October 2019.
- 21. Professor Claire Bradley, Licensed by Health Psychology Research Ltd. Diabetes treatment satisfaction questionnaire. 2015. https://www.healthpsychologyresearch.com/find-a-questionnaire?search_api_views_fulltext=DTSQ. Accessed August 26, 2019.
- 22. Daniel BK, Harland T. Higher Education Research Methodology: A Step-by-Step Guide to the Research Process. London, UK: Routledge; 2017. [Google Scholar]
- 23. Reddy M, Jugnee N, El Laboudi A, Spanudakis E, Anantharaja S, Oliver N. A randomized controlled pilot study of continuous glucose monitoring and flash glucose monitoring in people with type 1 diabetes and impaired awareness of hypoglycaemia. Diabet Med. 2018;35(4):483-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lawton J, Blackburn M, Allen J, et al. Patients’ and caregivers’ experiences of using continuous glucose monitoring to support diabetes self-management: qualitative study. BMC Endocr Disord. 2018;18(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pickup JC, Holloway MF, Samsi K. Real-time continuous glucose monitoring in type 1 diabetes: a qualitative framework analysis of patient narratives. Diabetes Care. 2015;38(4):544-550. [DOI] [PubMed] [Google Scholar]
- 26. Battelino T, Conget I, Olsen B, et al. The use and efficacy of continuous glucose monitoring in type 1 diabetes treated with insulin pump therapy: a randomised controlled trial. Diabetologia. 2012;55(12):3155-3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Beck RW, Riddlesworth T, Ruedy K, et al. Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections: the DIAMOND randomized clinical trial. JAMA. 2017;317(4):371-378. [DOI] [PubMed] [Google Scholar]
- 28. Pickup JC, Freeman SC, Sutton AJ. Glycaemic control in type 1 diabetes during real time continuous glucose monitoring compared with self monitoring of blood glucose: meta-analysis of randomised controlled trials using individual patient data. BMJ. 2011;343:d3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shivers JP, Mackowiak L, Anhalt H, Zisser H. “Turn it off!”: diabetes device alarm fatigue considerations for the present and the future. J Diabetes Sci Technol. 2013;7(3):789-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Forlenza GP, Argento NB, Laffel LM. Practical considerations on the use of continuous glucose monitoring in pediatrics and older adults and nonadjunctive use. Diabetes Technol Ther. 2017;19(S3):S13-S20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Marshall DC, Holloway M, Korer M, Woodman J, Brackenridge A, Hussain S. Do-it-yourself artificial pancreas systems in type 1 diabetes: perspectives of two adult users, a caregiver and three physicians. Diabetes Ther. 2019;10:1553-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hussain T, Akle M, Nagelkerke N, Deeb A. Comparative study on treatment satisfaction and health perception in children and adolescents with type 1 diabetes mellitus on multiple daily injection of insulin, insulin pump and sensor-augmented pump therapy [published online ahead of print 23 February 2017]. SAGE Open Med. 2017;5:2050312117694938 10.1177/2050312117694938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tang TS, Digby EM, Wright AM, et al. Real-time continuous glucose monitoring versus internet-based blood glucose monitoring in adults with type 2 diabetes: a study of treatment satisfaction. Diabetes Res Clin Pract 2014;106(3):481-486. [DOI] [PubMed] [Google Scholar]
- 34. Tansey M, Laffel L, Cheng J, et al. Satisfaction with continuous glucose monitoring in adults and youths with type 1 diabetes. Diabet Med. 2011;28(9):1118-1122. [DOI] [PubMed] [Google Scholar]
- 35. Bruttomesso D, Laviola L, Avogaro A, et al. The use of real time continuous glucose monitoring or flash glucose monitoring in the management of diabetes: a consensus view of Italian diabetes experts using the Delphi method. Nutr Metab Cardiovasc Dis. 2019;29(5):421-431. [DOI] [PubMed] [Google Scholar]